Effect of Aromatic Herbs and Spices Present in the Mediterranean Diet on the Glycemic Profile in Type 2 Diabetes Subjects: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Outcome Measures
2.3. Data Collection and Data Synthesis
2.4. Statistical Analysis
2.5. Quality Measures
3. Results
3.1. Study Selection
3.2. Participants and Main Study Characteristics
3.3. Aromatic Herb Supplementation
3.4. Changes in Glycemic Metabolism
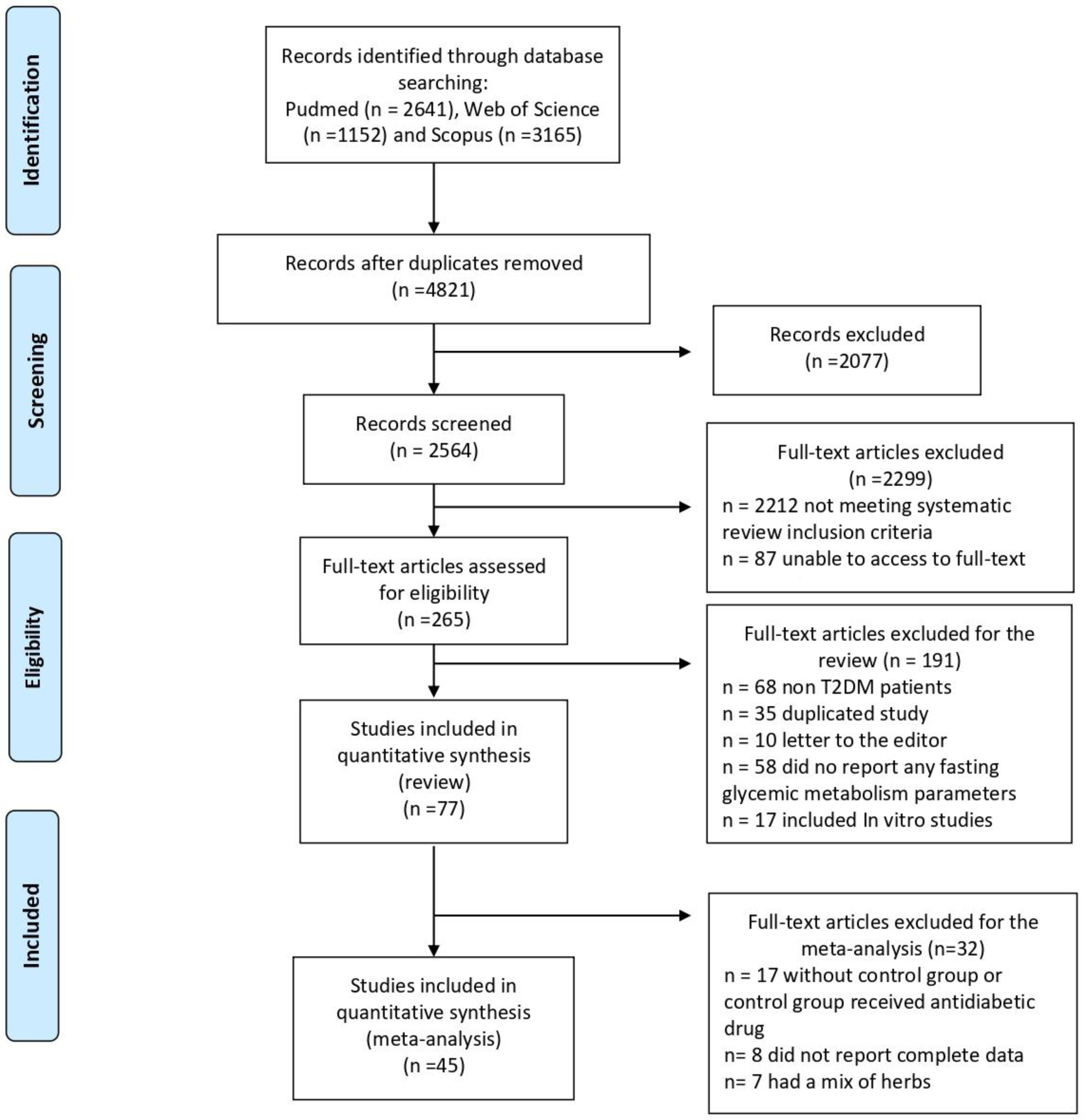
3.4.1. Fasting Glucose
3.4.2. HbA1c
3.4.3. Insulin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Holloway, S.; Polya, R.; Sloan, R.; Zhang, L.; Gregg, E.W.; Harrison, K.; Elvidge, J.; Jonsson, P.; Porter, T. Variations in Comorbidity Burden in People with Type 2 Diabetes over Disease Duration: A Population-Based Analysis of Real World Evidence. eClinicalMedicine 2022, 52, 101584. [Google Scholar] [CrossRef]
- Redondo, M.J.; Hagopian, W.A.; Oram, R.; Steck, A.K.; Vehik, K.; Weedon, M.; Balasubramanyam, A.; Dabelea, D. The Clinical Consequences of Heterogeneity within and between Different Diabetes Types. Diabetologia 2020, 63, 2040–2048. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef]
- Sumamo Schellenberg, E.; Dryden, D.M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients with and at Risk for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2013, 159, 543–551. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Estruch, R.; Salas-Salvadó, J. Towards an Even Healthier Mediterranean Diet. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1163–1166. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Corella, D.; Salas-salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort Profile: Design and Methods of the PREDIMED Study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef]
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martínez-González, M.A.; Castañer, O.; Bulló, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; et al. Mediterranean Diets and Metabolic Syndrome Status in the PREDIMED Randomized Trial. CMAJ Can. Med. Assoc. J. J. L’association Medicale Can. 2014, 186, E649–E657. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of Diabetes with Mediterranean Diets: A Subgroup Analysis of a Randomized Trial. Ann. Intern. Med. 2014, 160, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A Journey into a Mediterranean Diet and Type 2 Diabetes: A Systematic Review with Meta-Analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.I.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2018; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: https://www.R-project.org (accessed on 6 September 2023).
- Mateo-Gallego, R.; Madinaveitia-Nisarre, L.; Giné-Gonzalez, J.; María Bea, A.; Guerra-Torrecilla, L.; Baila-Rueda, L.; Perez-Calahorra, S.; Civeira, F.; Lamiquiz-Moneo, I. The Effects of High-Intensity Interval Training on Glucose Metabolism, Cardiorespiratory Fitness and Weight Control in Subjects with Diabetes: Systematic Review a Meta-Analysis. Diabetes Res. Clin. Pract. 2022, 190, 109979. [Google Scholar] [CrossRef]
- Kmet, L.M.; Lee, R.C.; Cook, L.S. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; AHFMR-HTA Initiative #13; Alberta Heritage Foundation for Medical Research (AHFMR): Edmonton, AB, USA, 2004. [Google Scholar]
- Lamiquiz-Moneo, I.; Giné-González, J.; Alisente, S.; Bea, A.M.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Jarauta, E.; Cenarro, A.; Civeira, F.; et al. Effect of Bergamot on Lipid Profile in Humans: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Lira Neto, J.C.G.; Damasceno, M.M.C.; Ciol, M.A.; de Freitas, R.W.J.F.; de Araújo, M.F.M.; Teixeira, C.R.d.S.; Carvalho, G.C.N.; Lisboa, K.W.S.C.; Marques, R.L.L.; Alencar, A.M.P.G.; et al. Efficacy of Cinnamon as an Adjuvant in Reducing the Glycemic Biomarkers of Type 2 Diabetes Mellitus: A Three-Month, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Am. Nutr. Assoc. 2022, 41, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Mang, B.; Wolters, M.; Schmitt, B.; Kelb, K.; Lichtinghagen, R.; Stichtenoth, D.O.; Hahn, A. Effects of a Cinnamon Extract on Plasma Glucose, HbA, and Serum Lipids in Diabetes Mellitus Type 2. Eur. J. Clin. Investig. 2006, 36, 340–344. [Google Scholar] [CrossRef]
- Mirfeizi, M.; Mehdizadeh Tourzani, Z.; Mirfeizi, S.Z.; Asghari Jafarabadi, M.; Rezvani, H.R.; Afzali, M. Controlling Type 2 Diabetes Mellitus with Herbal Medicines: A Triple-Blind Randomized Clinical Trial of Efficacy and Safety. J. Diabetes 2016, 8, 647–656. [Google Scholar] [CrossRef]
- Talaei, B.; Amouzegar, A.; Sahranavard, S.; Hedayati, M.; Mirmiran, P.; Azizi, F. Effects of Cinnamon Consumption on Glycemic Indicators, Advanced Glycation End Products, and Antioxidant Status in Type 2 Diabetic Patients. Nutrients 2017, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon Improves Glucose and Lipids of People with Type 2 Diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano Curcumin Supplementation Reduced the Severity of Diabetic Sensorimotor Polyneuropathy in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo- Controlled Clinical Trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Mir Taheri, M. The Effects of Ginger on Fasting Blood Sugar, Hemoglobin A1c, Apolipoprotein B, Apolipoprotein A-I and Malondialdehyde in Type 2 Diabetic Patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar] [PubMed]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.-A.; Najarzadeh, A.; Mozayan, M.R. The Effect of Ginger Powder Supplementation on Insulin Resistance and Glycemic Indices in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Complement. Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The Effect of Ginger (Zingiber officinale) on Glycemic Markers in Patients with Type 2 Diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hariri, M.; Abbasi, B. Effects of Cinnamon, Cardamom, Saffron, and Ginger Consumption on Markers of Glycemic Control, Lipid Profile, Oxidative Stress, and Inflammation in Type 2 Diabetes Patients. Rev. Diabet. Stud. 2014, 11, 258–266. [Google Scholar] [CrossRef]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Aeinehchi, A.; Sefid-Mooye Azar, P.; Hadi, A.; Ostadrahimi, A. Saffron Improves Life and Sleep Quality, Glycaemic Status, Lipid Profile and Liver Function in Diabetic Patients: A Double-Blind, Placebo-Controlled, Randomised Clinical Trial. Int. J. Clin. Pract. 2021, 75, e14334. [Google Scholar] [CrossRef]
- Darmian, M.A.; Hoseini, R.; Amiri, E.; Golshani, S. How combined and separate aerobic training and turmeric supplementation alter lipid profile and glycemic status? A clinical trial in middle-aged females with type 2 diabetes and hyperlipidemia. Int. Cardiovasc. Res. J. 2021, 15, 111–118. [Google Scholar]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The Effect of Ginger Consumption on Glycemic Status, Lipid Profile and Some Inflammatory Markers in Patients with Type 2 Diabetes Mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. Available online: https://www.tandfonline.com/doi/abs/10.3109/09637486.2014.880671?journalCode=iijf20 (accessed on 6 September 2023). [CrossRef]
- Makhdoomi Arzati, M.; Mohammadzadeh Honarvar, N.; Saedisomeolia, A.; Anvari, S.; Effatpanah, M.; Makhdoomi Arzati, R.; Yekaninejad, M.S.; Hashemi, R.; Djalali, M. The Effects of Ginger on Fasting Blood Sugar, Hemoglobin A1c, and Lipid Profiles in Patients with Type 2 Diabetes. Int. J. Endocrinol. Metab. 2017, 15, e57927. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.; Daryabeygi-Khotbehsara, R.; Mirmiran, P.; McVicar, J.; Hadi, V.; Soleimani, D.; Askari, G. Effect of Nigella Sativa Oil Extract on Cardiometabolic Risk Factors in Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2021, 35, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Kooshki, A.; Tofighiyan, T.; Rastgoo, N.; Rakhshani, M.H.; Miri, M. Effect of Nigella Sativa Oil Supplement on Risk Factors for Cardiovascular Diseases in Patients with Type 2 Diabetes Mellitus. Phytother. Res. 2020, 34, 2706–2711. [Google Scholar] [CrossRef]
- Sepahi, S.; Golfakhrabadi, M.; Bonakdaran, S.; Lotfi, H.; Mohajeri, S.A. Effect of Crocin on Diabetic Patients: A Placebo-Controlled, Triple-Blinded Clinical Trial. Clin. Nutr. ESPEN 2022, 50, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Sahebkar, A.; Aryaeian, N.; Pahlavani, N.; Fallah, S.; Moradi, N.; Abbasi, D.; Hosseini, A.F. Effects of Saffron Supplementation On Inflammation and Metabolic Responses in Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. Syndr. Obes. 2019, 12, 2107–2115. [Google Scholar] [CrossRef]
- Milajerdi, A.; Jazayeri, S.; Hashemzadeh, N.; Shirzadi, E.; Derakhshan, Z.; Djazayeri, A.; Akhondzadeh, S. The Effect of Saffron (Crocus Sativus L.) Hydroalcoholic Extract on Metabolic Control in Type 2 Diabetes Mellitus: A Triple-Blinded Randomized Clinical Trial. J. Res. Med. Sci. 2018, 23, 16. [Google Scholar] [CrossRef]
- Shahbazian, H.; Moravej Aleali, A.; Amani, R.; Namjooyan, F.; Cheraghian, B.; Latifi, S.M.; Bahrainian, S.; Ghadiri, A. Effects of saffron on homocysteine, and antioxidant and inflammatory biomarkers levels in patients with type 2 diabetes mellitus: A randomized double-blind clinical trial. Avicenna J. Phytomed. 2019, 9, 436–445. [Google Scholar]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Glycated Haemoglobin and Blood Pressure-Lowering Effect of Cinnamon in Multi-Ethnic Type 2 Diabetic Patients in the UK: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabet. Med. 2010, 27, 1159–1167. [Google Scholar] [CrossRef]
- Mobasseri, M.; Ostadrahimi, A.; Tajaddini, A.; Asghari, S.; Barati, M.; Akbarzadeh, M.; Nikpayam, O.; Houshyar, J.; Roshanravan, N.; Alamdari, N.M. Effects of Saffron Supplementation on Glycemia and Inflammation in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind, Placebo-Controlled Clinical Trial Study. Diabetes Metab. Syndr. 2020, 14, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Behrouz, V.; Dastkhosh, A.; Hedayati, M.; Sedaghat, M.; Sharafkhah, M.; Sohrab, G. The effect of crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes: A pilot study. Diabetol. Metab. Syndr. 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Mirkarimi, S.A.; Amini, M.; Mohtashami, R.; Kianbakht, S.; Fallah Huseini, H. Effects of Nigella Sativa L. Seed Oil in Type II Diabetic Patients: A Randomized, Double-Blind, Placebo—Controlled Clinical Trial. J. Med. Plants 2013, 12, 93–99. [Google Scholar]
- Heshmati, J.; Namazi, N.; Memarzadeh, M.-R.; Taghizadeh, M.; Kolahdooz, F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Food Res. Int. 2015, 70, 87–93. [Google Scholar] [CrossRef]
- Moravej Aleali, A.; Amani, R.; Shahbazian, H.; Namjooyan, F.; Latifi, S.M.; Cheraghian, B. The effect of hydroalcoholic Saffron (Crocus sativus L.) extract on fasting plasma glucose, HbA1c, lipid profile, liver, and renal function tests in patients with type 2 diabetes mellitus: A randomized double-blind clinical trial. Phytother Res. 2019, 33, 1648–1657. [Google Scholar] [CrossRef]
