Risk of Excess Maternal Folic Acid Supplementation in Offspring
Abstract
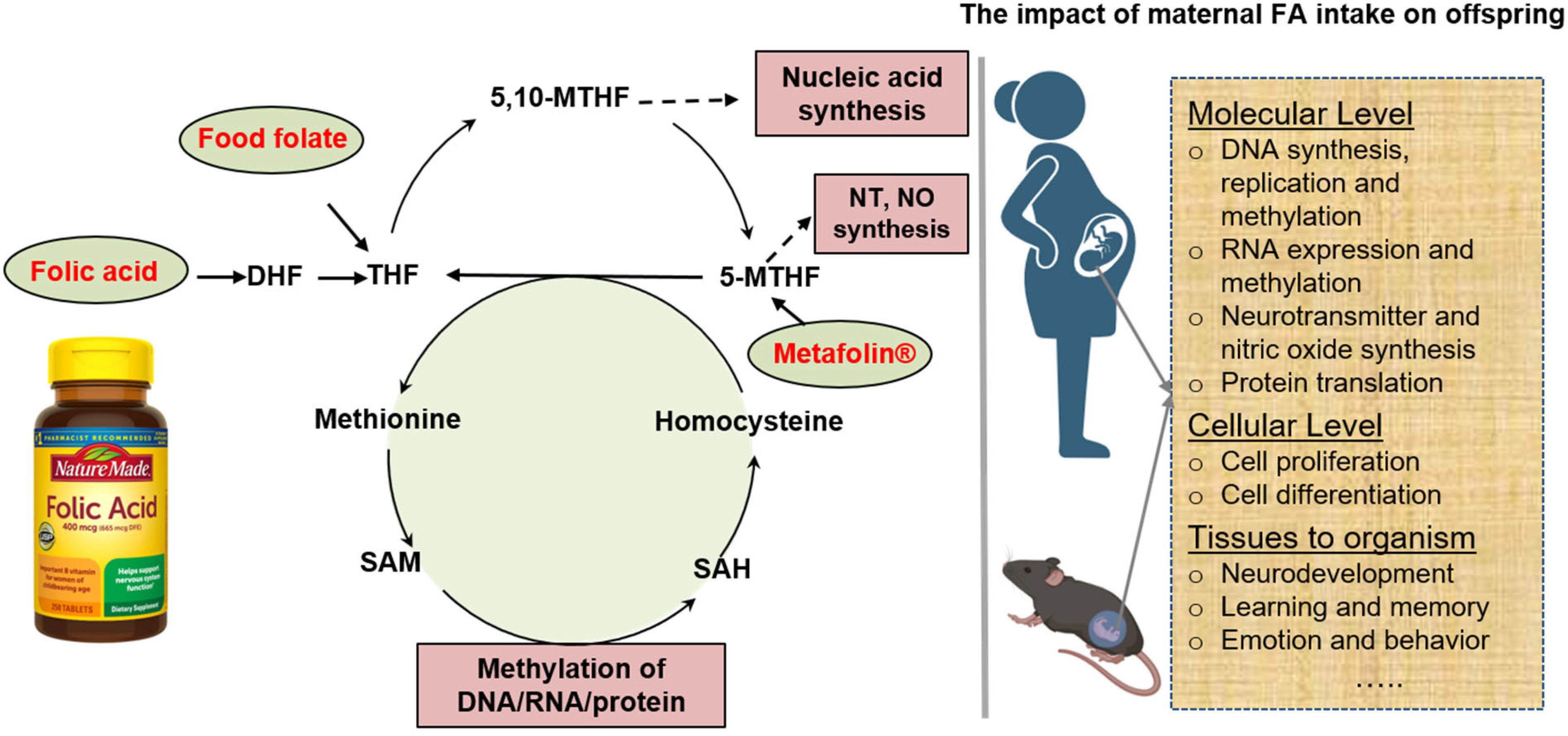
1. Introduction
2. Human Studies on the Effect of Maternal Folic Acid Supplementation
3. Influence of Periconceptional Folic Acid Supplementation in DNA Methylation in the Offspring
4. The Impact of Periconceptional Folic Acid Supplementation on the Neurodevelopment of Offspring
5. Dose-Dependent Association of Folic Acid Supplementation with the Incidence of ASD
6. Mouse Models to Study the Impact of High-Dose Maternal Folic Acid Supplementation
| Reference | FA Dosage | Duration | Gene Expression Changes | Behavioral Changes |
|---|---|---|---|---|
| Barua et al., 2015 [68] | 2 mg/kg 20 mg/kg | 1 week before gestation and throughout the pregnancy | Compared to the control, the P1 cerebellum of high FA group showed 1076 downregulated and 499 upregulated genes in male pups, and 4764 downregulated and 1511 upregulated genes in female pups, with 339 downregulated and 152 upregulated transcripts shared by both sexes. | NA |
| Barua et al., 2016 [69] | 2 mg/kg 20 mg/kg | 1 week prior to mating and throughout the entire period of gestation | RT-qPCR and Western blot analysis of P1 cerebral hemispheres in the high FA group showed sex-specific changes in transcription factors Nfix, Runx1, and Vgll2, DNA Methyltransferase Dnmt3b, the imprinted gene Dio3, H19, and Xist, and the candidate autism susceptible gene Auts2, Fmr1 at mRNA levels, and Gad1, Park2, and Hsp90 at protein levels. | NA |
| Bahous et al., 2017 [56] | 2 mg/kg 20 mg/kg | 5 weeks before mating, during pregnancy and lactation until P21 | RT-qPCR analysis of cortex and hippocampus in P21 male offspring showed decreased Dnmt3a mRNA expression in the high FA group. | No differences were observed in ladder beam, open field, and Y-maze tests in HFA male P21 pups. In the novel object recognition test, HFA male pups exhibited short-term memory impairment, indicated by less time spent with the novel object compared to the familiar object. |
| Henzel et al., 2017 [62] | 2.3 mg/kg 40 mg/kg | 6 weeks prior to mating | RNA-seq of hippocampus at 14 weeks showed 12 differentially expressed genes, and the changes were diminished at 12 months old. | No differences were observed in open field, context fear conditioning, object place recognition, and rotarod tests in 14-week-old high FA offspring. However, in the Morris water maze test, high FA offspring showed impaired cognitive flexibility in reversal learning tasks. |
| Chu et al., 2019 [60] | Control: 2 mg/kg MFA: 2.5× FA HFA: 10× FA | 1 week before mating, throughout pregnancy and lactation until P21 | RNA-seq of cortex in the male offspring identified 176 DEGs (103 upregulated, 73 downregulated) in the MFA group compared to the control, and 96 DEGs (34 upregulated, 62 down regulated) in the HFA group compared to the control. | Compared to the control, MFA male offspring at 2 months old showed no differences in motor ability and spatial memory, whereas they displayed elevated anxiety-like behavior, impaired social preference, motor learning, and spatial learning ability. The HFA male offspring at 2 months old exhibited only mild behavioral abnormalities, without an effect on social behavior or anxiety-like behavior in the elevated plus maze. |
| Yang et al., 2019 [70] | Control: 2 mg/kg MFA: 5 mg/kg | Throughout pregnancy and lactation | RT-qPCR and Western blot analysis of hippocampus at 7 weeks showed increased expression of PCNA, DCX, BDNF, and GR in the MFA group. | Compared to the control, MFA male offspring at 6 weeks showed increased spatial learning and memory with fewer fear-related behaviors, represented by increased center area entries and duration in the open field test, and increased frequency of entering open arms in the elevated plus maze. No differences were observed in the forced swimming test or tail suspension test. |
