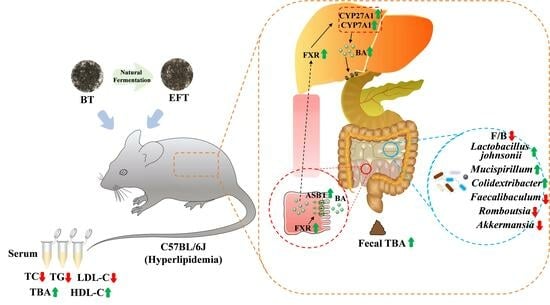
Microbial Fermentation Enhances the Effect of Black Tea on Hyperlipidemia by Mediating Bile Acid Metabolism and Remodeling Intestinal Microbes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tea Extracts
2.2. Analysis of the Main Components of Tea
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Animal Experiments
2.5. Biochemical Assays
2.6. Histopathological Staining
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Western Blot
2.9. Analysis of Gut Microbiota by 16S rRNA Sequencing
2.10. Statistical Analysis
3. Results
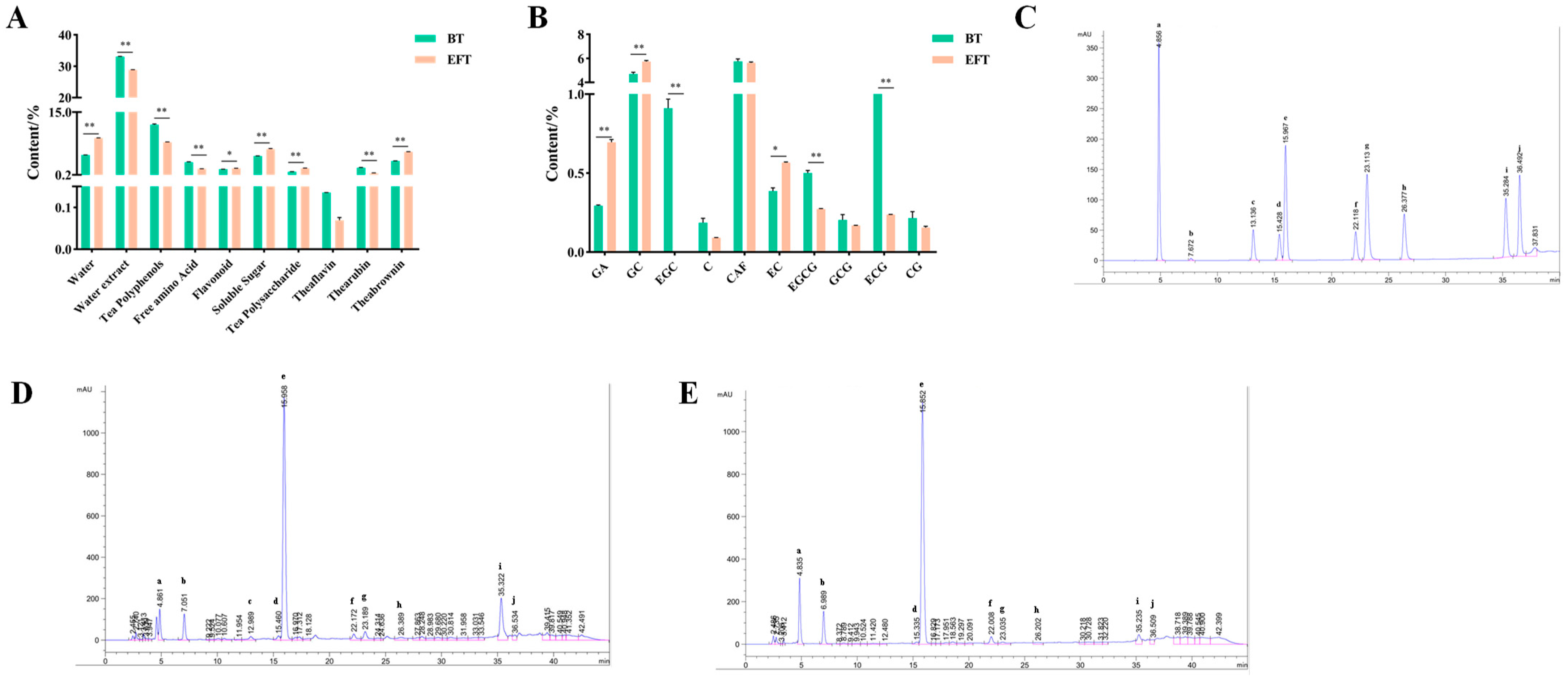
3.1. Analysis of the Main Components of BT and EFT
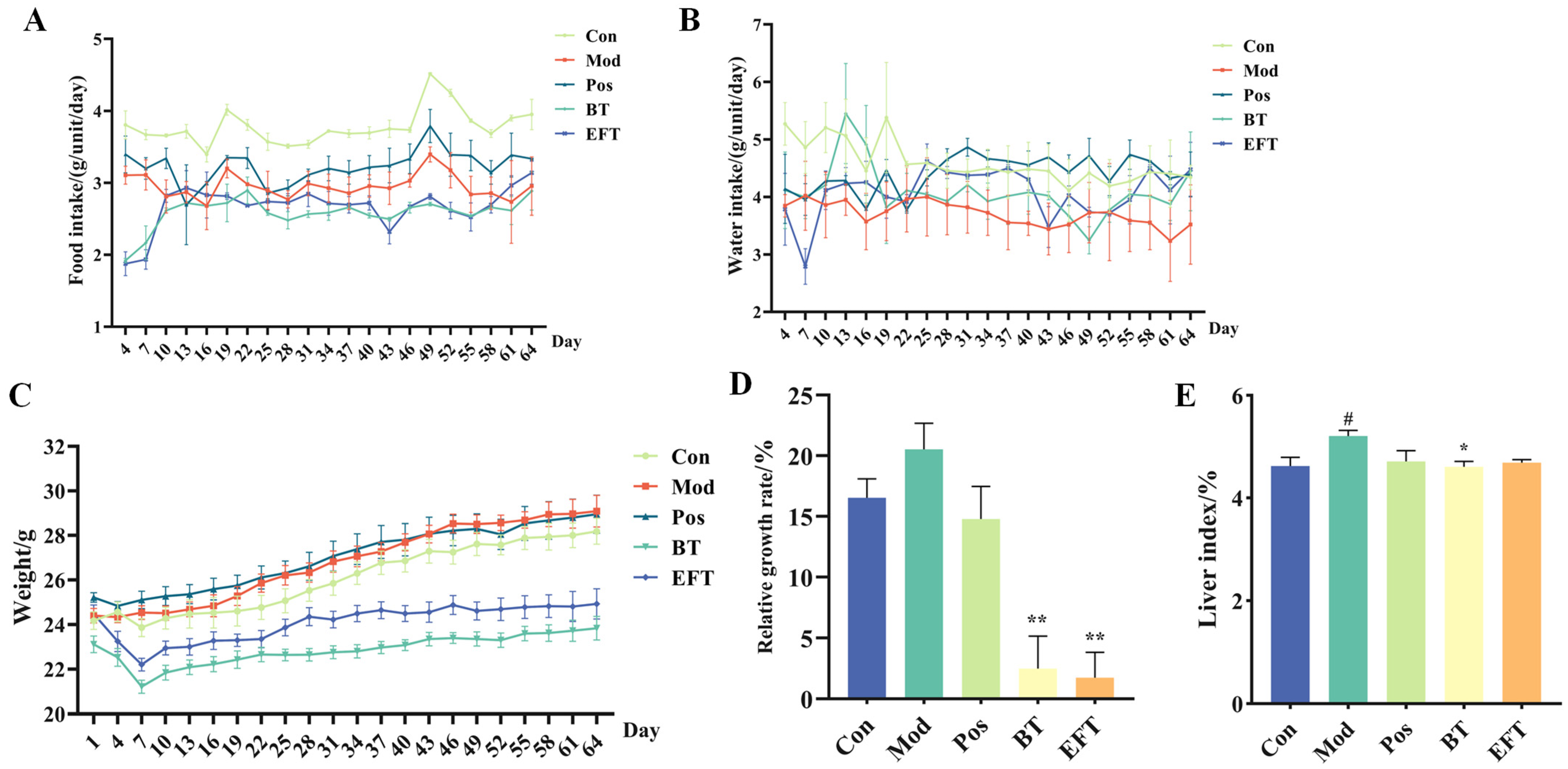
3.2. Effect of BT and EFT on Body Weight and Liver
3.3. Effect of BT and EFT on Lipid Level in Serum and Liver
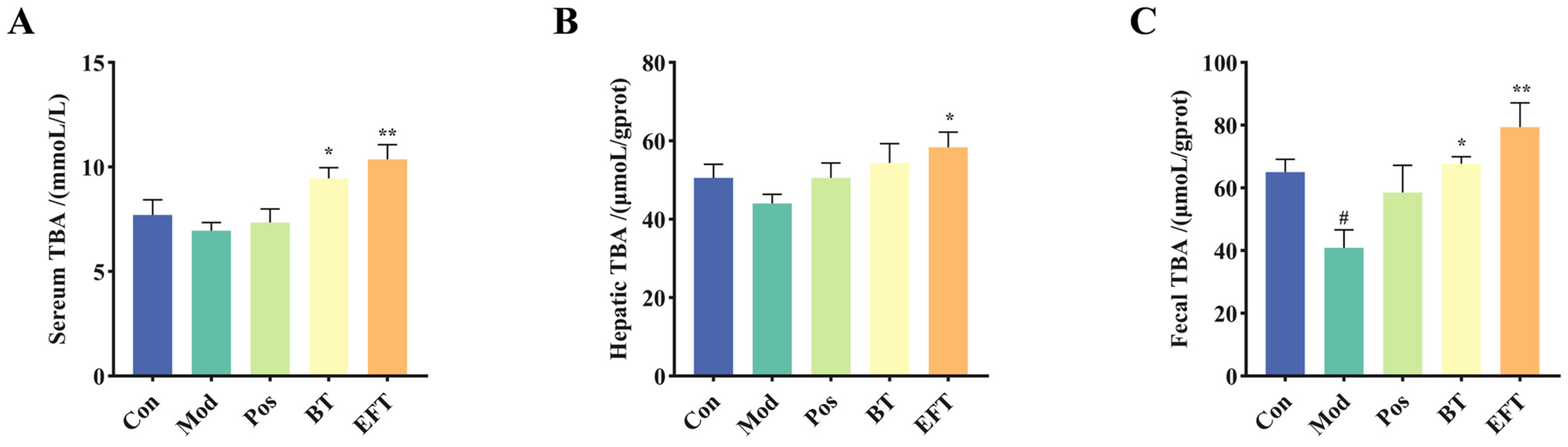
3.4. Effect of BT and EFT on TBA Level in Serum, Liver, and Feces
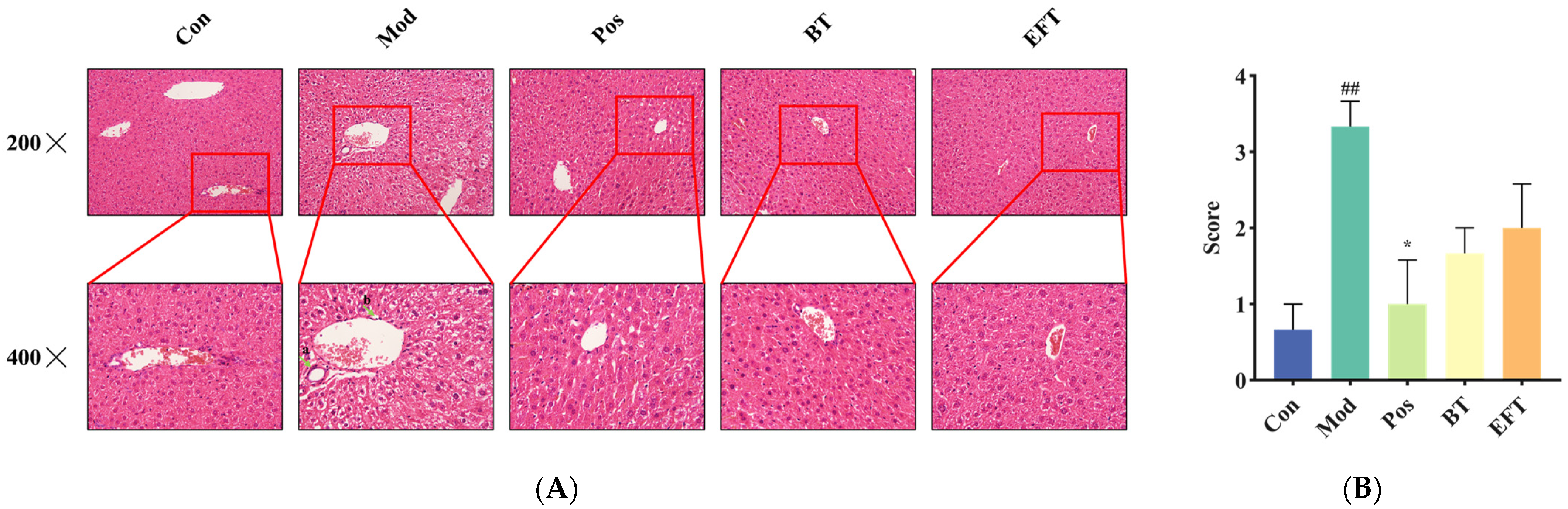
3.5. Analysis of Liver Histopathology
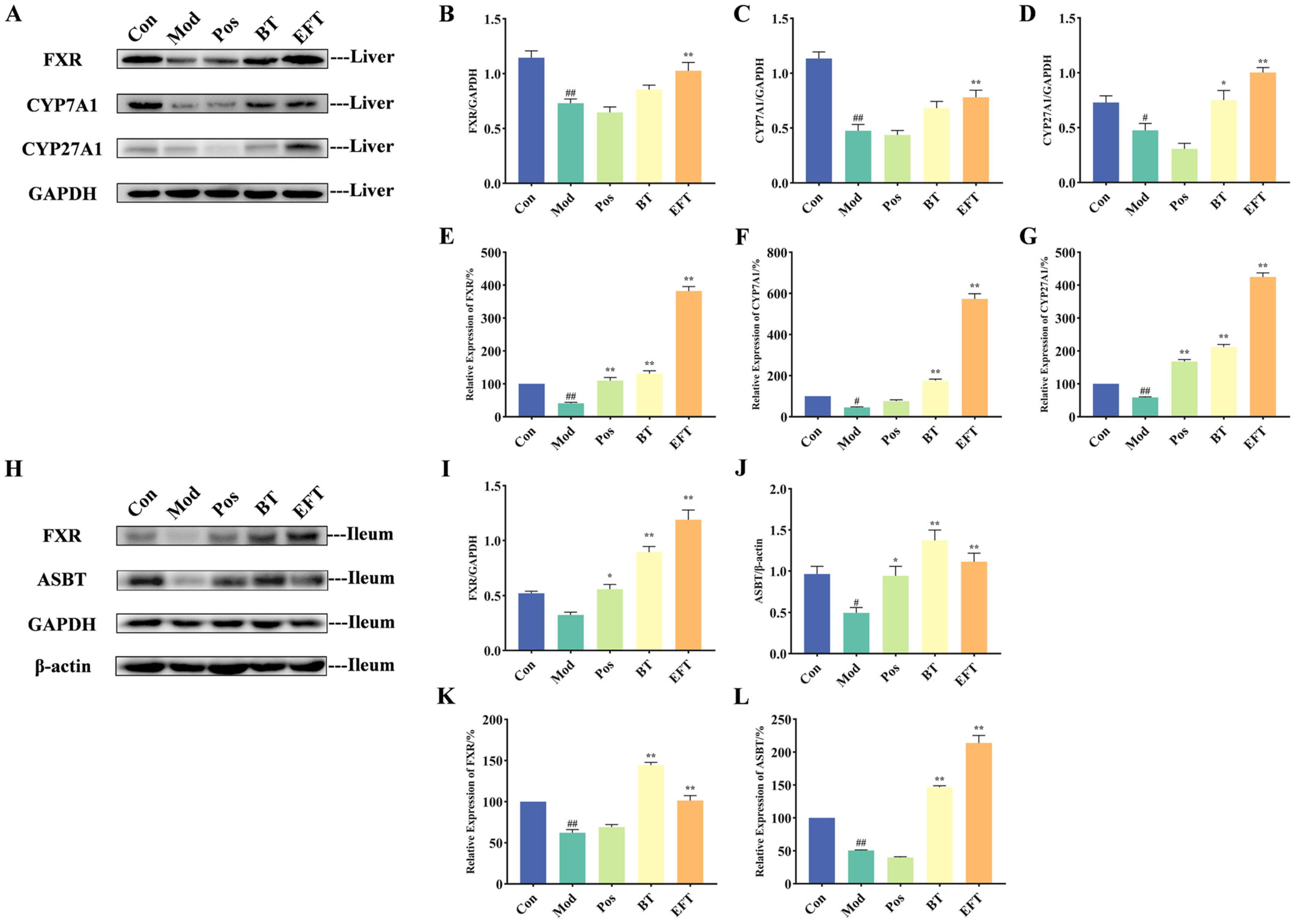
3.6. Effect of BT and EFT on Expression of Genes and Proteins in Bile Acid Metabolism Signaling Pathway
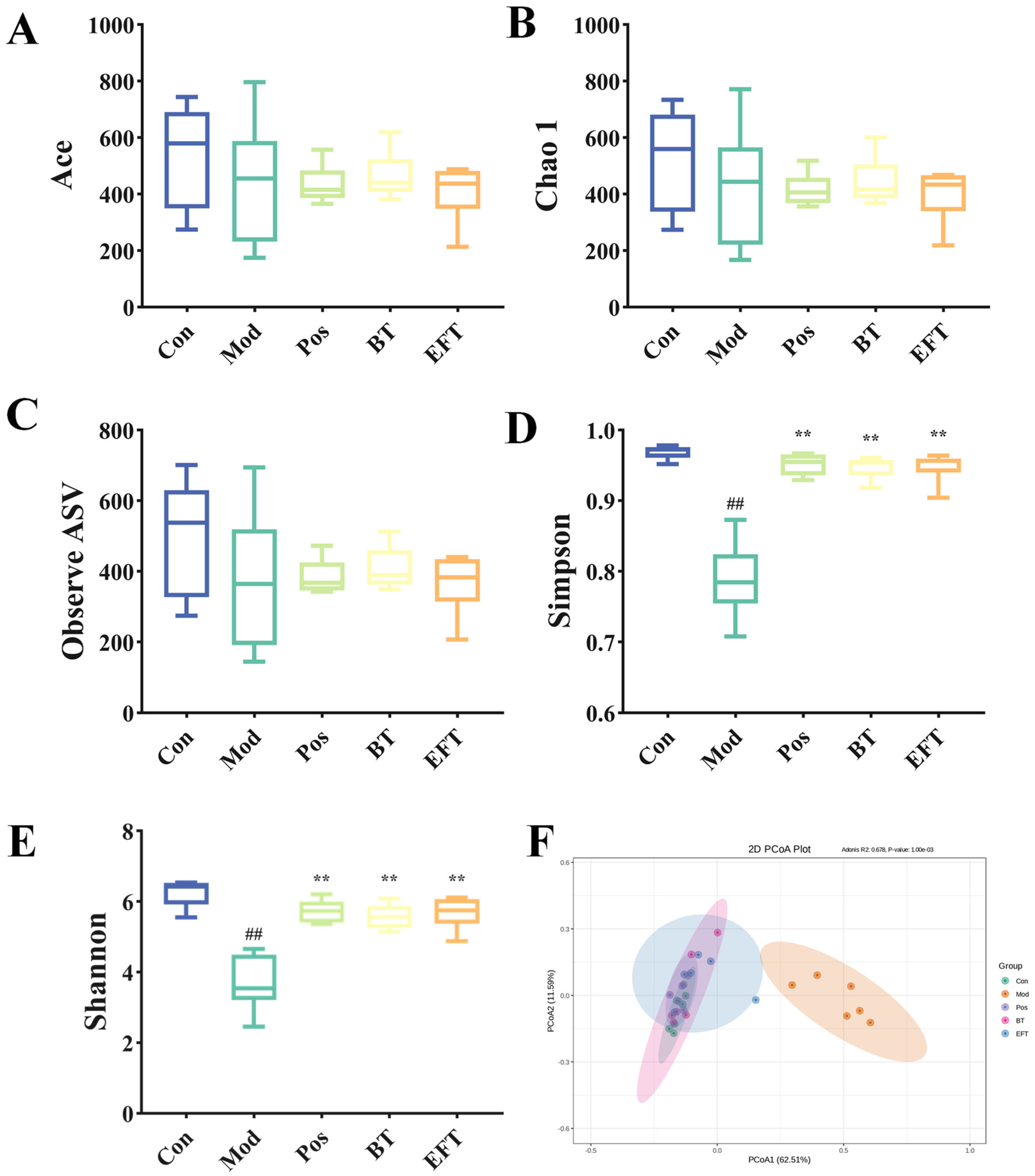
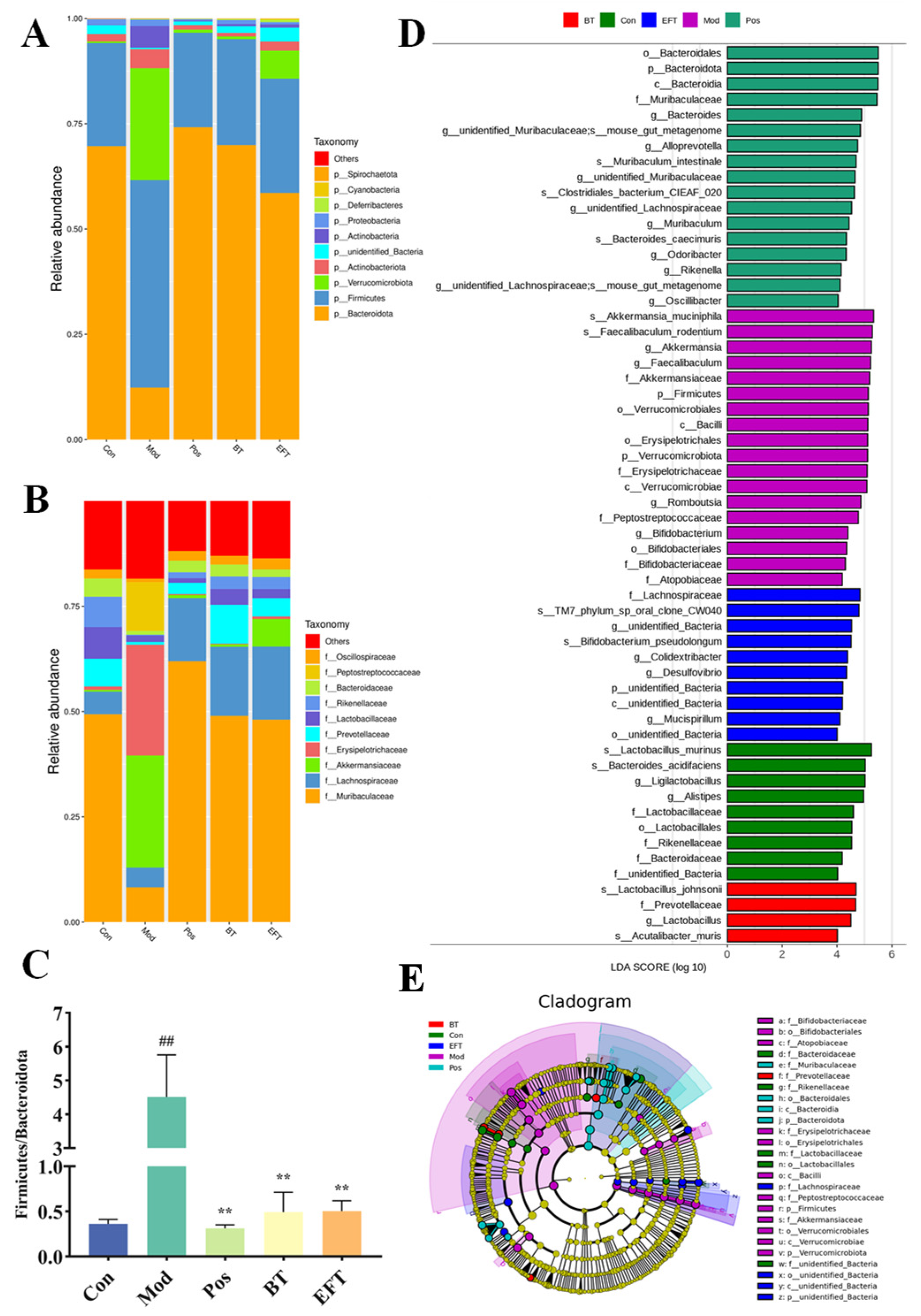
3.7. Effects of BT and EFT on the Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Manting, L.; Haihong, Z.; Jing, L.; Shaodong, C.; Yihua, L. The Model of Rat Lipid Metabolism Disorder Induced by Chronic Stress Accompanying High-Fat-Diet. Lipids Health Dis. 2011, 10, 153. [Google Scholar] [CrossRef]
- He, C.; Cheng, D.; Peng, C.; Li, Y.; Zhu, Y.; Lu, N. High-Fat Diet Induces Dysbiosis of Gastric Microbiota Prior to Gut Microbiota in Association with Metabolic Disorders in Mice. Front. Microbiol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Hsu, C.-Y.; Lee, H.-A.; Wang, W.-H.; Kurniawan, A.L.; Chao, J.C.-J. Dietary Patterns in Relation to Components of Dyslipidemia and Fasting Plasma Glucose in Adults with Dyslipidemia and Elevated Fasting Plasma Glucose in Taiwan. Nutrients 2019, 11, 845. [Google Scholar] [CrossRef]
- Miao, J.; Zang, X.; Cui, X.; Zhang, J. Autophagy, Hyperlipidemia, and Atherosclerosis. Adv. Exp. Med. Biol. 2020, 1207, 237–264. [Google Scholar] [CrossRef]
