Abstract
Due to reduced cost and accessibility, the use of genetic testing has appealed to health professionals for personalising nutrition advice. However, translation of the evidence linking polymorphisms, dietary requirements, and pathology risk proves to be challenging for nutrition and dietetic practitioners. Zinc status and polymorphisms of genes coding for zinc-transporters have been associated with chronic diseases. The present study aimed to systematically review the literature to assess whether recommendations for zinc intake could be made according to genotype. Eighteen studies investigating 31 Single Nucleotide Polymorphisms (SNPs) in relation to zinc intake and/or status were identified. Five studies examined type 2 diabetes; zinc intake was found to interact independently with two polymorphisms in the zinc-transporter gene SLC30A8 to affect glucose metabolism indicators. While the outcomes were statistically significant, the small size of the effect and lack of replication raises issues regarding translation into nutrition and dietetic practice. Two studies assessed the relationship of polymorphisms and cognitive performance; seven studies assessed the association between a range of outcomes linked to chronic conditions in aging population; two papers described the analysis of the genetic contribution in determining zinc concentration in human milk; and two papers assessed zinc concentration in plasma without linking to clinical outcomes. The data extracted confirmed a connection between genetics and zinc requirements, although the direction and magnitude of the dietary modification for carriers of specific genotypes could not be defined. This study highlights the need to summarise nutrigenetics studies to enable health professionals to translate scientific evidence into dietary recommendations.
1. Introduction
Genetic background can affect a person’s nutritional status. This may have the potential to modify an individual’s optimal nutrient requirements and risk of developing specific pathologic conditions [1]. Zinc (Zn) is an essential micronutrient that plays fundamental roles in several aspects of physiology, cellular metabolism, and gene expression [2]. In Australia, the current Recommended Dietary Intake (RDI) for Zn is 14 mg/day for men and 8 mg/day for women (19+ years), with an upper limit of 40 mg/day [3]. Similarly, in the US, the current Recommended Dietary Allowance for Zn is 11 mg/day for men and 8 mg/day for women (19+ years) [4]. Zn deficiency is the cause of a complex syndrome [5], more subtle and difficult to assess is marginal Zn deficiency; while currently not well-understood, it may be a contributing factor in chronic disease development [6]. Zn deficiency can arise from several causes including specific genetic background [7]. For example, mutations in the gene coding for the intestinal transporter SLC39A4 cause the inherited disorder Acrodermatitis Enteropathica (AE), a condition caused by the inability to absorb Zn and resulting in systemic deficiency that is resolved with lifelong Zn supplementation (1 mg/kg/day body weight) [8].
Zn crosses biological membranes with the aid of specialized trans-membrane proteins. More than 20 proteins coordinate their activity to maintain systemic and cellular Zn homeostasis; in mammals, ZnT (coded by SLC30A genes) and ZIP (coded by SLC39A genes) were identified for this role. Intracellular Zn concentration is buffered by Metallothioneins (MTs), a class of proteins with high affinity for metals [9]. With the completion of the human genome sequence, all Zn transporter genes were identified and several common polymorphisms and rare mutations were found to be associated with human pathologies [6,10].
Insulin metabolism in the pancreatic β-cells requires Zn [11], and intracellular Zn directly acts on insulin receptors’ intracellular pathways. A Single Nucleotide Polymorphism (SNP) in the gene coding for the Zn transporter SLC30A8 (rs13266634) has been identified as being associated with increasing the risk of developing type 2 diabetes (T2D); as such, modification of Zn requirements was hypothesized as a potential method to reduce the risk of T2D for rs13266634 carriers [12,13]. Another condition that is impacted by genetic polymorphisms in Zn transporters is mammary gland secretion of Zn in milk. Null mutations in genes coding for transporters expressed in mammary epithelium result in milk Zn concentrations that are insufficient to support the growth of babies, ultimately leading to poor infant health outcomes [8,14,15]. Zn status has been also associated with cognitive performance [16]. Genetic variations have been explored in cognitive studies and may also play a role in conditions such as Alzheimer’s Disease (AD) [17], a concept that is yet to be investigated in human studies [18]. Many studies have investigated genetic polymorphisms in regards to gene-nutrient interactions and nutrient status in the onset and progression of specific diseases. However, the question remains; is there sufficient evidence to make specific dietary and nutrition recommendations based on genotype? [19]. The aim of this paper was to systematically review the evidence to answer whether specific polymorphisms modify individual dietary Zn requirements and whether current evidence can inform clinical dietetic practice.
