The Potential Role of Nutrition in Overtraining Syndrome: A Narrative Review
Abstract
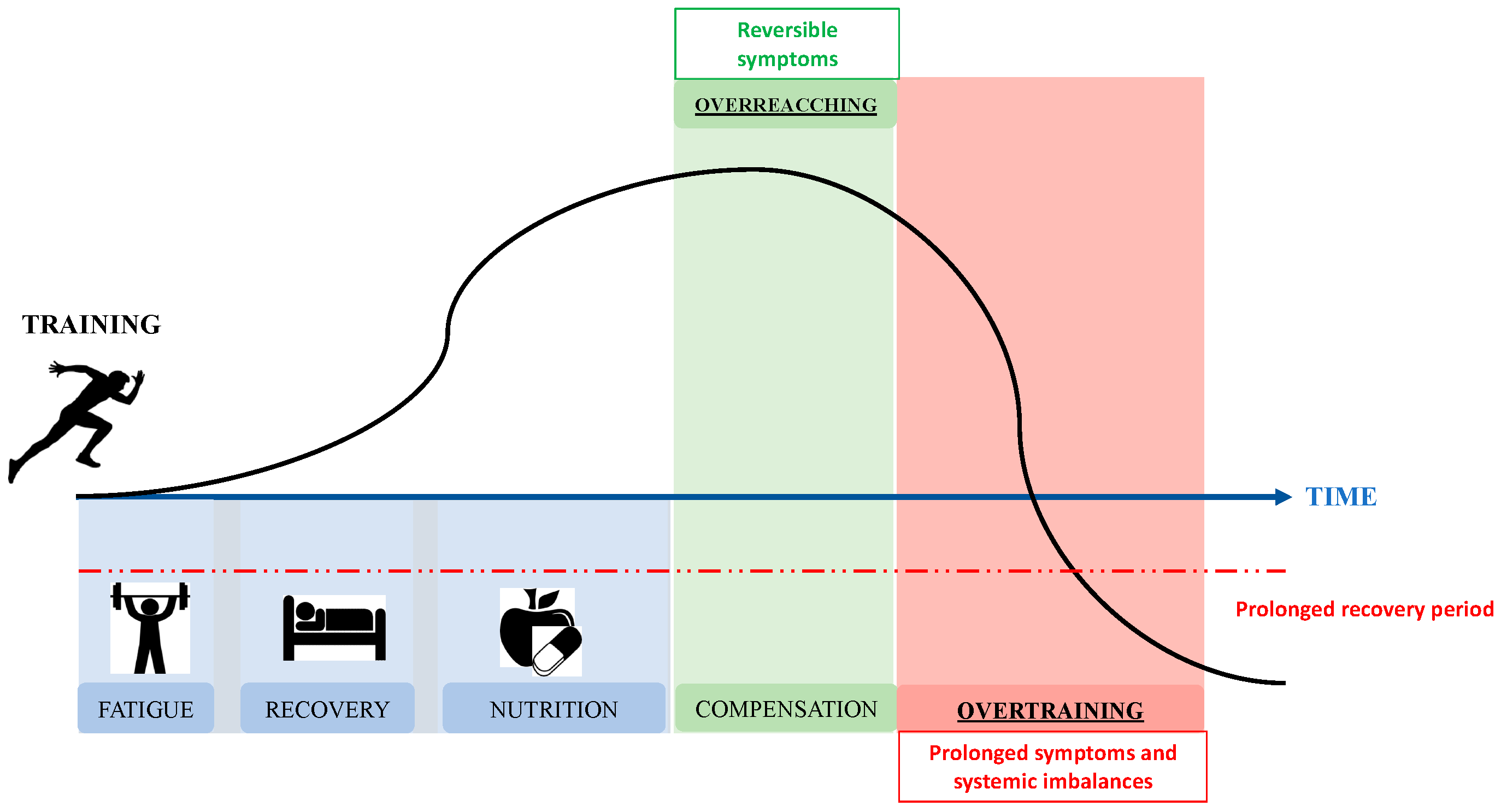
:1. Introduction
2. Pathophysiology of OTS
2.1. Cytokine Hypothesis
2.2. Glutamine Hypothesis
2.3. Central Fatigue Theory
2.4. Glycogen Hypothesis
2.5. Autonomic Nervous System Hypothesis
2.6. Oxidative Stress Hypothesis
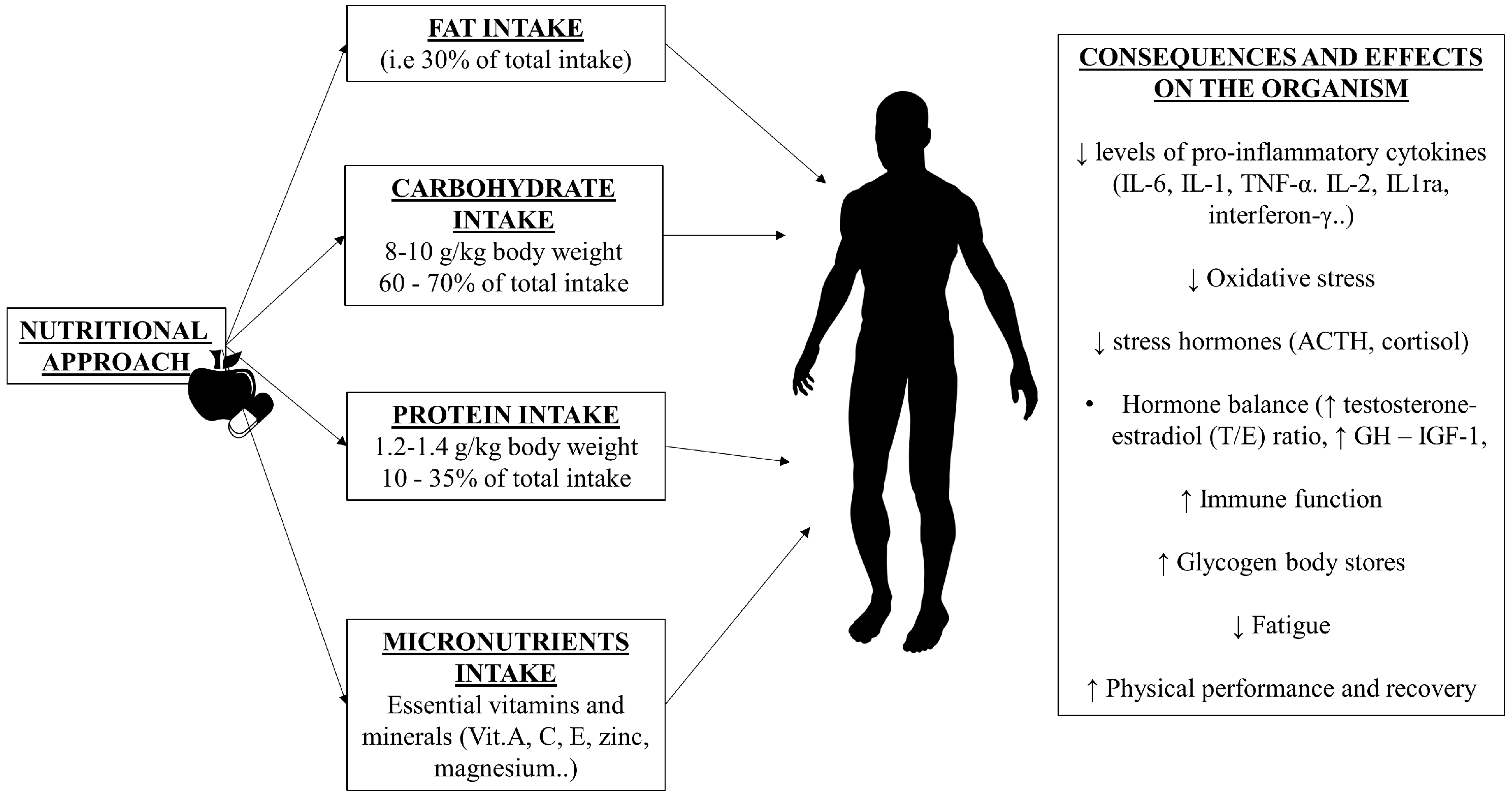
3. Dietary Intake in OTS
3.1. Dietary Fat
3.2. Dietary Protein
3.3. Dietary Carbohydrate
4. Micronutrients and OTS
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamlin, M.J.; Wilkes, D.; Elliot, C.A.; Lizamore, C.A.; Kathiravel, Y. Monitoring Training Loads and Perceived Stress in Young Elite University Athletes. Front. Physiol. 2019, 29, 10–34. [Google Scholar] [CrossRef]
- Kellmann, M. Preventing overtraining in athletes in high-intensity sports and stress/recovery monitoring. Scand. J. Med. Sci. Sports 2010, 20, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. European College of Sport Science, & American College of Sports Medicine. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [PubMed]
- Halson, S.L.; Jeukendrup, A.E. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004, 34, 967–981. [Google Scholar] [CrossRef]
- Carter, J.G.; Potter, A.W.; Brooks, K.A. Overtraining syndrome: Causes, consequences, and methods for prevention. J. Sport Hum. Perf. 2014, 2, 1–14. [Google Scholar]
- Budgett, R.; Newsholme, E.; Lehmann, M.; Sharp, C.; Jones, D.; Jones, T.; Peto, T.; Collins, D.; Nerukar, R.; White, P. Redefining the overtraining syndrome as the unexplained underperformance syndrome. Br. J. Sports Med. 2000, 34, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Nederhof, E.; Lemmink, K.A.P.M.; Visscher, C.; Meeusen, R.; Mulder, T. Psychomotor Speed: Possibly a new marker for overtraining syndrome. Sports Med. 2006, 36, 817–828. [Google Scholar] [CrossRef]
- Sims, S. The overtraining syndrome and endurance athletes. Strength Cond. J. 2001, 23, 45–46. [Google Scholar] [CrossRef]
- Kreider, R. Central Fatigue Hypothesis and Overtraining. In Overtraining in Sport; Kreider, R., Fry, A.C., O’Toole, M.L., Eds.; Human Kinetics: Champaign, IL, USA, 1998; pp. 309–334. [Google Scholar]
- Snyder, A. Overtraining and glycogen depletion hypothesis. Med. Sci. Sports Exerc. 1998, 30, 1146–1150. [Google Scholar] [CrossRef]
- Smith, L. Tissue trauma: The underlying cause of overtraining syndrome? J. Strength Cond. Res. 2004, 18, 185–193. [Google Scholar] [CrossRef]
- Smith, L. Cytokine hypothesis of overtraining: A physiological adaptation to excessive stress? Med. Sci. Sports Exerc. 2000, 32, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Blannin, A.K.; Robson, P.J.; Gleeson, M. Glutamine, exercise and immune function. Sports Med. 1998, 26, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Hsiao, Y.T.; Huang, W.C. Physiological and Psychological Effects of Treadmill Overtraining Implementation. Biology 2021, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.; MacKinnon, L.T.; Hanrahan, S. Mood states as an indication of staleness and recovery. Int. J. Sport Psychol. 1997, 28, 1–12. [Google Scholar]
- Koutedakis, Y.; Sharp, N.C. Seasonal variations of injury and overtraining in elite athletes. Clin. J. Sport Med. 1998, 8, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Raglin, J.; Sawamura, S.; Alexiou, S.; Hassmen, P. Training practice and staleness in 13–18-yearold swimmers: A cross-cultural study. Ped. Exerc. Sci. 2000, 12, 61–70. [Google Scholar] [CrossRef]
- Morgan, W.P.; O’Connor, P.; Sparling, P.; Pate, R.R. Psychological characterizations of the elite female distance runner. Int J Sports Med. 1987, 8, 124–131. [Google Scholar] [CrossRef]
- Matos, N.F.; Winsley, R.J.; Williams, C.A. Prevalence of non-functional overreaching/overtraining in young English athletes. Med. Sci. Sports Exerc. 2011, 43, 1287–1294. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Leonard, B.E. Inflammation, depression and dementia: Are they connected? Neurochem. Res. 2007, 32, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Dalgleish, A. Infection, immunoregulation, and cancer. Immunol. Rev. 2011, 240, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Sanchis-Gomar, F.; Martinez-Bello, V.; Gomez-Cabrera, M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Phillips, E.M. ACSM’s Exercise is Medicine™: A Clinician’s Guide to Exercise Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Eichner, E.R. Overtraining: Consequences and prevention. J. Sports Sci. 1995, 13, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.C. The role of training intensity in resistance exercise overtraining and overreaching. Overtrain. Sport 1998, 127, 107–127. [Google Scholar]