- Tang, Q.; Gao, L.; Tong, Z.; Li, W. Hyperlipidemia, COVID-19 and Acute Pancreatitis: A Tale of Three Entities. Am. J. Med. Sci. 2022, 364, 257–263. [Google Scholar] [CrossRef]
- Jia, X.; Xu, W.; Zhang, L.; Li, X.; Wang, R.; Wu, S. Impact of Gut Microbiota and Microbiota-Related Metabolites on Hyperlipidemia. Front. Cell Infect. Microbiol. 2021, 11, 634780. [Google Scholar] [CrossRef]
- Li, T.; Hu, S.; Pang, X.; Wang, J.; Yin, J.; Li, F.; Wang, J.; Yang, X.; Xia, B.; Liu, Y.; et al. The Marine-derived Furanone Reduces Intracellular Lipid Accumulation in Vitro by Targeting LXRα and PPARα. J. Cell Mol. Med. 2020, 24, 3384–3398. [Google Scholar] [CrossRef]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J.A. Healthy Properties of Green and White Teas: An Update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef]
- van Duynhoven, J.; Vaughan, E.E.; van Dorsten, F.; Gomez-Roldan, V.; de Vos, R.; Vervoort, J.; van der Hooft, J.J.; Roger, L.; Draijer, R.; Jacobs, D.M. Interactions of Black Tea Polyphenols with Human Gut Microbiota: Implications for Gut and Cardiovascular Health1234. Am. J. Clin. Nutr. 2013, 98, 1631S–1641S. [Google Scholar] [CrossRef]
- Liao, W.; Liu, S.; Chen, Y.; Kong, Y.; Wang, D.; Wang, Y.; Ling, T.; Xie, Z.; Khalilova, I.; Huang, J. Effects of Keemun and Dianhong Black Tea in Alleviating Excess Lipid Accumulation in the Liver of Obese Mice: A Comparative Study. Front. Nutr. 2022, 9, 849582. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The Modulatory Effect of Infusions of Green Tea, Oolong Tea, and Black Tea on Gut Microbiota in High-Fat-Induced Obese Mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef]
- Wen, L.; Sun, L.; Chen, R.; Li, Q.; Lai, X.; Cao, J.; Lai, Z.; Zhang, Z.; Li, Q.; Song, G.; et al. Metabolome and Microbiome Analysis to Study the Flavor of Summer Black Tea Improved by Stuck Fermentation. Foods 2023, 12, 3414. [Google Scholar] [CrossRef]
- Moon, K.; Lee, S.; Cha, J. Bacillus Subtilis Fermentation of Malva Verticillata Leaves Enhances Antioxidant Activity and Osteoblast Differentiation. Foods 2020, 9, 671. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, S.; Lee, S.-W.; Sin, H.-S.; Kim, S.-Y. Protective Effects of Fermented Paprika (Capsicum annuum L.) on Sodium Iodate-Induced Retinal Damage. Nutrients 2020, 13, 25. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Pan, Y.-Z.; Liu, Q.-J.; Li, X.