2. Materials and Methods
2.1. Search Methods
A literature search was conducted in October, 2015. The following search strategy was used to search Medline, Embase, and CENTRAL. This strategy was then adapted to search Web of Science, CINAHL and Scopus databases (S1). (See PRISMA checklist (S2)).
- Zinc.ti,ab.
- Zn.ti,ab.
- Zinc/
- Zinc compounds/
- Finger*.ti,ab.
- 1 OR 2 OR 3 OR 4
- 6 NOT 5
- (Polymorph* OR Allel* OR Genet* OR Geno* OR Gene OR Genes).ti,ab.
- Exp Polymorphism, Genetic/
- 8 OR 9
- Exp Cation Transport Proteins/
- (Zinc adj2 deficienc*).ti,ab.
- (Zn adj2 deficienc*).ti,ab.
- (Zinc adj2 transporter*).ti,ab.
- (Zn adj2 transporter*).ti,ab.
- 11 OR 12 OR 13 OR 14 OR 15
- 7 AND 10 AND 16
2.2. Inclusion Criteria
Studies were included if they were conducted in mammals, in particular humans, mice, and rats, if they investigated polymorphisms within the population, if they modulated plasma Zn concentrations or investigated whether this interaction was modulated by Zn status. Study designs that were observational, case-control, and clinical trials were eligible for inclusion in this review.
2.3. Exclusion Criteria
Studies were excluded that were not written in English. Studies were also excluded that were examining type 1 diabetes, conducted before 1995 (when the first gene coding for a Zn transporter was identified [20]), single case studies, book chapters, editorial letters, conference proceedings, and reviews.
2.4. Data Collection and Analysis
2.4.1. Selection of Studies
Three reviewers (KD, MA, and CM) independently screened the titles and abstracts of the studies retrieved by the above search strategy. Full articles were then retrieved and were independently assessed by the same three reviewers for inclusion using the criteria outlined above. Conflicts were discussed and decided upon as a group. Reference lists of relevant studies and reviews were also manually searched.
2.4.2. Data Extraction
An extraction table was developed to include information about study design, association of genetic variants with phenotypic traits, and other relevant outcomes. Three review authors (KD, MA, and CM) independently extracted the data into an extraction table which included zinc status method, genotyping method, polymorphisms identified, association between SNP, zinc status and biomarkers, association between SNP, zinc status, and disease state. The data were then cross-verified by another author (AD). Any discrepancies were resolved through group discussion. Corresponding authors for the papers [21,22,23,24] were contacted with requests for more information; unfortunately, we were unable to obtain answers to the questions we sought.
2.5. Quality Assessment
A suitable, validated quality assessment (QA) tool was not available for the types of studies and outcomes reviewed in this study. Therefore, an original quality assessment tool was created by merging and adapting the American Dietetic Association (ADA) quality criteria checklist for Randomized Control Trials (RCTs) and cohorts [25], the Cochrane risk of bias [26], and the STROBE statement checklist for observational studies [27] (Figure 1). The key domain that was included and considered particularly pertinent for this review was “polymorphisms are with the Hardy-Weinberg equilibrium” [28] and whether the association of the genetic variants with specific phenotypic traits was properly reported. The final checklist included 13 “yes” or “no” statements. Similar to the ADA quality criteria checklist and Cochrane risk of bias tool, each study was assigned as positive, neutral, or negative based on the number of “yes”, “no”, or “not applicable” answers. A study was assigned positive if the majority of statements received “yes”, or were assigned negative if the majority of statements received “no”. If a study had the same number of yes and no statements it was assigned neutral (Figure 1). Two reviewers (KD, MA, and AD) independently performed the quality assessment and any discrepancies were discussed as a group.
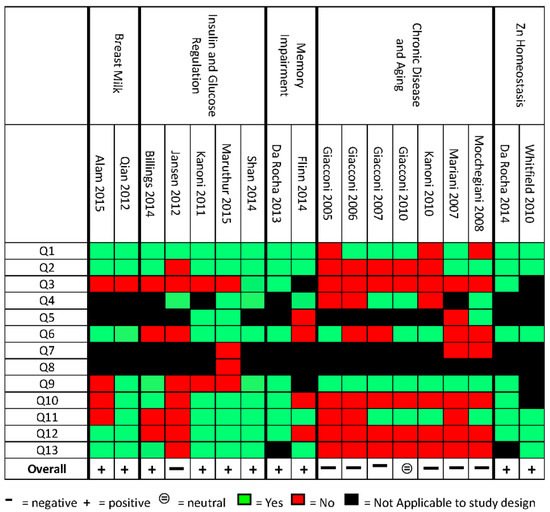
Figure 1.
