Abstract
Isothiocyanates are biologically active products resulting from the hydrolysis of glucosinolates predominantly present in cruciferous vegetables belonging to the Brassicaceae family. Numerous studies have demonstrated the diverse bioactivities of various isothiocyanates, encompassing anticarcinogenic, anti-inflammatory, and antioxidative properties. Nature harbors distinct isothiocyanate precursors, glucosinolates such as glucoraphanin and gluconastrin, each characterized by unique structures, physical properties, and pharmacological potentials. This comprehensive review aims to consolidate the current understanding of Moringa isothiocyanates, mainly 4-[(α-L-rhamnosyloxy) benzyl] isothiocyanate), comparing this compound with other well-studied isothiocyanates such as sulforaphane and phenyl ethyl isothiocyanates. The focus is directed toward elucidating differences and similarities in the efficacy of these compounds as agents with anticancer, anti-inflammatory, and antioxidative properties.
1. Introduction
Isothiocyanates, a class of compounds predominantly found in cruciferous plants such as broccoli, cauliflower, kale, cabbage, watercress, and others, impart these vegetables with their characteristic spicy and bitter taste. Epidemiological studies dating back to the early 1990s have indicated that an increased intake of these vegetables can yield beneficial health effects and has the potential to mitigate the risk of developing certain diseases. These hypotheses were subsequently corroborated by clinical studies [1,2]. Isothiocyanates emerge as biologically active products resulting from the hydrolysis of glucosinolates, secondary metabolites found in plants from the Brassicaceae family. The conversion of glucosinolates into bioactive forms occurs through the process of hydrolysis, catalyzed either by the endogenous enzyme myrosinase (β-thioglucosidase) or human gastrointestinal microbiota [3,4,5]. The activation of glucosinolates into their active metabolites takes place when plants are exposed to various stressors such as infections or mechanical damage. Consequently, the formation of isothiocyanates serves as a defensive mechanism protecting the plant from injury [6,7]. This intriguing observation prompted further investigations into isothiocyanates, aiming to unravel the underlying mechanisms facilitating plant survival in diverse conditions. Studies have revealed that isothiocyanates encompass a plethora of health benefits, including antidiabetic, anticancer, analgesic, and cardioprotective effects, the potential to treat neurological disorders, and regulation of thyroid gland function [8,9,10]. Thus, the purpose of this review is to consolidate current understanding about well-studied isothiocyanates such as sulforaphane and phenyl ethyl isothiocyanate, and to compare them with less investigated moringa isothiocyanates (MIC). The focus is directed toward elucidating differences and similarities in the anticancer, anti-inflammatory, and antioxidative efficacy of these compounds.
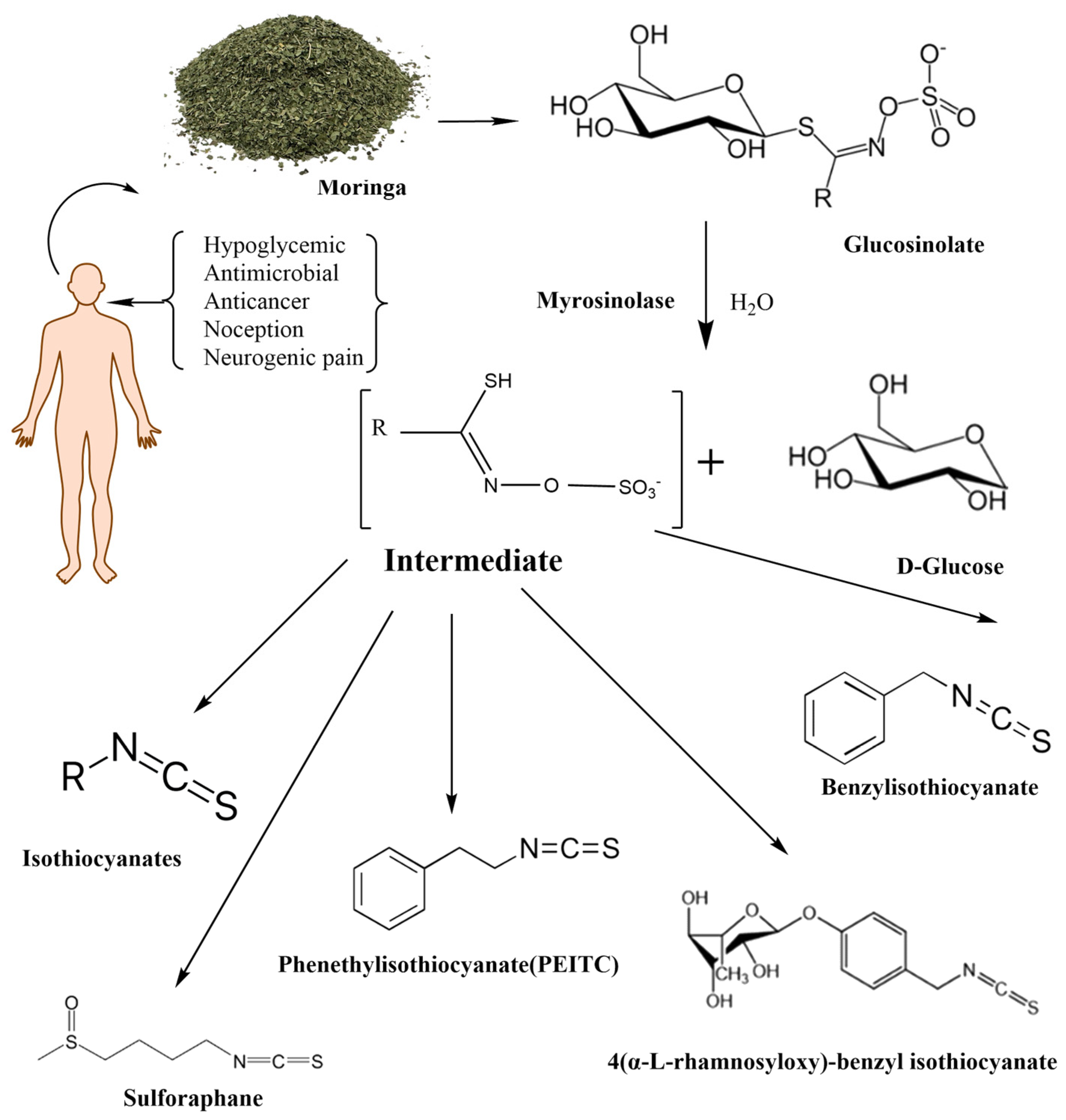
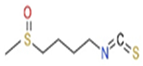
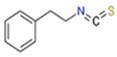
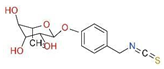
Mentioned representatives of this compound class, phenethyl isothiocyanate (PEITC or chemically 2-isothiocyanato ethylbenzene), sulforaphane (SFN or 1-isothiocyanato-4-methylsulfinylbutane), and MIC (4-[(α-L-rhamnosyloxy)benzyl] isothiocyanate), have been investigated (Figure 1). As illustrated in Table 1, these bioactive compounds exhibit variations in structure and physical properties.
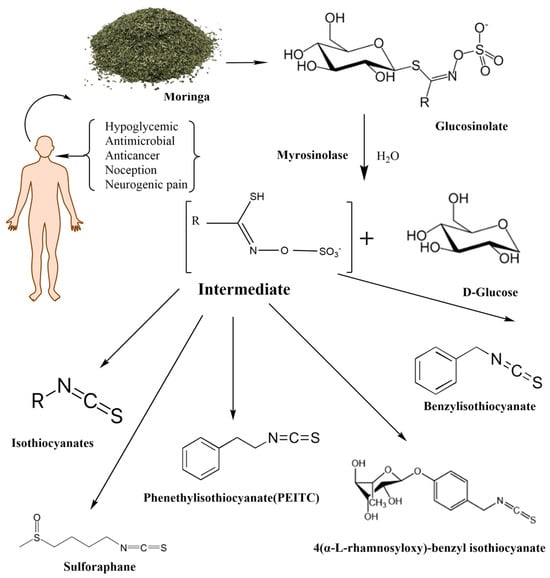
Figure 1.
The enzymatic reactions of glycosylate with myrosinase and their degradation products.

Table 1.
Comparison of the properties of sulforaphane, PEITC, and MIC according to the National Center for Biotechnology Information. PubChem Compound Summary for CID 5350, 16746, and 153557.