-H. Exposure Assessment of Lovastatin in Pu-Erh Tea. Int. J. Food Microbiol. 2013, 164, 26–31. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.-H.; Zhao, S.-S.; Yang, J.; Chen, L.; Wang, X.-Y.; Wang, Z.-Y.; Liu, H.-X. Identification and Spatial Visualization of Dysregulated Bile Acid Metabolism in High-Fat Diet-Fed Mice by Mass Spectral Imaging. Front. Nutr. 2022, 9, 858603. [Google Scholar] [CrossRef]
- Sun, X.; Winglee, K.; Gharaibeh, R.Z.; Gauthier, J.; He, Z.; Tripathi, P.; Avram, D.; Bruner, S.; Fodor, A.; Jobin, C. Microbiota-Derived Metabolic Factors Reduce Campylobacteriosis in Mice. Gastroenterology 2018, 154, 1751–1763.e2. [Google Scholar] [CrossRef]
- Safari, H.; Kaczorowski, N.; Felder, M.L.; Brannon, E.R.; Varghese, M.; Singer, K.; Eniola-Adefeso, O. Biodegradable, Bile Salt Microparticles for Localized Fat Dissolution. Sci. Adv. 2020, 6, eabd8019. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, S.; Yuan, M.; Zhang, M.; Tang, L.; Wang, P.; Liu, Y.; Xu, C.; Luo, P.; Gao, X. Fermented Rosa Roxburghii Tratt Juice Alleviates High-Fat Diet-Induced Hyperlipidemia in Rats by Modulating Gut Microbiota and Metabolites. Front. Pharmacol. 2022, 13, 883629. [Google Scholar] [CrossRef]
- Song, W.; Sun, L.-Y.; Zhu, Z.-J. Effects of Previous Kasai Surgery on Gut Microbiota and Bile Acid in Biliary Atresia with End-Stage Liver Disease. Front. Med. 2021, 8, 704328. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, Y.; Zhang, Y.; Yang, Y.; Su, W.; Yang, Y.; Sun, L.; Zhang, F.; Yu, J.; Wang, Y.; et al. Gut Microbiota Specifically Mediates the Anti-Hypercholesterolemic Effect of Berberine (BBR) and Facilitates to Predict BBR’s Cholesterol-Decreasing Efficacy in Patients. J. Adv. Res. 2021, 37, 197–208. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Sun, L.; Lai, X.; Li, Q.; Zhang, W.; Xiang, L.; Sun, S.; Cao, F. Six Types of Tea Reduce High-Fat-Diet-Induced Fat Accumulation in Mice by Increasing Lipid Metabolism and Suppressing Inflammation. Food Funct. 2019, 10, 2061–2074. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.; Chen, R.; Wen, S.; Li, Q.; Lai, X.; Zhang, Z.; Cao, F.; Sun, S. Chinese Tea Alleviates CCl4-Induced Liver Injury through the NF-κBorNrf2Signaling Pathway in C57BL-6J Mice. Nutrients 2022, 14, 972. [Google Scholar] [CrossRef]