- Rayavarapu, S.; Coley, W.; Nagaraju, K. Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr. Rheumatol. Rep. 2012, 14, 238–243. [Google Scholar] [CrossRef]
- Gabellec, M.M.; Griffais, R.; Fillion, G.; Haour, F. Expression of interleukin 1 alpha, interleukin 1 beta and interleukin 1 receptor antagonist mRNA in mouse brain: Regulation by bacterial lipopolysaccharide (LPS) treatment. Brain Res. Mol. 1995, 31, 122–130. [Google Scholar] [CrossRef]
- Layé, S.; Parnet, P.; Goujon, E.; Dantzer, R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res. Mol. 1994, 27, 157–162. [Google Scholar] [CrossRef]
- Layé, S.; Gheusi, G.; Cremona, S.; Combe, C.; Kelley, K.; Dantzer, R.; Parnet, P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R93–R98. [Google Scholar] [CrossRef]
- Romanatto, T.; Cesquini, M.; Amaral, M.E.; Roman, E.A.; Moraes, J.C.; Torsoni, M.A.; Cruz-Neto, A.P.; Velloso, L.A. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient--effects on leptin and insulin signaling pathways. Peptides 2007, 28, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; da Rocha, A.L.; Pauli, J.R.; Ropelle, E.R.; de Souza, C.T.; Cintra, D.E.; Sant’Ana, M.R.; da Silva, A.S. Excessive eccentric exercise leads to transitory hypothalamic inflammation, which may contribute to the low body weight gain and food intake in overtrained mice. Neuroscience 2015, 311, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; da Rocha, A.L.; Pereira, B.C.; Oliveira, L.D.; Morais, G.P.; Moura, L.P.; Ropelle, E.R.; Pauli, J.R.; da Silva, A.S. Excessive training is associated with endoplasmic reticulum stress but not apoptosis in the hypothalamus of mice. Appl. Physiol. Nutr. Metab. 2017, 42, 354–360. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, A.L.; Pinto, A.P.; Kohama, E.B.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.S. The proinflammatory effects of chronic excessive exercise. Cytokine 2019, 119, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Weeks, K.L.; Pretorius, L.; McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol. Ther. 2010, 128, 191–227. [Google Scholar] [CrossRef]
- da Rocha, A.L.; Teixeira, G.R.; Pinto, A.P.; de Morais, G.P.; Oliveira, L.D.C.; de Vicente, L.G.; da Silva, L.E.C.M.; Pauli, J.R.; Cintra, D.E.; Ropelle, E.R.; et al. Excessive training induces molecular signs of pathologic cardiac hypertrophy. J. Cell Physiol. 2018, 233, 8850–8861. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, N.; Pedersen, B.K. Exercise-induced immunosuppresion- plasma glutamine is not the link. J. Appl. Physiol. 2002, 93, 813–822. [Google Scholar] [CrossRef]
- Mackinnon, L.T.; Hooper, S.L. Plasma glutamine and upper respiratory tract infection during intensified training in swimmers. Med. Sci. Sports Exerc. 1996, 28, 285–290. [Google Scholar]
- Kreher, J.B.; Schwartz, J.B. Overtraining syndrome: A practical guide. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef]
- Angeli, A.; Minetto, M.; Dovio, A.; Paccotti, P. The overtraining syndrome in athletes: A stressrelated disorder. J. Endocrinol. Investig. 2004, 27, 603–612. [Google Scholar] [CrossRef]
- Lehmann, M.; Foster, C.; Keul, J. Overtraining in endurance athletes: A brief review. Med. Sci. Sports Exerc. 1993, 25, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.L.; Noakes, T.D.; Levy, W.; Smith, C.; Millar, R. Hypothalamic dysfunction in overtrained athletes. J. Clin. Endocrinol. Metab. 1985, 60, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Urhausen, A.; Kindermann, W. Diagnosis of overtraining—What tools do we have? Sports Med. 2002, 32, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; VanHeest, J.L. The unknown mechanism of the overtraining syndrome: Clues from depression and psychoneuroimmunology. Sports Med. 2002, 32, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.P.; Brown, D.R.; Raglin, J.S.; O’Connor, P.J.; Ellickson, K.A. Psychological monitoring of overtraining and staleness. Br. J. Sports Med. 1987, 21, 107–114. [Google Scholar] [CrossRef]