1.1. Phenethyl Isothiocyanate
Watercress (Nasturtium officinale) stands out as an easily accessible garden vegetable, abundant in gluconasturtiin—an aromatic glucosin olate featuring an ethyl chain linked to benzene in its radical. The hydrolysis of gluconasturtiin by myrosinase yields PEITC, an isothiocyanate with a phenylethyl radical attached to a nitrogen atom [11]. Upon activation, PEITC showcases neuro- and cardioprotective effects, along with antitumor and antimutagenic properties, contributing significantly to the prevention of carcinogenesis and other chronic degenerative diseases [12,13,14,15]. The pivotal mechanism driving PEITC bioactivity involves the activation of molecular pathways regulated by the transcription factors nuclear factor erythroid 2-related factor 2 (Nrf2) and heat shock factor 1 (HSF1). The sulfhydryl group of PEITC binds to cysteine residues in its protein targets, initiating the molecular process [16,17]. This review will delve further into the intricate interplay between PEITC and Nrf2, providing a comprehensive exploration of this critical mechanism.
1.2. Sulforaphane
Sulforaphane emerges as a metabolic product originating from the stable phytochemical glucoraphanin (GPN), a glucosinolate derived from dihomomethionine. The conversion of GPN to SFN transpires during the cutting or chewing of broccoli sprouts, a process that exposes GPN to the catalytic action of the myrosinase enzyme [18]. Similar to other isothiocyanates, SFN has demonstrated a myriad of health benefits, encompassing anti-inflammatory and antioxidant effects, inhibition of tumor cell growth, antidiabetic properties, cardioprotective effects, and other advantageous health impacts [19,20,21]. The isothiocyanate functional group within the SFN molecule emerges as the crucial pharmacophore, dictating its bioactivity [22]. SFN has garnered increased popularity in recent years, primarily due to its recognized anticancer potential. Research reports suggest that SFN induces apoptosis in tumor cells through both intrinsic and extrinsic apoptotic pathways [23]. Furthermore, SFN inhibits cancer initiation by modulating metabolic enzymes, leading to the reduction of carcinogen-activating phase I enzymes (e.g., decreasing CYP1A1 (cytochrome P450 family 1 subfamily A member 1) and CYP3A4 (cytochrome P450 family 3 subfamily A member 4) activity) and activation of carcinogen-detoxifying phase II enzymes [22,24].
1.3. Moringa Isothiocyanates
Moringa isothiocyanates are the least studied of the isothiocyanates. Similar to other isothiocyanates, they are found in a precursor form in Moringa oleifera, a tropical plant abundant in glucosinolates [25]. Moringa glucosinolates exhibit a unique structure with an additional sugar group. Four bioactive and relatively stable isothiocyanates are formed from Moringa glucosinolates, with 4-[(α-L-rhamnosyloxy) benzyl] isothiocyanate and 4-[(4′-O-acetyl-α-L-rhamnosyloxy)benzyl] isothiocyanate comprising over 95% of the total MIC, while others are present in smaller amounts [26]. Moringin (MG), as the most abundant MIC, results from myrosinase-catalyzed hydrolysis of glucomoringin (GMG). Alongside PEITC and SFN, MG has attracted attention across various research domains, including cancer, antimicrobial applications, neurodegenerative diseases, and more, owing to its anti-inflammatory and antioxidative effects (Figure 1) [27].
For instance, transcriptomic analysis revealed MG’s capacity to suppress inflammation and oxidative stress by reducing the expression of inflammatory cytokines, such as TNF-α (tumor necrosis factor α), IFN-α (interferon α), IL-1β (interleukin 1β), and IL-6 (interleukin 6), while simultaneously increasing Nrf2 gene expression and its nuclear accumulation. This resulted in a decrease in NF-κB translocation and its binding to promoter sites on responsive genes, coupled with a reduction in reactive oxygen species production. MG also demonstrated antioxidative characteristics, overcoming colchicine-induced oxidative stress in an Alzheimer’s disease rat model and improving the rats’ memory [12,13].
2. Isothiocyanate Metabolism
Upon hydrolyzation of glucosinolates in the gastrointestinal tract, isothiocyanates seamlessly enter the bloodstream and form associations with plasma proteins. This binding facilitates their traversal across the plasma membrane of cells, allowing entry into the cellular milieu. Once inside the cells, isothiocyanates engage in a reaction with glutathione, catalyzed by the enzyme glutathione S-transferase (GST). Subsequently, this conjugate is transported to the extracellular medium, where γ-glutamyl transferase and dipeptidase enzymatically dismantle its γ-glutamyl and glycyl components. The resulting metabolite then undergoes transportation to the liver, where it enters the mercapturic acid pathway. Within this pathway, N-acetyl transferases acetylate the metabolite, leading to the formation of the N-α-acetyl derivative or mercapturic acid. Finally, the formed derivatives are conveyed to the kidneys and actively expelled into the urine, completing the process of elimination from the body [28,29].
2.1. Metabolism of Phenethyl Isothiocyanate
PEITC demonstrates rapid absorption and notable bioavailability. The primary metabolic pathway for PEITC involves glutathione conjugation, leading to excretion in both urine and bile in the form of mercapturate. In the liver, the enzyme GST facilitates the addition of a glutathione (GSH) tail to PEITC, forming PEITC-GSH. Further metabolism occurs as γ-glutamyl transferase and dipeptidase act on the γ-glutamyl and glycyl moieties of PEITC-SG, respectively. The resulting cysteine conjugate undergoes N-acetylation of its cysteine radical through the action of N-acetyltransferase 2 (NAT2) and is eventually excreted as mercapturic acid in the urine [30,31].
2.2. Metabolism of Sulforaphane
Upon entering the body, SFN undergoes metabolism through the mercapturic acid pathway. The interaction involves the reactive electrophilic carbon from the isothiocyanate functional group (−N=C-S) and GSH, catalyzed by GST. The resulting SFN-GSH complex undergoes further transformations by enzymes such as γ-glutamyltranspeptidase, cysteinylglycinase, and N-acetyltransferase. The main metabolite formed is SFN-N-acetylcysteine. SFN exhibits high bioavailability, undergoes rapid metabolism, and is excreted from the body through urine. Once inside the cells, it accumulates [4,6].
2.3. Metabolism of Moringa Isothiocyanates
Although no data are available about the metabolic transformation of Moringa isothiocyanates, it is anticipated that they enter the metabolic pathway of mercapturic acid similarly to other isothiocyanates. As reported for PEITC and SFN, Moringa isothiocyanates undergo consecutive reactions involving GST, γ-glutamyl transferase, and dipeptidase. Eventually, NAT2 converts them into mercapturic acid, which is then excreted via the kidneys [4,6,29,30,31]. However, a recent study conducted in rats detected MIC in the serum without chemical or enzymatic degradation, indicating that MIC is protected from typical isothiocyanate metabolism and other metabolic modifications because of its unique glycosidic motif [32], but further confirmations are needed. Moreover, it was reported that the structural difference from other isothiocyanates does not impact MIC efficacy but rather contributes to their stability. As stated, purified MIC is a white crystalline powder stable at 25 °C, while SFN and PEITC are volatile, viscous liquids [33].
3. Chemical Structures of the Three Isothiocyanates
Functional similarity among isothiocyanates arises from the isothiocyanate moiety (−N=C=S), a functional group formed by substituting the oxygen in the isocyanate group with sulfur. This group forms hydroxyl bonds with sulfhydryl, hydroxy, and amine groups on targeted proteins. The newly formed bonds are reversible, meaning that the activity of isothiocyanates ends after the hydrolysis of proteins [34].
Moringa isothiocyanates exhibit solid and relatively stable properties at room temperature, as a result of the additional rhamnose sugar group attached to the isothiocyanate moiety. On the other hand, PEITC and SFN are volatile molecules available in liquid form [26,35]. Isothiocyanates are liposoluble molecules with the ability to cross the blood–brain barrier, giving them neuroprotective activity against conditions such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [36,37].
These plant-derived isothiocyanates are well-known for their antioxidative and anti-inflammatory activity, which may be responsible for various beneficial health effects reported in the literature.
4. Nrf2 Activation and Regulation of Oxidative Stress
The activation of the Nrf2 transcription factor and consequently the inhibition of nuclear factor kappa B (NF-κB) signaling resulting in protection from oxidative stress is one of the most investigated mechanisms for PEITC and SFN. Upon translocation into the nucleus, Nrf2 activates the antioxidant response element (ARE) and stimulates the transcription of antioxidant and cytoprotective enzymes [37,38]. The interaction between Nrf2 and ARE leads to the upregulation of heme oxygenase 1 (HO-1), an enzyme that catalyzes the degradation of heme into CO, bilirubin, and free iron. HO-1 inhibits the production of pro-inflammatory cytokines such as IL-6 and TNF-α while stimulating the secretion of anti-inflammatory interleukins, mainly interleukin 10 (IL-10). Moreover, the HO-1-mediated release of CO leads to inhibition of the NF-ĸB signaling pathway and thus, further decreases the expression of inflammatory molecules [39]. Nrf2 mediates the transcription of other detoxification and antioxidant genes, such as NAD(P)H-quinone oxidoreductase 1 (NQO1), glutathione S-transferases (GSTs), aldo-keto reductases (AKRs), aldose reductase (AR), γ-glutamyl peptidase (GGT), carboxylesterase (CES), UDP-glucuronosyltransferases (UGTs), thioredoxin (TXN), and glutamate-cysteine ligase catalytic/modifier subunits (GCLC/GCLM) [17], indicating the existence of a complex interplay between inflammation and oxidative stress where Nrf2 plays a central role.