- Liu, H.; Chen, R.; Wen, S.; Li, Q.; Lai, X.; Zhang, Z.; Sun, L.; Sun, S.; Cao, F. Tea (Camellia sinensis) Ameliorates DSS-Induced Colitis and Liver Injury by Inhibiting TLR4/NF-κB/NLRP3 Inflammasome in Mice. Biomed. Pharmacother. 2023, 158, 114136. [Google Scholar] [CrossRef]
- Wen, S.; Sun, L.; An, R.; Zhang, W.; Xiang, L.; Li, Q.; Lai, X.; Huo, M.; Li, D.; Sun, S. A Combination of Citrus Reticulata Peel and Black Tea Inhibits Migration and Invasion of Liver Cancer via PI3K/AKT and MMPs Signaling Pathway. Mol. Biol. Rep. 2020, 47, 507–519. [Google Scholar] [CrossRef]
- Wu, D.; Chen, R.; Zhang, W.; Lai, X.; Sun, L.; Li, Q.; Zhang, Z.; Cao, J.; Wen, S.; Lai, Z.; et al. Tea and Its Components Reduce the Production of Uric Acid by Inhibiting Xanthine Oxidase. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Xu, H.; Lyu, X.; Guo, X.; Yang, H.; Duan, L.; Zhu, H.; Pan, H.; Gong, F.; Wang, L. Distinct AMPK-Mediated FAS/HSL Pathway Is Implicated in the Alleviating Effect of Nuciferine on Obesity and Hepatic Steatosis in HFD-Fed Mice. Nutrients 2022, 14, 1898. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, Z.; Chen, H.; Pan, X.; Li, R.; Wu, D.; Hu, X.; Zhang, L.; Wu, H.; Li, X. Dynamic Analysis of Physicochemical Properties and Polysaccharide Composition during the Pile-Fermentation of Post-Fermented Tea. Foods 2022, 11, 3376. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients 2019, 11, 2909. [Google Scholar] [CrossRef]
- Wang, Y.; Gunewardena, S.; Li, F.; Matye, D.J.; Chen, C.; Chao, X.; Jung, T.; Zhang, Y.; Czerwiński, M.; Ni, H.-M.; et al. An FGF15/19-TFEB Regulatory Loop Controls Hepatic Cholesterol and Bile Acid Homeostasis. Nat. Commun. 2020, 11, 3612. [Google Scholar] [CrossRef]
- Hou, G.; Peng, W.; Wei, L.; Li, R.; Yuan, Y.; Huang, X.; Yin, Y. Lactobacillus Delbrueckii Interfere with Bile Acid Enterohepatic Circulation to Regulate Cholesterol Metabolism of Growing-Finishing Pigs via Its Bile Salt Hydrolase Activity. Front. Nutr. 2020, 7, 617676. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, C.; Liu, H.; Liu, M.; Wang, M.; Jiang, J.; Zhang, G.; Yang, C.; Huang, J.; Lou, Z. Linderae Radix Ethanol Extract Alleviates Diet-Induced Hyperlipidemia by Regulating Bile Acid Metabolism Through Gut Microbiota. Front. Pharmacol. 2021, 12, 627920. [Google Scholar] [CrossRef]