- Carvalho, G.C.N.; Lira-Neto, J.C.G.; de Araújo, M.F.M.; de Freitas, R.W.J.F.; Zanetti, M.L.; Damasceno, M.M.C. Eficacia del jengibre en la reducción de los niveles metabólicos de personas con diabetes: Un ensayo clínico aleatorizado. Rev. Latino-Am. Enferm. 2020, 28, e3369. [Google Scholar] [CrossRef]
- Davari, M.; Hashemi, R.; Mirmiran, P.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. Effects of Cinnamon Supplementation on Expression of Systemic Inflammation Factors, NF-kB and Sirtuin-1 (SIRT1) in Type 2 Diabetes: A Randomized, Double Blind, and Controlled Clinical Trial. Nutr. J. 2020, 19, 1. [Google Scholar] [CrossRef]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Efficacy of Turmeric as Adjuvant Therapy in Type 2 Diabetic Patients. Indian J. Clin. Biochem. 2015, 30, 180–186. [Google Scholar] [CrossRef]
- El Gayar, M.H.; Aboromia, M.M.M.; Ibrahim, N.A.; Hafiz, M.H.A. Effects of ginger powder supplementation on glycemic status and lipid profile in newly diagnosed obese patients with type 2 diabetes mellitus. Obes. Med. 2019, 14, 100094. [Google Scholar] [CrossRef]
- Rostamkhani, H.; Veisi, P.; Niknafs, B.; Jafarabadi, M.A.; Ghoreishi, Z. The Effect of Zingiber Officinale on Prooxidant-Antioxidant Balance and Glycemic Control in Diabetic Patients with ESRD Undergoing Hemodialysis: A Double-Blind Randomized Control Trial. BMC Complement. Med. Ther. 2023, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Niknafs, B.; Naseri, M.; Nouri, M.; Tarighat-Esfanjani, A. Effect of Nigella Sativa Oil on Oxidative Stress, Inflammatory, and Glycemic Control Indices in Diabetic Hemodialysis Patients: A Randomized Double-Blind, Controlled Trial. Evidence.-Based Complement. Altern. Med. 2022, 2022, 2753294. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Z.; Nasiruddin, M.; Khan, R.; Haque, S. Protective Role of Nigella Sativa in Diabetic Nephropathy: A Randomized Clinical Trial. Saudi J. Kidney Dis. Transplant. 2017, 28, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Sheng, H.; Wu, J.; Cheng, Y.; Zhu, J.; Chen, Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr. Res. 2012, 32, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P. Effectiveness of Cinnamon for Lowering Hemoglobin A1C in Patients with Type 2 Diabetes: A Randomized, Controlled Trial. J. Am. Board Fam. Med. 2009, 22, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Vanaie, A.; Shahidi, S.; Iraj, B.; Siadat, Z.D.; Kabirzade, M.; Shakiba, F.; Mohammadi, M.; Parvizian, H. Curcumin as a Major Active Component of Turmeric Attenuates Proteinuria in Patients with Overt Diabetic Nephropathy. J. Res. Med. Sci. 2019, 24, 77. [Google Scholar] [CrossRef] [PubMed]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E.J. Effects of Ginger (Zingiber officinale) on Plasma Glucose Level, HbA1c and Insulin Sensitivity in Type 2 Diabetic Patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Jangjo-Borazjani, S.; Dastgheib, M.; Kiyamarsi, E.; Jamshidi, R.; Rahmati-Ahmadabad, S.; Helalizadeh, M.; Iraji, R.; Cornish, S.M.; Mohammadi-Darestani, S.; Khojasteh, Z.; et al. Effects of Resistance Training and Nigella Sativa on Type 2 Diabetes: Implications for Metabolic Markers, Low-Grade Inflammation and Liver Enzyme Production. Arch. Physiol. Biochem. 2023, 129, 913–921. [Google Scholar] [CrossRef]
- Rajabi, A.; Khajehlandi, M.; Siahkuhian, M.; Akbarnejad, A.; Khoramipour, K.; Suzuki, K. Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus. Sports 2022, 10, 167. [Google Scholar] [CrossRef]
- Vanschoonbeek, K.; Thomassen, B.J.W.; Senden, J.M.; Wodzig, W.K.W.H.; van Loon, L.J.C. Cinnamon Supplementation Does Not Improve Glycemic Control in Postmenopausal Type 2 Diabetes Patients. J. Nutr. 2006, 136, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Adab, Z.; Eghtesadi, S.; Vafa, M.-R.; Heydari, I.; Shojaii, A.; Haqqani, H.; Arablou, T.; Eghtesadi, M. Effect of Turmeric on Glycemic Status, Lipid Profile, Hs-CRP, and Total Antioxidant Capacity in Hyperlipidemic Type 2 Diabetes Mellitus Patients. Phytother. Res. 2019, 33, 1173–1181. [Google Scholar] [CrossRef]
- Najmi, A.A.; Nasiruddin, M.; Khan, R.A.; Haque, S.F. Therapeutic effect of Nigella sativa in patients of poor glycemic control. Asian J. Pharm. Clin. Res. 2012, 5, 224–228. [Google Scholar]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.R.; Raju, Y.S.N.; Chandra, N. Effect of NCB-02, Atorvastatin and Placebo on Endothelial Function, Oxidative Stress and Inflammatory Markers in Patients with Type 2 Diabetes Mellitus: A Randomized, Parallel-Group, Placebo-Controlled, 8-Week Study. Drugs R D 2008, 9, 243–250. [Google Scholar] [CrossRef]
- Jaafarinia, A.; Kafami, B.; Sahebnasagh, A.; Saghafi, F. Evaluation of therapeutic effects of crocin in attenuating the progression of diabetic nephropathy: A preliminary randomized triple-blind placebo-controlled trial. BMC Complement. Med. Ther. 2022, 22, 262. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.N.C.; Lira-Neto, J.C.G.; Nunes, L.C.C.; Alencar, A.M.P.G.; Marques, R.L.L.; Damasceno, M.M.C. Effectiveness of ginger in the treatment of Type 2 Diabetes Mellitus: A pilot study of the randomized clinical trial type. Aquichan 2021, 21, e2115. [Google Scholar] [CrossRef]
- Hooshmand Moghadam, B.; Rashidlamir, A.; Attarzadeh Hosseini, S.R.; Gaeini, A.A.; Kaviani, M. The effects of saffron (Crocus sativus L.) in conjunction with concurrent training on body composition, glycaemic status, and inflammatory markers in obese men with type 2 diabetes mellitus: A randomized double-blind clinical trial. Br. J. Clin. Pharmacol. 2022, 88, 3256–3271. [Google Scholar] [CrossRef] [PubMed]
- Assaad-Khalil, S.; Elkafrawy, N.; Khaled, M.; Mogeib, O.; Badr, H.; Rashwan, A.; Youssef, M.; Eltamawy, K.; Mohamed, S. A Phase II, Randomized, Double-Blind, Double-Dummy, Active-Controlled Clinical Trial to Investigate the Efficacy and Safety of NW Low-Glu® in Patients Newly Diagnosed with Type 2 Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2022, 2022, 9176026. [Google Scholar] [CrossRef]
- Quirarte-Báez, S.M.; Zamora-Perez, A.L.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; Sosa-Macías, M.; Galaviz-Hernández, C.; Manríquez, G.G.G.; Lazalde-Ramos, B.P. A Shortened Treatment with Rosemary Tea (Rosmarinus officinalis) instead of Glucose in Patients with Diabetes Mellitus Type 2 (TSD). J. Popul. Ther. Clin. Pharmacol. 2019, 26, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.A.M.; El Wakeel, L.M.; Halawa, M.R.; Sabri, N.A.; El-Bahy, A.Z.; Singab, A.N. Effect of Nigella Sativa Oil versus Metformin on Glycemic Control and Biochemical Parameters of Newly Diagnosed Type 2 Diabetes Mellitus Patients. Endocrine 2019, 65, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Effect of Nigella Sativa Seeds on the Glycemic Control of Patients with Type 2 Diabetes Mellitus—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21675032/ (accessed on 19 September 2023).
- Hamdan, A.; Haji Idrus, R.; Mokhtar, M.H. Effects of Nigella Sativa on Type-2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4911. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Masud, T.; Uppal, A.M.; Naveed, A.K. Effects of Nigella sativa Oil on Some Blood Parameters in Type 2 Diabetes Mellitus Patients. Asian J. Chem. 2009, 21, 5373–5381. [Google Scholar]
- Ali, S.M.; Chen, P.; Sheikh, S.; Ahmad, A.; Ahmad, M.; Paithankar, M.; Desai, B.; Patel, P.; Khan, M.; Chaturvedi, A.; et al. Thymoquinone with Metformin Decreases Fasting, Post Prandial Glucose, and HbA1c in Type 2 Diabetic Patients. Drug Res. 2021, 71, 302–306. [Google Scholar] [CrossRef]
- Zarvandi, M.; Rakhshandeh, H.; Abazari, M.; Shafiee-Nick, R.; Ghorbani, A. Safety and Efficacy of a Polyherbal Formulation for the Management of Dyslipidemia and Hyperglycemia in Patients with Advanced-Stage of Type-2 Diabetes. Biomed. Pharmacother. 2017, 89, 69–75. [Google Scholar] [CrossRef]
- Banerji, S.; Banerjee, S. A formulation of grape seed, Indian gooseberry, turmeric and fenugreek helps controlling type 2 diabetes mellitus in advanced-stage patients. Eur. J. Integr. Med. 2016, 8, 645–653. [Google Scholar] [CrossRef]
- Faizal, P.; Suresh, S.; Satheesh Kumar, R.; Augusti, K.T. A study on the hypoglycemic and hypolipidemic effects of an ayurvedic drug Rajanyamalakadi in diabetic patients. Indian J. Clin. Biochem. 2009, 24, 82–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sousa, D.F.D.; Araújo, M.F.M.D.; de Mello, V.D.; Damasceno, M.M.C.; Freitas, R.W.J.F.D. Cost-Effectiveness of Passion Fruit Albedo versus Turmeric in the Glycemic and Lipaemic Control of People with Type 2 Diabetes: Randomized Clinical Trial. J. Am. Coll. Nutr. 2021, 40, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Iyer, U.M.; Desai, P.A.; Venugopal, S. Impact of Panchratna Juice in the Management of Diabetes Mellitus: Fresh vs. Processed Product. Int. J. Green Pharm. (IJGP) 2010, 4, 123–128. [Google Scholar] [CrossRef][Green Version]
- Kurian, G.A.; Manjusha, V.; Nair, S.S.; Varghese, T.; Padikkala, J. Short-term effect of G-400, polyherbal formulation in the management of hyperglycemia and hyperlipidemia conditions in patients with type 2 diabetes mellitus. Nutrition 2014, 30, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Chauhan, P.; Subramani, S.K.; Anand, A.; Borole, D.; Goswamy, H.; Gbks, P. Evaluation of “GSPF Kwath”: A Gymnema Sylvestre-Containing Polyherbal Formulation for the Treatment of Human Type 2 Diabetes Mellitus. Eur. J. Integr. Med. 2015, 7, 303–311. [Google Scholar] [CrossRef]
- Mani, V.; Iyer, U.; Mani, I.; Hsr Desikachar, U. Long-Term Effect of Cereal-Pulse Mix (Diabetic Mix) Supplementation on Serum Lipid Profile in Non-Insulin-Dependent Diabetes Mellitus Patients. J. Nutr. Environ. Med. 1997, 7, 163–168. Available online: https://www.tandfonline.com/doi/abs/10.1080/13590849762574 (accessed on 6 September 2023).
- Reddy, K.R.C. Effect of Chanaka Yoga as a Dietary Supplement in the Management of Type II Diabetes Mellitus Patients. Int. J. Green Pharm. (IJGP) 2016, 10, S223–S232. Available online: http://www.greenpharmacy.info/index.php/ijgp/article/view/787 (accessed on 6 September 2023).
- Sukandar, E.Y.; Permana, H.; Adnyana, I.K.; Sigit, J.I.; Ilyas, R.A.; Hasimun, P.; Mardiyah, D. Clinical Study of Turmeric (Curcuma longa L.) and Garlic (Allium sativum L.) Extracts as Antihyperglycemic and Antihyperlipidemic Agent in Type-2 Diabetes-Dyslipidemia Patients. Int. J. Pharmacol. 2010, 6, 456–463. [Google Scholar] [CrossRef]
- Sukandar, E.Y.; Sudjana, P.; Adnyana, I.K.; Setiawan, A.S.; Yuniarni, U. Recent Study of Turmeric in Combination with Garlic as Antidiabetic Agent. Procedia Chem. 2014, 13, 44–56. [Google Scholar] [CrossRef]
- Effect of a Polyherbal Formulation Cream on Diabetic Neuropathic Pain among Patients with Type 2 Diabetes—A Pilot Study—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5206872/ (accessed on 6 September 2023).
- Effect of Soya Beans Bread Fortified with Turmeric or Ginger on Diabesity. Available online: https://www.researchgate.net/publication/311266062_Effect_of_soya_beans_bread_fortified_with_turmeric_or_ginger_on_diabesity (accessed on 6 September 2023).