Quality Assessment (QA) results for each included study. Q1. Research question; Q2. Study Design; Q3. Subjects/patients bias; Q4. Group comparisons; Q5. Intervention/therapeutic description; Q6. Outcomes; Q7. Withdrawals; Q8. Blinding; Q9. Hardy-Weinberg equilibrium; Q10. Association between Zn intake or Zn status and Single Nucleotide Polymorphisms (SNPs); Q11. Appropriateness of statistics; Q12. Supported conclusions; Q13. Funding bias [29].
3. Results
3.1. Description of Included Studies
The result of the systematic search of the databases is shown in the PRISMA flowchart (Figure 2); eighteen studies matched the inclusion and exclusion criteria. Of these 18 studies, eight were observational cross-sectional [21,30,31,32,33,34,35], six were case control studies [23,36,37,38,39,40,41], three were non-randomised clinical control trials ([24,42,43], and one was a cross-sectional meta-analysis [22]. Nine separate genes with 31 SNPs (Table 1), and one study that looked at twins [34] were identified across the 18 studies. The studies selected were grouped according to the association between one or more polymorphisms with a phenotypic trait. Using this criterion, the studies were classified into the following five groups: polymorphisms in genes relating to Zn transporters for breastmilk, insulin and glucose regulation, cognitive performance, chronic disease in the ageing population, and Zn homeostasis (Table 1).
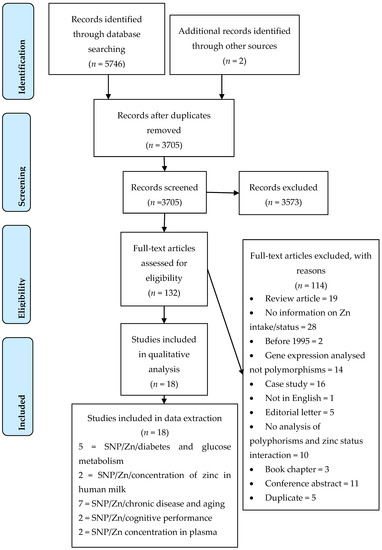
Figure 2.
PRISMA 2009 Flow Diagram adapted from [44].

Table 1.
Data extraction of each study identified. T1D, type 1 diabetes; T2D, type 2 diabetes.
The 18 studies were scored for their quality against a list of 13 items; a summary of the results of the quality assessment is summarised in Figure 1. Overall, the studies scored positively on the identification of the research questions and validation of methods; the studies identified as scoring negatively did so mainly in the selection of study participants, or providing conclusions that did not take into consideration the limitations of the study or study bias. Due to the lack of consistency across grouped studies, identification of outcomes, and methods of determining associations, conclusions on how to translate the information into recommendations in practice could not be drawn.
3.2. Does Dietary Zn Modulate the Effect of SLC30A2 Polymorphisms on Human Milk Zinc Content?
Two cross-sectional studies examined the association of polymorphisms within SLC30A2 gene and Zn content variability in human milk from a total of 794 healthy, breastfeeding mothers with children from single births, without complications. Zn concentrations in breastmilk were examined at 42 days and 4 months postpartum [28]. SLC30A2 SNPs found to be associated with variability in milk Zn concentrations are listed in Table 1. SNP rs-numbers were only reported in Qian et al. [30], whereas SNPs were identified as “novel” without associated rs-numbers in Alam et al. [28], which makes identifying common SNPs between the studies difficult. Qian et al. identified five SNPs in the Chinese mothers, with two associated with decreased concentrations and three with no association with Zn in breastmilk. In the US, Alam et al. identified 12 polymorphisms in mothers, of which two variants were associated with decreased concentrations of Zn in breastmilk. Low concentration of Zn in breastmilk were defined as 21.5 µmol/L [30], and less than 1 mg per L (15.3 µmol/L) [28]. Neither study reported whether maternal dietary Zn intake or plasma Zn modulated Zn concentrations of milk in mothers carrying polymorphisms associated with low breastmilk Zn concentrations [28,30]. Both of these studies were assessed by QA rating P (Figure 1).
3.3. Does Dietary Zn Modulate the Association between Gene Variants and Glucose Metabolism Traits in Relation to Type-2 Diabetes?
Five independent studies examined the association between SNPs for the SLC30A8 gene, Zn intake, the risk of T2D diabetes, and glucose metabolism biomarkers (Table 1). Three out of five studies examined the SNP rs13266634 [36,41,43]; of these studies, all but Jansen et al. [36] confirmed the increased risk of T2D associated with the T allele of this SNP (Table 1). While Jansen and colleagues showed contradicting results, the small sample size may have not allowed the association to be significant, and this was discussed by the authors. When the effect of dietary Zn intake was investigated in carriers of the T allele for the SNP rs13266634, Shan et al. [41] reported a reversed risk for T2D with high plasma Zn concentration (third highest tertile ≥ 197.58 µg/dL). Maruthur et al. also reported that after Zn supplementation for 14 days carriers of the T allele experienced increased insulin response to glucose by 15% and 14% at 5 and 10 minutes, respectively, compared with individuals carrying the CC genotype [43].
