Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Baseline Assessments
2.4. PGX Supplementation
2.5. Post-Intervention Assessments
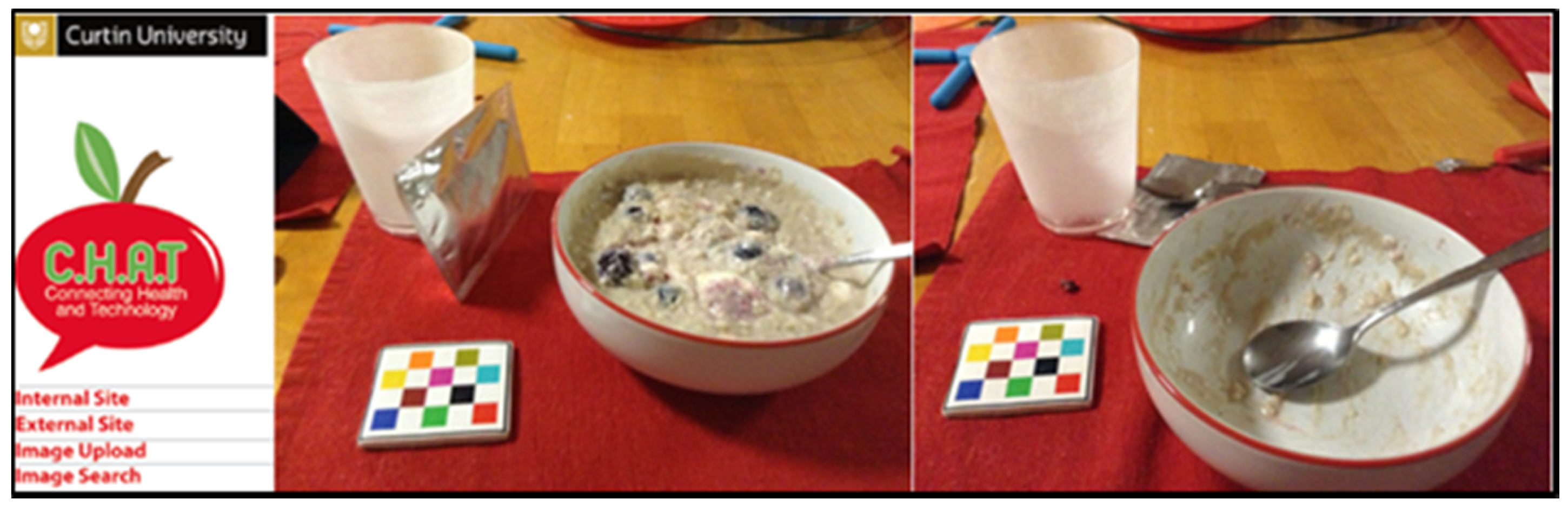
2.6. Dietary Analysis
2.7. Statistical Analysis
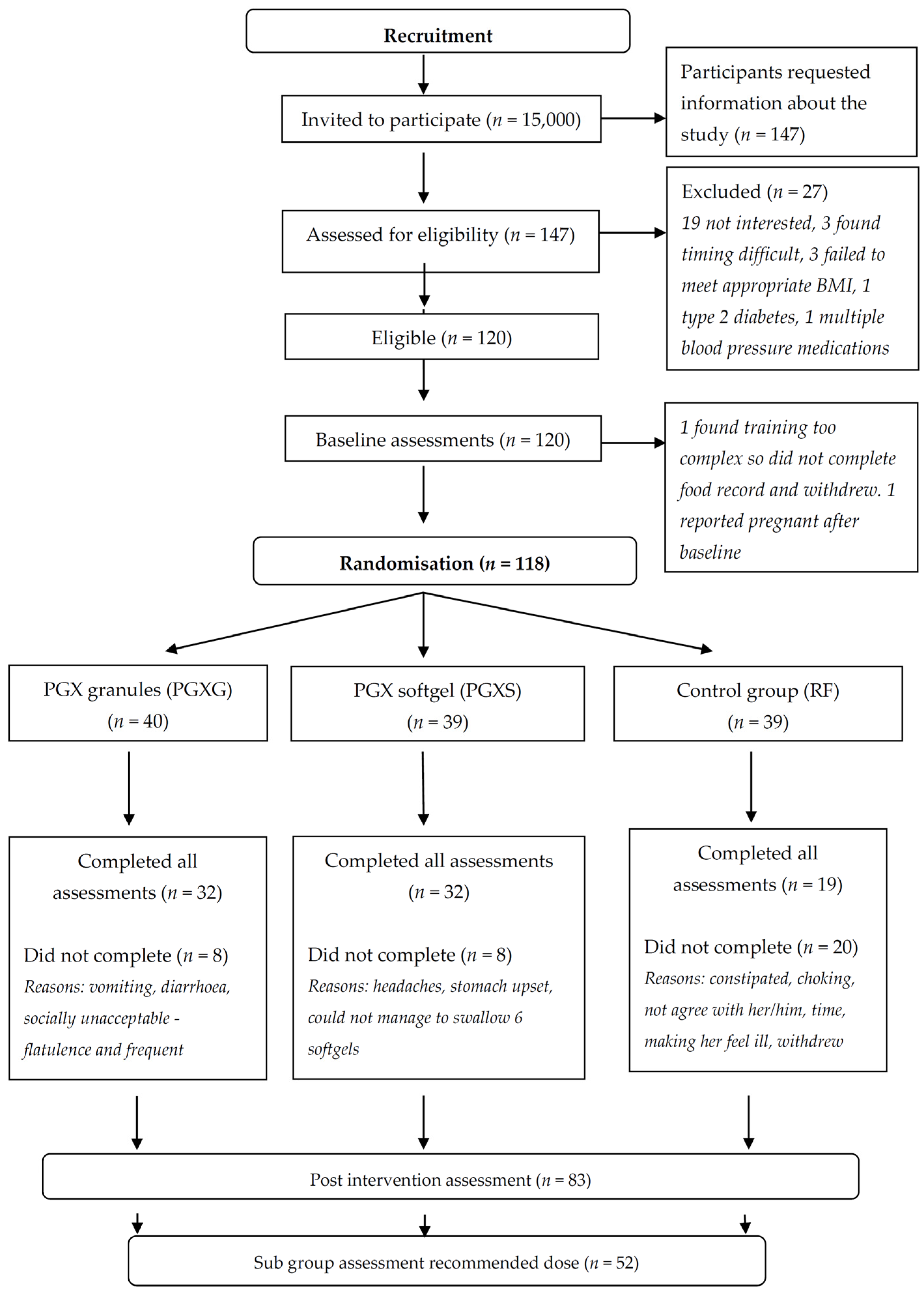
3. Results
3.1. Baseline Characteristics
3.1.1. Per-Protocol Analysis
3.1.2. Subgroup Analysis
3.2. Effect of Intervention on Body Weight and Body Composition
3.2.1. Per-Protocol Analysis
3.2.2. Subgroup Analysis
3.3. Number of Eating Occasions and Food Group Servings
3.3.1. Per-Protocol Analysis
3.3.2. Subgroup Analysis
3.4. Adverse Events
4. Discussion
Limitation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swinburn, B.; Kraak, V.; Rutter, H.; Vandevijvere, S.; Lobstein, T.; Sacks, G.; Gomes, F.; Marsh, T.; Magnusson, R. Strengthening of accountability systems to create healthy food environments and reduce global obesity. Lancet 2015, 385, 2534–2545. [Google Scholar] [CrossRef]
- Liese, A.D.; Krebs-Smith, S.M.; Subar, A.F.; George, S.M.; Harmon, B.E.; Neuhouser, M.L.; Boushey, C.J.; Schap, T.E.; Reedy, J. The Dietary Patterns Methods Project: Synthesis of findings across cohorts and relevance to dietary guidance. J. Nutr. 2015, 145, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Tayyem, R.F.; Bawadi, H.A.; Shehadah, I.; Agraib, L.M.; AbuMweis, S.S.; Al-Jaberi, T.; Al-Nusairr, M.; Bani-Hani, K.E.; Heath, D.D. Dietary patterns and colorectal cancer. Clin. Nutr. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. A place for dietary fiber in the management of the metabolic syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Jensen, M.G. Dietary fibres in the regulation of appetite and food intake. Importance of viscosity. Appetite 2011, 56, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kacinik, V.; Lyon, M.; Purnama, M.; Reimer, R.A.; Gahler, R.; Green, T.J.; Wood, S. Effect of PGX, a novel functional fibre supplement, on subjective ratings of appetite in overweight and obese women consuming a 3-day structured, low-calorie diet. Nutr. Diabetes 2011, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Yamaguchi, H.; Eller, L.K.; Lyon, M.R.; Gahler, R.J.; Kacinik, V.; Juneja, P.; Wood, S. Changes in visceral adiposity and serum cholesterol with a novel viscous polysaccharide in Japanese adults with abdominal obesity. Obesity 2013, 21, E379–E387. [Google Scholar] [PubMed]