- Nganou-Gnindjio, C.N.; Ngati Nyonga, D.; Wafeu, G.S.; Nga, E.N.; Sobngwi, E. Cardiometabolic effects of Zingiber Officinale Roscoe extracts in Type 2 diabetic Cameroonians patients after six weeks of add-on Therapy: A single clinical-arm trialE ffets cardio-métaboliques d’extraits de zingiber officinale roscoe chez les patients camerounais diabétiques de type 2 après six semaines de supplémentation: Essai clinique à bras unique. Ann. Cardiol. Angeiol. 2022, 71, 160–165. [Google Scholar] [CrossRef]
- Yu, X.; Xu, L.; Zhou, Q.; Wu, S.; Tian, J.; Piao, C.; Guo, H.; Zhang, J.; Li, L.; Wu, S.; et al. The Efficacy and Safety of the Chinese Herbal Formula, JTTZ, for the Treatment of Type 2 Diabetes with Obesity and Hyperlipidemia: A Multicenter Randomized, Positive-Controlled, Open-Label Clinical Trial. Int. J. Endocrinol. 2018, 2018, 9519231. [Google Scholar] [CrossRef]
- Blevins, S.M.; Leyva, M.J.; Brown, J.; Wright, J.; Scofield, R.H.; Aston, C.E. Effect of Cinnamon on Glucose and Lipid Levels in Non Insulin-Dependent Type 2 Diabetes. Diabetes Care 2007, 30, 2236–2237. [Google Scholar] [CrossRef]
- Mirmiran, P.; Davari, M.; Hashemi, R.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. A randomized controlled trial to determining the effect of cinnamon on the plasma levels of soluble forms of vascular adhesion molecules in type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2019, 73, 1605–1612. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2019, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tanzidi-Roodi, O.; Jafari, F.; AkbariRad, M.; Asili, J.; Elyasi, S. Evaluation of a New Herbal Formulation (Viabet®) Efficacy in Patients with Type 2 Diabetes as an Adjuvant to Metformin: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Herb. Med. 2023, 37, 100617. [Google Scholar] [CrossRef]
- Wainstein, J.; Stern, N.; Heller, S.; Boaz, M. Dietary cinnamon supplementation and changes in systolic blood pressure in subjects with type 2 diabetes. J. Med. Food 2011, 14, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, A.; Nagabhusahnam, K.; Mundkur, L.; Paulose, S. A Randomized, Double-Blind Clinical Trial of a Herbal Formulation (GlycaCare-II) for the Management of Type 2 Diabetes in Comparison with Metformin. Diabetol. Metab. Syndr. 2021, 13, 132. [Google Scholar] [CrossRef]
- Rao, A.S.; Hegde, S.; Pacioretty, L.M.; DeBenedetto, J.; Babish, J.G. Nigella Sativa and Trigonella Foenum-Graecum Supplemented Chapatis Safely Improve HbA1c, Body Weight, Waist Circumference, Blood Lipids, and Fatty Liver in Overweight and Diabetic Subjects: A Twelve-Week Safety and Efficacy Study. J. Med. Food 2020, 23, 905–919. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Jarvill-Taylor, K.J.; Anderson, R.A.; Graves, D.J. A Hydroxychalcone Derived from Cinnamon Functions as a Mimetic for Insulin in 3T3-L1 Adipocytes. J. Am. Coll. Nutr. 2001, 20, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Imparl-Radosagevich, J.; Deas, S.; Polansky, M.M.; Baedke, D.A.; Ingebritsen, T.S.; Anderson, R.A.; Graves, D.J. Regulation of PTP-1 and Insulin Receptor Kinase by Fractions from Cinnamon: Implications for Cinnamon Regulation of Insulin Signalling. Horm. Res. 1998, 50, 177–182. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the Active Principle of Turmeric (Curcuma Longa), Ameliorates Diabetic Nephropathy in Rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Wani, K.; Kaul-Ghanekar, R.; Asmita, P.; Ogale, S. From micron to nano-curcumin by sophorolipid co-processing: Highly enhanced bioavailability, fluorescence, and anti-cancer efficacy. RSC Adv. 2014, 4, 60334–60341. [Google Scholar] [CrossRef]
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of Curcuma Longa (Turmeric) on Postprandial Plasma Glucose and Insulin in Healthy Subjects. Nutr. J. 2010, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Targeting Inflammation-Induced Obesity and Metabolic Diseases by Curcumin and Other Nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Na, L.-X.; Li, Y.; Pan, H.-Z.; Zhou, X.-L.; Sun, D.-J.; Meng, M.; Li, X.-X.; Sun, C.-H. Curcuminoids Exert Glucose-Lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Anti-Hyperglycemic and Insulin Sensitizer Effects of Turmeric and Its Principle Constituent Curcumin. Int. J. Endocrinol. Metab. 2014, 12, e18081. [Google Scholar] [CrossRef]
- Alappat, L.; Awad, A.B. Curcumin and Obesity: Evidence and Mechanisms. Nutr. Rev. 2010, 68, 729–738. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Solyom, A.M.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Fresh Organically Grown Ginger (Zingiber officinale): Composition and Effects on LPS-Induced PGE2 Production. Phytochemistry 2004, 65, 1937–1954. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially Processed Dry Ginger (Zingiber officinale): Composition and Effects on LPS-Stimulated PGE2 Production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef]