The hypothesis that more variants of SLC30A8 are associated with impaired glucose metabolism was explored by Billings and co-workers in a study that sequenced the exome of the gene in 380 subjects classified as possessing an increased risk of developing T2D [21]. This study identified 44 novel SLC30A8 variants; four of which demonstrated a positive association, and one that showed a negative association with pancreatic β-cell function biomarkers (Table 1). The authors concluded that dietary Zn intake did not modify the genetic predisposition to T2D, suggesting a limited role for dietary manipulation in affecting risk in relation to the SNPs identified. The study’s main limitation was a lack of description of a method to measure dietary intake which prevented the results from properly supporting the conclusion (Table 1 and Figure 1). The authors were contacted on this matter, however, we did not obtain a response.
The fifth study was a cross-sectional meta-analysis on 14 cohorts including a total of 46,021 individuals of European ancestry with fasting glucose <6 mmol/L [22]. The association of dietary zinc intake with fasting glucose, and the interaction with 20 genetic variants known to be related to glucose metabolism traits was examined. This study identified the SLC30A8 SNP rs11558471, where carriers of the A allele have increased fasting glucose. An association was observed with the SNP rs11558471, fasting glucose, and total Zn intake, suggesting that Zn intake has an inverse association with fasting glucose plasma concentration in carriers of the A allele for that SNP. Fasting glucose of individuals carrying the A allele of rs11558471 responded to increased Zn intake compared with GG genotype carriers; for each milligram of Zn intake per day, a reduction of −0.0017 mmol/L fasting glucose was reported (Table 1) [22]. Quality of the meta-analysis was assessed as positive, however, the rationale for examining the specific SNP was not reported.
3.4. Does Dietary Zn Modulate the Effect of Gene Variants on Cognitive Performance?
Two studies, one in humans and one in mice, were identified that examined Zn, genes, and cognition. The human study examined the interaction between SNPs for the SLC30A3 gene, cognitive performance, and Zn dietary intake in 240 healthy people over 50 years of age, but with memory deficit [32]. The authors reported a gene-nutrient interaction for SLC30A3 rs73924411 and serum Zn concentration (Table 1). Carriers of the T allele displayed higher memory score than the CC genotype carriers when serum Zn concentration was below the recommended concentration (<0.70 mg/L = 0.01 mmol/L). This suggests a possible neurotoxicity effect of Zn for T allele carriers with serum Zn > 0.70 mg/L = 0.01 mmol/L.
The mouse model study examined late-onset AD, and analysed the interaction between Zn dietary intake and the isoform ε4 of the apolipoprotein E (ApoE) human gene [37] corresponding to SNP rs7412 in the NCBI database. The authors tested whether high Zn intake worsened the spatial memory of mice in which the endogen APOE gene was replaced with the isoform ε4 of human ApoE. The ApoE isoform ε4 is over-represented in AD patients and promotes the binding of Zn to amyloid plaques [45]. Although in a mouse model, Flinn’s group showed that high Zn intake was associated with worse performance in tests aimed at evaluating spatial memory in carriers of the ApoE isoform ε4 [37]. However, the authors did not report actual Zn intake, as the mice were allowed to drink Zn-spiked water ad-libitum.
Both these studies reported that high Zn intake, in association with specific gene variants, significantly impaired the memory scores of older adults and spatial memory in an AD mouse model, respectively. Flinn’s study scored negative in the QA assessment mainly because the lack of reporting of Zn intake. Da Rocha et al., although with some limitations, was assessed as positive in the QA (Figure 1) [32,37].
3.5. Does Dietary Zn Modulate the Association between Gene Variants and the Development of Chronic Diseases in the Aging Population?
Seven studies aimed to identify carriers of genetic polymorphisms that would benefit from Zn supplementation to support healthy ageing [23,24,35,38,39,40,46]. Unlike the other studies reported in this review, Zn and healthy ageing have not been associated with Zn transporter genes, but rather genes that are associated with inflammation and oxidative stress. Three papers investigated the association between Interleukin-6 (IL-6) SNP rs1800795 and serum Zn in a group of older adults, but did not show any conclusive relationship (Table 1) [24,35,46]. All papers in this group reported on SNPs in relation to separate outcomes, often in small sample sizes. Differing targets and the lack of consistency in reporting across the group prevents reaching a clinically relevant conclusion (Table 1) [23,35,38,39,40,42]. These studies were all assessed negative or neutral in the QA score (Figure 1).