- Solah, V.; Brand-Miller, J.; Atkinson, F.; Gahler, R.; Kacinik, V.; Lyon, M.; Wood, S. Dose-response effect of a novel functional fibre, PolyGlycopleX, PGX, on satiety. Appetite 2014, 77, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Solah, V.A.; O’Mara-Wallace, B.; Meng, X.; Gahler, R.J.; Kerr, D.A.; James, A.P.; Fenton, H.K.; Johnson, S.K.; Wood, S. Consumption of the Soluble Dietary Fibre Complex PolyGlycopleX® Reduces Glycaemia and Increases Satiety of a Standard Meal Postprandially. Nutrients 2016, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.; Van Den Borne, J.J.G.C.; De Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristensen, M.; Mars, M.; Schols, H.A.; Feskens, E.J.M. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. 2011, 12, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.; Green, H. Dietary fibre and satiety. Nutr. Bull. 2007, 32, 32–42. [Google Scholar] [CrossRef]
- Mann, J.I.; Cummings, J.H. Possible implications for health of the different definitions of dietary fibre. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary fibre and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C.; Atkinson, F.S.; Gahler, R.J.; Kacinik, V.; Lyon, M.R; Wood, S. Effects of PGX, a novel functional fibre, on acute and delayed postprandial glycaemia. Eur. J. Clin. Nutr. 2010, 64, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C.; Atkinson, F.S.; Gahler, R.J.; Kacinik, V.; Lyon, M.R.; Wood, S. Effects of added PGX®, a novel functional fibre, on the glycaemic index of starchy foods. Br. J. Nutr. 2012, 108, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R. Energy intake and obesity: Ingestive frequency outweighs portion size. Physiol. Behav. 2015, 134, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Aljuraiban, G.S.; Chan, Q.; Griep, L.M.O.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.S.; INTERMAP Research Group. The impact of eating frequency and time of intake on nutrient quality and Body Mass Index: The INTERMAP Study, a Population-Based Study. J. Acad. Nutr. Diet. 2015, 115, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Subar, A.F. Dietary Assessment Methodology. In Nutrition in the Prevention and Treatment of Disease, 3rd ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Meng, X.; Kerr, D.A.; Zhu, K.; Devine, A.; Solah, V.A.; Wright, J.; Binns, C.W.; Prince, R.L. Under-reporting of energy intake in elderly Australian women is associated with a higher body mass index. J. Nutr. Health Aging 2013, 17, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Zhu, F.; Khanna, N.; Boushey, C.J.; Delp, E.J. Combining global and local features for food identification in dietary assessment. IEEE Trans. Image Process. 2011, 2011, 1789–1792. [Google Scholar] [PubMed]