All isothiocyanates react with Nrf2 in the same way. The electrophilic center of isothiocyanates interacts with thiol (-SH) groups of cysteine residues in Kelch-like ECH-associated protein 1 (Keap1), forming a stable bond between sulfur and carbon in a process called Michael addition. This leads to the release of Nrf2 from its complex with Keap1, stabilization of Nrf2 protein, and its translocation to the nucleus [40,41,42]. It is claimed that SFN, PEITC, and MIC have a high affinity for Keap1 [42]; however, their dissociation constant (Kd) has not been determined yet. Thus, the isothiocyanates–Keap1 interaction might be dependent on other factors, such as the dose of isothiocyanate used in a reaction, the time of exposure, the type of cells exposed to isothiocyanates, or other factors.
4.1. Role of Phenethyl Isothiocyanate in Nrf2 Activation and Activities
Phenethyl isothiocyanate has been shown to activate Nrf2 in both in vitro and in vivo studies. Ernst et al. (2011) explained that PEITC increases the phosphorylation of extracellular signal-regulated (ERK1/2) kinases 1/2, which are responsible for Nrf2 phosphorylation and translocation to the nucleus [40]. In a study by Eisa et al. (2021), the nephroprotective role of PEITC in diabetic mice was found to be Nrf2-dependent, stimulating the expression of downstream targets such as HO-1 and γ-GCS (gamma glutamate-cysteine), proteins that counteract oxidative stress [43].
However, other anti-inflammatory mechanisms of PEITC have also been proposed. In obese mice treated with PEITC, higher levels of NF-κB, lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1), and cyclooxygenase-2 (COX-2) were observed compared to the control group. PEITC supplementation was able to decrease the expression levels of NF-κB, LOX-1, and COX-2, ameliorating obesity-induced inflammation via mTOR/PPARγ/AMPK signaling (mammalian target of rapamycin/peroxisome proliferator-activated receptor gamma/adenosine-monophosphate activated-protein kinase) [40,44]. PEITC has also shown beneficial effects against bacterial and viral infections by suppressing Toll-like receptor signaling. This leads to the downregulation of NF-κB and interferon regulatory factor 3 (IRF3), ultimately inhibiting interferon β (IFNβ) and interferon-inducible protein-10 (IP-10), which are genes involved in infection-triggered inflammation [45].
4.2. Role of Sulforaphane in Nrf2 Activation and Activities
Sulforaphane has been associated with the modulation of Nrf2/Keap and NF-κB, contributing to protection against cardiovascular-related inflammation, atherosclerosis, hypertension, diabetes mellitus, cardiomyopathy, and heart failure [12].
For instance, Pan et al. (2023) demonstrated that SFN can reduce vascular remodeling in hypoxic pulmonary hypertension by reactivating Nrf2, decreasing the activity of effector T cells, and suppressing the production of inflammatory molecules such as TNF-α and IL-6. Additionally, SFN enhanced antioxidative defense mechanisms by improving superoxide dismutase (SOD) activity and increasing total glutathione levels [46]. The anti-inflammatory properties of SFN were also observed in an animal model of diabetic cardiomyopathy [47,48]. Mice with diabetes treated with SFN for 4 months showed lower risks of developing cardiac dysfunction, oxidative damage, inflammation, fibrosis, and hypertrophy compared to a control group [47,48]. Similarly, Sun and colleagues reported that SFN-mediated activation of Nrf2 signaling can prevent cardiomyopathy in mice with streptozotocin-induced hyperglycemia in an AMPKα2-dependent manner. AMPKα2 is an immune-suppressive protein that downregulates the activation of NF-κB signaling [49].
4.3. Role of Moringa Isothiocyanates in Nrf2 Activation and Activities
Most studies on Moringa isothiocyanates focus on their anti-inflammatory activities, demonstrating beneficial roles in conditions such as diabetic nephropathy, microbial infections, and neuroprotection [10,50,51]. The anticipated immune-suppressive mechanism of MIC was found to be Nrf2-dependent. For instance, exposing human renal proximal tubule HK-2 cells to increasing doses of MG (1.25–5 μM) resulted in the upregulation of Nrf2 gene expression, particularly NQO1, HO-1, and GCLC, while inhibiting transforming growth factor beta 1 (TGFβ1). Consequently, MG exhibited a dual action by suppressing inflammation and reducing oxidative stress in the treated cells through the common isothiocyanate mechanism of action [50]. Likewise, MG demonstrated the ability to prevent renal injury in diabetes-bearing mice by repressing ROS and malondialdehyde (MDA) levels, while enhancing the production and secretion of defensive proteins like GSH, SOD, and catalase (CAT). At the molecular level, this was associated with the induction of Nrf2 signaling and the inhibition of NF-κB activity [52]. Additionally, the neuroprotective effects of MG were observed in various neurodegenerative disorders, including Parkinson’s Disease, Alzheimer’s Disease, Huntington’s Disease, and Amyotrophic Lateral Sclerosis, where once again, Nrf2 activation played a central role [10]. The documented beneficial effects of isothiocyanates against various cancers highlight the proposed induction of both intrinsic and extrinsic apoptotic pathways as the most crucial underlying mechanism.
5. Antitumor Activities of Isothiocyanates
5.1. Antitumor Activities of Phenethyl Isothiocyanate
Phenethyl isothiocyanate is one of the most studied isothiocyanates with well-established antitumor characteristics. In vitro studies have demonstrated that PEITC induces oxidative damage in cervical cancer cells, triggering caspase-3 mediated apoptosis [53]. Similarly, colon cancer cells treated with PEITC, either alone or in combination with irinotecan, experienced higher levels of ROS and Ca2+ compared to the control. Authors explained that in a state of oxidative stress, cancer cells activate apoptosis as a defense mechanism, along with the Nrf2 pathway, which promotes the production of antioxidative proteins such as HO-1 and GSH [54]. Consequently, PEITC and/or irinotecan were able to indirectly reduce tumor growth and progression by shifting the balance of oxidation–reduction reactions to the left.
The potential of PEITC to impair the cancer cell cycle and inhibit cell viability and proliferation in four human osteosarcoma cell lines has been clearly demonstrated [55]. Multiple cell death modalities were detected, including ferroptosis, apoptosis, and autophagy, as a result of increased oxidative stress and GSH depletion. These findings were confirmed in a xenograft osteosarcoma mouse model treated with 30 mg/kg of PEITC [55].
In a mouse transgenic model of prostate cancer, a diet with 0.05% PEITC led to a reduction in tumor incidence through several mechanisms. PEITC inhibited the cell cycle in cancer cells, reduced inflammation, and disrupted cancer-related signaling [56]. Similar results were reported by Stan and colleagues in 2014, where PEITC inhibited the growth of prostatic cancer cells both in vitro and in vivo. The effect was dose-dependent, with 7 μmol/L proposed as IC50. PEITC suppressed tumor growth in vivo by stimulating G2/M phase cell cycle arrest and apoptosis. The antiapoptotic proteins B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-XL) were downregulated in treated mice, while the expression of the proapoptotic protein Bcl-2 homologous antagonist killer (Bak) was increased [57].
Recent data indicated that PEITC can reduce the tumor’s potential to metastasize. For instance, Zhang et al. (2021) noted the ability of PEITC to reduce breast cancer metastasis via epigenetic reactivation of the tumor suppressor gene cadherin (CDH1). Reactivated CDH1 suppressed the Wnt/β-catenin pathway, which confers breast cancer stem cell properties in breast cancer cells [58]. Similar PEITC activity was observed in colorectal cancer stem cells (CSC), where it significantly reduced stem cell properties, such as clonogenicity and the expression of pluripotent factors in vitro. When PEITC-pretreated CSC were inoculated in mice, a significant reduction in tumor growth and progression was observed, in contrast to the group where mice inoculated with CSC were treated with PEITC. Thus, the authors concluded that PEITC might contribute to the prevention or delay of colorectal cancer growth by inhibiting stem cells [59].