| Yang et al., 2021 [61] | 2 mg/kg MFA: 2.5× FA | 1 week before mating, throughout pregnancy and lactation until P21 | RNA-seq analysis of P21 cortex showed 115 DEGs (36 upregulated, and 79 downregulated) in the MFA female group compared to the control, with 39 DEGs (16 upregulated and 23 downregulated) overlapping with the DEGs identified in the MFA male group. | Compared to the control, the MFA female offspring at 2 months old showed decreased exploratory activity and increased anxiety-like behavior in the open field test, and impaired motor coordination in the rotarod test. No differences were found in the three-chamber social approach, social novelty test, or the elevated plus maze test. |
| Harlan De Crescenzo et al., 2021 [63] | 0 mg/kg 2 mg/kg 20 mg/kg | 2 weeks before mating, during pregnancy and lactation until P21 | Western blot analysis of P0 brain and liver tissues showed no significant changes in Mthfr protein levels. | Offspring aged 4–10 weeks in the high FA group exhibited increased anxiety-like behavior in the elevated plus maze, reduced exploratory behavior and potential increase in anxiety in the open field test, and reduced marble burying in the marble burying test. No significant differences were noted in the novel object recognition and 3-chambered social approach tests. |
| Cosín-Tomás et al., 2020 [71] | 2 mg/kg 10 mg/kg | 1 month before mating, during pregnancy and lactation until P30 | Western blot analysis of P30 cortex and liver in the high FA group showed a decreased expression of Mthfr protein levels in the liver, but not in the cortex. | The high FA offspring aged 3 weeks showed hyperactivity-like behaviors without notable differences in the anxiety levels in the open field test, and short-term memory impairment in the novel object recognition test. No differences were observed in grip strength or social interaction. |
| Luan et al., 2021 [57] | 2 mg/kg 10 mg/kg | 1 month before mating, during pregnancy until E17.5 | Microarray analysis of the E17.5 placenta showed 186 DEGs in males and 274 DEGs in females, with only seven genes common between two sexes. RT-qPCR analysis confirmed changes in 29 genes associated with angiogenesis, receptor biology, and neurodevelopment. Western blot analysis did not show significant differences in MTHFR expression in the placenta. | NA |
| Luan et al., 2022 [58] | 2 mg/kg 10 mg/kg | 1 month before mating, during pregnancy and lactation until P30 | Microarray analysis of the E17.5 cortex in the high FA group identified 274 DEGs (114 downregulated, 160 upregulated) in males, and 354 DEGs (177 downregulated, 177 upregulated) in females, with only 5 commonly changed genes in both sexes. Microarray analysis of the P30 cortex in the high FA group identified 599 DEGs (357 downregulated, 242 upregulated) in males and 419 DEGs (202 downregulated, 217 upregulated) in females, with only 15 genes shared in both sexes. | NA |
7. Gene Expression Changes Induced by Maternal High Folate Supplementation
8. Developmental and Behavioral Abnormalities Induced by Maternal High Folate Supplementation
9. Metabolic and Reproduction Disorders Induced by Maternal High Folate Supplementation
10. Discussion and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Berry, R.J.; Bailey, L.; Mulinare, J.; Bower, C. Fortification of flour with folic acid. Food Nutr. Bull. 2010, 31 (Suppl. S1), S22–S35. [Google Scholar] [CrossRef]