- Tao, W.; Liu, W.; Wang, M.; Zhou, W.; Xing, J.; Xu, J.; Pi, X.; Wang, X.; Lu, S.; Yang, Y. Dendrobium Officinale Polysaccharides Better Regulate the Microbiota of Women Than Men. Foods 2022, 11, 1641. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea Aroma Formation from Six Model Manufacturing Processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-Erh Tea Attenuates Hypercholesterolemia via Modulation of Gut Microbiota and Bile Acid Metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef]
- Zhu, M.; Ouyang, J.; Zhou, F.; Zhao, C.; Zhu, W.; Liu, C.; Huang, P.; Li, J.; Tang, J.; Zhang, Z.; et al. Polysaccharides from Fu Brick Tea Ameliorate Obesity by Modulating Gut Microbiota and Gut Microbiota-Related Short Chain Fatty Acid and Amino Acid Metabolism. J. Nutr. Biochem. 2023, 118, 109356. [Google Scholar] [CrossRef]
- Zhu, M.-Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.-M.; Xu, W.; Li, J.; Lin, H.-Y.; Zhang, Z.; Xiao, J.-B.; et al. Microbial Bioconversion of the Chemical Components in Dark Tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Huang, D.-W.; Chang, W.-C.; Yang, H.-J.; Wu, J.S.-B.; Shen, S.-C. Gallic Acid Alleviates Hypertriglyceridemia and Fat Accumulation via Modulating Glycolysis and Lipolysis Pathways in Perirenal Adipose Tissues of Rats Fed a High-Fructose Diet. Int. J. Mol. Sci. 2018, 19, 254. [Google Scholar] [CrossRef]
- Murase, T.; Misawa, K.; Haramizu, S.; Hase, T. Catechin-Induced Activation of the LKB1/AMP-Activated Protein Kinase Pathway. Biochem. Pharmacol. 2009, 78, 78–84. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, M.-S.; Chang, E.; Jung, S.; Ko, H.; Lee, E.; Lee, S.; Kim, C.-T.; Kim, I.-H.; Kim, Y. Tartary Buckwheat Extract Attenuated the Obesity-Induced Inflammation and Increased Muscle PGC-1a/SIRT1 Expression in High Fat Diet-Induced Obese Rats. Nutrients 2019, 11, 654. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, N.; Zhao, W.; Su, J.; Liang, M.; Xie, Z.; Wu, X.; Li, Q. (-)-Epicatechin Regulates Blood Lipids and Attenuates Hepatic Steatosis in Rats Fed High-Fat Diet. Mol. Nutr. Food Res. 2017, 61, 1700303. [Google Scholar] [CrossRef]