Additionally, PEITC is often combined with other natural cytotoxic molecules or chemotherapeutics for a more pronounced antitumor effect. Results show that the combination of PEITC, indole-3-carbinol, xanthohumol, and resveratrol is more efficient in the activation of Nrf2 and NF-κB inhibition in pancreatic cancer cells than either of the substances alone [60]. Kasukabe and colleagues investigated the combination of cotylenin A and PEITC in pancreatic cancer cells resistant and non-resistant to gemcitabine and found that the combined treatment synergistically induced the generation of ROS, leading to more pronounced cancer cell death in both cell lines [61]. Finally, Li et al. (2016) demonstrated the ability of PEITC to reverse the resistance of biliary tract cancer cells to cisplatin [62].
5.2. Antitumor Activities of Sulforaphane
Numerous in vivo animal studies have indicated the antitumor potential of SFN. For instance, a study on rats demonstrated that SFN significantly reduces the proliferation of triple-negative breast cancer cells. Moreover, it diminished the number of stem-like cancer cells responsible for resistance to chemotherapy and radiotherapy [63]. Similarly, SFN exhibited a dose-dependent suppression of proliferation, induction of apoptosis, and reduction of lymph metastasis in female breast tumor-bearing mice [64]. The anti-tumor efficacy of SFN was observed in transgenic pancreatic cancer mice treated with 50 mg/kg of SFN for 120 days [65].
In lung tumors, SFN prevents the epithelial–mesenchymal transition, a process involved in all stages of lung cancer development and progression. This resulted in decreased invasiveness and migratory capacity of lung cancer cells via induction of MAPK/ERK signaling (mitogen-activated protein kinase/extracellular-signal-regulated kinase signaling) [66]. Li et al. (2020) identified the activation of caspase-mediated apoptosis as the key mechanism of SFN-induced tumor regression. However, they also noted that the activation of Nrf2 could stimulate autophagy, a process that helps tumors survive in a nutrition-depleted environment [67]. Lu et al. (2021) demonstrated that SFN, at a dose of 5 mg/kg b.w., induces tumor-protective autophagy, and the addition of an autophagy inhibitor increased SFN sensitivity in esophageal squamous cell carcinoma [68]. Conversely, Byun et al. showed that 5 mg/kg b.w. of SFN reduced the size of colon tumors by increasing the activity of proteins involved in the cell cycle. In vitro analysis revealed that SFN decreased cellular levels of glutathione, leading to increased ROS production in cancer cells. Elevated ROS levels further activated Nrf2 signaling and the stress-activated kinase, p38, ultimately resulting in cell cycle arrest and apoptosis [69].
Additionally, some studies demonstrated that SFN can increase cancer cell sensitivity to commonly used antitumor drugs. For example, DNA damage-induced apoptosis was more pronounced in breast cancer cells treated with a combination of cisplatin and SFN than in cells treated with only one of the mentioned drugs [70]. Similarly, combinational treatment of imatinib (IM) and SFN stimulated apoptosis of IM-resistant leukemia stem cells (LSCs). Mechanistic studies showed that combined treatment induced ROS production and subsequent activation of apoptotic molecules, namely, caspase 3, poly (ADP-ribose) polymerase (PARP), and Bcl-2-associated X protein (Bax), while Bcl-2 expression was inhibited [71]. However, even with the well-known and proven antitumor activity of SFN, Rai et al. (2020) found that independent application of SFN contributed to tumor size reduction only when combined with paclitaxel. The authors proposed that SFN could be administered as an adjunct to chemotherapy to reduce side effects [72].
5.3. Antitumor Activities of Moringa Isothiocyanates
Although least investigated, MIC has been shown to inhibit cancer growth and promote apoptosis in cancer cells. Notably, MG and one of its acetylated isomers were demonstrated to induce NQO1 enzyme activity as effectively as SFN in hepatocellular carcinoma cells, suggesting that MG acts as an Nrf2 activator as well [73].
Additionally, other MIC-mediated antitumor mechanisms have been recognized as important. Xie et al. (2022) demonstrated that MG induces apoptosis in renal cancer cells both in vitro and in vivo by increasing the Bax/Bcl-2 ratio and inducing cell cycle arrest [74]. In malignant astrocytoma cells, MG stimulated the apoptotic process through p53 and Bax activation and Bcl-2 inhibition. Furthermore, the induction of oxidative stress and subsequent activation of the Nrf2 transcription factor further contributed to tumor cell death [75].
The effects of MG on glioma cells were shown to be time- and dose-dependent, as reported by Xie et al. (2023). They found that MG stimulated tumor cell apoptosis in a manner similar to the previously explained mechanisms without causing harm to normal human gastric mucosal cells [76]. MIC-mediated activation of apoptotic genes, including caspase, p53, Akt/MAPK, and Bax of the proapoptotic Bcl family, has also been observed in human prostate [77], liver [78], and neuroblastoma cancer cells [79].
5.4. Anticancer and Cancerogenic Potential of Isothiocyanates
Although current data supports the hypothesis of anti-cancerogenic properties of isothiocyanates, some differences between them were described in the literature. For example, Yuan et al. (2013) compared the antitumor potential of SFN and PEITC in vitro in human glioblastoma cells, as well as in vivo in a murine orthotropic glioblastoma tumor model. While both SFN and PEITC were able to inhibit proliferation and stimulate the apoptosis of three different glioblastoma cell lines, in vivo studies showed that only SFN was able to decrease the tumor weight at a dose of 12.5 mg/kg daily [80]. On the contrary, prostate cancer cells were more sensitive to PEITC than SFN. PEITC was able to inhibit cell replication at a dose of 10 μM while the same effects were seen when prostate cancer cells were treated with 40 μM of SFN [81]. Next, SFN was shown to stimulate apoptosis and suppress the metastasis of bladder cancer cells, both in vitro [82] and in vivo [83] in a dose-dependent manner. While ≥20 μM SFN decreases cell viability and migration of bladder cancer cells, a low concentration of SFN (1–5 μM) promotes their proliferation and migration [84]. Similarly, dual results were seen when bladder cancer was treated with PEITC. The administration of 0.01% to 0.05% of PEITC significantly increased the incidences of papillary or nodular hyperplasia, dysplasia, and transitional cells in rat urinary bladder carcinomas, while doses higher than 0.05% showed antitumor effects [85]. Additionally, when 0.1% PEITC was given to 6-week-old F344 rats for 1, 2, 3, and 7 days, a significant reduction in urinary pH levels compared to the normal control was detected, together with an increase in the thickness of the urinary bladder urothelium and occurrence of inflammation, vacuolation, erosion, and apoptosis/single cell necrosis in the urinary bladder lesion. Histopathological simple hyperplasia was observed when 0.1% PEITC is administered for 14 days, suggesting that the use of PEITC may induce continuous proliferation of bladder epithelial cells, especially in the early stage of bladder carcinogenesis and thus, should be used with caution [81]. When it comes to MIC, current data suggest only positive anticancer effects in bladder cancer [86,87]. However, this may not be a realistic scenario as MIC are the least investigated isothiocyanates and thus, require further investigation to confirm these results.
Overall, the anti-cancerogenic potential of isothiocyanates might depend on several factors including the type and dose of isothiocyanate used in the treatment, as well as the type of cancer.
6. Neuroprotective Properties of Isothiocyanates
6.1. Neuroprotective Properties of Phenethyl Isothiocyanate
Phenyl ethyl isothiocyanate has emerged as a promising protective agent in the context of neurodegeneration, showcasing neuroprotective properties through its modulation of various molecular pathways associated with oxidative stress, inflammation, and apoptosis. Experimental models have demonstrated the potential of PEITC to attenuate neurodegenerative processes, presenting a novel avenue for therapeutic intervention [37]. The neuroprotective activity of PEITC is realized through the activation of Nrf2 and its downstream cytoprotective pathways [17]. Despite promising potential, a limited number of studies have explored the use of PEITC in neurodegenerative diseases.
Wang et al. (2019) conducted a study showing that PEITC could promote the repair of injured sensory neurons [88]. However, it is important to note that conflicting findings exist in the literature. One study indicated that PEITC may induce dose-dependent neurotoxic effects in the brain. In an experiment, pregnant adult rats were treated with 15, 60, and 120 mg/kg of PEITC from the 7th to the 16th week of gestation. The study observed impairment of neuro-behavioral development and learning abilities in the offspring exposed to 60 and 120 mg/kg of PEITC, while 15 mg/kg showed no neurotoxic effects [89]. These conflicting results underscore the need for further research to delineate the nuanced effects of PEITC on neurodegenerative processes and to establish safe and effective therapeutic protocols.