- Harray, A.J.; Boushey, C.J.; Pollard, C.M.; Delp, E.J.; Ahmad, Z.; Dhaliwal, S.S.; Mukhtar, S.A.; Kerr, D.A. A novel dietary assessment method to measure a healthy and sustainable diet using the Mobile Food Record: Protocol and methodology. Nutrients 2015, 7, 5375–5395. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.A.; Pollard, C.M.; Howat, P.; Delp, E.J.; Pickering, M.; Kerr, K.R.; Dhaliwal, S.S.; Pratt, I.S.; Wright, J.; Boushey, C.J. Connecting Health and Technology (CHAT): Protocol of a randomized controlled trial to improve nutrition behaviours using mobile devices and tailored text messaging in young adults. BMC Public Health 2012, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.A.; Harray, A.J.; Pollard, C.M.; Dhaliwal, S.S.; Delp, E.J.; Howat, P.A.; Pickering, M.R.; Ahmad, Z.; Meng, X.; Pratt, I.S.; et al. The connecting health and technology study: A 6-month randomized controlled trial to improve nutrition behaviours using a mobile food record and text messaging support in young adults. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Bosch, M.; Khanna, N.; Boushey, C.J.; Delp, E.J. Multiple hypotheses image segmentation and classification with application to dietary assessment. IEEE J. Biomed. Health Inform. 2015, 19, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Norton, K.; Olds, T. Anthropometrica; University of New South Wales Press: Sydney, Australia, 2000. [Google Scholar]
- National Health and Medical Research Council. Eat for Health. Australian Dietary Guidelines Summary. Canberra: Australian Government, Department of Health and Ageing. 2013. Available online: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/n55a_australian_dietary_guidelines_susumma_131014.pdf (accessed on 2 February 2015). [Google Scholar]
- Lyon, M.; Wood, S.; Pelletier, X.; Donazzolo, Y.; Gahler, R.; Bellisle, F. Effects of a 3-month supplementation with a novel soluble highly viscous polysaccharide on anthropometry and blood lipids in nondieting overweight or obese adults. J. Hum. Nutr. Diet. 2011, 24, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ho, S.; Gahler, R.J.; Wood, S. Effect on body weight and composition in overweight/obese Australian adults over 12 months consumption of two different types of fibre supplementation in a randomized trial. Nutr. Metab. 