- van der Linden, I.J.; Nguyen, U.; Heil, S.G.; Franke, B.; Vloet, S.; Gellekink, H.; den Heijer, M.; Blom, H.J. Variation and expression of dihydrofolate reductase (DHFR) in relation to spina bifida. Mol. Genet. Metab. 2007, 91, 98–103. [Google Scholar] [CrossRef]
- Appling, D.R. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 1991, 5, 2645–2651. [Google Scholar] [CrossRef]
- Blakley, R.L. The interconversion of serine and glycine: Participation of pyridoxal phosphate. Biochem. J. 1955, 61, 315–323. [Google Scholar] [CrossRef]
- Goyette, P.; Sumner, J.S.; Milos, R.; Duncan, A.M.; Rosenblatt, D.S.; Matthews, R.G.; Rozen, R. Human methylenetetrahydrofolate reductase: Isolation of cDNA mapping and mutation identification. Nat. Genet. 1994, 7, 551. [Google Scholar] [CrossRef]
- Shane, B. Folate and vitamin B12 metabolism: Overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food Nutr. Bull. 2008, 29 (Suppl. S2), S5–S16; discussion S17–S19. [Google Scholar] [CrossRef]
- Pietrzik, K.; Bailey, L.; Shane, B. Folic acid and L-5-methyltetrahydrofolate: Comparison of clinical pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2010, 49, 535–548. [Google Scholar] [CrossRef]
- Griffith, T.M.; Chaytor, A.T.; Bakker, L.M.; Edwards, D.H. 5-Methyltetrahydrofolate and tetrahydrobiopterin can modulate electrotonically mediated endothelium-dependent vascular relaxation. Proc. Natl. Acad. Sci. USA 2005, 102, 7008–7013. [Google Scholar] [CrossRef]
- Miyan, J.; Buttercase, C.; Beswick, E.; Miyan, S.; Moshkdanian, G.; Naz, N. Folate Related Pathway Gene Analysis Reveals a Novel Metabolic Variant Associated with Alzheimer’s Disease with a Change in Metabolic Profile. Metabolites 2022, 12, 475. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.H. Folic Acid supplementation and pregnancy: More than just neural tube defect prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Ouyang, F.; Longnecker, M.P.; Venners, S.A.; Johnson, S.; Korrick, S.; Zhang, J.; Xu, X.; Christian, P.; Wang, M.C.; Wang, X. Preconception serum 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane and B-vitamin status: Independent and joint effects on women’s reproductive outcomes. Am. J. Clin. Nutr. 2014, 100, 1470–1478. [Google Scholar] [CrossRef][Green Version]
- De Wals, P.; Tairou, F.; Van Allen, M.I.; Uh, S.H.; Lowry, R.B.; Sibbald, B.; Evans, J.A.; Van den Hof, M.C.; Zimmer, P.; Crowley, M.; et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 2007, 357, 135–142. [Google Scholar] [CrossRef]
- Surén, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013, 309, 570–577. [Google Scholar] [CrossRef]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef]
- Wang, G.; Hu, F.B.; Mistry, K.B.; Zhang, C.; Ren, F.; Huo, Y.; Paige, D.; Bartell, T.; Hong, X.; Caruso, D.; et al. Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations with Child Metabolic Health. JAMA Pediatr. 2016, 170, e160845. [Google Scholar] [CrossRef]
- Wang, H.; Mueller, N.T.; Li, J.; Sun, N.; Huo, Y.; Ren, F.; Wang, X. Association of Maternal Plasma Folate and Cardiometabolic Risk Factors in Pregnancy with Elevated Blood Pressure of Offspring in Childhood. Am. J. Hypertens. 2017, 30, 532–540. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Virdi, S.; Jadavji, N.M. The Impact of Maternal Folates on Brain Development and Function after Birth. Metabolites 2022, 12, 876. [Google Scholar] [CrossRef]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.; Rosenberg, I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Hughes, J.P.; Lacher, D.A.; Bailey, R.L.; Berry, R.J.; Zhang, M.; Yetley, E.A.; Rader, J.I.; Sempos, C.T.; Johnson, C.L. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J. Nutr. 2012, 142, 886–893. [Google Scholar] [CrossRef]