- Budgett, R.; Hiscock, N.; Arida, R.M.; Castell, L.M. The effects of the 5-HT2C agonist m-chlorophenylpiperazine on elite athletes with unexplained underperformance syndrome (overtraining). Br. J. Sports Med. 2010, 44, 280–283. [Google Scholar] [CrossRef]
- Castell, L.M.; Poortmans, J.R.; Leclercq, R.; Brasseur, M.; Duchateau, J.; Newsholme, E.A. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 47–53. [Google Scholar] [CrossRef]
- Costill, D.L.; Flynn, M.G.; Kirwan, J.P.; Houmard, J.A.; Mitchell, J.B.; Thomas, R.; Park, S.H. Effects of repeated days of intensified training on muscle glycogen and swimming performance. Med. Sci. Sports Exerc. 1988, 20, 249–254. [Google Scholar] [CrossRef]
- Alghannam, A.F.; Ghaith, M.M.; Alhussain, M.H. Regulation of Energy Substrate Metabolism in Endurance Exercise. Int. J. Environ. Res. Public Health 2021, 7, 4963. [Google Scholar] [CrossRef]
- Gleeson, M. Biochemical and immunological markers of over-training. J. Sports Sci. Med 2002, 1, 31–41. [Google Scholar]
- Kentta, G.; Hassmen, P. Overtraining and recovery: A conceptual model. Sports Med. 1998, 26, 1–16. [Google Scholar] [CrossRef]
- Pichot, V.; Roche, F.; Gaspoz, F.E. Relation between heart rate variability and training load in middle-distance runners. Med. Sci. Sports Exerc. 2000, 32, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Fry, R.W.; Grove, J.R.; Morton, A.R.; Zeroni, P.M.; Gaudieri, S.; Keast, D. Psychological and immunological correlates of acute overtraining. Br. J. Sports Med. 1994, 28, 241–246. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Liu, Y.; Zhang, Z. Effects of Exercise-Induced ROS on the Pathophysiological Functions of Skeletal Muscle. Oxid. Med. Cell Longev. 2021, 2021, 3846122. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Kajaia, T.; Maskhulia, L.; Chelidze, K.; Akhalkatsi, V.; McHedlidze, T. Implication of relationship between oxidative stress and antioxidant status in blood serum. Georgian Med. News 2018, 284, 71–76. [Google Scholar]
- Lewis, N.A.; Redgrave, A.; Homer, M.; Burden, R.; Martinson, W.; Moore, B.; Pedlar, C.R. Alterations in Redox Homeostasis During Recovery from Unexplained Underperformance Syndrome in an Elite International Rower. Int. J. Sports Physiol. Perform. 2018, 13, 107–111. [Google Scholar] [CrossRef]
- Tanskanen, M.; Atalay, M.; Uusitalo, A. Altered oxidative stress in overtrained athletes. J. Sports Sci. 2010, 28, 309–317. [Google Scholar] [CrossRef]
- Friedl, K.E.; Moore, R.J.; Hoyt, R.W.; Marchitelli, L.J.; Martinez-Lopez, L.E.; Askew, E.W. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J. Appl. Physiol. 2000, 88, 1820–1830. [Google Scholar] [CrossRef]
- Nindl, B.C.; Barnes, B.R.; Alemany, J.A.; Frykman, P.N.; Shippee, R.L.; Friedl, K.E. Physiological consequences of U.S. Army Ranger training. Med. Sci. Sports Exerc. 2007, 39, 1380–1387. [Google Scholar] [CrossRef]
- Szivak, T.K.; Kraemer, W.J. Physiological Readiness and Resilience: Pillars of Military Preparedness. J. Strength Cond. Res. 2015, 29, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.E.; Arrington, L.J.; Turcotte, L.P.; Kelly, K.R. Hormonal balance and nutritional intake in elite tactical athletes. Steroids 2019, 152, 108504. [Google Scholar] [CrossRef]
- Lowery, L.; Forsythe, C.E. Protein and overtraining: Potential applications for free-living athletes. J. Int. Soc. Sports Nutr. 2006, 3, 42–50. [Google Scholar] [CrossRef]
- Woods, A.L.; Garvican-Lewis, L.A.; Lundy, B.; Rice, A.J.; Thompson, K.G. New approaches to determine fatigue in elite athletes during intensified training: Resting metabolic rate and pacing profile. PLoS ONE 2017, 12, e0173807. [Google Scholar] [CrossRef] [PubMed]
- Melin, A.K.; Heikura, I.A.; Tenforde, A.; Mountjoy, M. Energy Availability in Athletics: Health, Performance, and Physique. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Barley, O.R.; Chapman, D.W.; Blazevich, A.J.; Abbiss, C.R. Acute Dehydration Impairs Endurance Without Modulating Neuromuscular Function. Front. Physiol. 2018, 9, 1562. [Google Scholar] [CrossRef]
- Jones, L.C.; Cleary, M.A.; Lopez, R.M.; Zuri, R.E.; Lopez, R. Active dehydration impairs upper and lower body anaerobic muscular power. J. Strength Cond. Res. 2008, 22, 455–463. [Google Scholar] [CrossRef]
- Casa, D.J. Fundamentals of thermal physiology, performance implications, and dehydration. J. Athl. Train. 1999, 34, 246–252. [Google Scholar]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef]