- Shanmugam, K.R.; Mallikarjuna, K.; Kesireddy, N.; Sathyavelu Reddy, K. Neuroprotective Effect of Ginger on Anti-Oxidant Enzymes in Streptozotocin-Induced Diabetic Rats. Food Chem. Toxicol. 2011, 49, 893–897. [Google Scholar] [CrossRef] [PubMed]
- González, V. Gliflozinas: Más que antidiabéticos orales. Una breve revisión de la literatura. Rev. Urug. Cardiol. 2021, 36, e36112. [Google Scholar] [CrossRef]
- Hernández Mijares, A. Inhibidores de la DPP-4 frente a análogos del receptor de GLP-1 tras el fracaso de la monoterapia con metformina en la diabetes tipo 2. Av. Diabetol. 2010, 26, 200–202. [Google Scholar] [CrossRef]
- Kord, M.T.; Poorrajab, F.; Ardekani, J.M.; Azari, M.; Raeissi, A. Ginger Accelerates GLUT4 Translocation to the Cell Membrane of C2C12 Myotubes. Med. J. Tabriz Univ. Med. Sci. Health Serv. 2016, 38, 34–41. [Google Scholar]
- Zhang, X.F.; Tan, B.K. Effects of an Ethanolic Extract of Gynura Procumbens on Serum Glucose, Cholesterol and Triglyceride Levels in Normal and Streptozotocin-Induced Diabetic Rats. Singap. Med. J. 2000, 41, 9–13. [Google Scholar]
- Isa, Y.; Miyakawa, Y.; Yanagisawa, M.; Goto, T.; Kang, M.-S.; Kawada, T.; Morimitsu, Y.; Kubota, K.; Tsuda, T. 6-Shogaol and 6-Gingerol, the Pungent of Ginger, Inhibit TNF-Alpha Mediated Downregulation of Adiponectin Expression via Different Mechanisms in 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 2008, 373, 429–434. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A Review on Therapeutic Potential of Nigella Sativa: A Miracle Herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Mahmoodi, M.R.; Mohammadizadeh, M. Therapeutic Potentials of Nigella Sativa Preparations and Its Constituents in the Management of Diabetes and Its Complications in Experimental Animals and Patients with Diabetes Mellitus: A Systematic Review. Complement. Ther. Med. 2020, 50, 102391. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Legoabe, L.J.; Montesano, D.; Zengin, G. Nigella sativa L. and Its Active Compound Thymoquinone in the Clinical Management of Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 12111. [Google Scholar] [CrossRef]
- Giannoulaki, P.; Kotzakioulafi, E.; Chourdakis, M.; Hatzitolios, A.; Didangelos, T. Impact of Crocus sativus L. on Metabolic Profile in Patients with Diabetes Mellitus or Metabolic Syndrome: A Systematic Review. Nutrients 2020, 12, 1424. [Google Scholar] [CrossRef]
- Correia, A.G.D.S.; Alencar, M.B.; Dos Santos, A.N.; da Paixão, D.C.B.; Sandes, F.L.F.; Andrade, B.; Castro, Y.; de Andrade, J.S. Effect of Saffron and Fenugreek on Lowering Blood Glucose: A Systematic Review with Meta-Analysis. Phytother. Res. 2023, 37, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and Spices-Biomarkers of Intake Based on Human Intervention Studies—A Systematic Review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Zare, V.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. Antidiabetic Potential of Saffron and Its Active Constituents. J. Cell Physiol. 2019, 234, 8610–8617. [Google Scholar] [CrossRef] [PubMed]
- Moini Jazani, A.; Karimi, A.; Nasimi Doost Azgomi, R. The Potential Role of Saffron (Crocus sativus L.) and Its Components in Oxidative Stress in Diabetes Mellitus: A Systematic Review. Clin. Nutr. ESPEN 2022, 48, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, F.; Hajiaghaalipour, F.; Yusof, A.; Muniandy, S.; Hosseini, S.A.; Heydari, S.; Salim, L.Z.A.; Azarbayjani, M.A. Saffron with Resistance Exercise Improves Diabetic Parameters through the GLUT4/AMPK Pathway in-Vitro and in-Vivo. Sci. Rep. 2016, 6, 25139. [Google Scholar] [CrossRef] [PubMed]
- Nasimi Doost Azgomi, R.; Karimi, A.; Zarshenas, M.M.; Moini Jazani, A. The Mechanisms of Saffron (Crocus sativus’) on the Inflammatory Pathways of Diabetes Mellitus: A Systematic Review. Diabetes Metab. Syndr. 2022, 16, 102365. [Google Scholar] [CrossRef] [PubMed]
- Vafaeipour, Z.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Effect of Saffron, Black Seed, and Their Main Constituents on Inflammatory Cytokine Response (Mainly TNF-α) and Oxidative Stress Status: An Aspect on Pharmacological Insights. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 2241–2259. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value. 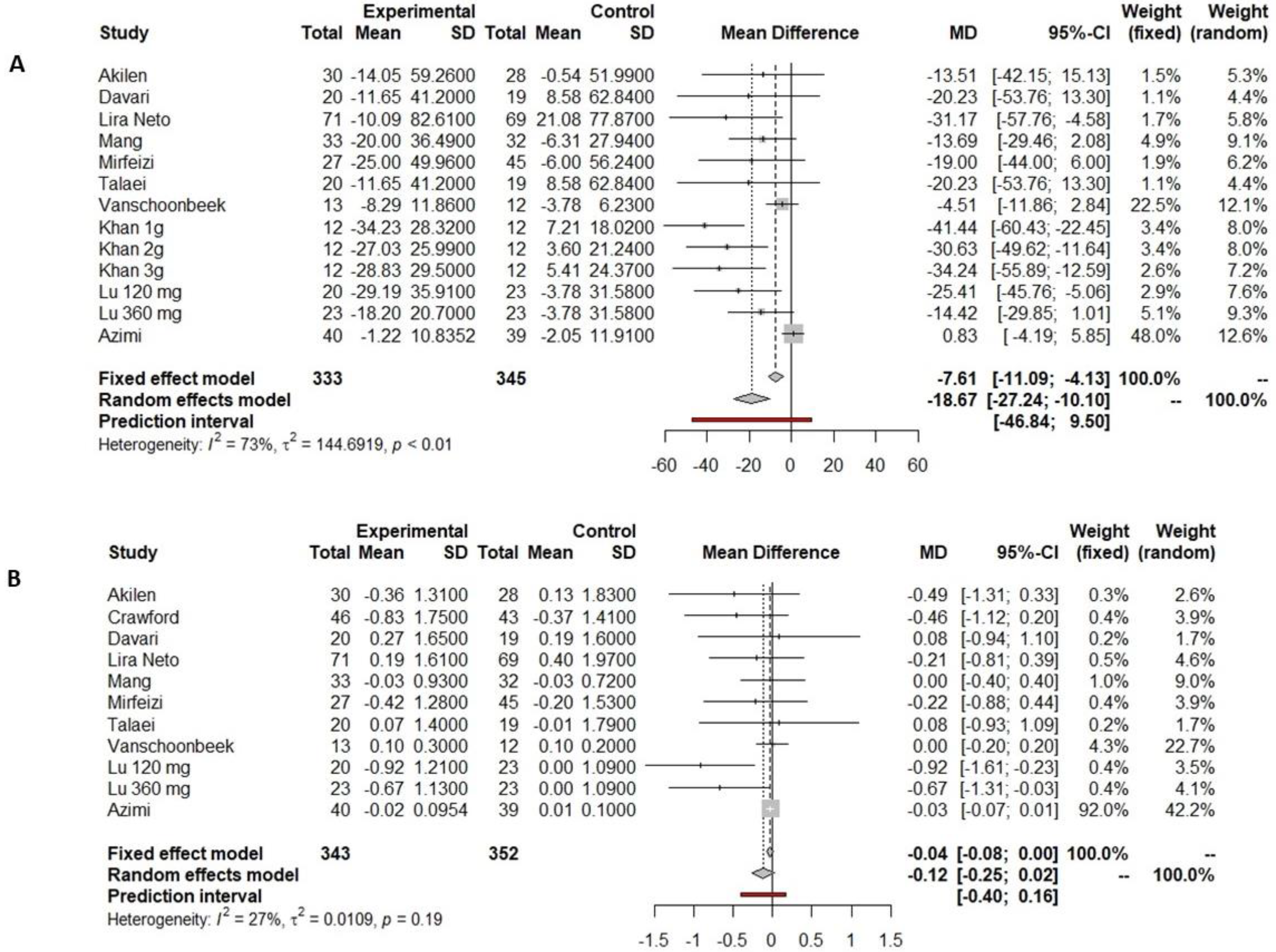
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.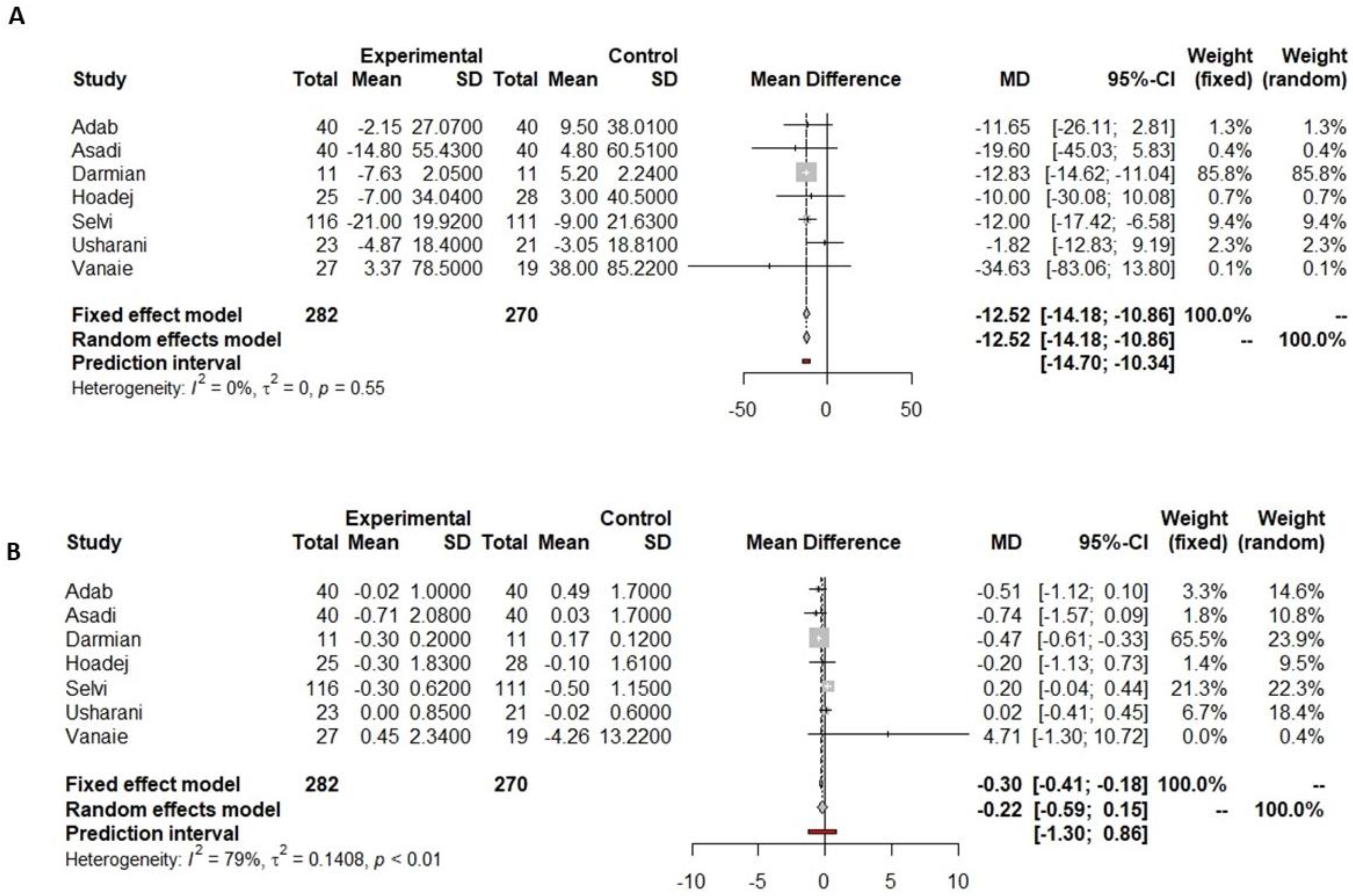
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.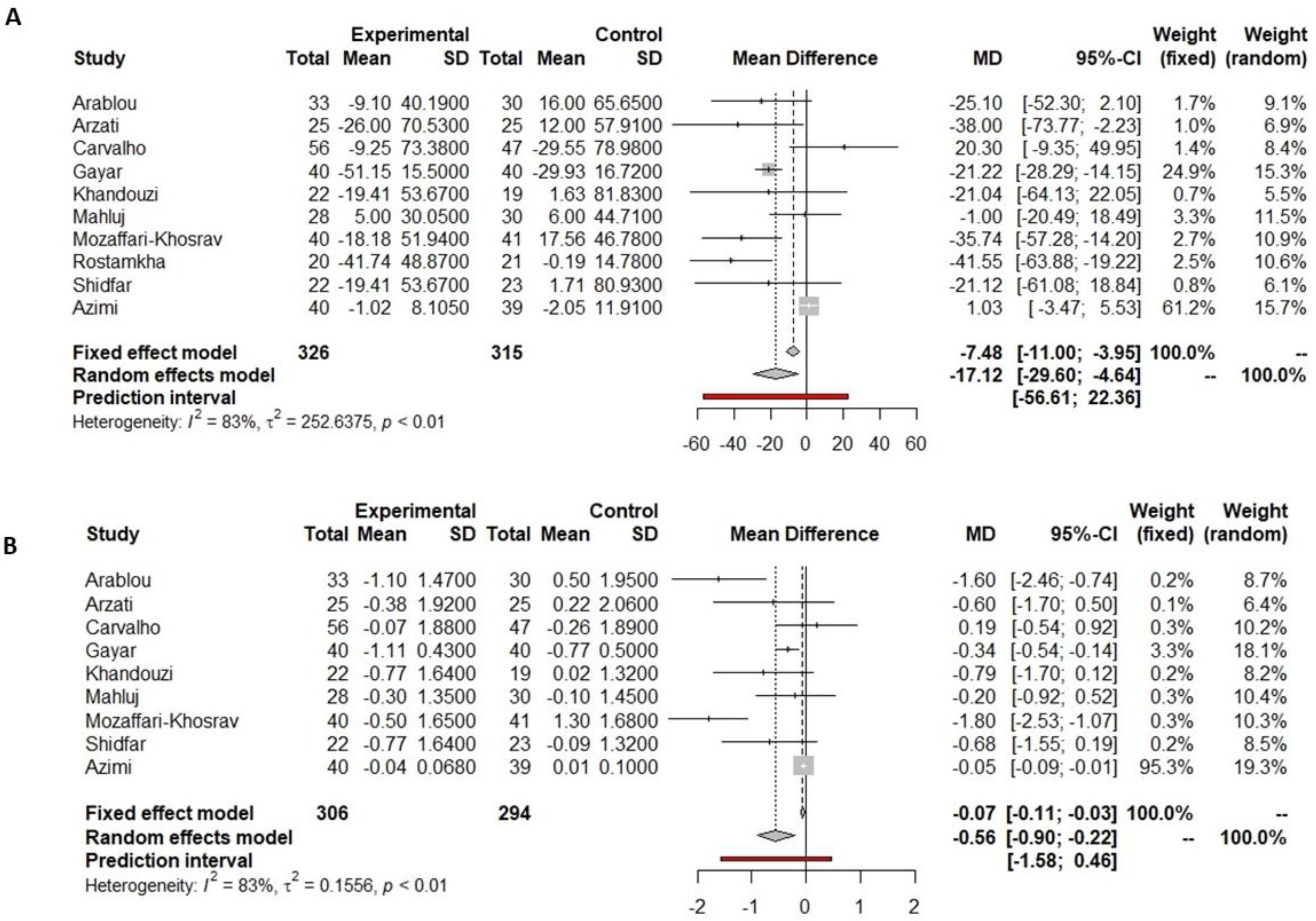
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.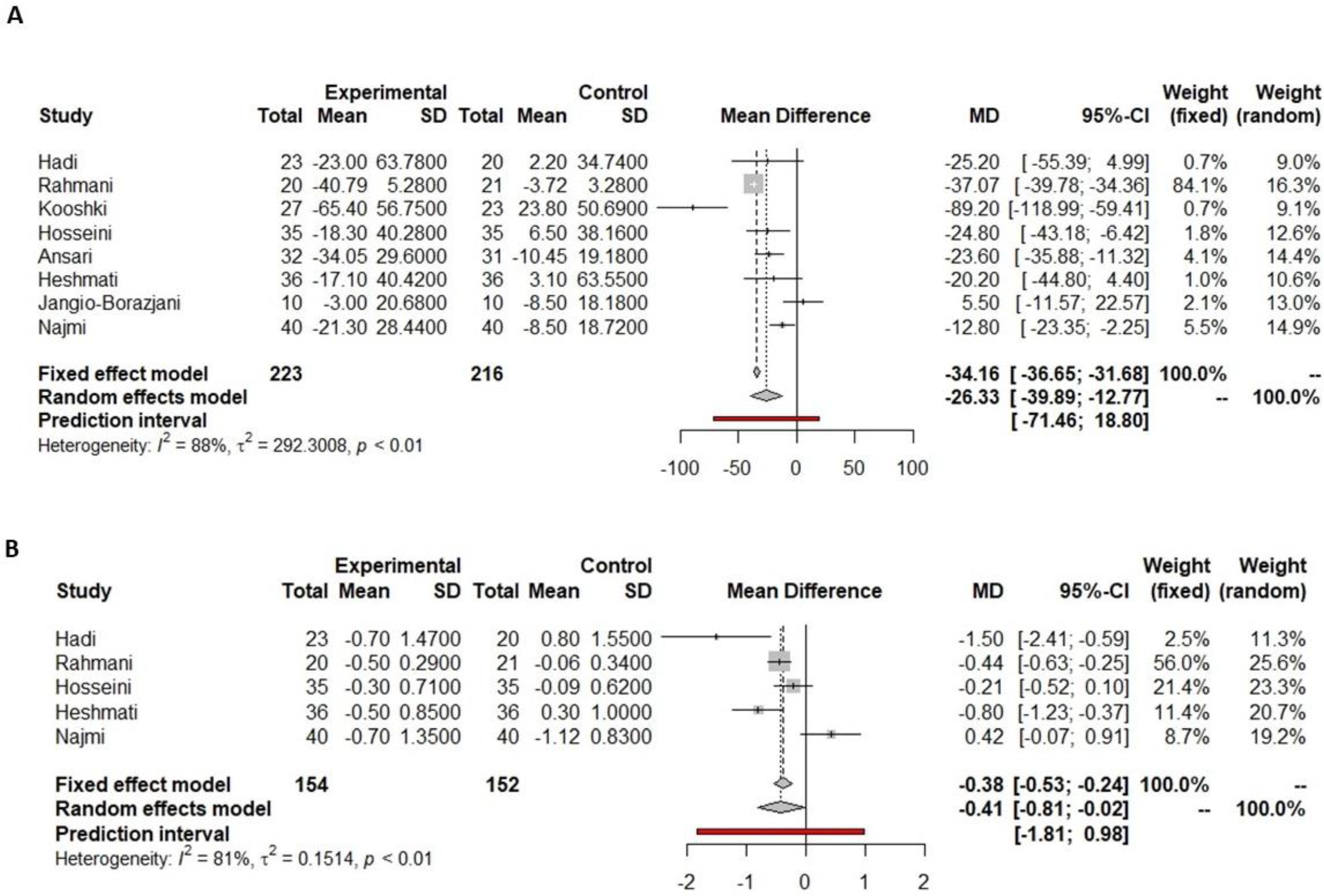
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.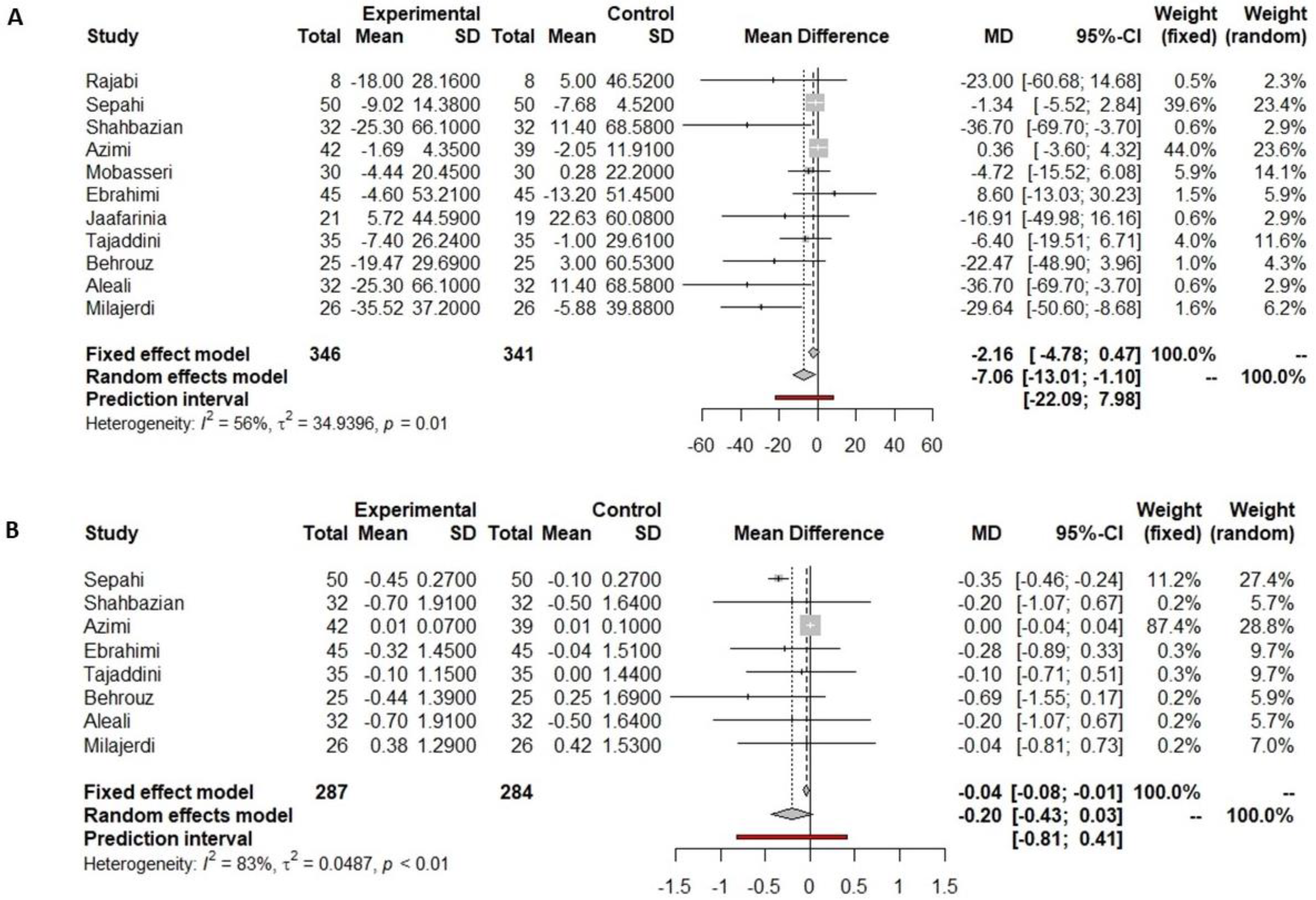
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.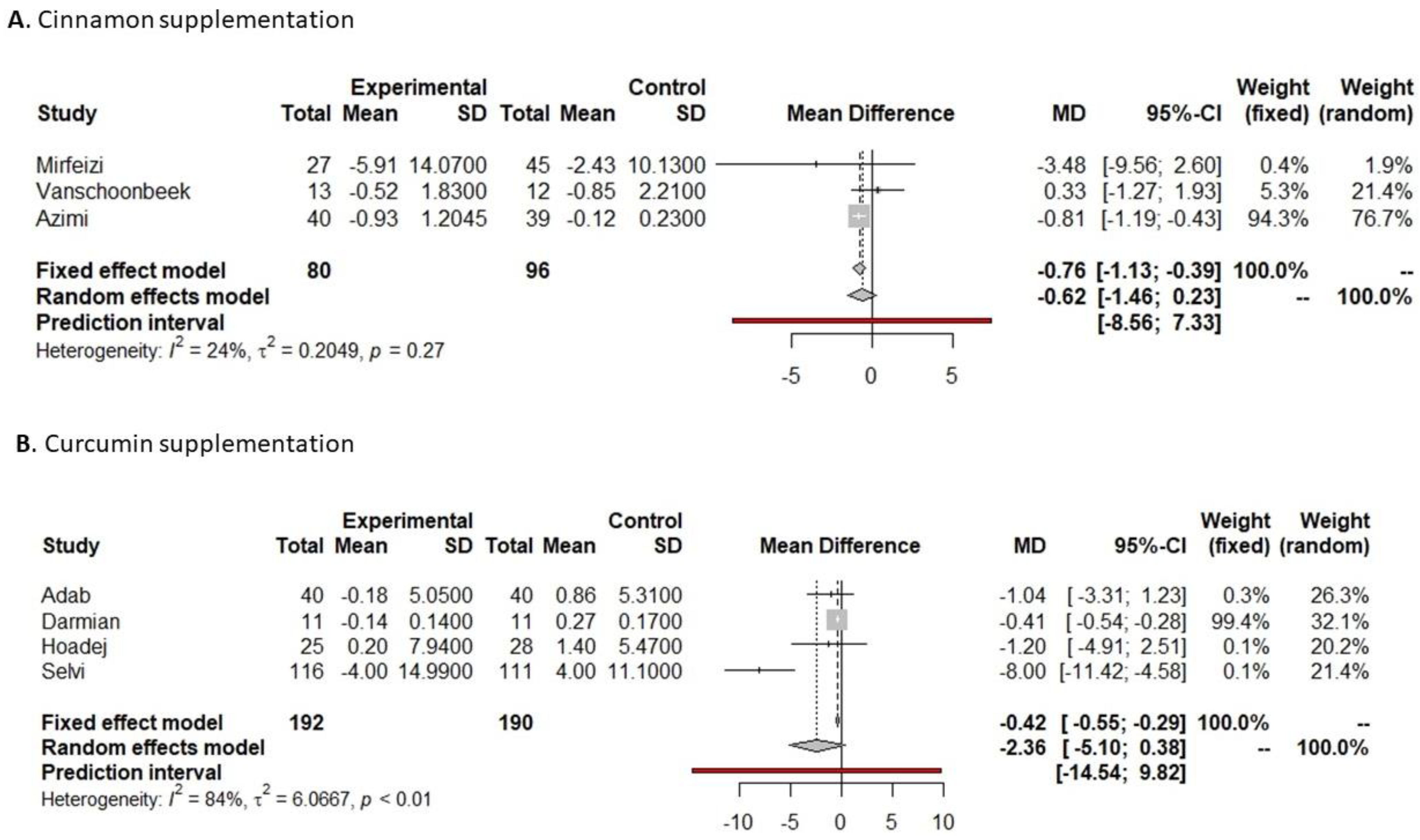
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.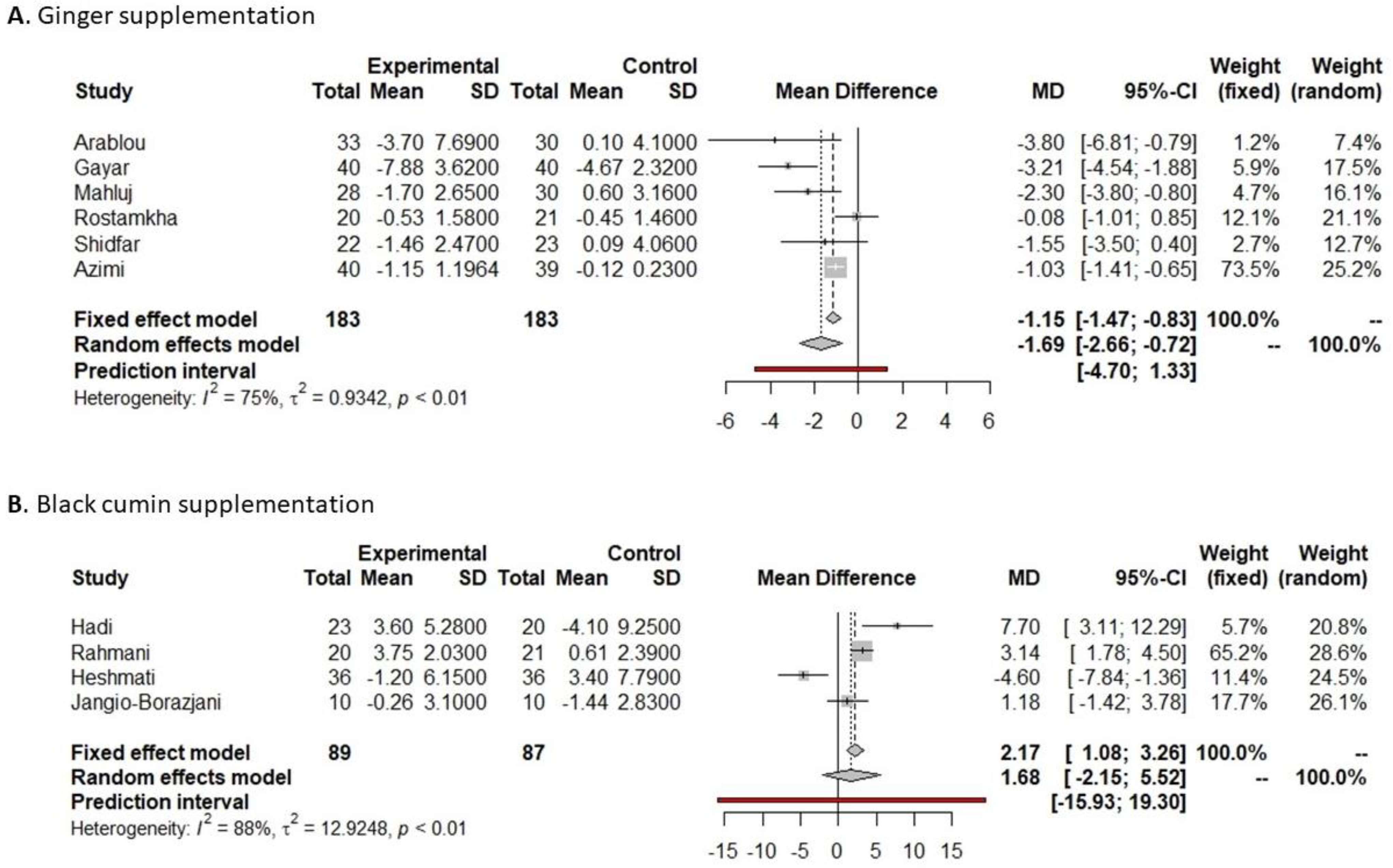
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.