3.6. Do Polymorphisms in Genes Involved in Zn Transport and Metabolism Affect Plasma Zn Concentration?
Two of the 18 papers identified did not explore the interaction between polymorphisms in Zn metabolism and dietary Zn in relation to a clinical biomarker/disease state, but they investigated the influence of polymorphisms only on plasma Zn concentrations and whether this is affected by dietary Zn intake [33,34]. Da Rocha et al. looked at the influence of SNPs rs11126936 and rs73924411 in the gene SLC20A3 and found that Zn serum concentrations were significantly lower in carriers of C allele of rs11126936 compared to T carriers (p = 0.014). When subjects were grouped by serum Zn concentrations the CC genotype was more frequently observed in subjects with low Zn serum. Da Rocha and colleagues did not find any association between serum Zn concentration and rs73924411. This finding needs to be evaluated against another study from the same group that found a significant association between rs73924411 and Zn concentrations in relation to cognitive impairment scores [32]. This suggests the interaction is only observed in relation to cognitive impairment. Finally, Whitfield et al. [34] investigated to what extent Zn concentrations in erythrocytes are influenced by genetic effects by obtaining samples from twins. The group used model-fitting and grouped twins according to zygosity in order to establish to what extent the Zn concentration variation was due to genetic or environmental factors including diet. They concluded that 20% of the variation in Zn concentration is due to genetic factors [34].
4. Discussion
The completion of the Human Genome Project held the promise of resolving the complexity of individual response to diet and one-size fits all public health guidelines, and to reveal the role of genetics in shaping nutritional requirements [1,19]. Zn exemplifies this paradigm; this essential micronutrient is distributed throughout all organs, Zn absorption and metabolism involves numerous gene products, and it is expected that genetic variability is capable of affecting requirements [6].
The Zn transporter SLC30A8 is a gene expressed in insulin secreting pancreas β-cells; SLC30A8 SNP rs13266634 has been associated with increased risk of T2D [31,47,48,49,50]. The discovery of a relationship between Zn transporter gene variants and T2D led to the hypothesis that Zn intake may affect insulin and/or glucose metabolism [51], thus making SLC30A8 polymorphisms good candidates to modify Zn requirements. This systematic review highlighted the role of Zn intake and its interaction with SLC30A8 SNPs. In the meta-analysis performed by Kanoni et al. 2011, the gene dependent nutrient interaction with glucose metabolism markers was confirmed. Kanoni et al. demonstrated that total Zn intake has a stronger inverse association with fasting glucose concentration in individuals carrying the glucose-raising A allele of rs11558471. The interaction resulted in a reduction of 0.024 mmol/L in blood sugar concentration per 1 mg of Zn. The normal range for blood glucose concentration is considered 4–8 mmol/L; this finding, while interesting, may not be easily utilised in clinical practice due to the relatively small effects [22]. SLC30A8 has been linked to pancreatic islets function [12], but this was the first time that rs11558471 was investigated in the context of glucose metabolism outcomes. rs11558471 was reported to be in strong linkage disequilibrium with rs13266634, although no further explanation was offered on why this SNP was selected. Different SNPs on SLC30A8 were tested by Shan’s group, showing that the risk of T2D and impaired glucose regulation could be attenuated by increasing plasma Zn concentrations in CC carriers of rs13266634. These observations support the concept that Zn intervention could play a role in T2D, and that Zn recommendations may benefit from being personalised according to SLC30A8 genotypes. This study was assessed positive on quality, however, its use in clinical practice is limited, as it does not provide indications on the magnitude of an intervention (i.e., within or above the RDI) [41]. Jansen and collaborators reported rs13266634 in the same gene and presented conflicting results, reporting a lack of association with glucose metabolism biomarkers, possibly due to the small sample analysed [36]. This highlights the importance of further larger scale research projects that seek to clarify any gene-nutrient interactions and provide clear understanding of any intervention requirements.