- Murray, R. Dehydration, hyperthermia, and athletes: Science and practice. J. Athl. Train. 1996, 31, 248–252. [Google Scholar] [PubMed]
- Cadegiani, F.A.; Kater, C.E. Eating, Sleep, and Social Patterns as Independent Predictors of Clinical, Metabolic, and Biochemical Behaviors Among Elite Male Athletes: The EROS-PREDICTORS Study. Front. Endocrinol. 2020, 26, 414. [Google Scholar] [CrossRef] [PubMed]
- Nindl, B.C.; Leone, C.D.; Tharion, W.J.; Johnson, R.F.; Castellani, J.W.; Patton, J.F.; Montain, S.J. Physical performance responses during 72 h of military operational stress. Med. Sci. Sports Exerc. 2002, 34, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, L.E.; Rose, R.M.; Jennings, J.R. Suppression of plasma testosterone levels and psychological stress. A longitudinal study of young men in Officer Candidate School. Arch. Gen. Psychiatry 1972, 26, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Henning, P.C.; Scofield, D.E.; Spiering, B.A.; Staab, J.S.; Matheny, R.W., Jr.; Smith, M.A.; Bhasin, S.; Nindl, B.C. Recovery of endocrine and inflammatory mediators following an extended energy deficit. J. Clin. Endocrinol. Metab. 2014, 99, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, J.T.; Feng, X.; Pendergast, D.R. Effects of dietary fat and endurance exercise on plasma cortisol, prostaglandin E2, interferon-gamma and lipid peroxides in runners. J. Am. Coll. Nutr. 2001, 20, 529–536. [Google Scholar] [CrossRef]
- Lambert, E.V.; Speechly, D.P.; Dennis, S.C.; Noakes, T.D. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 287–293. [Google Scholar] [CrossRef]
- Muoio, D.M.; Leddy, J.J.; Horvath, P.J.; Awad, A.B.; Pendergast, D.R. Effect of dietary fat on metabolic adjustments to maximal VO2 and endurance in runners. Med. Sci. Sports Exerc. 1994, 26, 81–88. [Google Scholar] [CrossRef]
- Horvath, P.J.; Eagen, C.K.; Fisher, N.M.; Leddy, J.J.; Pendergast, D.R. The effects of varying dietary fat on performance and metabolism in trained male and female runners. J. Am. Coll. Nutr. 2000, 19, 52–60. [Google Scholar] [CrossRef]
- Bishop, N.C.; Blannin, A.K.; Walsh, N.P.; Robson, P.J.; Gleeson, M. Nutritional aspects of immunosuppression in athletes. Sports Med. 1999, 28, 151–176. [Google Scholar] [CrossRef]
- Hämäläinen, E.K.; Adlercreutz, H.; Puska, P.; Pietinen, P. Decrease of serum total and free testosterone during a low-fat high-fibre diet. J. Steroid. Biochem. 1983, 18, 369–370. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 13, 601939. [Google Scholar] [CrossRef]
- Hämäläinen, E.; Adlercreutz, H.; Puska, P.; Pietinen, P. Diet and serum sex hormones in healthy men. J. Steroid. Biochem. 1984, 20, 459–464. [Google Scholar] [CrossRef]
- Dorgan, J.F.; Judd, J.T.; Longcope, C.; Brown, C.; Schatzkin, A.; Clevidence, B.A.; Campbell, W.S.; Nair, P.P.; Franz, C.; Kahle, L.; et al. Effects of dietary fat and fiber on plasma and urine androgens and estrogens in men: A controlled feeding study. Am. J. Clin. Nutr. 1996, 64, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Abdelhamid, A.; Bunn, D.; Brown, T.; Summerbell, C.D.; Skeaff, C.M. Effects of total fat intake on body weight. Cochrane Database Syst. Rev. 2015, 2015, CD011834. [Google Scholar] [CrossRef]
- Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series, No. 916; World Health Organization: Geneva, Switzerland, 2003.
- Nations, Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition Paper 91; Food and Agriculture Organization of the United: Rome, Italy, 2010.
- Poffé, C.; Ramaekers, M.; Van Thienen, R.; Hespel, P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. Physiol. J. 2019, 597, 3009–3027. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise&Sports Nutrition Review Update: Research&Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar]
- Nieman, D.C.; Pedersen, B.K. Exercise and immune function: Recent developments. Sports Med. 1999, 28, 73–80. [Google Scholar] [CrossRef]
- Bopp, M.J.; Houston, D.K.; Lenchik, L.; Easter, L.; Kritchevsky, S.B.; Nicklas, B.J. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J. Am. Diet. Assoc. 2008, 108, 1216–1220. [Google Scholar] [CrossRef]
- Cintineo, H.P.; Arent, M.A.; Antonio, J.; Arent, S.M. Effects of Protein Supplementation on Performance and Recovery in Resistance and Endurance Training. Front. Nutr. 2018, 5, 83. [Google Scholar] [CrossRef]