 Indicated results of fixed effect model,
Indicated results of fixed effect model,  indicated results of random effects models and
indicated results of random effects models and  indicated prediction interval of predictive value.
indicated prediction interval of predictive value.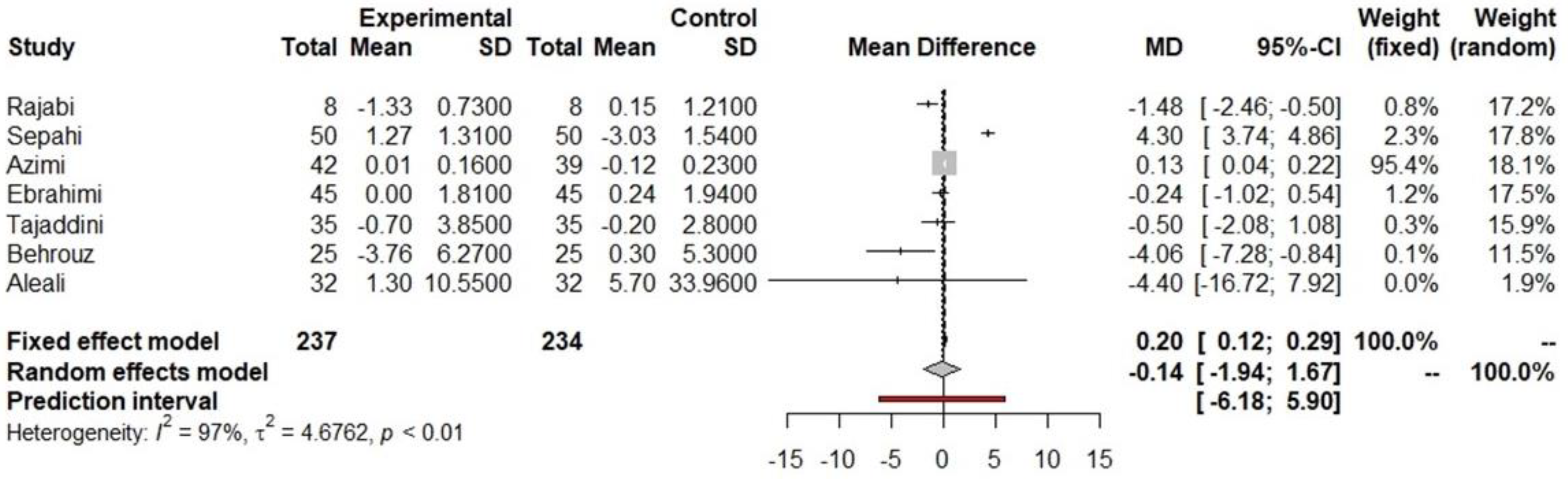
| First Author, Year of Publication | N | Participants | Male, n (%) | Age, Years | Study Design | Intervention Dosage mg/day (Number of Subjects) | Duration (Days) | Body Weight (kg) | Glucose (mg/dL) | HbA1c (%) | Insulin (UI/µL) | Quality Checklist Mean | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | Baseline | Final | |||||||||
| Akilen et al., 2010 [43] | 58 | T2D subjects treated with oral hypoglycemic agents, 18 years of age or older. Patients treated with insulin therapy, those with chronic disease, and pregnant or lactating women were excluded. | 11 (36.6) | 54.90 ± 10.14 | Prospective, randomized, placebo-controlled, double-blind clinical trial. These patients were randomly assigned to placebo (n = 28) or cinnamon (n = 30) groups. | Cinnamon group (N = 30): received cinnamon capsules (500 mg) per day | 84 days | 87.6 ± 17.5 | 84.7 ± 16.4 | 159 ± 62.2 | 145 ± 55.9 | 8.22 ± 1.16 | 7.86 ± 1.42 * | NR | NR | 0.785 |
| 15 (53.6) | 54.43 ± 12.53 | Placebo group (N = 28): received placebo capsules (500 mg) per day | 87.52 ± 20.24 | 87.02 ± 18.88 | 158 ± 46.7 | 157 ± 56.0 | 8.55 ± 1.82 | 8.68 ± 1.83 | NR | NR | ||||||
| Davari et al., 2020 [50] | 39 | Newly diagnosed T2D subjects, age 25–75 years, BMI 18–30 kg/m2, and T2D-diagnosed for less than 8 years. Pregnancy or patients with chronic disease were excluded. | 8 (40%) | Randomized, double-blind, placebo-controlled clinical trial. All patients were randomized into two groups: cinnamon and control group. | Cinnamon group (N = 20): received three capsules of 1 g cinnamon extract (3 g of cinnamon per day) | 56 days | 73.75 ± 10.74 | NR | 183.85 ± 36.16 | 172.20 ± 44.86 | 10.04 ± 1.30 | 10.31 ± 1.86 | 9.85 (7.92–19.22) | 12.10 (10.65–18.45) | 0.661 | |
| 7 (36.8%) | Control group (N = 19): received three capsules of microcrystalline cellulose | 77.15 ± 15.63 | NR | 190.57 ± 70.58 | 199.15 ± 49.86 | 10.11 ± 1.49 | 10.30 ± 1.70 | 10.60 (8.80–17.30) | 12.20 (9.30–14.20) | |||||||
| Lira Neto et al., 2022 [22] | 140 | T2D non-insulin subjects, age 18–80 years, and HbA1c > 6.0%. Patients with chronic disease, pregnancy, or allergic reaction to cinnamon were excluded. | 51 (71.8) | 61.7 (11.7) | Randomized, triple-blind, placebo-controlled clinical trial. All patients were randomized into two groups: cinnamon and control. | Cinnamon group (N = 71): received 3 g/day of cinnamon in capsules | 90 days | NR | NR | 10.3 (4.59) | 9.77 (4.58) * | 8.5 (2.3) | 8.3 (2.2) | −0.01 (−12.20, 7.20) | 0.857 | |
| 46 (66.7) | 60.8 (10.8) | Control group (N = 69): received placebo; capsules were identical in both groups | NR | NR | 9.00 (3.84) | 10.17 (4.68) | 8.0 (1.8) | 8.4 (2.1) | −0.40 (−7.20, 11.30) | |||||||
| Mang et al., 2006 [23] | 79 | T2D non-insulin treatment. | 21 (63.6) | 62.8 ± 8.37 | Randomized, placebo-controlled, double-blind design study. All patients were randomized into two groups: cinnamon and placebo. | Cinnamon group (N = 33): received 1 g of cinnamon per day in capsules | 121 days | NR | NR | 9.26 ± 2.26 | 8.15 ± 1.65 * | 6.86 ± 1.00 | 6.83 ± 0.83 | NR | NR | 0.411 |
| 23 (71.9) | 63.7 ± 7.17 | Placebo group (N = 32): received placebo capsules (microcrystalline cellulose) | NR | NR | 8.66 ± 1.47 | 8.31 ± 1.62 | 6.71 ± 0.73 | 6.68 ± 0.70 | NR | NR | ||||||
| Mirfeizi et al., 2016 [24] | 105 | T2D non-insulin-therapy subjects, with FBS > 140 mg/dL and HbA1c > 7%. Patients with chronic disease or with specific dietary needs or pregnancy were excluded. | 3 (11.1) | 52 ± 13 | Multicenter stratified randomization (triple-blind) placebo-controlled. All patients were randomized into three parallel groups: cinnamon, Caucasian whortleberry, and placebo. | Cinnamon group (N = 27): received 1000 mg per day of cinnamon in capsules | 84 days | 28.4 ± 3.27 | 27.8 ± 3.01 * | 180 ± 56 | 155 ± 40 * | 8.52 ± 1.32 | 8.10 ± 1.24 * | 21.6 ± 15.7 | 15.7 ± 11.4 * | 0.786 |
| 9 (30) | 55 ± 10 | Caucasian whortleberry (N = 30): received 1000 mg/day of whortleberry | 28.6 ± 3.27 | 28.3 ± 3.69 | 199 ± 79 | 154 ± 39 * | 8.80 ± 1.60 | 8.20 ± 1.41 * | 22.5 ± 24.2 | 12.7 ± 8.68 * | ||||||
| 11 (24.4) | 54 ± 12 | Placebo group (N = 45): received 1000 mg/day of starch capsules | 28.9 ± 4.45 | 28.8 ± 4.33 | 172 ± 53 | 166 ± 59 | 8.58 ± 1.38 | 8.38 ± 1.65 | 20.0 ± 11.1 | 17.6 ± 8.67 | ||||||
| Talaei et al., 2017 [25] | 39 | T2D non-insulin-therapy subjects, FBS: <180 mg/dL, and T2D history < 8 years. Pregnancy, consumption of specific medicines, or chronic disease were excluded. | 8 (40) | 58.90 ± 7.93 | Double-blind, randomized, placebo-controlled clinical trial. All patients were randomized into two groups: placebo and intervention. | Intervention group (N = 20): received three capsules of 1 g of cinnamon per capsule (3 g of cinnamon/day) | 56 days | 73.8 ± 10.7 | NR | 184 ± 36.2 | 172 ± 44.9 | 10.0 ± 1.30 | 10.1 ± 1.49 | 9.85 (7.92–19.2) | 12.10 (10.7–18.5) | 0.512 |
| 7 (36.8) | 56.26 ± 9.46 | Placebo group (N = 19): received three capsules with microcrystalline cellulose as placebo per day | 77.2 ± 15.6 | NR | 191 ± 70.6 | 199 ± 49.9 | 10.3 ± 1.86 | 10.3 ± 1.70 | 10.6 (8.80–17.3) | 12.2 (9.30–14.2) | ||||||
| Vanschoonbeek et al., 2006 [62] | 25 | Postmenopausal T2D women, non-insulin-dependent, and with stable medication for last 3 months. | 0 (0) | 64 ± 2 | Double-blind, placebo-controlled trial. All patients were randomized into two groups: placebo and cinnamon. | Placebo group (N = 13): received 1500 mg/d placebo (wheat flour) | 42 days | NR | NR | 149 ± 5.95 | 145 ± 6.49 | 7.1 ± 0.2 | 7.2 ± 0.2 | 15.5 ± 2.16 | 14.62 ± 2.25 | 0.444 |
| 0 (0) | 62 ± 2 | Cinnamon group (N = 12): received 1500 mg/d of cinnamon capsules (Cinnamomum cassia) | NR | NR | 151 ± 10.6 | 143 ± 12.8 | 7.4 ± 0.3 | 7.5 ± 0.3 | 15.3 ± 1.81 | 14.8 ± 1.84 | ||||||
| Khan et al., 2003 [26] | 60 | T2D non-insulin subjects, age > 40 years, and FBS 140–400 mg/dL. Patients who were taking other medicine for other health conditions were excluded. | 30 (50) | 52.0 ± 6.87 | Randomized clinical trial. All participants were divided into six groups: three received different gr of cinnamon, while another three-groups received placebo. | Group 1 (N = 12): received 1 g of cinnamon capsule per day | 40 days | NR | NR | 209 ± 30.6 | 175 ± 25.2 | NR | NR | NR | NR | 0.356 |
| Group 2 (N = 12): received 2 g of cinnamon capsule per day | NR | NR | 205 21.6 | 178 ± 28.8 | NR | NR | NR | NR | ||||||||
| Group 3 (N = 12): received 3 g of cinnamon capsule per day | NR | NR | 234 ± 25.2 | 205 ± 32.4 | NR | NR | NR | NR | ||||||||
| 52.0 ± 5.85 | Group 4 (N = 12): received 1 capsule of placebo | NR | NR | 220 ± 18.0 | 227 ± 18.0 | NR | NR | NR | NR | |||||||
| Group 5 (N = 12): received 2 capsules of placebo | NR | NR | 223 ± 18.0 | 227 ± 23.4 | NR | NR | NR | NR | ||||||||
| Group 6 (N = 12): received 3 capsules of placebo | NR | NR | 301 ± 25.2 | 306 ± 23.4 | NR | NR | NR | NR | ||||||||
| Lu et al., 2012 [56] | 66 | T2D subjects with HbA1c > 7% and FBS > 8.0 mmol/L. | 8(40) | 62.4 ± 7.9 | Randomized, double-blinded clinical study. All participants were randomly divided into 3 groups: placebo, low-dosage, and high-dosage groups. All patients were taking gliclazide (30 mg/day). | Low-dosage group (N = 20): received 120 mg of cinnamon capsule per day | 84 days | NR | NR | 11.2 ± 2.21 | 9.59 ± 1.66 * | 8.92 ± 1.35 | 8.00 ± 1.00 * | NR | NR | 0.511 |
| 8 (34.8) | 58.9 ± 6.4 | High-dosage group (N = 23): received 360 mg of cinnamon capsule per day | NR | NR | 9.00 ± 1.23 | 7.99 ± 1.05 * | 8.90 ± 1.24 | 8.23 ± 0.99 * | NR | NR | ||||||
| 9 (39.1) | 60 ± 5.9 | Placebo group (N = 23): received placebo capsules | NR | NR | 8.92 ± 1.21 | 8.71 ± 2.01 | 8.93 ± 1.14 | 8.93 ± 1.04 | NR | NR | ||||||
| Crawford et al., 2009 [57] | 89 | T2D subjects with HbA1c > 7%. Pregnancy, age < 18 years, and allergy to cinnamon were exclusion criteria. | 32 (58) | 60.5 ± 10.7 | Randomized clinical trial. Enrolled subjects were randomized into two groups: cinnamon (C. cassia) and control group. | Cinnamon group (N = 46): received capsules (500 mg each) of Cinnamomum cassia; they were instructed to take 2 capsules daily | 90 days | 31.9 ± 6.4 | NR | NR | NR | 8.47 ± 1.8 | 7.64 ± 1.7 * | NR | NR | 0.536 |
| 32 (59) | 59.9 ± 9.2 | Control group (N = 43): did not receive any supplementation | 32.9 ± 6.4 | NR | NR | NR | 8.28 ± 1.3 | 7.91 ± 1.5 | NR | NR | ||||||
| Adab et al., 2019 [63] | 80 | Hyperlipidemic T2D patients, FBS < 200 mg/dL, HbA1C > 6%, TG > 150 mg/dL, or LDL-c > 100 mg/dL, BMI: 20–35 kg/m2, no insulin therapy, and no use of polyphenols or multivitamin supplements. | 19 (48.7) | 54.76 ± 6.00 | Randomized, double-blind clinical trial. Eligible patients were randomly divided into two groups: the intervention (n = 40) and placebo (n = 40) groups. | Intervention group: received 2100 mg turmeric powder (three 700 mg turmeric capsules after main meals) | 56 days | 76.9 ± 10.4 | 75.1 ± 9.96 * | 134 ± 25.6 | 132 ± 28.33 | 7.06 ± 1.01 | 7.04 ± 0.98 | 7.29 ± 4.92 | 7.11 ± 5.17 | 0.911 |
| 17 (47.2) | 55.66 ± 8.64 | Placebo group: received 2100 mg corn starch flour as placebo (three 700 mg capsules after main meals) | 74.6 ± 17.0 | 76.7 ± 14.4 | 130 ± 33.0 | 139 ± 41.6 | 6.79 ± 1.08 | 7.28 ± 1.59 * | 7.29 ± 4.77 | 8.15 ± 5.72 | ||||||
| Asadi et al., 2019 [27] | 80 | T2D not insulin-dependent patients, aged 30–60 years, and BMI 25 to 39.9 kg/m2. Patients with chronic disease, pregnancy, or lactating were excluded. | 5 (12.5) | 53.3 (6.5) | Double-blind randomized, parallel, placebo-controlled clinical trial study conducted using intervention and placebo groups. | Intervention group (N = 40): received 80 mg of nano-curcumin capsules | 56 days | 77.4 (10.9) | 77.1 (10.9) | 166 (52.3) | 151 (58.1) * | 8.89 (2.18) | 8.18 (1.96) * | NR | NR | 0.856 |
| 5 (12.5) | 54.6 (6.2) | Placebo group: received 80 mg of polysorbate | 75.9 (12.4) | 75.9 (12.2) | 185 (58.3) | 190 (62.5) | 9.19 (1.68) | 9.22 (1.72) | NR | NR | ||||||
| Darmian et al., 2021 [34] | 42 | T2D non-insulin-dependent (type II) diabetes, HbA1C > 6, Triglycerides (TG) > 150 mg/dL, LDL > 100 mg/dL, and BMI = 25–30 kg/m2. | NR | 43.02 ± 3.04 | Single-blind, randomized, placebo-controlled study. Subjects were randomly assigned to four groups, namely AT + TS, AT + placebo, TS, and control + placebo. The participants in the AT group were required to exercise at home three times per week. Each training session included 20 min at 60% of HRmax, 40 min at 75% of HRmax, and a 10 min cool-down. HRmax was calculated as = 220 – age. | Group AT+ TS (N = 11): received 2100 mg capsules containing turmeric powder daily | 56 days | 73.1 ± 2.91 | 69.2 ± 3.22 * | 153 ± 1.75 | 135 ± 2.36 * | 7.68 ± 0.48 | 6.93 ± 0.64 * | 6.69 ± 0.13 | 5.98 ± 0.19 * | 0.786 |
| NR | 42.13 ± 2.39 | Group AT+ placebo (N = 11): received 2100 mg capsules containing cornstarch flour daily | 75.1 ± 2.07 | 72.2 ± 1.01 * | 155 ± 1.48 | 142 ± 2.11 * | 7.93 ± 0.69 | 7.06 ± 0.45 * | 6.59 ± 0.08 | 6.28 ± 0.05 * | ||||||
| NR | 44.33 ± 1.23 | Group TS (N = 11): received 2100 mg capsules containing turmeric powder daily | 74.1 ± 2.68 | 72.2 ± 1.76 * | 155 ± 2.04 | 147 ± 2.06 * | 7.70 ± 0.22 | 7.40 ± 0.16 * | 6.55 ± 0.16 | 6.41 ± 0.06 * | ||||||
| NR | 44.22 ± 3.07 | Group control + placebo (N = 11): received 2100 mg capsules containing cornstarch flour daily | 75.1 ± 3.20 | 78.4 ± 4.21 * | 153 ± 2.50 | 159 ± 1.84 * | 7.75 ± 0.13 | 7.92 ± 0.11 * | 6.63 ± 0.18 | 6.90 ± 0.13 * | ||||||
| Hodaei et al., 2019 [28] | 53 | T2D not insulin-dependent patients, aged 40–70 years old, and BMI 18.5–35 kg/m2. Patients with chronic disease and multivitamin supplements were excluded. | 15 (61.6) | 58 ± 8 | Randomized, double-blind, placebo-controlled trial. All patients were randomized into two groups: curcumin group and placebo. All patients were followed-up by phone every 15 days. | Curcumin group (n = 25) received three capsules of 500 mg of curcumin; 21 subjects of this group completed the trial | 70 days | 78 ± 13.28 | 77 ± 13.6 * | 160 ± 35 | 153 ± 33 * | 11.3 ± 1.6 | 11 ± 2 | 9.2 ± 9 | 9.4 ± 6 | 0.878 |
| 11 (39.1) | 60 ± 7 | Placebo group (n = 28) received three capsules of placebo (444 mg of cooked rice flour); 23 subjects of this group completed the trial | 74.04 ± 11.5 | 74.23 ± 12.3 | 144 ± 40.6 | 147 ± 40.4 | 11.2 ± 1.3 | 11.1 ± 1.8 | 8.3 ± 6 | 9.7 ± 4.7 | ||||||