- Gallo, L.A.; Steane, S.E.; Young, S.L.; de Jersey, S.; Schoenaker, D.; Borg, D.J.; Lockett, J.; Collins, C.E.; Perkins, A.V.; Kumar, S.; et al. Dietary supplements, guideline alignment and biochemical nutrient status in pregnancy: Findings from the Queensland Family Cohort pilot study. Matern. Child. Nutr. 2024, 20, e13589. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- CDC. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm. Rep. 1992, 41, 1–7. [Google Scholar]
- Administration, F.D. Food standards: Amendment of standards of identity for enriched grain products to require addition of folic acid. Fed. Regist. 1996, 61, 8781–8797. [Google Scholar]
- Stamm, R.A.; Houghton, L.A. Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients 2013, 5, 3920–3947. [Google Scholar] [CrossRef]
- Honein, M.A.; Paulozzi, L.J.; Mathews, T.J.; Erickson, J.D.; Wong, L.Y. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001, 285, 2981–2986. [Google Scholar] [CrossRef]
- McNulty, B.; McNulty, H.; Marshall, B.; Ward, M.; Molloy, A.M.; Scott, J.M.; Dornan, J.; Pentieva, K. Impact of continuing folic acid after the first trimester of pregnancy: Findings of a randomized trial of Folic Acid Supplementation in the Second and Third Trimesters. Am. J. Clin. Nutr. 2013, 98, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.M.; Elango, R.; Devlin, A.M.; Hutcheon, J.A.; Karakochuk, C.D. Human milk unmetabolized folic acid is increased following supplementation with synthetic folic acid as compared to (6S)-5-methyltetrahydrofolic acid. Sci. Rep. 2023, 13, 11298. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Jaddoe, V.W.; Hofman, A.; Steegers-Theunissen, R.P.; Steegers, E.A. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: The Generation R Study. Br. J. Nutr. 2009, 102, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, H.; Dowling, K.F.; Huntington, F.C.; Rodriguez-Thompson, A.; Soare, T.W.; Beard, L.M.; Lee, H.; Blossom, J.C.; Gollub, R.L.; Susser, E.; et al. Association of Prenatal Exposure to Population-Wide Folic Acid Fortification with Altered Cerebral Cortex Maturation in Youths. JAMA Psychiatry 2018, 75, 918–928. [Google Scholar] [CrossRef]
- Yusuf, K.K.; Salihu, H.M.; Wilson, R.; Mbah, A.; Sappenfield, W.; Bruder, K.; Wudil, U.J.; Aliyu, M.H. Folic Acid Intake, Fetal Brain Growth, and Maternal Smoking in Pregnancy: A Randomized Controlled Trial. Curr. Dev. Nutr. 2019, 3, nzz025. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.A.; Cassidy, T.; McLaughlin, M.; Pentieva, K.; McNulty, H.; Walsh, C.P.; Lees-Murdock, D. Folic Acid Supplementation throughout pregnancy: Psychological developmental benefits for children. Acta Paediatr. 2018, 107, 1370–1378. [Google Scholar] [CrossRef]
- McNulty, H.; Rollins, M.; Cassidy, T.; Caffrey, A.; Marshall, B.; Dornan, J.; McLaughlin, M.; McNulty, B.A.; Ward, M.; Strain, J.J.; et al. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: A follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med. 2019, 17, 196. [Google Scholar] [CrossRef]
- Caffrey, A.; McNulty, H.; Rollins, M.; Prasad, G.; Gaur, P.; Talcott, J.B.; Witton, C.; Cassidy, T.; Marshall, B.; Dornan, J.; et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: An 11-year follow-up from a randomised controlled trial. BMC Med. 2021, 19, 73. [Google Scholar] [CrossRef]
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr. Perinat. Epidemiol. 2009, 23, 199–206. [Google Scholar] [CrossRef]