- MacNeil, B.; Hoffman-Goetz, L.; Kendall, A.; Houston, M.; Arumugam, Y. Lymphocyte proliferation responses after exercise in men: Fitness, intensity, and duration effects. J. Appl. Physiol. 1991, 70, 179–184. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Toft, A.D. Effects of exercise on lymphocytes and cytokines. Br. J. Sports Med. 2000, 34, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Brendle, D.; Henson, D.A.; Suttles, J.; Cook, V.D.; Warren, B.J.; Butterworth, D.E.; Fagoaga, O.R.; Nehlsen-Cannarella, S.L. Immune function in athletes versus non-athletes. Int. J. Sports Med. 1995, 16, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.M.; Newsholme, E.A. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition 1997, 13, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Shewchuk, L.D.; Baracos, V.E.; Field, C.J. Dietary L-glutamine does not improve lymphocyte metabolism or function in exercise trained rats. Med. Sci. Sports Exerc. 1997, 29, 474–481. [Google Scholar] [CrossRef]
- Crewther, B.; Keogh, J.; Cronin, J.; Cook, C. Possible stimuli for strength and power adaptation: Acute hormonal responses. Sports Med. 2006, 36, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.J.; Castracane, V.D.; Hollander, D.B.; Tryniecki, J.L.; Bamman, M.M.; O’Neal, S.; Hebert, E.P.; Kraemer, R.R. Hormonal responses from concentric and eccentric muscle contractions. Med. Sci. Sports Exerc. 2003, 35, 937–943. [Google Scholar] [CrossRef]
- Hayes, L.D.; Grace, F.M.; Baker, J.S.; Sculthorpe, N. Resting steroid hormone concentrations in lifetime exercisers and lifetime sedentary males. Aging Male 2015, 18, 22–26. [Google Scholar] [CrossRef]
- Shaner, A.A.; Vingren, J.L.; Hatfield, D.L.; Budnar, R.G., Jr.; Duplanty, A.A.; Hill, D.W. The acute hormonal response to free weight and machine weight resistance exercise. J. Strength Cond. Res. 2014, 28, 1032–1040. [Google Scholar] [CrossRef]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Hormonal response to a non-exercise stress test in athletes with overtraining syndrome: Results from the Endocrine and metabolic Responses on Overtraining Syndrome (EROS)—EROS-STRESS. J. Sci. Med. Sport 2018, 21, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Darcy, J. GH and ageing: Pitfalls and new insights. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.B.; Dixit, M.; Neginskaya, M.; Nagaraj, K.; Pavlov, E.; Werner, H.; Yakar, S. Effects of GH/IGF on the Aging Mitochondria. Cells 2020, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef]
- Moøller, N.; Joørgensen, J.O.L. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Wurzburger, M.I.; Prelevic, G.M.; Sonksen, P.H.; Balint-Peric, L.A.; Wheeler, M. The effect of recombinant human growth hormone on regulation of growth hormone secretion and blood glucose in insulin-dependent diabetes. J. Clin. Endocrinol. Metab. 1993, 77, 267–272. [Google Scholar]
- Tarnopolsky, M. Protein requirements for endurance athletes. Nutrition 2004, 20, 662–668. [Google Scholar] [CrossRef]
- Fielding, R.A.; Parkington, J. What are the dietary protein requirements of physically active individuals? New evidence on the effects of exercise on protein utilization during post-exercise recovery. Nutr. Clin. Care 2002, 5, 191–196. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Acad. Nutr. Diet. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Layman, D.K. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr. Metab. 2009, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Gastmann, U.A.; Lehmann, M.J. Overtraining and the BCAA hypothesis. Med. Sci. Sports Exerc. 1998, 30, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Fang, C.C.; Lee, Y.H.; Yang, M.T.; Chan, K.H. Effects of 4-Week Creatine Supplementation Combined with Complex Training on Muscle Damage and Sport Performance. Nutrients 2018, 10, 1640. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Avelar, A.; Kassiano, W.; Nunes, J.P.; Schoenfeld, B.J.; Aguiar, A.F.; Trindade, M.C.C.; Silva, A.M.; Sardinha, L.B.; Cyrino, E.S. Creatine supplementation does not influence the ratio between intracellular water and skeletal muscle mass in resistance-trained men. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M. High-Quality Carbohydrates and Physical Performance: Expert Panel Report. Nutr. Today 2018, 53, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Kiens, B.; Ivy, J.L. Carbohydrates and fat for training and recovery. J. Sports Sci. 2004, 22, 15–30. [Google Scholar] [CrossRef]