| Selvi et al., 2013 [51] | 60 | T2D subjects with T2D diagnosed < 2 years. | 30 (100) | 46.8 ± 6.1 | Open-label randomized clinical trial. All T2D patients were randomized into two groups: one treatment only with metformin and another with metformin + turmeric. | Group 1: T2D subjects’ treatment with metformin (500 mg) twice a day | 28 days | 24.1 ± 3.26 kg/m2 † | NR | 111 ± 24 | 102 ± 18 * | 7.8 ± 0.5 | 7.5 ± 0.7 | 23 ± 16.4 | 19 ± 13 | 0.515 |
| 30 (100) | 47 ± 7.17 | Group 2: T2D subjects’ treatment with metformin (500 mg) twice a day + turmeric capsules (2 g/day). | 23.4 ± 3.03 kg/m2 † | NR | 116 ± 23 | 95 ± 11.4 * | 7.9 ± 1.3 | 7.4 ± 0.9 * | 18 ± 9.9 | 22 ± 12 | ||||||
| Usharani et al., 2008 [65] | 72 | T2D subjects aged 21–80 years and taking stable T2D medications for 2 months. Uncontrolled T2D, smoking, or patients with other chronic diseases were excluded. | 11 (47.8) | 55.52 ± 10.76 | Randomized, parallel-group, placebo-controlled trial. Subjects were randomized into NCB-02 (new formula with curcumin), atorvastatin, or placebo. | NCB-02 group (N = 23): received new formulation with curcumin, demethoxy curcumin, and bisdemethoxy; this capsule contained curcumin 150 mg; they received it twice per day | 56 days | 63.6 ± 10.7 | NR | 155 ± 17.9 | 150 ± 18.8 | 8.04 ± 0.85 | 8.04 ± 0.85 | NR | NR | 0.452 |
| 12 (52.2) | 50.47 ± 10.35 | Atorvastatin (N = 23): received 10 mg of atorvastatin daily | 64.6 ± 9.27 | NR | 161 ± 19.7 | 158 ± 16.5 | 8.30 ± 0.86 | 8.29 ± 0.81 | NR | NR | ||||||
| 11 (52.4) | 49.75 ± 8.18 | Placebo (N = 21): two capsules daily | 61.5 ± 8.63 | NR | 161 ± 20.0 | 158 ± 17.4 | 7.82 ± 0.57 | 7.80 ± 0.62 | NR | NR | ||||||
| Vanaie et al., 2019 [58] | 46 | T2D patients on oral antidiabetic drugs or insulin, age ≥ 18 years, overt proteinuria, eGFR ≥ 30 mL/min/1.73 m2, and controlled blood pressure. | 16 [59%] | 59 ± 6.25 | Randomized, double-blind, controlled trial. Patients were randomized into two groups (curcumin and placebo). | Curcumin group (N = 27): the patients received 500 mg curcumin capsule three times/day after meal (1500 mg/day) | 56 days | NR | NR | 184 ± 75.4 | 187 ± 81.3 | 9.46 ± 2.25 | 9.91 ± 2.42 | NR | NR | 0.570 |
| 11 [58%] | 61 ± 10.80 | Placebo group (N = 19): the patients received a placebo capsule with a similar packing | NR | NR | 176 ± 73.0 | 214 ± 93.6 | 13.0 ± 14.17 | 8.75 ± 2.17 | NR | NR | ||||||
| Arablou et al., 2014 [35] | 70 | T2D non-insulin-dependent subjects, HbA1C 7–10%, BMI 20–35 kg/m2, no pregnancy, no use of tobacco or alcohol, and no chronic disease. | 8 (24.2) | 52.6 ± 8.4 | Double-blinded, placebo-controlled clinical trial. Participants allocated randomly into two groups receiving ginger or placebo. | Ginger group (N = 33): received two capsules per day, which contained 1600 mg of ginger | 84 days | 66.2 ± 8.2 | 66.1 ± 8.2 | 131 ± 42.5 | 122 ± 37.4 | 8.4 ± 1.6 | 7.3 ± 1.3 * | 8.3 ± 8.3 | 4.6 ± 1.4 * | 0.714 |
| 7 (23.3) | 52.0 ± 9.0 | Control group (N = 30): received placebo capsules (containing wheat flour) | 66.1 ± 7.8 | 66.0 ± 7.7 | 129 ± 62.5 | 145 ± 68.4 | 8.1 ± 1.5 | 8.6 ± 2.2 | 6.9 ± 4.6 | 7.0 ± 3.3 | ||||||
| Arzati et al., 2017 [36] | 50 | T2D not insulin-dependent patients, BMI 18.5–35 kg/m2, and age 30–60 years. | 9 (34.8) | 51.7 ± 8.5 | Double-blind placebo-controlled trial study. All T2D subjects were randomly allocated to 2 groups of intervention and placebo. | Intervention group (N = 25): received 2000 mg per day of ginger capsules | 70 days | 78.4 ± 11.7 | 77.9 ± 11.2 | 170 ± 74.8 | 144 ± 65.3 | 7.30 ± 1.90 | 6.92 ± 1.93 | NR | NR | 0.676 |
| 7 (27.3) | 49.6 ± 8.6 | Control group (N = 25): received 2000 mg per day of placebo supplements | 76.7 ± 14.2 | 76.7 ± 14.0 | 161 ± 49.0 | 173 ± 63.9 | 7.50 ± 2.03 | 7.72 ± 2.08 | NR | NR | ||||||
| Carvalho et al., 2020 [49] | 103 | T2D subjects, with HbA1c 6–10%, with oral hypoglycemic agents. | 31 (30.1%) | 58.64 ± 11.11 | Double-blind, parallel, randomized control trial. All patients were divided into two groups: control and intervention. | Control group (N = 56): received 600 mg per day of cellulose supplement in capsules | 84 days | NR | NR | 185 ± 74.2 | 176 ± 72.6 * | 8.36 ± 1.89 | 8.29 ± 1.86 | NR | NR | 0.832 |
| Intervention group (N = 47): received 600 mg per day of ginger supplement | NR | NR | 204 ± 88.2 | 174 ± 64.1 * | 8.40 ± 1.96 | 8.14 ± 1.81 | NR | NR | ||||||||
| El Gayar et al., 2019 [52] | 80 | T2DM newly diagnosed subjects, HbA1c < 9%, and BMI ≥ 30 kg/m2. Pregnancy and patients with chronic disease were excluded. | 19 (47.5) | 46.35 ± 9.53 | A randomized, single-blind, placebo-controlled clinical trial. Subjects were randomly divided into two groups: ginger and placebo groups. All patients had to maintain a diet and constant PA. | Ginger group (N = 40): consumed three capsules daily, each capsule containing 600-mg of ginger powder (total daily dosage was 1.8 g) + 1000 mg of metformin | 56 days | 32.4 ± 1.51 kg/m2 † | 31.8 ± 1.21 * kg/m2 † | 172 ± 17.9 | 121 ± 9.06 * | 8.05 ± 0.46 | 6.94 ± 0.38 * | 20.7 ± 4.14 mIU/L | 12.9 ± 2.59 * mIU/L | 0.748 |
| 22 (55) | 46.10 ± 8.66 | Placebo group (N = 40): received three placebo capsules (wheat flour) + 1000 mg of metformin | 32.3 ± 1.39 kg/m2 † | 32.3 ± 1.39 kg/m2 † | 182 ± 18.8 | 152 ± 13.2 * | 8.03 ± 0.54 | 7.26 ± 0.45 * | 17.9 ± 2.50 | 13.2 ± 2.08 * | ||||||
| Khandouzi et al., 2015 [29] | 41 | T2D non-insulin therapy patients, aged 20–60 years, with T2D diagnosis for more than 2 years. Patients with chronic disease were excluded. | 5 (22.7) | 45.20 ± 7.64 | Randomized, double-blind, placebo-controlled clinical trial. Patients were divided randomly into two groups: experimental and control. | Experimental group (N = 22): received 2 g/day of ginger powder supplement in capsules | 84 days | No significant differences in BMI at the beginning and the end of the study in both groups | 162 ± 58.0 | 142 ± 47.9 * | 7.37 ± 1.86 | 6.60 ± 1.26 * | NR | NR | 0.643 | |
| 9 (47.4) | 47.10 ± 8.31 | Control group (N = 19): received 2 g/day of lactose supplement, as placebo | 155 ± 81.8 | 157 ± 81.8 * | 7.30 ± 1.31 | 7.32 ± 1.32 | NR | NR | ||||||||
| Mahluj et al., 2013 [59] | 64 | T2D subjects with normal blood pressure, aged 38–65 years, and mean BMI 29.5 kg/m2. | 14 (43.8) | 49.2 ± 5.1 | Randomized, double-blind, placebo-controlled trial. All participants were randomized into two groups: intervention and placebo. | Intervention group (N = 28 completed study): received one tablet of ginger twice a day (2 g/day) immediately after lunch and dinner | 56 days | 79.3 ± 11.8 | 79.1 ± 11.4 | 142 ± 34 | 147 ± 23 | 7.0 ± 1.3 | 6.7 ± 1.4 | 12.7 ± 2.9 | 11.0 ± 2.3 * | 0.714 |
| 16 (50) | 53.1 ± 7.9 | Placebo group (N = 30 patients completed study): received one tablet of placebo twice a day | 76.8 ± 14.5 | 76.9 ± 14.1 | 153 ± 47 | 159 ± 42 | 6.9 ± 1.4 | 6.8 ± 1.5 | 11.5 ± 3.0 | 12.1 ± 3.3 | ||||||
| Mozaffari-Khosravi et al., 2014 [30] | 88 | T2D non-insulin subjects for at least 10 years, FBS < 180, no pregnancy or lactation, no autoimmune or chronic disease, BMI < 40 kg/m2, and no consumption of lipid-lowering drugs. | 13 (32.5) | 49.83 ± 7.23 | Randomized, double-blind, placebo-controlled trial. The patients were categorized into 2 groups of ginger (GG) and placebo (PG). | Ginger group (N = 40): consumed daily 3 one-gram capsules containing ginger powder, after taking meals | 56 days | 28.1 ± 5.29 kg/m2 † | 28.1 ± 5.33 kg/m2 † | 171 ± 54.91 | 153 ± 48.34 * | 8.2 ± 1.6 | 7.7 ± 1.7 * | NR | NR | 0.732 |
| 18 (43.9) | 51.05 ± 7.70 | Placebo group (N = 41): consumed daily 3 cellulose microcrystalline capsules, after taking meals | 28.51 ± 4.95 kg/m2 † | 28.53 ± 0.03 kg/m2 † | 136 ± 40.53 | 154 ± 50.57 | 6.9 ± 1.3 | 8.2 ± 1.9 * | NR | NR | ||||||
| Rostamkhani et al., 2023 [53] | 41 | T2D subjects with end-stage renal disease who were on hemodialysis, aged > 18 years, free of any acute gastrointestinal issues, thyroid abnormalities, gallstones, or a history of ginger sensitivity. | 11 (50%) | 60.05 ± 11.12 | Randomized, double-blind, controlled parallel-group study. The participants were allocated into intervention and control groups. | Intervention group (N = 20): received four capsules with 500 mg of ginger per day (2000 mg of ginger powder daily) | 56 days | 69.7 ± 10.8 | 69.8 ± 10.4 | 175 ± 56.1 | 133 ± 33.2 * | NR | NR | 11.2 ± 1.68 | 10.6 ± 1.47 | 0.818 |
| 12 (54.5%) | 59.64 ± 10.69 | Control group (N = 21): received four placebo capsules containing starch | 74.6 ± 14.3 | 74.4 ± 15.2 | 150 ± 34.0 | 157 ± 34.5 | NR | NR | 10.5 ± 1.54 | 10.1 ± 1.37 | ||||||
| Shidfar et al., 2015 [31] | 45 | T2D non-insulin and non-smoking subjects, age 20–60 years, BMI < 30 kg/m2, and HbA1c 6–8%. Patients with chronic disease, pregnancy, or multivitamin supplementation were excluded. | NR | 45.2 ± 7.64 | Double-blind, parallel, randomized clinical trial. The patients were stratified by sex and BMI and randomly assigned into two groups: ginger or placebo. | Ginger group (N = 22): received 3 g of powdered ginger capsules daily (each capsule contained 1 g) | 84 days | 81.2 ± 13.25 | 80.0 ± 13.2 | 162 ± 58 | 142 ± 47.9 * | 7.37 ± 1.86 | 6.60 ± 1.26 * | 5.97 ± 2.76 | 4.51 ± 2.01 * | 0.712 |
| NR | 47.1 ± 8.31 | Placebo group (N = 23): received 3 g of daily placebo (lactose) capsules | 78.5 ± 14.1 | 78.2 ± 13.4 | 155 ± 81.8 | 157 ± 81.8 | 7.39 ± 1.31 | 7.30 ± 1.32 | 6.43 ± 3.98 | 6.52 ± 4.14 | ||||||
| Hadi et al., 2021 [37] | 43 | T2D subjects with BMI of 25–35 kg/m2, aged 30–60 years, non-smokers, not currently receiving insulin therapy, and did not have history of other diseases. | 10 (43.5) | 51.4 ± 9.2 | Double-blind randomized, controlled clinical trial was conducted among two groups (intervention and control) running in parallel. | Intervention group (N = 23): received two soft gel capsules containing 500 mg of Nigella sativa per day | 56 days | 28.4 ± 4.4 kg/m2 † | 27.6 ± 4.09 * kg/m2 † | 190 ± 71.5 | 167 ± 51.0 * | 7.9 ± 1.6 | 7.2 ± 1.3 * | 8.2 ± 3.2 | 11.8 ± 6.1 | 0.723 |
| 10 (50) | 56.00 ± 3.4 | Control group (N = 20): received daily two soft gel capsules containing oil or sunflower oil | 28.8 ± 8.1 kg/m2 † | 29.6 ± 7.7 kg/m2 † | 154 ± 35.7 | 156 ± 33.7 | 7.7 ± 1.5 | 8.5 ± 1.6 | 16.6 ± 10.6 | 12.5 ± 6.4 | ||||||
| Rahmani et al., 2022 [54] | 41 | T2D hemodialysis subjects aged 20 to 60 years, BMI 18.5 to 30 kg/m2, three HD sessions per week, six months on HD, and willingness to participate in the study. Exclusion criteria were pregnancy or lactation and cigarette smoking, among others. | 12 (60.0) | 49.60 (8.75) | Randomized, double-blinded, placebo-controlled, parallel-group clinical trial. Patients were divided into two groups: Nigella sativa group (NS) or placebo group using random allocation software. All patients were requested not to change their PA and diet during the study. | Nigella sativa group (N = 20): received two g/d of NS oil soft gel capsules (one capsule, twice daily) | 84 days | 79.2 ± 12.55 | NR | 190.70 ± 6.08 | 149.91 (2.68) * | 8.26 ± 0.33) | 7.76 ± 0.23 * | 15.9 ± 2.07 | 19.7 ± 1.98 * | 0.761 |
| 11 (52.4) | 48.57 (10.5) | Placebo group (N = 21): received the same amount of paraffin oil; both NS oil and paraffin oil capsules were packaged in dark containers with similar colors, smells, and appearances; each container included 30 capsules | 78.4 ± 10.99 | NR | 157 ± 3.43 | 153 ± 3.10 | 8.38 ± 0.37) | 8.32 ± 0.31) | 19.4 ± 2.49 | 20.0 ± 2.28 | ||||||
| Kooshki et al., 2019 [38] | 50 | T2D patients aged 35–64 years old and BMI of 25–34 kg/m2. Subjects with infection diseases, renal or thyroid diseases, hepatitis, cancer, or stroke; those on cholesterol-lowering drugs or insulin were excluded. | 7 (25.9) | 52.30 (9.43) | Randomized, double-blind clinical trial study. Patients were divided into two groups: intervention or placebo. Subjects were advised not to change their dietary habits, PA, and drug regimens. The 24 h food recall and PA questionnaires were evaluated. | Intervention group (N = 27): received 1000 mg N. sativa oil as two capsules, each containing 500 mg N. sativa oil, daily | 56 days | 29.01 (3.48) kg/m2 † | NR | 219 ± 64 | 153.6 ± 44.2 * | NR | NR | NR | NR | 0.747 |
| 9 (39.1) | 55.91 (8.98) | Placebo group (N = 23): received two placebo capsules containing medium-chain triglyceride oils at lunch and dinner | 28.1 (4.45) kg/m2 † | NR | 173 ± 47.2 | 196 ± 53.3 | NR | NR | NR | NR | ||||||