2016, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Pelletier, X.; Carabin, I.G.; Lyon, M.; Gahler, R.; Parnell, J.A.; Woods, S. Increased plasma PYY levels following supplementation with functional fiber PolyGlycopleX in healthy adults. Eur. J. Clin. Nutr. 2010, 64, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.M.; Pulker, C.; Meng, X.; Denham, F.; Solah, V.; Scott, J.A.; Kerr, D.A. Cereal foods consumption trends and factors associated with changing intake, among Western Australian adults, 1995 to 2012. FASEB J. 2016, 30, 409. [Google Scholar]
- Hill, J.O. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am. J. Clin. Nutr. 2009, 89, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Castellanos, V.H.; Pelkman, C.L.; Thorwart, M.L.; Rolls, B.J. Energy density of foods affects energy intake in normal-weight women. Am. J. Clin. Nutr. 1998, 67, 412–420. [Google Scholar]
- Bell, E.A.; Rolls, B.J. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am. J. Clin. Nutr. 2001, 73, 1010–1018. [Google Scholar] [PubMed]
- Solah, V.A.; Meng, X.; Wood, S.; Gahler, R.J.; Kerr, D.A.; James, A.P.; Pal, S.; Fenton, H.K.; Johnson, S.K. Effect of training on the reliability of satiety evaluation and use of trained panellists to determine the satiety effect of dietary fibre: A randomised controlled trial. PLoS ONE 2015, 10, e012. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.K.; Solah, V.A.; Johnson, S.K.; Meng, X.; Kerr, D.A.; James, A.P.; Fenton, H.K.; Gahler, R.J.; Wood, S. Effects of a viscous-fibre supplemented evening meal and the following un-supplemented breakfast on post-prandial satiety responses in healthy women. Physiol. Behav. 2016, 154, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Castellanos, V.H.; Halford, J.C.; Kilara, A.; Panyam, D.; Pelkman, C.L.; Smith, G.P.; Thorwart, M.L. Volume of food consumed affects satiety in men. Am. J. Clin. Nutr. 1998, 67, 1170–1177. [Google Scholar] [PubMed]
- Rolls, B.J. The relationship between dietary energy density and energy intake. Physiol. Behav. 2009, 97, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ello-Martin, J.A.; Roe, L.S.; Ledikwe, J.H.; Beach, A.M.; Rolls, B.J. Dietary energy density in the treatment of obesity: A year-long trial comparing 2 weight-loss diets. Am. J. Clin. Nutr. 2007, 85, 1465–1477. [Google Scholar] [PubMed]