- Chiang, J.Y. Recent Advances in Understanding Bile Acid Homeostasis. F1000Res 2017, 6, 2029. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Wang, S.; Zhang, Q.; Zhao, J.; Zhang, H.; Narbad, A.; Tian, F.; Zhai, Q.; Chen, W. Cholestasis: Exploring the Triangular Relationship of Gut Microbiota-Bile Acid-Cholestasis and the Potential Probiotic Strategies. Gut Microbes 2023, 15, 2181930. [Google Scholar] [CrossRef]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef]
- Larsen, I.S.; Jensen, B.A.H.; Bonazzi, E.; Choi, B.S.Y.; Kristensen, N.N.; Schmidt, E.G.W.; Süenderhauf, A.; Morin, L.; Olsen, P.B.; Hansen, L.B.S.; et al. Fungal Lysozyme Leverages the Gut Microbiota to Curb DSS-Induced Colitis. Gut Microbes 2021, 13, 1988836. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-Microbiota Interaction-Mediated Resistance to Inflammatory Bowel Disease in Pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef]
- Ijssennagger, N.; van Rooijen, K.S.; Magnúsdóttir, S.; Ramos Pittol, J.M.; Willemsen, E.C.L.; de Zoete, M.R.; Baars, M.J.D.; Stege, P.B.; Colliva, C.; Pellicciari, R.; et al. Ablation of Liver Fxr Results in an Increased Colonic Mucus Barrier in Mice. JHEP Rep. 2021, 3, 100344. [Google Scholar] [CrossRef]
- Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Wang, C.; Ye, B.; Lv, L.; Ye, Q.; Xie, S.; Hu, G.; Zou, J. Dietary Antimicrobial Peptides Improve Intestinal Function, Microbial Composition and Oxidative Stress Induced by Aeromonas Hydrophila in Pengze Crucian Carp (Carassius Auratus Var. Pengze). Antioxidants 2022, 11, 1756. [Google Scholar] [CrossRef]
- Du, L.; Zhang, Y.; Ji, S.; Wang, L.; Zhao, X.; Yan, S.; Xiao, X.; Li, S. Mechanisms of Zhenwu Decoction for the Treatment of Renal Fibrosis at Various Stages: What Is the Role of Corynebacterium? Front. Microbiol. 2022, 13, 913465. [Google Scholar] [CrossRef]
- Yang, G.; Hong, E.; Oh, S.; Kim, E. Non-Viable Lactobacillus Johnsonii JNU3402 Protects against Diet-Induced Obesity. Foods 2020, 9, 1494. [Google Scholar] [CrossRef]
- Rodríguez-Lara, A.; Plaza-Díaz, J.; López-Uriarte, P.; Vázquez-Aguilar, A.; Reyes-Castillo, Z.; Álvarez-Mercado, A.I. Fiber Consumption Mediates Differences in Several Gut Microbes in a Subpopulation of Young Mexican Adults. Nutrients 2022, 14, 1214. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, F.; Zhang, W.; Xia, W.; Chen, Y.; Liu, Y.; Fan, Z.; Zhang, Y.; Yang, Y. Cholesterol-Lowering Effect of Bile Salt Hydrolase from a Lactobacillus Johnsonii Strain Mediated by FXR Pathway Regulation. Food Funct. 