| Hosseini et al., 2013 [46] | 70 | T2D patients with FBG 140–180 mg/dL, body weight 55–75 kg, age 34–63 years, taking no more than 500 mg metformin. | 14 (40) | 48.74 ± 7.33 | Randomized double-blind study. Patients were divided into two groups: N. sativa and placebo group. | N. sativa group (N = 35): received 5 mL daily N. sativa oil | 84 days | 30.8 (3.55) kg/m2 † | 29.52 (3.50) * kg/m2 † | 180 ± 31.8 | 162 ± 45.3 * | 8.82 ± 0.73 | 8.52 ± 0.68 * | NR | NR | 0.464 |
| 16 (46) | 50.72 ± 5.69 | Placebo group (N = 35): received 5 mL daily mineral oil (placebo) | 30.92 (3.67) kg/m2 † | 31.12 (3.73) kg/m2 † | 180 ± 32.3 | 186 ± 42.1 | 8.79 ± 0.55 | 8.70 ± 0.67 | NR | NR | ||||||
| Ansari et al., 2017 [55] | 63 | T2D subjects with CKD (Stage 3 and 4) due to diabetic nephropathy aged 20–60 years were included. Pregnant females, patients on dialysis, terminally sick, immune-deficient, or having severe renal pathology were excluded. | NR | 48.09 | Prospective, randomized, parallel-group, and open-label study. T2D patients were randomized into two groups: control and intervention. | Control group (N = 31): received conservative management (insulin, torsemide, telmisartan, iron, calcium, Vitamin D3, and erythropoietin) of diabetic nephropathy | 84 days | NR | NR | 138 ± 33.1 | 104 ± 9.30 * | NR | NR | NR | NR | 0.416 |
| NR | 53.27 | Intervention group (N = 32) received conservative management along with N. sativa oil (2.5 mL, per orally, once daily) | NR | NR | 114 ± 22.0 | 104 ± 13.2 * | NR | NR | NR | NR | ||||||
| Heshmati et al., 2015 [47] | 80 | T2D patients aged 30–60 years old, T2D diagnosed for more than six months and taking antidiabetic medications. Patients with CVD, renal, hepatic, or pancreatic diseases were excluded. | 16 (45.7) | 45.3 ± 6.5 | Double-blind, placebo-controlled, randomized clinical trial. Patients were randomly divided into two groups: the intervention group received Nigella sativa oil soft gel capsules, and the control group received the placebo oil. | Intervention group (N = 36): received 3 g/day Nigella sativa oil soft gel capsules (one three times a day) | 84 days | 77.7 ± 11.4 | 74.8 ± 11.3 * | 183 ± 42.1 | 166 ± 38.5 * | 8.3 ± 0.9 | 7.8 ± 0.8 * | 12.2 ± 7.1 mg/dL | 11.0 ± 3.3 mg/dL | 0.947 |
| 17 (48.6) | 47.5 ± 8.0 | Control group (N = 36): received sunflower oil as placebo; both NS oil and sunflower capsules were provided for subjects in similar opaque bottles | 76.6 ± 13.7 | 77.3 ± 14.0 | 202 ± 63.9 | 205 ± 63.2 | 8.3 ± 1.0 | 8.6 ± 1.0 * | 10.3 ± 9.0 mg/dL | 13.7 ± 4.6 mg/dL | ||||||
| Jangjo-Borazjani et al., 2023 [60] | 40 | T2D middle-aged women without previous CVD. The exclusion criteria included previous or current insulin therapy, history of cardiovascular disease, conditions that would preclude physical activity, and use of antioxidant, anti-inflammatory, and corticosteroid medicines. | 0 (0) | 43.23 ± 3.45 | Randomized, double-blind clinical trial. Subjects were randomly assigned to 4 groups: resistance training + Nigella sativa (RN), Nigella sativa (NS), resistance training + placebo (RP), and control group (CO). Subjects of the RN and RP groups performed resistance training 3 days per week. Each session comprised a 10 min warm-up, 45 min resistance training, and a 10 min cool-down. | RN group (training + Nigella supplementation) (N = 10): received four N. sativa capsules (500 ± 10 mg), taking 2 g of N. sativa per day | 56 days | 76.3 ± 12.58 | 66.0 ± 4.59 | 142 ± 21.1 | 117 ± 12.3 * | NR | NR | 11.0 ± 4.19 | 5.76 ± 2.48 * | 0.607 |
| 44.2 ± 4 | NS group (Nigella supplementation) (N = 10): received four N. sativa capsules (500 ± 10 mg), taking 2 g of N. sativa per day | 66.6 ± 6.61 | 66.77 ± 6.08 | 132.40 ± 23.63 | 129.40 ± 14.81 * | NR | NR | 10.23 ± 3.53 | 9.97 ± 2.25 * | |||||||
| 44.13 ± 1.19 | RP group (training + placebo) (N = 10): received four capsules with maltodextrin (500 ± 10 mg) as a placebo per day | 74.5 ± 12.75 | 72.99 ± 6.67 | 118.30 ± 17.45 | 119.3 ± 8.43 | NR | NR | 6.92 ± 2.95 | 7.40 ± 1.37 | |||||||
| 42.9 ± 3.2 | Control group (N = 10): received four capsules with maltodextrin (500 ± 10 mg) as a placebo per day | 70.64 ± 7.02 | 69.34 ± 4.98 | 150.70 ± 19.20 | 142.20 ± 16.94 | NR | NR | 11.55 ± 2.91 | 10.11 ± 2.75 | |||||||
| Najmi et al., 2012 [64] | 80 | Newly detected patients of metabolic syndrome with T2D (HbA1C > 7%), aged 20–70 years. The exclusion criteria were pregnancy, T1D, CVD, impaired liver function test, chronic renal disease, or familial dyslipidemia. | 52 (65) | 20–70 years | Open-label randomized controlled study. Patients were randomly divided into two groups (n = 40 each). In group I (Std group), patients received metformin and atorvastatin. In group II (NSO group), patients received Nigella sativa as add-on therapy. | Std group (N = 40): received metformin 500 mg twice a day and atorvastatin 10 mg once a day | 56 days | NR | NR | 165.6 ± 32.6 | 144.3 ± 12.9 * | 8.11 ± 0.83 | 6.99 ± 0.83 * | NR | NR | 0.381 |
| NSO group (N = 40): received 500 mg capsule of Nigella sativa as add-on therapy; aspirin 150 mg once a day was given in both group | NR | NR | 144.2 ± 21.6 | 135.7 ± 11.6 | 7.71 ±0.73 | 7.18 ± 0.70 | NR | NR | ||||||||
| Rajabi et al., 2022 [61] | 32 | Obese women with T2DM without CVD and musculoskeletal disorders, HbA1c < 9.9%, no diabetic complications, no regular AT, no smoking, DM history less of than 5 years, and a maximum of one type of oral antidiabetic tablet a day. | 0 (0) | 51.5 ± 6.16 | Participants were divided into four groups: saffron + training (ST) (n = 8). | Powdered saffron (400 mg) was placed in capsules and used for two months | 56 days | 81.0 ± 5.01 | 77.6 ± 6.37 | 185 ± 30 | 128 ± 32 ** | NR | NR | 7.72 ± 1.92 | 5.00 ± 1.25 1 | 0.536 |
| 57.62 ± 6.81 | Placebo + training (PT) (n = 8). | Placebo capsules containing 400 mg of wheat flour and used for two months | 81.9 ± 3.30 | 80.1 ± 3.47 | 194 ± 42 | 175 ± 3 ** | NR | NR | 8.51 ± 1.45 | 6.75 ± 0.95 ** | ||||||
| 54.12 ± 7.37 | Saffron supplementation (SS) (n = 8). | Powdered saffron (400 mg) was placed in capsules and used for two months | 81.5 ± 6.91 | 79.6 ± 7.47 | 190 ± 31 | 172 ± 7 | NR | NR | 8.13 ± 0.75 | 6.80 ± 0.70 ** | ||||||
| 56.87 ± 5.11 | Placebo (P) (n = 8). | Placebo capsules containing 400 mg of wheat flour and used for two months | 87.0 ± 5.90 | 87.2 ± 6.32 | 215 ± 42 | 220 ± 50 | NR | NR | 8.95 ± 1.10 | 9.10 ± 1.30 | ||||||
| Sepahi et al., 2022 [39] | 150 | Patients with DM2 who did not use insulin, not well-controlled diabetes mellitus, age > 18, and HbA1c > 7. Patients with CKD and/or hepatic failure and mothers during pregnancy or lactating periods were excluded from the study. | 22 (44) | 57.58 ± 1.0 | Placebo-controlled triple-blinded clinical trial, where DM2 participants were divided into three groups: 50 subjects received saffron. | The saffron tablets contained 15 mg saffron. Crocin, placebo, and saffron tablets were prepared in a similar shape, color, and size, stored in a dark container, and coded by a pharmacist | 84 days | NR | NR | 171 ± 9.41 | 162 ± 16.6 | 7.92 ± 0.2 | 7.47 ± 0.31 * | 10.9 ± 0.94 | 12.2 ± 1.5 | 0.795 |
| 21 (42) | 57.16 ± 1.5 | 50 subjects received crocin. | The crocin tablets contained 15 mg crocin | NR | NR | 185 ± 12.1 | 164 ± 14.4 * | 8 ± 0.22 | 7.46 ± 0.25 * | 11.5 ± 1.13 | 10.8 ± 1.34 | |||||
| 25 (50) | 56.92 ± 1.9 | 50 subjects received placebo. | The placebo tablets contained 15 mg placebo | NR | NR | 161 ± 4.33 | 154 ± 4.69 * | 7.84 ± 0.23 | 7.74 ± 0.3 | 14.4 ± 1.60 | 11.4 ± 1.48 * | |||||
| Shahbazian et al., 2019 [42] | 64 | T2DM patients aged 30–65 years old, using oral hypoglycemic agents, having FBS ≥ 126 mg/dL and an HbA1c ≥ 7%. The exclusion criteria included pregnancy or lactating, chronic T2DM complications, or insulin treatment, among others. | 11 (34.4) | 52.4 ± 13 | Randomized double-blind clinical trial. All T2D patients included were randomized into two groups: saffron and control group. A 24 h dietary recall questionnaire was completed. The patients were asked not to change their diet, medication, and physical activity. | Control group (N = 32) received two placebo capsules per day; these placebo capsules contained lactose, magnesium stearate, and starch | 84 days | 27.5 ± 4.2 kg/m2 † | NR | 177 ± 60.1 | 189 ± 74.7 | 8.80 ± 1.8 | 8.3 ± 1.4 | NR | NR | 0.818 |
| Saffron group (N = 32) received two capsules (each 15 mg saffron) per day (30 mg/day) | 28.8 ± 4.0 kg/m2 † | NR | 173 ± 73.9 | 148 ± 53.5 * | 8.9 ± 2.0 | 8.2 ± 1.8 * | NR | NR | ||||||||
| Azimi et al., 2014 [32] | 204 | Subjects with T2D (FBS ≥ 126 mg/dL), aged ≥30 years, BMI ≥ 25 kg/m2, not on insulin therapy, and not taking medications except metformin or glibenclamide. Exclusion criteria included pregnancy, starting insulin therapy, or consumption of cinnamon, cardamom, ginger, or saffron during the running period. | 16 (0.40) | 54.15 ± 1.0 | Parallel, randomized, single-blind, placebo- controlled clinical trial. Before intervention, all participants were included in a three-week run-in period to match their tea consumption. The patients were randomly assigned to four intervention groups, cardamom, cinnamon, ginger, and saffron, and one control group. | Cinnamon group (N = 40) received 3 g cinnamon in three glasses of black tea | 56 days | 75.6 ± 1.20 | 75.3 ± 1.20 | 359 ± 10.8 | 358 ± 10.9 * | 7.89 ± 0.10 | 7.87 ± 0.09 | 11.4 ± 0.17 | 11.3 ± 0.17 | 0.714 |
| 17 (40.5) | 51.59 ± 1.3 | Cardamon group (N = 42) received 3 g cardamom in three glasses of black tea | 78.6 ± 1.20 | 78.5 ± 1.20 | 361 ± 12.3 | 359 ± 12.04 | 7.89 ± 0.10 | 7.87 ± 0.10 | 11.2 ± 0.20 | 11.2 ± 0.19 | ||||||
| 16 (0.38) | 57.02 ± 1.0 | Saffron group (N = 42) received 1 g saffron in three glasses of black tea | 82.0 ± 1.0 | 81.9 ± 0.99 | 358 ± 4.30 | 357 ± 4.39 | 7.73 ± 0.07 | 7.74 ± 0.07 | 11.0 ± 0.15 | 11.0 ± 0.15 | ||||||
| 15 (0.37) | 55.21 ± 1.1 | Ginger group (N = 41) received 3 g ginger in three glasses of black tea | 79.4 ± 0.9 | 79.2 ± 0.96 | 367 ± 8.09 | 366 ± 8.12 | 7.94 ± 0.069 | 7.90 ± 0.067 | 11.8 ± 0.15 | 11.6 ± 0.18 | ||||||
| 15 (0.38) | 53.64 ± 1.3 | Control group (N = 39): received 1 g placebo in three glasses of black tea | 78.7 ± 1.2 | 78.5 ± 1.1 | 355 ± 11.9 | 353 ± 12.0 | 7.50 ± 0.10 | 7.51 ± 0.10 | 11.0 ± 0.22 | 10.9 ± 0.22 | ||||||
| Mobasseri et al., 2020 [44] | 60 | T2D subjects with FBS> 126 mg/dL, HbA1c > 6.5%, with BMI 25 to 35 kg/m2, and having T2DM for at least six months and using antidiabetic drugs. Exclusion criteria were using insulin and hormone replacement therapy and using any antioxidant supplements, among others. | NR | 50.57 ± 9.88 | Randomized, double-blind, placebo-controlled clinical Trial (allocation ratio 1:1) was carried out with 60 T2D patients. These 60 patients were randomly allocated to one of the two treatment groups: saffron group (n = 30) and placebo group (n = 30). All the patients were asked to keep their dietary intake or PA as usual. | Saffron group (N = 30) received 100 mg/day saffron capsules (1 capsule) per day | 56 days | 83.0 ± 11.47 | 135 ± 19.6 | 131 ± 21.2 * | NR | NR | NR | NR | 0.818 | |
| NR | 51.63 ± 11.30 | Control group (N = 30) received starch capsules (1 capsule) per day | 85.4 ± 14.2 | 135 ± 21.3 | 135 ± 23.0 | NR | NR | NR | NR | |||||||
| Ebrahimi et al., 2019 [40] | 80 | T2D subjects, aged 30–70 years, HbA1c 6.5–10%, taking no nutritional supplements, no smoking, alcohol abuse, and BMI 20–35 kg/m2. The exclusion criteria included insulin therapy and changes in drug treatment or PA. | 20 (50) | 55.2 ± 7.3 | Prospective, double-blind, placebo-controlled, randomized study. Subjects were randomly allocated to the saffron supplement group (n = 45) or placebo group (n = 45). | Saffron group received daily a tablet containing 100 mg saffron twice a day | 84 days | 75.4 ± 12.8 | 74.2 ± 12.9 * | 167 ± 53.7 | 162 ± 52.7 | 8.01 ± 1.40 | 7.69 ± 1.49 * | 4.70 ± 1.7 pmol/L | 4.70 ± 1.9 pmol/L | 0.773 |
| 16 (38) | 53 ± 10.6 | Control group received daily the same amount of placebo (maltodextrin) | 80.3 ± 12.9 | 78.8 ± 18.1 | 161 ± 51.1 | 148 ± 51.8 | 7.38 ± 1.53 | 7.34 ± 1.48 | 4.47 ± 1.8 pmol/L | 4.71 ± 2.05 pmol/L | ||||||
| Jaafarinia et al., 2022 [66] | 40 | T2D patients aged ≥ 18 years, 5 years of history of T2DM, HbA1c < 8%, SBP < 160 or DBP < 100 mmHg, SCr levels ≤ 2 mg/dL, oral hypoglycemic or insulin treatment, or hypercholesterolemia within a statin. Exclusion criteria included eGFR < 30 mL/min/1.73 m2, CVD, alcohol dependency, or cigarette smoking among, others. | 11 (57.90) | 62.68 ± 9.84 | Randomized, triple-blind, placebo-controlled, 2-arm, parallel-group, phase 2 clinical trial using a 1:1 ratio of allocation. Saffron group included 22 subjects, while 22 subjects were included in placebo group. Three patients, one from the saffron group and two from the placebo group, dropped out of the intervention study. | Saffron group (N = 21): patients received one tablet of crocin 15 mg daily | 90 days | 27.2 ± 3.86 kg/m2 † | 27.0 ± 3.95 kg/m2 † | 141 ± 36.7 | 146 ± 49.6 | NR | NR | NR | NR | 0.909 |