- Manfredi, T.G.; Fielding, R.A.; O’Reilly, K.P.; Meredith, C.N.; Lee, H.Y.; Evans, W.J. Plasma creatine kinase activity and exercise-induced muscle damage in older men. Med. Sci. Sports Exerc. 1991, 23, 1028–1034. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Costill, D.L.; Houmard, J.A.; Flynn, M.G.; Fink, W.J.; Beltz, J.D. Influence of carbohydrate ingestion on counterregulatory hormones during prolonged exercise. Int. J. Sports Med. 1990, 11, 33–36. [Google Scholar] [CrossRef]
- Murray, R.; Paul, G.L.; Seifert, J.G.; Eddy, D.E. Responses to varying rates of carbohydrate ingestion during exercise. Med. Sci. Sports Exerc. 1991, 23, 713–718. [Google Scholar] [CrossRef]
- Hadley, A.R.; Tran, L.T.; Fagoaga, O.R.; Nehlsen-Cannarella, S.L.; Yellon, S.M. Sex differences in photoperiod control of antigen-specific primary and secondary humoral immunity in Siberian Hamsters. J. Neuroimmunol. 2002, 128, 39–48. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C. Special feature for the Olympics: Effect of exercise on the immune system: Modification of immune responses to exercise by carbohydrate, glutamine and anti-oxidant supplements. Immunol. Cell Biol. 2000, 78, 554–561. [Google Scholar]
- Cadegiani, F.A.; Kater, C.E. Basal hormones and biochemical markers as predictors of OTS: Results from the Endocrine and metabolic Responses on Overtraining Syndrome (EROS) study—EROS-BASAL. J. Athl. Train. 2019, 54, 906–914. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef]
- Patrice, F.; Céline, K.; Defour, J.P. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar]
- Suh, B.; Shin, D.W.; Kwon, H.M.; Yun, J.M.; Yang, H.K.; Ahn, E.; Lee, H.; Park, J.H.; Cho, B. Elevated neutrophil to lymphocyte ratio and ischemic stroke risk in generally healthy adults. PLoS ONE 2017, 12, e0183706. [Google Scholar] [CrossRef]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef]
- Hofman, D.L.; van Buul, V.J.; Brouns, F.J. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Rombaldi, A.J.; Leite, C.F.; Hartleben, C.P.; Medeiros, T.H. Effects of carbohydrate supplementation and different types of exercise training on blood cells concentrations. Rev. Bras. Med. Esporte 2013, 19, 200–203. [Google Scholar] [CrossRef]
- Khorshidi-Hosseini, M.; Nakhostin-Roohi, B. Effect of glutamine and maltodextrin acute supplementation on anaerobic power. Asian J. Sports Med. 2013, 4, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ghazzawi, H.A.; Hussain, M.A.; Raziq, K.M.; Alsendi, K.K.; Alaamer, R.O.; Jaradat, M.; Alobaidi, S.; Al Aqili, R.; Trabelsi, K.; Jahrami, H. Exploring the Relationship between Micronutrients and Athletic Performance: A Comprehensive Scientific Systematic Review of the Literature in Sports Medicine. Sports 2023, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Khanna, D. The Role of Vitamin C in Human Immunity and Its Treatment Potential Against COVID-19: A Review Article. Cureus 2023, 15, e33740. [Google Scholar] [CrossRef]
- Thompson, S.H. Characteristics of the female athlete triad in collegiate cross-country runners. J. Am. Coll. Health 2007, 56, 129–136. [Google Scholar] [CrossRef]
- Li, S.; Fasipe, B.; Laher, I. Potential harms of supplementation with high doses of antioxidants in athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef]
- Holzhauer, P. Micronutrient therapy with selenium, vitamin C, and L carnitine in gynecological oncology: Areas of application and studies. Gynakologe 2017, 50, 15–21. [Google Scholar] [CrossRef]
- De Brito, E.; Teixeira, A.D.O.; Righi, N.C.; Paulitcth, F.D.S.; da Silva, A.M.V.; Signori, L.U. Vitamins C and E Associated with Cryotherapy in the Recovery of the Inflammatory Response After Resistance Exercise: A Randomized Clinical Trial. J. Strength Cond. Res. 2022, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, H.; Karczemna, A.; Włodarek, D. Nutrition for Female Soccer Players—Recommendations. Medicina 2020, 56, 28. [Google Scholar] [CrossRef]