- Polidori, D.; Sanghvi, A.; Seeley, R.J.; Hall, K.D. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity 2016, 24, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
| Grains | Vegetable | Fruit | Meats | Dairy | Junk Food | Fibre * | ||||||||
| Bread | Porridge cooked | Muesli/Oats raw | Rice Pasta cooked | Beef Lamb Pork | Chicken | Fish | Milk | Cheese | Meat pie | Donut or Cake | PGXS | PGXG | ||
| 40 | 120 | 30 | 75–120 (1/2 cup) | 75 | 150 | 65 | 80 | 100 | 250 mL | 40 | 60 | 40 | 3.8 | 4.4 |
| All Participants | PGXS (n = 40) | PGXG (n = 40) | RF (n = 40) |
|---|---|---|---|
| Men | 9 | 10 | 9 |
| Women | 31 | 30 | 31 |
| Mean ± SD | |||
| Age (years) | 42.2 ± 16.0 | 46.5 ± 14.0 | 43.3 ± 16.8 |
| Height (cm) | 167.4 ± 9.1 | 167.3 ± 9.0 | 166.4 ± 7.9 |
| Weight (kg) | 82.7 ± 16.8 | 80.9 ± 16.6 | 81.3 ± 17.7 |
| Waist (cm) | 89.8 ± 12.8 | 90.7 ± 12.1 | 88.4 ± 14.3 |
| Body Mass Index (BMI, kg/m2) | 29.4 ± 4.8 | 28.7 ± 4.4 | 29.2 ± 4.8 |
| Eating occasions per day | 5.4 ± 2.8 | 6.3 ± 2.0 | 4.8 ± 2.1 |
| Food group servings (mean daily serves ± SD) | |||
| Fruit (150 g) | 0.8 ± 0.8 | 1.0 ± 1.0 | 1.1 ± 1.2 |
| Vegetable (75 g) | 2.4 ± 1.5 | 2.6 ± 1.4 | 2.5 ± 1.1 |
| Grain (cereal) (40 g bread, 75–120 g cooked rice, pasta etc. or 500 kJ) | 3.8 ± 1.8 | 4.3 ± 2.2 | 4 .0 ± 1.5 |
| Dairy (250 mL milk, 40 g cheese or 500–600 kJ) | 1.3 ± 0.9 | 1.6 ± 0.8 | 1.3 ± 0.8 |
| Junk food (60 g meat pie or hot chips, 40 g donut or cake) | 3.5 ± 1.9 | 3.1 ± 1.6 | 3.0 ± 1.7 |
| Meat (65 g meat, 100 g fish or 500–699 kJ) | 1.0 ± 0.7 | 1.6 ± 0.8 | 1.4 ± 0.7 |
| Alcohol (150 mL) | 0.5 ± 0.8 | 0.4 ± 0.5 | 0.1 ± 0.2 |
| All Participants | PGXS (n = 17) | PGXG (n = 18) | RF (n = 17) | p Value |
|---|---|---|---|---|
| Body weight (kg) | 76.5 ± 15.9 | 87.7 ± 20.2 | 78.3 ± 15.0 | 0.62 |
| BMI (kg/m2) | 27.2 ± 4.5 | 28.7 ± 5.2 | 28.3 ± 5.2 | 0.64 |
| Waist (cm) | 84.8 ± 12.2 | 89.2 ± 20.4 | 87.1 ± 13.8 | 0.59 |
| Eating occasions per day | 7.4 ± 2.5 | 6.0 ± 2.0 | 5.5 ± 2.6 | p > 0.05 |
| All Participants | PGXS (n = 32) | PGXG (n = 32) | RF (n = 19) |
|---|---|---|---|
| Body weight (kg) | 0.47 ± 1.85 | −0.49 ± 0.34 | −0.03 ± 0.58 |
| BMI (kg/m2) | 0.15 ± 0.65 | −0.17 ± 0.13 | 0.01 ± 0.20 |
| Waist (cm) | −0.17 ± 2.92 | −2.50 ± 0.60 p = 0.03 | −1.3 ± 1.0 |
| Eating occasions per day | −0.60 ± 1.5 | −0.82 ± 1.28 p = 0.01 | −0.22 ± 1.72 |
| Food group servings (mean daily serves ± SD) | |||
| Fruit (150 g) 1 | −0.2 ± 0.76 | 0.08 ± 0.7 | −0.18 ± 0.75 |
| Vegetable (75 g) 1 | −0.07 ± 1.11 | −0.34 ± 1.22 | −0.23 ± 0.64 |