| 12 (57.14) | 63.86 ± 10.62 | Placebo group (N = 19): patients received one tablet of crocin 15 mg daily | 27.3 ± 3.34 kg/m2 † | 27.2 ± 3.44 kg/m2 † | 137 ± 57.2 | 159 ± 62.6 * | NR | NR | NR | NR | ||||||
| Tajaddini et al., 2021 [33] | 70 | T2D subjects with BMI 25–35 kg/m2, aged 30–60 years. Insulin treatment, hormone replacement therapy and consumption of dietary or antioxidant supplements, history of surgery, serious illness, pregnancy, or lactation were excluded. | 15 (50.0) | 50.5 ± 9.8 | Double-blind, randomized, placebo-controlled clinical trial. Seventy participants were randomly allocated to two groups: control (N = 35) and saffron group (N = 35). Both patients and assessors were blind to the allocation. | Saffron group (N = 35) received a capsule with 100 mg saffron powder per day, which should be taken daily before a meal | 56 days | 82.7 ± 11.3 | 82.4 ± 11.1 | 138 ± 21.6 | 131 ± 29.2 * | 7.7 ± 1.2 | 7.6 ± 1.1 | 7.3 ± 3.8 | 6.6 ± 3.9 * | 0.843 |
| 13 (43.3) | 51.8 ± 10.9 | Control group (N = 35) received a capsule with 100 mg of maltodextrin per day, which should be taken daily before a meal | 84.6 ± 14.4 | 84.3 ± 13.8 | 134 ± 29.2 | 133 ± 30.0 | 7.5 ± 1.6 | 7.5 ± 1.2 | 7.1 ± 2.7 | 6.9 ± 2.8 | ||||||
| Behrouz et al., 2020 [45] | 50 | T2D subjects, aged 30–70 years, BMI 18.5–30 kg/m2, and taking oral hypoglycemic agents. Insulin, herbal and/or nutritional supplements, glucocorticoids, and non-steroid anti-inflammatory drugs within 3 months, uncontrolled diabetes (HbA1c ≥ 8.5%), and patients with chronic diseases were excluded. | 4 (16) | 57.08 ± 7.41 | Randomized, double-blind, single-center, parallel-group, controlled clinical trial. Patients were selected using a simple sampling procedure and stratified (1:1) into two groups randomly: crocin group (n = 25) or the placebo group (n = 25). Three subjects in saffron and two in control group dropped out of the study. | Saffron group: two tablets of 15 mg crocin were administered orally (15 mg/day) | 84 days | 77.1 ± 10.2 | NR | 149 ± 30.1 | 129 ± 29.31 * | 7.80 ± 1.29 | 7.36 ± 1.47 | 17.3 ± 7.14 (mU/L) | 13.5 ± 4.62 * (mU/L) | 0.869 |
| 3 (12) | 59.86 ± 9.46 | Control group: two tablets of 0 mg crocin were administered orally; placebo tablets were similar to the crocin supplements in terms of the size, color, shape, smell, and distribution bottles | 74.18 ± 7.97 | NR | 157.18 ± 63.29 | 160.18 ± 57.34 | 7.61 ± 1.62 | 7.86 ± 1.75 | 15.0 ± 5.52 (mU/L) | 15.3± 5.04 (mU/L) | ||||||
| Aleali et al., 2019 [48] | 64 | T2D patients aged 30–65 years, taking oral hypoglycemic medicines and without diabetic complications. Pregnancy and breastfeeding, chronic complications of diabetes, insulin treatment, CVD history, smoking, alcohol intake, and anticoagulant therapy were excluded. | 8 (25) | 53.5 ± 9.9 | Double-blind clinical trial. T2D patients were randomized into two groups: saffron group and control group. Saffron or placebo capsules were given for 2 weeks. Patients were followed by either telephone or face-to-face contact. Two 24 h food recall questionnaires were completed. | Saffron group (N = 32): received two capsules per day (in total, 30 mg saffron) | 84 days | 28.8 ± 4.0 kg/m2 † | NR | 173 ± 73.9 | 148 ± 53.5 * | 8.9 ± 2.0 | 8.2 ± 1.8 * | 12.5 ± 9.9 | 13.8 ± 11.1 | 0.738 |
| 11 (34.4) | 52.4 ± 13 | Control group (N = 32): received two placebo capsules that were identical to the main capsules | 27.5 ± 4.2 kg/m2 † | NR | 177 ± 60.1 | 189 ± 74.7 | 8.8 ± 1.8 | 8.3 ± 1.4 | 12.3 ± 8.2 | 18.0 ± 37.3 | ||||||
| Milajerdi et al., 2017 [41] | 54 | T2D subjects, aged 40–65 years, BMI 18.5–30 kg/m2. Smoking patients, insulin medications, uncontrolled blood glucose, high PA, pregnant, lactating, and those women who had planned for pregnancy were excluded. | 6 (23.1) | 54.57 ± 6.96 | Randomized triple-blind clinical trial. Fifty-four T2D patients were randomized into two groups: saffron and control group. One person from the control and one from the saffron group left the study. Participants were asked not to change their diet, PA, or drugs during the intervention. | Saffron group (N = 26) received two capsules twice a day (in the morning and evening); each capsule contained 15 mg of saffron | 56 days | 63.1 ± 31.6 | NR | 164 ± 40.9 | 129 ± 31.9 * | 6.37 ± 1.30 | 6.75 ± 1.28 | NR | NR | 0.839 |
| 6 (23.1) | 55.42 ± 7.58 | Control group (N = 26) received two capsules twice a day (in the morning and evening); each capsule contained 15 mg of placebo | 66.3 ± 9.01 | NR | 160 ± 38.4 | 154 ± 41.2 | 6.83 ± 1.36 | 7.25 ± 1.65 | NR | NR | ||||||
| Instruments | Objective Sufficiently Described | Study Design | Method of Subject | Comparison Group | Random Allocation | Blinding of Investigators | Blinding of Subjects | Outcome and Exposure Measure(s) | Sample Size | Analytic Methods | Estimate of Variance | Controlling for Confounding | Results Reported | Conclusion Supported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akilen et al., 2010 [43] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Davari et al., 2020 [50] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Lira Neto et al., 2022 [22] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Mang et al., 2006 [23] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Mirfeizi et al., 2016 [24] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Talei et al., 2017 [25] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Vanschoobeek et al., 2006 [62] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Khan et al., 2003 [26] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Lu et al., 2012 [56] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Crawford et al., 2009 [57] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Adab et al., 2018 [63] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Asadia et al., 2019 [27] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Darmian et al., 2021 [34] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Hodaei et al., 2019 [28] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Selvi et al., 2013 [51] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Usharani et al., 2008 [65] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Vanaie et al., 2019 [58] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Arablou et al., 2014 [35] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Arzati et al., 2017 [36] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Carvalho et al., 2020 [49] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| El Gayar et al., 2019 [52] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Khandouzi et al., 2015 [29] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Mahlujl et al., 2013 [59] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Mozaffari-khosravi et al., 2014 [30] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Rostamkhani et al., 2023 [53] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Shidfar et al., 2015 [31] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Hadi et al., 2020 [37] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Rahmani et al., 2022 [54] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Kooshki et al., 2019 [38] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Hosseini et al., 2013 [46] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Ansari et al., 2017 [55] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Heshmati et al., 2015 [47] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Jangjo-Borazjani et al., 2021 [60] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Najmi et al., 2012 [64] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Rajabi et al., 2022 [61] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Sepahi et al., 2022 [39] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Shahbazian et al., 2019 [42] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Azimi et al., 2014 [32] |  |  |  |  |  | NA |  |  |  |  |  |  |  |  |
| Mobasseri et al., 2020 [44] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Ebrahimi et al., 2019 [40] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Jaafarinia et al., 2022 [66] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Tajaddini et al., 2021 [33] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Behrouz et al., 2020 [45] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Aleali et al., 2019 [48] |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Milajerdi et al., 2017 [41] |  |  |  |  |  | NA | NA |  |  |  |  |  |  |  |
 Yes (2 points);
Yes (2 points);  Partial (1 point);
Partial (1 point);  No (0 points); NA denotes “Not applicable”. A complete description of the issues included in the quality assessment is as follows: (1) Question or objective sufficiently described? (2) Study design evident and appropriate? (3) Method of subject and comparison group selection or source of information and input variables described and appropriate? (4) Subject and comparison group (if applicable) characteristics sufficiently described? (5) If interventional and random allocation was possible, was it reported? (6) If interventional and blinding of investigators was possible, was it reported? (7) If interventional and blinding of subjects was possible, was it reported? (8) Outcome and (if applicable) exposure measure(s) well defined and robust to measurement, misclassification bias? Means of assessment reported? (9) Sample size appropriate? (10) Analytic methods described, justified, and appropriate? (11) Some estimate of variance is reported for the main results? (12) Controlling for confounding? (13) Results reported in sufficient detail? (14) Conclusion supported by the results?
No (0 points); NA denotes “Not applicable”. A complete description of the issues included in the quality assessment is as follows: (1) Question or objective sufficiently described? (2) Study design evident and appropriate? (3) Method of subject and comparison group selection or source of information and input variables described and appropriate? (4) Subject and comparison group (if applicable) characteristics sufficiently described? (5) If interventional and random allocation was possible, was it reported? (6) If interventional and blinding of investigators was possible, was it reported? (7) If interventional and blinding of subjects was possible, was it reported? (8) Outcome and (if applicable) exposure measure(s) well defined and robust to measurement, misclassification bias? Means of assessment reported? (9) Sample size appropriate? (10) Analytic methods described, justified, and appropriate? (11) Some estimate of variance is reported for the main results? (12) Controlling for confounding? (13) Results reported in sufficient detail? (14) Conclusion supported by the results?Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garza, M.C.; Pérez-Calahorra, S.; Rodrigo-Carbó, C.; Sánchez-Calavera, M.A.; Jarauta, E.; Mateo-Gallego, R.; Gracia-Rubio, I.; Lamiquiz-Moneo, I. Effect of Aromatic Herbs and Spices Present in the Mediterranean Diet on the Glycemic Profile in Type 2 Diabetes Subjects: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 756. https://doi.org/10.3390/nu16060756
Garza MC, Pérez-Calahorra S, Rodrigo-Carbó C, Sánchez-Calavera MA, Jarauta E, Mateo-Gallego R, Gracia-Rubio I, Lamiquiz-Moneo I. Effect of Aromatic Herbs and Spices Present in the Mediterranean Diet on the Glycemic Profile in Type 2 Diabetes Subjects: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(6):756. https://doi.org/10.3390/nu16060756
Chicago/Turabian StyleGarza, María Carmen, Sofía Pérez-Calahorra, Carmen Rodrigo-Carbó, María Antonia Sánchez-Calavera, Estíbaliz Jarauta, Rocío Mateo-Gallego, Irene Gracia-Rubio, and Itziar Lamiquiz-Moneo. 2024. "Effect of Aromatic Herbs and Spices Present in the Mediterranean Diet on the Glycemic Profile in Type 2 Diabetes Subjects: A Systematic Review and Meta-Analysis" Nutrients 16, no. 6: 756. https://doi.org/10.3390/nu16060756
APA StyleGarza, M. C., Pérez-Calahorra, S., Rodrigo-Carbó, C., Sánchez-Calavera, M. A., Jarauta, E., Mateo-Gallego, R., Gracia-Rubio, I., & Lamiquiz-Moneo, I. (2024). Effect of Aromatic Herbs and Spices Present in the Mediterranean Diet on the Glycemic Profile in Type 2 Diabetes Subjects: A Systematic Review and Meta-Analysis. Nutrients, 16(6), 756. https://doi.org/10.3390/nu16060756