6.2. Neuroprotective Properties of Sulforaphane
Sulforaphane emerges as the most extensively studied isothiocyanate concerning the prevention of neurodegenerative diseases. Notably, SFN has demonstrated a capacity to counteract amyloid β (Aβ) aggregation in Alzheimer’s disease, with this effect being correlated with the activation of Nrf2 [90]. In cellular studies, Bahn et al. treated SH-SY5Y cells with 1 μM of SFN, resulting in the overexpression of Nrf2 and reduced transcription levels of beta-secretase 1 (BACE1) and BACE1 antisense RNA (BACE1-AS), proteins implicated in amyloidogenic processes [91]. SFN-mediated reduction of oxidative stress and prevention of neural cell damage have shown protective effects in Parkinson’s disease, with the mechanism being Nrf2-ARE-dependent [92]. The observed neuroprotective effects of SFN have been further confirmed in in vivo settings. For instance, Zhang et al. (2015) highlighted the potential therapeutic use of SFN in Alzheimer’s disease. In an Alzheimer’s mouse model, SFN exerted neuroprotective effects by shielding the brain from Aβ-deposits responsible for the degeneration of neurons and synapses. SFN restored endogenous antioxidants in the brain and regulated the activity of glutathione peroxidase (GPX). Additionally, SFN demonstrated a possible anxiolytic effect by influencing reduced locomotor activity in diseased mice [93]. Regular oral administration of SFN at a daily dose of 10 to 50 mg/kg prevented memory impairment characteristic of Alzheimer’s disease and reduced levels of tau, phosphorylated tau, and Aβ-protein—key pathophysiological factors in this disease. SFN achieved these effects by influencing their production and clearance, and increasing the activity of heat shock protein 70 (HSP70) and the C-terminus of the HSP70-interacting protein [94]. Furthermore, SFN demonstrated the ability to reduce depressive-like behavior in rats, possibly stemming from memory loss, through effects on serotonin metabolism and transport [95].
In vivo studies by Morroni et al. (2013), Jazwa et al. (2011), and Zhou et al. (2016) confirmed the neuroprotective activity of SFN for the treatment of Parkinson’s disease. SFN exhibited a neuroprotective effect, preventing the degeneration of dopaminergic neurons and significantly improving impaired motor function, coordination, and balance. This effect was attributed to the increased antioxidant potential of the substantia nigra and the inhibition of apoptosis, with SFN enhancing brain antioxidant protection by activating Nrf2 and subsequently the antioxidant enzymes HO-1 and NQO1 [96,97,98].
Yoo et al. (2019) and Li et al. (2013) conducted studies in mice with autoimmune encephalomyelitis to explore the potential of SFN as an adjunctive therapy for multiple sclerosis. SFN demonstrated preventive effects against neuronal degeneration due to its antioxidant and anti-inflammatory properties. The activation of Nrf2 led to increased synthesis and activity of antioxidant enzymes, reducing the number of antigen-specific Th17 cells critical for the development of autoimmune encephalopathy. Additionally, SFN improved the integrity of the blood–brain barrier (BBB) by inhibiting oxidative stress, reducing the expression of matrix metalloproteinase-9 (MMP-9), the tissue inhibitor of metalloproteinase, and consequently protecting levels of the proteins claudin-5 and occludin, which are crucial for maintaining the integrity of the BBB [99,100].
6.3. Neuroprotective Properties of Moringa Isothiocyanates
Moringa isothiocyanates play a crucial role as antioxidants, effectively neutralizing free radicals and mitigating oxidative stress within the brain. Through the enhancement of the cellular antioxidant defense system, MIC demonstrate a capacity to prevent oxidative damage to neurons, contributing to overall neuroprotection [8]. In parallel to PEITC, MIC exhibit anti-inflammatory effects by suppressing the production of inflammatory cytokines and mediators in the brain [101,102]. Notably, a study conducted by Onasanwo and colleagues observed the potential pharmacological role of Moringa oleifera isothiocyanates in preventing the loss of neuronal cells and managing Alzheimer’s disease. They emphasized the critical role of MIC in reducing oxido-inflammatory stress, restoring cholinergic transmission through acetylcholinesterase inhibition, and maintaining neuronal integrity in the brains of mice with exogenously induced neurodegeneration [103].
Furthermore, recent research suggests that treatment with Moringa extract improves anxiety-like behavior, hyperactivity, cognitive learning, and memory impairments in mice with developed Alzheimer’s disease. This improvement is attributed to the inhibition of BACE1 and asparagine endopeptidase (AEP), as well as the upregulation of insulin-degrading enzyme (IDE), neprilysin (NEP), and low-density lipoprotein receptor-related protein 1 (LRP1) levels [104]. The same research group previously demonstrated that MIC alleviate hyperphosphorylation and Aβ pathology in a rat model with induced Alzheimer’s disease-like pathology [105]. Additionally, MIC have been shown to promote the expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), which play a crucial role in neuronal survival, growth, and maintenance, thereby contributing to the preservation of cognitive function and preventing neurodegeneration. In a study by Purwoningsih and colleagues, mice treated with Moringa oleifera seed oil exhibited amelioration of anxiety-like and depression-like symptoms, as well as memory improvement. This was linked to increased mRNA expression of BDNF, inhibition of acetylcholinesterase (AChE) activity, and prevention of the rise in malondialdehyde levels in the brain [106]. Furthermore, Moringa oleifera seed extract shows beneficial effects on congenital aspects. In mice with scopolamine-induced learning and memory impairment, MIC mediated the enhancement of the cholinergic neurotransmission system and neurogenesis through the activation of Akt, ERK1/2, and cAMP response element-binding protein (CREB) signaling pathways, resulting in a reduction of amnesia-related symptoms [107].
Another neuroprotective mechanism of MIC is linked to mitochondrial protection, as demonstrated by Gonzales-Burgos et al. (2021). Their investigation revealed the protective effects of MIC on an H2O2-induced oxidative stress model in human neuroblastoma cells. In vitro assays indicated that in addition to antioxidative activity involving the reduction of free radicals, a decrease in lipid peroxidation, and enhanced glutathione levels, MIC prevented mitochondrial dysfunction by regulating calcium levels and increasing mitochondrial membrane potential [108].
7. Conclusions
Isothiocyanates are inherent natural products that attain bioactivity through the hydrolysis of their precursors, known as glucosinolates. The functional similarity among them arises from the pharmacophore—the isothiocyanate moiety, which interacts effectively with sulfhydryl groups on targeted proteins. A distinguishing characteristic of Moringa isothiocyanates lies in their enhanced stability, attributed to an additional sugar group in their structure.
The activation of Nrf2 stands as a pivotal mechanism of action for all isothiocyanates, playing a key role in both antioxidative and anti-inflammatory activities. PEITC, SFN, and MIC collectively exhibit numerous health benefits, ranging from cardio- and nephroprotection to anticancer activity. The small, liposoluble nature of PEITC and SFN enables them to traverse the BBB, facilitating neuroprotective activity. Moreover, current data suggest that, although bigger and less liposoluble, MIC possesses neuroprotective effects, as shown by their ability to reduce oxido-inflammatory stress in neuronal cells. The significant health benefits of isothiocyanates cannot be underestimated and prompt questions on how their potency as antioxidants or anticancer agents varies with their structure. As shown in Table 1, SFN has a simple hydrocarbon backbone with the (−N=C=S) functional group, while PEITC has a phenyl ring. Contrary to others, MIC have a phenyl ring and a rhamnose sugar which confer stability at room temperature [26]. If structural differences influence physical properties, how much influence do they have on function? This question and more are worth exploring as we expand our understanding of the significant health benefits locked in cruciferous vegetables.