| Grain (cereal) | 0.21 ± 1.73 | −0.79 ± 1.66 | −0.51 ± 1.23 |
| Dairy | 0.11 ± 0.63 | −0.22 ± 0.64 | 0.07 ± 0.35 |
| Junk food | −0.14 ± 2.00 | −0.57 ± 1.29 | 0.28 ± 2.12 |
| Meat | 0.08 ± 0.59 | 0.01 ± 0.79 | −0.09 ± 0.78 |
| Alcohol | −0.22 ± 0.73 | 0.17 ± 0.99 | −0.02 ± 0.26 |
| Fibre (3.8–4.4 g) 1 | 1.89 ± 0.91 | 2.17 ± 0.71 | 2.35 ± 0.58 |
| All Participants | PGXS (n = 17) | PGXG (n = 18) | RF (n = 17) |
|---|---|---|---|
| Body weight (kg) | 0.22 ± 1.61 | −1.4 ± 0.10 p < 0.01 | −0.03 ± 0.58 |
| BMI (kg/m2) | 0.07 ± 0.59 | −0.5 ± 0.10 p < 0.01 | 0.01 ± 0.20 |
| Waist (cm) | −1.04 ± 2.28 | −1.2 ± 1.00 | −1.3 ± 1.0 |
| Eating occasions per day | −1.3 ± 1.9 | −1.4 ± 1.20 p < 0.01 | −0.22 ± 1.72 |
| Food group servings (mean daily serves ± SD) | |||
| Fruit (150 g) | −0.43 ± 0.59 | −0.63 ± 0.57 p = 0.022 | −0.18 ± 0.75 |
| Vegetable (75 g) | −0.35 ± 0.96 | −0.82 ± 1.31 | −0.23 ± 0.64 |
| Grain (cereal) | −0.93 ± 1.47 | −1.52 ± 1.84 p = 0.019 | −0.51 ± 1.23 |
| Dairy | −0.05 ± 0.56 | −0.59 ± 0.50 p = 0.012 | 0.07 ± 0.35 |
| Junk food | −0.17 ± 1.48 | −0.76 ± 0.85 | 0.28 ± 2.12 |
| Meat | −0.06 ± 0.62 | 0.18 ± 0.90 | −0.09 ± 0.78 |
| Alcohol | −0.50 ± 0.98 | 0.11 ± 0.32 | −0.02 ± 0.26 |
| Fibre supplement serves (serving size 4.5–5 g) | 2.6 ± 0.47 | 2.82 ± 0.24 | 2.35 ± 0.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solah, V.A.; Kerr, D.A.; Hunt, W.J.; Johnson, S.K.; Boushey, C.J.; Delp, E.J.; Meng, X.; Gahler, R.J.; James, A.P.; Mukhtar, A.S.; et al. Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals. Nutrients 2017, 9, 149. https://doi.org/10.3390/nu9020149
Solah VA, Kerr DA, Hunt WJ, Johnson SK, Boushey CJ, Delp EJ, Meng X, Gahler RJ, James AP, Mukhtar AS, et al. Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals. Nutrients. 2017; 9(2):149. https://doi.org/10.3390/nu9020149
Chicago/Turabian StyleSolah, Vicky A., Deborah A. Kerr, Wendy J. Hunt, Stuart K. Johnson, Carol J. Boushey, Edward J. Delp, Xingqiong Meng, Roland J. Gahler, Anthony P. James, Aqif S. Mukhtar, and et al. 2017. "Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals" Nutrients 9, no. 2: 149. https://doi.org/10.3390/nu9020149
APA StyleSolah, V. A., Kerr, D. A., Hunt, W. J., Johnson, S. K., Boushey, C. J., Delp, E. J., Meng, X., Gahler, R. J., James, A. P., Mukhtar, A. S., Fenton, H. K., & Wood, S. (2017). Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals. Nutrients, 9(2), 149. https://doi.org/10.3390/nu9020149