The potential associations of genetic polymorphisms with cognitive performance in relation to Zn intake were investigated in two of the selected studies [32,37]. The SLC30A3 gene was identified in 1996 and raised major interest, as the corresponding protein was shown to transport Zn into pre-synaptic vesicles of glutamatergic neurones of the cerebral cortex and hippocampus, key regions for the memory formation and with a role in the development of amyloid β plaques in AD [52,53]. Da Rocha and colleagues analysed the role of SLC30A3 rs73924411 in memory [32]. Another study analysed another genetic variation associated with AD, the ApoE isoform ε4 [54]. Both reports suggested that carriers of T allele of SLC30A3 rs73924411 and ApoE isoform ε4 (rs7412 in NCBI), respectively, could benefit from Zn plasma concentration on the lower side of the recommended cut-off and suggested that what is considered the optimal concentration could have a neurotoxic effect for the carriers of these polymorphisms. Those observations can lead to the conclusion that individuals with these genotypes would be better off in terms of cognitive performance with Zn intakes lower than the recommended amounts. Although one of these reports analysed a small sample [32] and the other did not report Zn intake correctly [54], they are examples of where one size fits all nutrient recommendations may not be appropriate. The quest to slow down the inevitable process of aging and the development of diseases associated with it has included the attempt to optimise diet to support the metabolic needs of elderly people. Seven papers indicated that there may be a potential interaction between markers of chronic disease in the elderly and the variation of Zn intake, with outcomes being dependent on genetic variations. However, these findings should be treated with caution until further research that explicitly quantifies the association of Zn intake and genotype, not just Zn status, is conducted. Therefore, no conclusions can be drawn at this time in relation to clinical practice.
5. Perspectives
Healthcare professionals need to be able to translate genetic information in the context of the health priorities of patients, to take into consideration the scenario of a patient carrying one or more SNPs in genes affecting Zn metabolism in different organs and the possibility of the results of these different genotypes modifying the risk of two pathologies in opposite/different ways. This would ideally require bioinformatics tools to evaluate the function and effect size of each relevant variant, along with clinical information. To develop dietary recommendations that incorporate information of common polymorphisms, more studies need to be conducted looking at the association of genetic variants, disease biomarkers, and dietary intake. The majority of the studies included in this report analysed the association of only two out of three of these factors, making it difficult to draw a conclusion about personalised dietary recommendations based on genotypes. Moreover, a reliable biomarker to assess Zn status is still an open debate; serum concentration is generally used, but this measure is affected by several transient factors, such as infections and acute inflammation [55].
A number of studies identified in this review confirmed the concept of genetic makeup modifying the response to Zn and possibly Zn requirements; however, we are still left with a number of questions such as what is the optimal Zn intake for specific genetic variations? Should the advice on Zn intake change in relation to a reduction in disease risk or to be used as a therapeutic tool to make treatments more effective? Most studies did not assess dietary Zn intake, so while associations between Zn status and SNPs may have been identified, there is no mention of whether current dietary recommendations are appropriate for different genotypes for general good health or in relation to disease outcomes. Another point of reflection is that most studies were performed on small cohorts, in specific sub groups of the population, not representing the variability present in the human species. These questions are important for research in the current environment, where more nutrigenetic tests are being developed and advertised as tools to tailor dietary advice; the public have increasing access to these tests through both a range of healthcare professionals and the internet. While tests may correctly report on the associations between genetics and health effects, they do not take into consideration the effect size and the direction of the intervention. The limited clear evidence on how variations affect dietary requirements for general good health, therapeutic treatments, and disease reduction, raises the question of deciding when it is useful to use the results to modify nutrient intake advice. Healthcare professionals with appropriate knowledge in both genetics and nutrition are required to help individuals understand whether and how information within nutrigenetic tests should be used to inform dietary intake. Limitations of this systematic study include the lack of homogeneity between the studies selected, which prevented the possibility of performing a meta-analysis, thereby making it difficult to reach a decisive conclusion that provides clear directions for application.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/9/2/148/s1, Figure S1: Databases’ search strategy, Figure S2: PRISMA checklist.
Acknowledgments
We thank Anne Yung for assisting with the initial database searches.