Author Contributions
Conceptualization, writing, and editing, J.B.O.; literature research, writing—original draft preparation, D.B. Writing and editing, U.N. and O.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge USDA NIFA AFRI Award 2023-67017-39267 for funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ARE | Antioxidant response element |
| GMG | Glucomoringin |
| HSF1 | Heat Shocking Factor 1 |
| GSH | Glutathione S-transferases |
| GGT | γ Glutamyl Peptidase |
| MG | Moringin |
| MIC | Moringa Isothiocyanate |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PEITC | Phenyl ethyl isothiocyanate |
| GPN | Glucoraphanin |
| CYP1A1 | Cytochrome P450 family 1 subfamily A member 1 |
| CYP3A4 | Cytochrome P450 family 3 subfamily A member 4 |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin 6 |
| TGFB | Transforming growth factor-beta |
| Bcl-XL | B-cell lymphoma-extra large |
| Bak | Bcl-2 homologous antagonist killer |
| CADH1 | Cadherin |
| MAPK/ERK | Mitogen-activated protein kinase/extracellular-signal-regulated kinase signaling |
| PARP | Poly (ADP-ribose) polymerase |
| BAX | Bcl-2-associated X protein |
| BACE1 | Beta-secretase 1 |
| BACE1-AS | BACE1 antisense RNA |
| MMP-9 | Matrix metalloproteinase 9 |
| CAT | Catalase |
| AEP | Asparagine endopeptidase |
| IDE | Insulin-degrading enzyme |
| NEP | Neprilysin |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
| AChE | Acetylcholinesterase |
| CREB | cAMP response element-binding protein |
References
- Verhoeven, D.T.; Goldbohm, R.A.; van Poppel, G.; Verhagen, H.; van den Brandt, P.A. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 733–748. [Google Scholar]
- Conaway, C.C.; Yang, Y.M.; Chung, F.L. Isothiocyanates as cancer chemopreventive agents: Their biological activities and metabolism in rodents and humans. Curr. Drug Metab. 2002, 3, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Bozic, D.; Baralić, K.; Živančević, K.; Miljaković, E.A.; Ćurčić, M.; Antonijević, B.; Djordjević, A.B.; Bulat, Z.; Zhang, Y.; Yang, L.; et al. Predicting sulforaphane-induced adverse effects in colon cancer patients via in silico investigation. Biomed. Pharmacother. 2022, 146, 112598. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Mao, X.; Du, M. Metabolism, absorption, and anti-cancer effects of sulforaphane: An update. Crit. Rev. Food Sci. Nutr. 2022, 62, 3437–3452. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qi, Y.; Zhao, J.; Jiang, H.; Chen, X.; Ren, J. Synthesis and biological evaluation of sulforaphane derivatives as potential antitumor agents. Eur. J. Med. Chem. 2013, 64, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Iahtisham-Ul-Haq; Khan, S.; Awan, K.A.; Iqbal, M.J. Sulforaphane as a potential remedy against cancer: Comprehensive mechanistic review. J. Food Biochem. 2022, 46, e13886. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef]
- Shoaib, S.; Khan, F.B.; Alsharif, M.A.; Malik, M.S.; Ahmed, S.A.; Jamous, Y.F.; Uddin, S.; Tan, C.S.; Ardianto, C.; Tufail, S.; et al. Reviewing the Prospective Pharmacological Potential of Isothiocyanates in Fight against Female-Specific Cancers. Cancers 2023, 15, 2390. [Google Scholar] [CrossRef]
- Mundkar, M.; Bijalwan, A.; Soni, D.; Kumar, P. Neuroprotective potential of Moringa oleifera mediated by NF-kB/Nrf2/HO-1 signaling pathway: A review. J. Food Biochem. 2022, 46, e14451. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Sousa, A.S.; Reis, C.A.; Pintado, M.M. Phenylethyl Isothiocyanate: A Bioactive Agent for Gastrointestinal Health. Molecules 2022, 27, 794. [Google Scholar] [CrossRef]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef] [PubMed]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Saxena, R.; Sinclair, E.; Fu, Y.; Jacobs, A.; Dyba, M.; Wang, X.; Cruz, I.; Berry, D.; Kallakury, B.; et al. Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth. Cell Death Differ. 2016, 23, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Smerák, P.; Polívková, Z.; Stetina, R.; Bártová, J.; Bárta, I. Antimutagenic effect of phenethyl isothiocyanate. Cent. Eur. J. Public Health 2009, 17, 86–92. [Google Scholar] [CrossRef]
- Keum, Y.S.; Owuor, E.D.; Kim, B.R.; Hu, R.; Kong, A.N. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC). Pharm. Res. 2003, 20, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Dayalan Naidu, S.; Suzuki, T.; Yamamoto, M.; Fahey, J.W.; Dinkova-Kostova, A.T. Phenethyl Isothiocyanate, a Dual Activator of Transcription Factors NRF2 and HSF1. Mol. Nutr. Food Res. 2018, 62, e1700908. [Google Scholar] [CrossRef]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef]
- Liang, J.; Hänsch, G.M.; Hübner, K.; Samstag, Y. Sulforaphane as anticancer agent: A double-edged sword? Tricky balance between effects on tumor cells and immune cells. Adv. Biol. Regul. 2019, 71, 79–87. [Google Scholar] [CrossRef]
- Rafiei, H.; Ashrafizadeh, M.; Ahmadi, Z. MicroRNAs as novel targets of sulforaphane in cancer therapy: The beginning of a new tale? Phytother. Res. 2020, 34, 721–728. [Google Scholar] [CrossRef]
- Sestili, P.; Fimognari, C. Cytotoxic and Antitumor Activity of Sulforaphane: The Role of Reactive Oxygen Species. Biomed. Res. Int. 2015, 2015, 402386. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Akter, S.; Lin, C.-N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phyther. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef] [PubMed]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Shi, Y.; Liu, H.; Panjwani, A.A.; Warrick, C.R.; Olson, M.E. A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies. Nutrients 2019, 11, 1547. [Google Scholar] [CrossRef] [PubMed]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, G.; De Petrocellis, L.; Schiano Moriello, A.; Bertoli, S.; Leone, A.; Battezzati, A.; Mazzini, S.; Bassoli, A. Moringin, A Stable Isothiocyanate from Moringa oleifera, Activates the Somatosensory and Pain Receptor TRPA1 Channel In Vitro. Molecules 2020, 25, 976. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef]
- Esteve, M. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Ioannides, C.; Konsue, N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab. Rev. 2015, 47, 356–373. [Google Scholar] [CrossRef]
- Morris, M.E.; Dave, R.A. Pharmacokinetics and pharmacodynamics of phenethyl isothiocyanate: Implications in breast cancer prevention. AAPS J. 2014, 16, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Kaschula, C.H.; Hunter, R. Chapter 1—Synthesis and Structure–Activity Relations in Allylsulfide and Isothiocyanate Compounds from Garlic and Broccoli Against In Vitro Cancer Cell Growth; Atta-ur-Rahman, Ed.; Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 1–43. ISSN 1572-5995. ISBN 9780444637499. [Google Scholar] [CrossRef]
- Wolff, K.; Jaja-Chimedza, A.; Kim, Y.; Waterman, C.; Poulev, A.; Raskin, I.; Ribnicky, D. Moringa isothiocyanate-1 is bioaccessible and bioavailable as a stable unmodified compound. Phytochem. Lett. 2020, 38, 33–38. [Google Scholar] [CrossRef]
- Wang, F.; Bao, Y.; Zhang, C.; Zhan, L.; Khan, W.; Siddiqua, S.; Ahmad, S.; Capanoglu, E.; Skalicka-Woźniak, K.; Zou, L.; et al. Bioactive components and anti-diabetic properties of Moringa oleifera Lam. Crit. Rev. Food Sci. Nutr. 2022, 62, 3873–3897. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Xu, Y.M.; Lau, A.T.Y. Anti-Cancer and Medicinal Potentials of Moringa Isothiocyanate. Molecules 2021, 26, 7512. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Adkins, C.; Lockman, P.; Srivastava, S.K. Metastasis of Breast Tumor Cells to Brain Is Suppressed by Phenethyl Isothiocyanate in a Novel In Vivo Metastasis Model. PLoS ONE 2013, 8, e67278. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Montaut, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia 2015, 106, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Ernst, I.M.; Wagner, A.E.; Schuemann, C.; Storm, N.; Höppner, W.; Döring, F.; Stocker, A.; Rimbach, G. Allyl-, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol. Res. 2011, 63, 233–240. [Google Scholar] [CrossRef]