2022, 13, 725–736. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Luo, D.; Lin, L.; Lu, X.; Gao, J.; Xiao, C.; Zhao, M. Effect of Bergamot and Laoxianghuang Polysaccharides on Gut Microbiota Derived from Patients with Hyperlipidemia: An Integrative Analysis of Microbiome and Metabolome during In Vitro Fermentation. Foods 2022, 11, 2039. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic Acid-Induced Gut Microbiota Improves Metabolic Endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef]
- Ma, Q.; Zhai, R.; Xie, X.; Chen, T.; Zhang, Z.; Liu, H.; Nie, C.; Yuan, X.; Tu, A.; Tian, B.; et al. Hypoglycemic Effects of Lycium Barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice via Modulating Gut Microbiota. Front. Nutr. 2022, 9, 916271. [Google Scholar] [CrossRef]
- Huang, H.-C.; Hsu, S.-J.; Chang, C.-C.; Chuang, C.-L.; Hou, M.-C.; Lee, F.-Y. Effects of PCSK-9 Inhibition by Alirocumab Treatments on Biliary Cirrhotic Rats. Int. J. Mol. Sci. 2022, 23, 7378. [Google Scholar] [CrossRef]
- Li, R.; Yi, X.; Yang, J.; Zhu, Z.; Wang, Y.; Liu, X.; Huang, X.; Wan, Y.; Fu, X.; Shu, W.; et al. Gut Microbiome Signatures in the Progression of Hepatitis B Virus-Induced Liver Disease. Front. Microbiol. 2022, 13, 916061. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, X.; Liu, Z.; Zhang, L.; Fang, X.; Sun, J.; Zhang, Z.; Sun, Y. Sodium Alginate Prevents Non-Alcoholic Fatty Liver Disease by Modulating the Gut–Liver Axis in High-Fat Diet-Fed Rats. Nutrients 2022, 14, 4846. [Google Scholar] [CrossRef]
- Dang, J.T.; Mocanu, V.; Park, H.; Laffin, M.; Hotte, N.; Karmali, S.; Birch, D.W.; Madsen, K.L. Roux-En-Y Gastric Bypass and Sleeve Gastrectomy Induce Substantial and Persistent Changes in Microbial Communities and Metabolic Pathways. Gut Microbes 2022, 14, 2050636. [Google Scholar] [CrossRef]
- Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Hidalgo-García, L.; Molina-Tijeras, J.A.; García, F.; Pischel, I.; Romero, M.; Duarte, J.; Diez-Echave, P.; Rodríguez-Cabezas, M.E.; et al. The Antioxidant Activity of Thymus Serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants 2022, 11, 1073. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Zeng, J.-L.; Ho, S.-T.; Xu, J.-W.; Lin, I.-H.; Wu, J.