- Purcell, L.; Society, C.P. Paediatric Sports and Exercise Medicine Section Sport nutrition for young athletes. Paediatr. Child Health 2013, 18, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, H.; Tanaka, M.; Nozaki, S.; Mizuno, K.; Tahara, T.; Ataka, S.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Daily oral administration of crocetin attenuates physical fatigue in human subjects. Nutr. Res. 2009, 29, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gaeini, A.A.; Rahnama, N.; Hamedinia, M.R. Effects of vitamin E supplementation on oxidative stress at rest and after exercise to exhaustion in athletic students. J. Sport Med. Phys. Fit 2006, 46, 458. [Google Scholar]
- Wierzejska, R.E. Dietary Supplements-For Whom? The Current State of Knowledge about the Health Effects of Selected Supplement Use. Int. J. Environ. Res. Public Health 2021, 18, 8897. [Google Scholar] [CrossRef]
- Erickson, J.M.; Mawson, A.R. Possible role of endogenous retinoid (Vitamin A) toxicity in the pathophysiology of primary biliary cirrhosis. J. Theor. Biol. 2000, 206, 47–54. [Google Scholar] [CrossRef]
- Carlsohn, A.; Braun, H.; Großhauser, M.; König, D.; Lampen, A.; Mosler, S.; Nieß, A.; Oberritter, H.; Schäbethal, K.; Schek, A.; et al. Position of the working group sports nutrition of the German Nutrition Society (DGE): Minerals and vitamins in sports nutrition. Dtsch. Z. Sportmed. 2020, 71, 208–215. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Vano, M.; Gargiulo, B.; Caiazza, M.; Amodio, F.; Coto, I.; D’Alicandro, G.; et al. The Biological Role of Vitamins in Athletes’ Muscle, Heart and Microbiota. Int. J. Environ. Res. Public Health 2022, 19, 1249. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Kochanowicz, A.; Piskorska, E.; Niespodziński, B.; Siódmiak, J.; Buśko, K.; Stankiewicz, B.; Olszewska-Słonina, D.; Antosiewicz, J. Serum levels of bone formation and resorption markers in relation to vitamin D status in professional gymnastics and physically active men during upper and lower body high-intensity exercise. J. Int. Soc. Sports Nutr. 2021, 18, 29. [Google Scholar] [CrossRef]
- Keen, D.A.; Constantopoulos, E.; Konhilas, J.P. The impact of post-exercise hydration with deep-ocean mineral water on rehydration and exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 17. [Google Scholar] [CrossRef]
- Peeling, P.; Fulton, S.K.; Binnie, M.; Goodman, C. Training environment and Vitamin D status in athletes. Int. J. Sports Med. 2013, 34, 248–252. [Google Scholar] [CrossRef]
- Vallieres, F.; Tremblay, A.; St-Jean, L. Study of the energy balance and the nutritional status of highly trained female swimmers. Nutr. Res. 1989, 9, 699–703. [Google Scholar] [CrossRef]
- Benson, J.; Gillien, D.M.; Bourdet, K.; Loosli, A.R. Inadequate Nutrition and Chronic Calorie Restriction in Adolescent Ballerinas. Phys. Sportsmed. 1985, 13, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Aminaei, M.; Shamsi, E.H.; Nikoei, R. The impact of eight weeks of calcium intake and vitamin D along with TRX exercise on body composition and lipid profiles of overweight women. Obes. Med. 2020, 19, 100249. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Bysani, C.; Venkatraman, J.T.; Tomar, V.; Zhao, W. Increased TGF-beta and decreased oncogene expression by omega-3 fatty acids in the spleen delays onset of autoimmune disease in B/W mice. J. Immunol. 1994, 152, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.V.; Lundsgaard, A.M.; Christensen, P.M.; Christensen, L.; Randers, M.B.; Mohr, M.; Nybo, L.; Kiens, B.; Fritzen, A.M. Nutritional optimization for female elite football players—Topical review. Scand. J. Med. Sci. Sport 2022, 32, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef]
- Kopp-Woodroffe, S.A.; Manore, M.M.; Dueck, C.A.; Skinner, J.S.; Matt, K.S. Energy and nutrient status of amenorrheic athletes participating in a diet and exercise training intervention program. Int. J. Sport Nutr. 1999, 9, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Vincent, J.B.; Neggers, Y.; McClung, J. Roles of Chromium(III), Vanadium, Iron, and Zinc in Sports Nutrition. In Nutrition and Enhanced Sports Performance; Elsevier: Amsterdam, The Netherlands, 2018; pp. 653–664. [Google Scholar]
- IAlaunyte, I.; Stojceska, V.; Plunkett, A. Iron and the female athlete: A review of dietary treatment methods for improving iron status and exercise performance. J. Int. Soc. Sport Nutr. 2015, 12, 38. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Martínez, A.C.; Seco-Calvo, J. The Role of Selenium Mineral Trace Element in Exercise: Antioxidant Defense System, Muscle Performance, Hormone Response, and Athletic Performance. A Systematic Review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Barrientos, G.; Alves, J.; Pradas, F.; Robles, M.C.; Muñoz, D.; Maynar, M. Association between Parameters Related to Oxidative Stress and Trace Minerals in Athletes. Sustainability 2020, 12, 4966. [Google Scholar] [CrossRef]