Author Contributions
C.M. and M.M.A. conceived the study. K.J.D., M.M.A. and C.M. performed the database searches and wrote the first draft of the manuscript; A.L.D., M.M.A. and K.J.D. assessed the quality of the selected studies. A.L.D. assisted with the final editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Kang, J.X. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J. Nutrigenet. Nutrigenom. 2016, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B. Dietary factors affecting trace element absorption in infants. Acta Paediatr. Scand. Suppl. 1989, 351, 109–113. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Nutrient Reference Values. 2006. Available online: http://www.nrv.gov.au (accessed on 24 May 2016). [Google Scholar]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Miale, A., Jr.; Farid, Z.; Sandstead, H.H.; Schulert, A.R. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J. Lab. Clin. Med. 1963, 61, 537–549. [Google Scholar] [PubMed]
- Devirgiliis, C.; Zalewski, P.D.; Perozzi, G.; Murgia, C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat. Res. 2007, 622, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Krebs, N.F. Zinc deficiency: A special challenge. J. Nutr. 2007, 137, 1101–1105. [Google Scholar] [PubMed]
- Ackland, M.L.; Michalczyk, A. Zinc deficiency and its inherited disorders—A review. Genes Nutr. 2006, 1, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Zinc and its transporters, pancreatic beta-cells, and insulin metabolism. Vitam. Horm. 2014, 95, 365–390. [Google Scholar] [PubMed]
- Rutter, G.A.; Chimienti, F. SLC30A8 mutations in type 2 diabetes. Diabetologia 2015, 58, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, W.; Wang, L.; Gu, S.; Dong, S.; Chen, M.; Jiang, X. Association of SLC30A8 gene polymorphism with type 2 diabetes, evidence from 46 studies: A meta-analysis. Endocrine 2016, 53, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hennigar, S.R.; Alam, S.; Nishida, K.; Kelleher, S.L. Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation. J. Biol. Chem. 2015, 290, 13064–13078. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Vespignani, I.; Rami, R.; Perozzi, G. The Znt4 mutation inlethal milk mice affects intestinal zinc homeostasis through the expression of other Zn transporters. Genes Nutr. 2006, 1, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Parncutt, J.; Lal, V.; James, S.; Hare, D.; Doble, P.; Bush, A.I. Metal chaperones prevent zinc-mediated cognitive decline. Neurobiol. Dis. 2015, 81, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Parncutt, J.M.; Finkelstein, D.I.; Bush, A.I. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010, 30, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem. Int. 2013, 62, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, J. Personalised nutrition: How far has nutrigenomics progressed? Eur. J. Clin. Nutr. 2013, 67, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Findley, S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [PubMed]
- Billings, L.K.; Jablonski, K.A.; Ackerman, R.J.; Taylor, A.; Fanelli, R.R.; McAteer, J.B.; Franks, P.W. The Influence of Rare Genetic Variation in SLC30A8 on Diabetes Incidence and beta-Cell Function. J. Clin. Endocrinol. Metab. 2014, 99, E926–E930. [Google Scholar] [CrossRef] [PubMed]
- Kanoni, S.; Nettleton, J.A.; Hivert, M.F.; Ye, Z.; Van Rooij, F.J.; Shungin, D.; Gustafsson, S. Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant: A 14-cohort meta-analysis. Diabetes 2011, 60, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Cipriano, C.; Muti, E.; Costarelli, L.; Malavolta, M.; Caruso, C.; Mocchegiani, E. Involvement of-308 TNF-α and 1267 Hsp70–2 polymorphisms and zinc status in the susceptibility of coronary artery disease (CAD) in old patients. Biogerontology 2006, 7, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Neri, S.; Cattini, L.; Mocchegiani, E.; Malavolta, M.; Dedoussis, G.V.; Facchini, A. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: Interactive influence of +647 MT1a and -174 IL-6 polymorphic alleles. Exp. Gerontol. 2008, 43, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Evidence Analysis Manual: Steps in the ADA Evidence Analysis Process; American Dietetic Association: Chicago, IL, USA, 2008. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- STROBE Statement. Checklist of items that should be included in reports of observational studies (STROBE initiative). Int. J. Public Health 2008, 53, 3–4. [Google Scholar]
- Salanti, G.; Amountza, G.; Ntzani, E.E.; Ioannidis, J.P. Hardy-Weinberg equilibrium in genetic association studies: An empirical evaluation of reporting, deviations, and power. Eur. J. Hum. Genet. 2005, 13, 840–848. [Google Scholar] [CrossRef] [PubMed]
- A Logo for Human Rights. ONE WORLD EQUAL RIGHTS. Available online: http://www.humanrightslogo.net/en/submission/one-world-%E2%80%93-equal-rights (accessed on 15 February 2017).