-H. Djulis Hull Improves Insulin Resistance and Modulates the Gut Microbiota in High-Fat Diet (HFD)-Induced Hyperglycaemia. Antioxidants 2021, 11, 45. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Zhao, Q.; Wang, Y.; Li, C.; Zou, L.; Hu, Y. Effects of Tartary Buckwheat Protein on Gut Microbiome and Plasma Metabolite in Rats with High-Fat Diet. Foods 2021, 10, 2457. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, L.; Shao, L.; Wang, Y.; Huang, Z.; Huang, X.; Li, C.; Wu, A.; Liu, Z.; Fan, X.; et al. Dynamic Alterations of the Gut Microbial Pyrimidine and Purine Metabolism in the Development of Liver Cirrhosis. Front. Mol. Biosci. 2022, 8, 811399. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Chleilat, F.; Schick, A.; Deleemans, J.M.; Ma, K.; Alukic, E.; Wong, J.; Noye Tuplin, E.W.; Nettleton, J.E.; Reimer, R.A. Paternal High Protein Diet Modulates Body Composition, Insulin Sensitivity, Epigenetics, and Gut Microbiota Intergenerationally in Rats. FASEB J. 2021, 35, e21847. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wen, L.; Li, Q.; Chen, R.; Wen, S.; Lai, X.; Lai, Z.; Cao, J.; Zhang, Z.; Hao, M.; et al. Microbial Fermentation Enhances the Effect of Black Tea on Hyperlipidemia by Mediating Bile Acid Metabolism and Remodeling Intestinal Microbes. Nutrients 2024, 16, 998. https://doi.org/10.3390/nu16070998
Sun L, Wen L, Li Q, Chen R, Wen S, Lai X, Lai Z, Cao J, Zhang Z, Hao M, et al. Microbial Fermentation Enhances the Effect of Black Tea on Hyperlipidemia by Mediating Bile Acid Metabolism and Remodeling Intestinal Microbes. Nutrients. 2024; 16(7):998. https://doi.org/10.3390/nu16070998
Chicago/Turabian StyleSun, Lingli, Lianghua Wen, Qiuhua Li, Ruohong Chen, Shuai Wen, Xingfei Lai, Zhaoxiang Lai, Junxi Cao, Zhenbiao Zhang, Mengjiao Hao, and et al. 2024. "Microbial Fermentation Enhances the Effect of Black Tea on Hyperlipidemia by Mediating Bile Acid Metabolism and Remodeling Intestinal Microbes" Nutrients 16, no. 7: 998. https://doi.org/10.3390/nu16070998
APA StyleSun, L., Wen, L., Li, Q., Chen, R., Wen, S., Lai, X., Lai, Z., Cao, J., Zhang, Z., Hao, M., Cao, F., & Sun, S. (2024). Microbial Fermentation Enhances the Effect of Black Tea on Hyperlipidemia by Mediating Bile Acid Metabolism and Remodeling Intestinal Microbes. Nutrients, 16(7), 998. https://doi.org/10.3390/nu16070998