- Mel’Nikov, A.A.; Vikulov, A.D. Relationship between mineral metabolism and blood rheology in athletes. Fiziol. Cheloveka 2003, 29, 48–56. [Google Scholar]
- Sone, R.; Nakazawa, S.; Ohishi, K. Efficacy of mineral-rich antioxidant supplements on oxidative stress markers and exercise performance. Gazz. Med. Ital. Arch. Sci. Med. 2022, 181, 295–302. [Google Scholar] [CrossRef]
- Williams, M.H. Dietary Supplements and Sports Performance: Minerals. J. Int. Soc. Sports Nutr. 2005, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.L.; Albracht-Schulte, K.; Robert-McComb, J.J. Micronutrient deficiency in athletes and inefficiency of supplementation: Is low energy availability a culprit? PharmaNutrition 2020, 14, 100229. [Google Scholar] [CrossRef]
- Serra, M.C.; Beavers, K.M. Essential and nonessential micronutrients and sport. In Nutritional Supplements in Sports and Exercise, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Burgos-Jara, C.; Cerda-Kohler, H.; Aedo-Muñoz, E.; Miarka, B. Eccentric Resistance Training: A Methodological Proposal of Eccentric Muscle Exercise Classification Based on Exercise Complexity, Training Objectives, Methods, and Intensity. Appl. Sci. 2023, 13, 7969. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Rehabilitation Nutrition for Injury Recovery of Athletes: The Role of Macronutrient Intake. Nutrients 2020, 12, 2449. [Google Scholar] [CrossRef]
- Noakes, T.D. What Is the Evidence That Dietary Macronutrient Composition Influences Exercise Performance? A Narrative Review. Nutrients 2022, 14, 862. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 863. [Google Scholar] [CrossRef]
- Whittaker, J.; Harris, M. Low-carbohydrate diets and men’s cortisol and testosterone: Systematic review and meta-analysis. Nutr. Health 2022, 28, 543–554, Correction in Nutr. Health 2022, 28, 783. [Google Scholar] [CrossRef]
- Food and Nutrition Board (FNB) of the Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fibre, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005; Available online: https://nap.nationalacademies.org/catalog/10490/dietary-reference-intakes-for-energy-carbohydrate-fiber-fat-fatty-acids-cholesterol-protein-and-amino-acids (accessed on 22 November 2023).
- Jannas-Vela, S.; Espinosa, A.; Candia, A.A.; Flores-Opazo, M.; Peñailillo, L.; Valenzuela, R. The Role of Omega-3 Polyunsaturated Fatty Acids and Their Lipid Mediators on Skeletal Muscle Regeneration: A Narrative Review. Nutrients 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef]
- Leong, W.Y.A.; Ngiam, J.N.; Tan, R.S.; Lim, S.L.; Poh, K.K. Controversies and discrepancies in the effect of dietary fat and cholesterol on cardiovascular risk. Singapore Med. J. 2021, 62, 56–62. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
la Torre, M.E.; Monda, A.; Messina, A.; de Stefano, M.I.; Monda, V.; Moscatelli, F.; Tafuri, F.; Saraiello, E.; Latino, F.; Monda, M.; et al. The Potential Role of Nutrition in Overtraining Syndrome: A Narrative Review. Nutrients 2023, 15, 4916. https://doi.org/10.3390/nu15234916
la Torre ME, Monda A, Messina A, de Stefano MI, Monda V, Moscatelli F, Tafuri F, Saraiello E, Latino F, Monda M, et al. The Potential Role of Nutrition in Overtraining Syndrome: A Narrative Review. Nutrients. 2023; 15(23):4916. https://doi.org/10.3390/nu15234916
Chicago/Turabian Stylela Torre, Maria Ester, Antonietta Monda, Antonietta Messina, Maria Ida de Stefano, Vincenzo Monda, Fiorenzo Moscatelli, Francesco Tafuri, Emma Saraiello, Francesca Latino, Marcellino Monda, and et al. 2023. "The Potential Role of Nutrition in Overtraining Syndrome: A Narrative Review" Nutrients 15, no. 23: 4916. https://doi.org/10.3390/nu15234916
APA Stylela Torre, M. E., Monda, A., Messina, A., de Stefano, M. I., Monda, V., Moscatelli, F., Tafuri, F., Saraiello, E., Latino, F., Monda, M., Messina, G., Polito, R., & Tafuri, D. (2023). The Potential Role of Nutrition in Overtraining Syndrome: A Narrative Review. Nutrients, 15(23), 4916. https://doi.org/10.3390/nu15234916