- Alam, S.; Hennigar, S.R.; Gallagher, C.; Soybel, D.I.; Kelleher, S.L. Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction. J. Mammary Gland Biol. Neoplasia 2015, 20, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Wang, B.; Tang, N.; Zhang, W.; Cai, W. Polymorphisms of SLC30A2 and selected perinatal factors associated with low milk zinc in Chinese breastfeeding women. Early Hum. Dev. 2012, 88, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, T.J.; Blehm, C.J.; Bamberg, D.P.; Fonseca, T.L.R.; Tisser, L.A.; de Oliveira Junior, A.A.; Fiegenbaum, M. The effects of interactions between selenium and zinc serum concentration and SEP15 and SLC30A3 gene polymorphisms on memory scores in a population of mature and elderly adults. Genes Nutr. 2014, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, T.J.; Korb, C.; Schuch, J.B.; Bamberg, D.P.; de Andrade, F.M.; Fiegenbaum, M. SLC30A3 and SEP15 gene polymorphisms influence the serum concentrations of zinc and selenium in mature adults. Nutr. Res. 2014, 34, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.B.; Dy, V.; McQuilty, R.; Zhu, G.; Heath, A.C.; Montgomery, G.W.; Martin, N.G. Genetic Effects on Toxic and Essential Elements in Humans: Arsenic, Cadmium, Copper, Lead, Mercury, Selenium, and Zinc in Erythrocytes. Environ. Health Perspect. 2010, 118, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Kanoni, S.; Dedoussis, G.V.; Herbein, G.; Fulop, T.; Varin, A.; Jajte, J.; Giacconi, R. Assessment of gene-nutrient interactions on inflammatory status of the elderly with the use of a zinc diet score—ZINCAGE study. J. Nutr. Biochem. 2010, 21, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Rosenkranz, E.; Overbeck, S.; Warmuth, S.; Mocchegiani, E.; Giacconi, R.; Rink, L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J. Nutr. Biochem. 2012, 23, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Flinn, J.M.; Bozzelli, P.L.; Adlard, P.A.; Railey, A.M. Spatial memory deficits in a mouse model of late-onset Alzheimer’s disease are caused by zinc supplementation and correlate with amyloid-beta levels. Front. Aging Neurosci. 2014, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Cipriano, C.; Muti, E.; Costarelli, L.; Maurizio, C.; Saba, V.; Mocchegiani, E. Novel -209A/G MT2A polymorphism in old patients with type 2 diabetes and atherosclerosis: Relationship with inflammation (IL-6) and zinc. Biogerontology 2005, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Kanoni, S.; Mecocci, P.; Malavolta, M.; Richter, D.; Pierpaoli, S.; Piacenza, F. Association of MT1A haplotype with cardiovascular disease and antioxidant enzyme defense in elderly Greek population: Comparison with an Italian cohort. J. Nutr. Biochem. 2010, 21, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Muti, E.; Malavolta, M.; Cipriano, C.; Costarelli, L.; Bernardini, G.; Mocchegiani, E. The +838 C/G MT2A polymorphism, metals, and the inflammatory/immune response in carotid artery stenosis in elderly people. Mol. Med. 2007, 13, 388–395. [Google Scholar] [CrossRef]
- Shan, Z.L.; Bao, W.; Zhang, Y.; Rong, Y.; Wang, X.; Jin, Y.; Liu, L. Interactions Between Zinc Transporter-8 Gene ( SLC30A8) and Plasma Zinc Concentrations for Impaired Glucose Regulation and Type 2 Diabetes. Diabetes 2014, 63, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Giacconi, R.; Costarelli, L.; Muti, E.; Cipriano, C.; Tesei, S.; Gasparini, N. Zinc deficiency and IL-6−174G/C polymorphism in old people from different European countries: Effect of zinc supplementation. ZINCAGE study. Exp. Gerontol. 2008, 43, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Maruthur, N.M.; Clark, J.M.; Fu, M.; Kao, W.L.; Shuldiner, A.R. Effect of zinc supplementation on insulin secretion: Interaction between zinc and SLC30A8 genotype in Old Order Amish. Diabetologia 2015, 58, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Zincage, C. Zinc, metallothioneins, longevity: Effect of zinc supplementation on antioxidant response: A zincage study. Rejuvenation Res. 2008, 11, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Balkau, B. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research; Saxena, R.; Voight, B.F.; Lyssenko, V.; Burtt, N.P.; de Bakker, P.I.; Chen, H.; Roix, J.J.; Kathiresan, S.; Hirschhorn, J.N.; et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007, 316, 1331–1336. [Google Scholar] [PubMed]
- Zeggini, E.; Weedon, M.N.; Lindgren, C.M.; Frayling, T.M.; Elliott, K.S.; Lango, H.; Barrett, J.C. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007, 316, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Weedon, M.N.; Lindgren, C.M.; Frayling, T.M.; Elliott, K.S.; Lango, H.; Barrett, J.C. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007, 316, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Giacconi, R.; Malavolta, M. Zinc signalling and subcellular distribution: Emerging targets in type 2 diabetes. Trends Mol. Med. 2008, 14, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; Quaife, C.J.; Findley, S.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.M.; Ghura, S.; Koster, K.P.; Liakaite, V.; Maienschein-Cline, M.; Kanabar, P.; Green, S.J. APOE-modulated Abeta-induced neuroinflammation in Alzheimer’s disease: Current landscape, novel data, and future perspective. J. Neurochem. 2015, 133, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040S–2051S. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).