- Keum, Y.S. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: Implications of posttranslational modifications. Ann. N. Y. Acad. Sci. 2011, 1229, 184–189. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Hwang, Y.; Liu, H.; Wang, X.J.; Zhang, Y.; Stephenson, K.K.; Boronina, T.N.; Cole, R.N.; Dinkova-Kostova, A.T.; Talalay, P.; et al. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc. Natl. Acad. Sci. USA 2010, 107, 9590–9595. [Google Scholar] [CrossRef] [PubMed]
- Eisa, N.H.; Khodir, A.E.; El-Sherbiny, M.; Elsherbiny, N.M.; Said, E. Phenethyl isothiocyanate attenuates diabetic nephropathy via modulation of glycative/oxidative/inflammatory signaling in diabetic rats. Biomed. Pharmacother. 2021, 142, 111666. [Google Scholar] [CrossRef]
- Gwon, M.H.; Yun, J.M. Phenethyl Isothiocyanate Improves Lipid Metabolism and Inflammation via mTOR/PPARγ/AMPK Signaling in the Adipose Tissue of Obese Mice. J. Med. Food 2021, 24, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.J.; Park, S.J.; Eom, S.H.; Gu, G.J.; Kim, S.H.; Youn, H.S. Phenethyl isothiocyanate regulates inflammation through suppression of the TRIF-dependent signaling pathway of Toll-like receptors. Life Sci. 2013, 92, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, R.; Pei, Y.; Wang, D.; Wu, N.; Ji, Y.; Tang, Q.; Liu, L.; Cheng, K.; Liu, Q.; et al. Sulforaphane alleviated vascular remodeling in hypoxic pulmonary hypertension via inhibiting inflammation and oxidative stress. J. Nutr. Biochem. 2023, 111, 109182. [Google Scholar] [CrossRef] [PubMed]
- Egbujor, M.C.; Petrosino, M.; Zuhra, K.; Saso, L. The Role of Organosulfur Compounds as Nrf2 Activators and Their Antioxidant Effects. Antioxidants 2022, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Cheng, Y.; Wu, H.; Kong, L.; Wang, S.; Xu, Z.; Zhang, Z.; Tan, Y.; Keller, B.B.; Zhou, H.; et al. Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy. Diabetes 2017, 66, 529–542. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Guo, H.; Zhang, J.; Ma, T.; Zheng, Y.; Zhang, Z.; Cai, L. Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function. Metabolism 2020, 102, 154002. [Google Scholar] [CrossRef]
- Cheng, D.; Gao, L.; Su, S.; Sargsyan, D.; Wu, R.; Raskin, I.; Kong, A.N. Moringa Isothiocyanate Activates Nrf2: Potential Role in Diabetic Nephropathy. AAPS J. 2019, 21, 31. [Google Scholar] [CrossRef]
- Sailaja, B.S.; Aita, R.; Maledatu, S.; Ribnicky, D.; Verzi, M.P.; Raskin, I. Moringa isothiocyanate-1 regulates Nrf2 and NF-κB pathway in response to LPS-driven sepsis and inflammation. PLoS ONE 2021, 16, e0248691. [Google Scholar] [CrossRef]
- Chen, L.; Fan, D.; Guo, F.; Deng, J.; Fu, L. The Effect of Moringa Isothiocyanate-1 on Renal Damage in Diabetic Nephropathy. Iran. J. Kidney Dis. 2023, 17, 245–254. [Google Scholar] [PubMed]
- Shoaib, S.; Tufail, S.; Sherwani, M.A.; Yusuf, N.; Islam, N. Phenethyl Isothiocyanate Induces Apoptosis Through ROS Generation and Caspase-3 Activation in Cervical Cancer Cells. Front. Pharmacol. 2021, 12, 673103. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Chueh, F.S.; Ma, Y.S.; Chou, Y.C.; Chen, J.C.; Liao, C.L.; Huang, Y.P.; Peng, S.F. Phenethyl isothiocyanate and irinotecan synergistically induce cell apoptosis in colon cancer HCT 116 cells in vitro. Environ. Toxicol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Shang, P. β-Phenethyl Isothiocyanate Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the MAPK Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 5021983. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, S.; Sargsyan, D.; Yin, R.; Kuo, H.C.; Peter, R.; Wang, L.; Hudlikar, R.; Liu, X.; Kong, A.N. DNA methylome, transcriptome, and prostate cancer prevention by phenethyl isothiocyanate in TRAMP mice. Mol. Carcinog. 2021, 60, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Stan, S.D.; Singh, S.V.; Whitcomb, D.C.; Brand, R.E. Phenethyl isothiocyanate inhibits proliferation and induces apoptosis in pancreatic cancer cells in vitro and in a MIAPaca2 xenograft animal model. Nutr. Cancer 2014, 66, 747–755. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Hao, M. Phenethyl isothiocyanate reduces breast cancer stem cell-like properties by epigenetic reactivation of CDH1. Oncol. Rep. 2021, 45, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Lim, E.; Cho, Y.S.; Nho, C.W. Cancer-preventive effect of phenethyl isothiocyanate through tumor microenvironment regulation in a colorectal cancer stem cell xenograft model. Phytomedicine 2021, 84, 153493. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Cykowiak, M.; Szaefer, H.; Kleszcz, R.; Baer-Dubowska, W. Combination of xanthohumol and phenethyl isothiocyanate inhibits NF-κB and activates Nrf2 in pancreatic cancer cells. Toxicol. In Vitro 2020, 65, 104799. [Google Scholar] [CrossRef]
- Kasukabe, T.; Honma, Y.; Okabe-Kado, J.; Higuchi, Y.; Kato, N.; Kumakura, S. Combined treatment with cotylenin A and phenethyl isothiocyanate induces strong antitumor activity mainly through the induction of ferroptotic cell death in human pancreatic cancer cells. Oncol. Rep. 2016, 36, 968–976. [Google Scholar] [CrossRef]
- Li, Q.; Zhan, M.; Chen, W.; Zhao, B.; Yang, K.; Yang, J.; Yi, J.; Huang, Q.; Mohan, M.; Hou, Z.; et al. Phenylethyl isothiocyanate reverses cisplatin resistance in biliary tract cancer cells via glutathionylation-dependent degradation of Mcl-1. Oncotarget 2016, 7, 10271–10282. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane Suppresses the Growth of Triple-negative Breast Cancer Stem-like Cells In vitro and In vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, S.; Yoshizawa, K.; Uehara, N.; Miki, H.; Sasaki, T.; Kuro, M.; Lai, Y.C.; Kimura, A.; Yuri, T.; Tsubura, A. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncol. Rep. 2011, 26, 603–608. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Z.; Zhou, C.; Chen, K.; Li, X.; Wang, Z.; Wu, Z.; Ma, J.; Ma, Q.; Duan, W. Activation of Nrf2 by Sulforaphane Inhibits High Glucose-Induced Progression of Pancreatic Cancer via AMPK Dependent Signaling. Cell. Physiol. Biochem. 2018, 50, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.Q.; Ge, M.M.; Zhang, Q.; Wang, X.Q.; Zhu, J.Y.; Xie, C.F.; Li, X.T.; Zhong, C.Y.; Han, H.Y. Sulforaphane inhibits epithelial-mesenchymal transition by activating extracellular signal-regulated kinase 5 in lung cancer cells. J. Nutr. Biochem. 2019, 72, 108219. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ren, Y.; Yang, L.; Jia, A.; Hu, Y.; Zhao, Y.; Zhao, W.; Yu, B.; Zhao, W.; Zhang, J.; et al. Inhibiting autophagy enhances sulforaphane-induced apoptosis via targeting NRF2 in esophageal squamous cell carcinoma. Acta Pharm. Sin. B 2021, 11, 1246–1260. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Li, Y.; Min, H.; Zhao, X.; Zhang, Y.; Qi, Y.; Shi, J.; Qi, S.; Bao, Y.; et al. Sulforaphane Mediates Glutathione Depletion via Polymeric Nanoparticles to Restore Cisplatin Chemosensitivity. ACS Nano 2019, 13, 13445–13455. [Google Scholar] [CrossRef]
- Lin, L.C.; Yeh, C.T.; Kuo, C.C.; Lee, C.M.; Yen, G.C.; Wang, L.S.; Wu, C.H.; Yang, W.C.; Wu, A.T. Sulforaphane potentiates the efficacy of imatinib against chronic leukemia cancer stem cells through enhanced abrogation of Wnt/β-catenin function. J. Agric. Food Chem. 2012, 60, 7031–7039, Erratum in: J. Agric. Food Chem. 2013, 61, 5410. Erratum in: J. Agric. Food Chem. 2014, 62, 2457. [Google Scholar] [CrossRef]
- Rai, R.; Gong Essel, K.; Mangiaracina Benbrook, D.; Garland, J.; Daniel Zhao, Y.; Chandra, V. Preclinical Efficacy and Involvement of AKT, mTOR, and ERK Kinases in the Mechanism of Sulforaphane against Endometrial Cancer. Cancers 2020, 12, 1273. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched Moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Qian, Y.Y.; Yang, Y.; Peng, L.J.; Mao, J.Y.; Yang, M.R.; Tian, Y.; Sheng, J. Isothiocyanate from Moringa oleifera Seeds Inhibits the Growth and Migration of Renal Cancer Cells by Regulating the PTP1B-dependent Src/Ras/Raf/ERK Signaling Pathway. Front. Cell Dev. Biol. 2022, 9, 790618. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells. Fitoterapia 2016, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, M.R.; Hu, X.; Hong, Z.S.; Bai, Y.Y.; Sheng, J.; Tian, Y.; Shi, C.Y. Moringa oleifera Lam. Isothiocyanate Quinazolinone Derivatives Inhibit U251 Glioma Cell Proliferation through Cell Cycle Regulation and Apoptosis Induction. Int. J. Mol. Sci. 2023, 24, 11376. [Google Scholar] [CrossRef] [PubMed]
- Abd Karim, N.A.; Adam, A.H.B.; Jaafaru, M.S.; Rukayadi, Y.; Abdull Razis, A.F. Apoptotic Potential of Glucomoringin Isothiocyanate (GMG-ITC) Isolated from Moringa oleifera Lam Seeds on Human Prostate Cancer Cells (PC-3). Molecules 2023, 28, 3214. [Google Scholar] [CrossRef] [PubMed]
- Antonini, E.; Iori, R.; Ninfali, P.; Scarpa, E.S. A Combination of Moringin and Avenanthramide 2f Inhibits the Proliferation of Hep3B Liver Cancer Cells Inducing Intrinsic and Extrinsic Apoptosis. Nutr. Cancer 2018, 70, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumeci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-κB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Ren, X.; Jaboin, J.; McConathy, J.; Rich, K.M. Isothiocyanates promote cell death and sensitize glioblastoma cells to radiation. Cancer Res. 2013, 73, 1598. [Google Scholar] [CrossRef]
- Hać, A.; Brokowska, J.; Rintz, E.; Bartkowski, M.; Węgrzyn, G.; Herman-Antosiewicz, A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur. J. Nutr. 2020, 59, 1421–1432. [Google Scholar] [CrossRef]
- Wang, F.; Liu, P.; An, H.; Zhang, Y. Sulforaphane suppresses the viability and metastasis, and promotes the apoptosis of bladder cancer cells by inhibiting the expression of FAT-1. Int. J. Mol. Med. 2020, 46, 1085–1095, Erratum in: Int. J. Mol. Med. 2022, 50, 93. [Google Scholar] [CrossRef]
- Huang, L.; He, C.; Zheng, S.; Wu, C.; Ren, M.; Shan, Y. AKT1/HK2 Axis-mediated Glucose Metabolism: A Novel Therapeutic Target of Sulforaphane in Bladder Cancer. Mol. Nutr. Food Res. 2022, 66, e2100738. [Google Scholar] [CrossRef] [PubMed]
- Mastuo, T.; Miyata, Y.; Yuno, T.; Mukae, Y.; Otsubo, A.; Mitsunari, K.; Ohba, K.; Sakai, H. Molecular Mechanisms of the Anti-Cancer Effects of Isothiocyanates from Cruciferous Vegetables in Bladder Cancer. Molecules 2020, 25, 575. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Hirose, M.; Sugiura, S.; Cui, L.; Imaida, K.; Ogiso, T.; Shirai, T. Dose-dependent promotion by phenylethyl isothiocyanate, a known chemopreventer, of two-stage rat urinary bladder and liver carcinogenesis. Nutr. Cancer 2001, 40, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.R.; Amin, H.A.; Sultan, A.A. The protective effect of Moringa oleifera leaves against cyclophosphamide-induced urinary bladder toxicity in rats. Tissue Cell 2015, 47, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Jeyachitra, A.; Padma, P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011, 49, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, W.; Li, B.; Chen, X.; Zhang, Y.; Chen, C.; Yu, M.; Xiu, Y.; Li, W.; Cao, J.; et al. PEITC promotes neurite growth in primary sensory neurons via the miR-17-5p/STAT3/GAP-43 axis. J. Drug Target. 2019, 27, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Liu, H.; Geng, G.; Yu, Z.; Xu, H. Changes on neurobehavioral development of the offspring in rats prenataly exposed by phenethyl isothiocyanate. Wei Sheng Yan Jiu 2011, 40, 36–39. (In Chinese) [Google Scholar]
- Hou, T.T.; Yang, H.Y.; Wang, W.; Wu, Q.Q.; Tian, Y.R.; Jia, J.P. Sulforaphane Inhibits the Generation of Amyloid-β Oligomer and Promotes Spatial Learning and Memory in Alzheimer’s Disease (PS1V97L) Transgenic Mice. J. Alzheimers Dis. 2018, 62, 1803–1813. [Google Scholar] [CrossRef]
- Bahn, G.; Park, J.-S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef]
- Bao, B.; Zhang, M.Q.; Chen, Z.Y.; Wu, X.B.; Xia, Z.B.; Chai, J.Y.; Yin, X.P. Sulforaphane prevents PC12 cells from oxidative damage via the Nrf2 pathway. Mol. Med. Rep. 2019, 19, 4890–4896. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, Q.W.; Zhu, C.X.; Zhao, Y.; Liu, L.; Yang, J.; An, L. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid β deposits and peroxidation in mice with Alzheimer-like lesions. Am. J. Alzheimers Dis. Other Dement. 2015, 30, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, B.R.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-β and Tau in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, e1800240. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, C.; Quan, M.; Li, T.; Jia, J. Sulforaphane Reverses the Amyloid-β Oligomers Induced Depressive-Like Behavior. J. Alzheimers Dis. 2020, 78, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Morroni, F.; Tarozzi, A.; Sita, G.; Bolondi, C.; Zolezzi Moraga, J.M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 2013, 36, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernández-Ruiz, J.; Cuadrado, A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, B.; Wang, X.; Wu, L.; Yang, Y.; Cheng, X.; Hu, Z.; Cai, X.; Yang, J.; Sun, X.; et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci. Rep. 2016, 6, 32206. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.H.; Kim, M.J.; Kim, J.; Sung, J.J.; Park, S.T.; Ahn, S.W. The Anti-Inflammatory Effect of Sulforaphane in Mice with Experimental Autoimmune Encephalomyelitis. J. Korean Med. Sci. 2019, 34, e197. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Liu, J.; Li, R.; Liu, Q.; Xie, X.H.; Ge, X.L.; Zhang, J.; Song, X.J.; Wang, Y.; et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013, 250, 239–249. [Google Scholar] [CrossRef]
- Ghimire, S.; Subedi, L.; Acharya, N.; Gaire, B.P. Moringa oleifera: A Tree of Life as a Promising Medicinal Plant for Neurodegenerative Diseases. J. Agric. Food Chem. 2021, 69, 14358–14371. [Google Scholar] [CrossRef]
- Azlan, U.K.; Khairul Annuar, N.A.; Mediani, A.; Aizat, W.M.; Damanhuri, H.A.; Tong, X.; Yanagisawa, D.; Tooyama, I.; Wan Ngah, W.Z.; Jantan, I.; et al. An insight into the neuroprotective and anti-neuroinflammatory effects and mechanisms of Moringa oleifera. Front. Pharmacol. 2023, 13, 1035220. [Google Scholar] [CrossRef]
- Onasanwo, S.A.; Adamaigbo, V.O.; Adebayo, O.G.; Eleazer, S.E. Moringa oleifera-supplemented diet protect against cortico-hippocampal neuronal degeneration in scopolamine-induced spatial memory deficit in mice: Role of oxido-inflammatory and cholinergic neurotransmission pathway. Metab. Brain Dis. 2021, 36, 2445–2460. [Google Scholar] [CrossRef] [PubMed]
- Mahaman, Y.A.R.; Feng, J.; Huang, F.; Salissou, M.T.M.; Wang, J.; Liu, R.; Zhang, B.; Li, H.; Zhu, F.; Wang, X. Moringa oleifera Alleviates Aβ Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice. Nutrients 2022, 14, 4284. [Google Scholar] [CrossRef] [PubMed]
- Mahaman, Y.A.R.; Huang, F.; Wu, M.; Wang, Y.; Wei, Z.; Bao, J.; Salissou, M.T.M.; Ke, D.; Wang, Q.; Liu, R.; et al. Moringa oleifera Alleviates Homocysteine-Induced Alzheimer’s Disease-Like Pathology and Cognitive Impairments. J. Alzheimers Dis. 2018, 63, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Purwoningsih, E.; Arozal, W.; Lee, H.J.; Barinda, A.J.; Sani, Y.; Munim, A. The Oil Formulation Derived from Moringa oleifera Seeds Ameliorates Behavioral Abnormalities in Water-immersion Restraint Stress Mouse Model. J. Exp. Pharmacol. 2022, 14, 395–407. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, W.S.; Suo, D.Q.; Li, Y.; Peng, L.; Xu, L.X.; Zeng, K.Y.; Ren, T.; Wang, Y.; Zhou, Y.; et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Front. Pharmacol. 2018, 9, 389. [Google Scholar] [CrossRef]
- González-Burgos, E.; Ureña-Vacas, I.; Sánchez, M.; Gómez-Serranillos, M.P. Nutritional Value of Moringa oleifera Lam. Leaf Powder Extracts and Their Neuroprotective Effects via Antioxidative and Mitochondrial Regulation. Nutrients 2021, 13, 2203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).