- Veena, S.R.; Krishnaveni, G.V.; Srinivasan, K.; Wills, A.K.; Muthayya, S.; Kurpad, A.V.; Yajnik, C.S.; Fall, C.H. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10-year-old children in South India. J. Nutr. 2010, 140, 1014–1022. [Google Scholar] [CrossRef]
- Roth, C.; Magnus, P.; Schjølberg, S.; Stoltenberg, C.; Surén, P.; McKeague, I.W.; Davey Smith, G.; Reichborn-Kjennerud, T.; Susser, E. Folic acid supplements in pregnancy and severe language delay in children. JAMA 2011, 306, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Papadopoulou, E.; Koutra, K.; Roumeliotaki, T.; Georgiou, V.; Stratakis, N.; Lebentakou, V.; Karachaliou, M.; Vassilaki, M.; Kogevinas, M. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: The mother-child cohort ‘Rhea’ study in Crete, Greece. Public. Health Nutr. 2012, 15, 1728–1736. [Google Scholar] [CrossRef]
- Villamor, E.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr. Perinat. Epidemiol. 2012, 26, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.T.; Dyer, R.A.; King, D.J.; Richardson, K.J.; Innis, S.M. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE 2012, 7, e43448. [Google Scholar] [CrossRef]
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 2013, 177, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, Y.; Li, Y.; Zhang, Y.; Zhang, Y.; Jiang, Y.; Chen, X.; Wang, L.; Yan, W. Maternal folate levels during pregnancy and children’s neuropsychological development at 2 years of age. Eur. J. Clin. Nutr. 2020, 74, 1585–1593. [Google Scholar] [CrossRef]
- Irvine, N.; England-Mason, G.; Field, C.J.; Letourneau, N.; Bell, R.C.; Giesbrecht, G.F.; Kinniburgh, D.W.; MacDonald, A.M.; Martin, J.W.; Dewey, D. Associations between maternal folate status and choline intake during pregnancy and neurodevelopment at 3–4 years of age in the Alberta Pregnancy Outcomes and Nutrition (APrON) study. J. Dev. Orig. Health Dis. 2023, 14, 402–414. [Google Scholar] [CrossRef]
- Tamura, T.; Goldenberg, R.L.; Chapman, V.R.; Johnston, K.E.; Ramey, S.L.; Nelson, K.G. Folate status of mothers during pregnancy and mental and psychomotor development of their children at five years of age. Pediatrics 2005, 116, 703–708. [Google Scholar] [CrossRef]
- Wehby, G.L.; Murray, J.C. The effects of prenatal use of folic acid and other dietary supplements on early child development. Matern. Child. Health J. 2008, 12, 180–187. [Google Scholar] [CrossRef]
- Raghavan, R.; Selhub, J.; Paul, L.; Ji, Y.; Wang, G.; Hong, X.; Zuckerman, B.; Fallin, M.D.; Wang, X. A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am. J. Clin. Nutr. 2020, 112, 1304–1317. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Obermann-Borst, S.A.; Kremer, D.; Lindemans, J.; Siebel, C.; Steegers, E.A.; Slagboom, P.E.; Heijmans, B.T. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS ONE 2009, 4, e7845. [Google Scholar] [CrossRef]
- Hoyo, C.; Murtha, A.P.; Schildkraut, J.M.; Jirtle, R.L.; Demark-Wahnefried, W.; Forman, M.R.; Iversen, E.S.; Kurtzberg, J.; Overcash, F.; Huang, Z.; et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011, 6, 928–936. [Google Scholar] [CrossRef]
- Haggarty, P.; Hoad, G.; Campbell, D.M.; Horgan, G.W.; Piyathilake, C.; McNeill, G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr. 2013, 97, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ondičová, M.; Irwin, R.E.; Thursby, S.J.; Hilman, L.; Caffrey, A.; Cassidy, T.; McLaughlin, M.; Lees-Murdock, D.J.; Ward, M.; Murphy, M.; et al. Folic acid intervention during pregnancy alters DNA methylation, affecting neural target genes through two distinct mechanisms. Clin. Epigenetics 2022, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Yetley, E.A.; Lacher, D.A.; Bailey, R.L.; Johnson, C.L. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J. Nutr. 2015, 145, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Bahous, R.H.; Jadavji, N.M.; Deng, L.; Cosín-Tomás, M.; Lu, J.; Malysheva, O.; Leung, K.Y.; Ho, M.K.; Pallàs, M.; Kaliman, P.; et al. High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring. Hum. Mol. Genet. 2017, 26, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Leclerc, D.; Cosín-Tomás, M.; Malysheva, O.V.; Wasek, B.; Bottiglieri, T.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Methyl Metabolism and in Sex-Specific Placental Transcription Changes. Mol. Nutr. Food Res. 2021, 65, e2100197. [Google Scholar] [CrossRef]
- Luan, Y.; Cosín-Tomás, M.; Leclerc, D.; Malysheva, O.V.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Sex-Specific Gene Expression in Brain of Young Mice and Embryos. Nutrients 2022, 14, 1051. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Chadman, K.K.; Kuizon, S.; Buenaventura, D.; Stapley, N.W.; Ruocco, F.; Begum, U.; Guariglia, S.R.; Brown, W.T.; Junaid, M.A. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS ONE 2014, 9, e101674. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Li, L.; Jiang, Y.; Tan, J.; Ji, J.; Zhang, Y.; Jin, N.; Liu, F. Excess Folic Acid Supplementation Before and During Pregnancy and Lactation Activates Fos Gene Expression and Alters Behaviors in Male Mouse Offspring. Front. Neurosci. 2019, 13, 313. [Google Scholar] [CrossRef]
- Yang, X.; Sun, W.; Wu, Q.; Lin, H.; Lu, Z.; Shen, X.; Chen, Y.; Zhou, Y.; Huang, L.; Wu, F.; et al. Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring. Nutrients 2021, 14, 66. [Google Scholar] [CrossRef]
- Henzel, K.S.; Ryan, D.P.; Schröder, S.; Weiergräber, M.; Ehninger, D. High-dose maternal folic acid supplementation before conception impairs reversal learning in offspring mice. Sci. Rep. 2017, 7, 3098. [Google Scholar] [CrossRef]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or Excess Folic Acid Supply During Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb. Cortex 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Chadman, K.K.; Flory, M.J.; Brown, W.T.; Junaid, M.A. Single-base resolution of mouse offspring brain methylome reveals epigenome modifications caused by gestational folic acid. Epigenet. Chromatin 2014, 7, 3. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Front. Neurosci. 2016, 10, 168. [Google Scholar] [CrossRef]
- Heid, M.K.; Bills, N.D.; Hinrichs, S.H.; Clifford, A.J. Folate deficiency alone does not produce neural tube defects in mice. J. Nutr. 1992, 122, 888–894. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kuizon, S.; Chadman, K.K.; Brown, W.T.; Junaid, M.A. Microarray analysis reveals higher gestational folic Acid alters expression of genes in the cerebellum of mice offspring-a pilot study. Brain Sci. 2015, 5, 14–31. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a sex-Specific Manner in Mouse Offspring. J. Mol. Neurosci. 2016, 58, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, S.; Liu, J.; Feng, Y.; Qi, F.; Zhao, R. DNA Hypomethylation of GR Promoters is Associated with GR Activation and BDNF/AKT/ERK1/2-Induced Hippocampal Neurogenesis in Mice Derived From Folic-Acid-Supplemented Dams. Mol. Nutr. Food Res. 2019, 63, e1801334. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Tomás, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. [Google Scholar] [CrossRef]
- Ryan, D.P.; Henzel, K.S.; Pearson, B.L.; Siwek, M.E.; Papazoglou, A.; Guo, L.; Paesler, K.; Yu, M.; Müller, R.; Xie, K.; et al. A paternal methyl donor-rich diet altered cognitive and neural functions in offspring mice. Mol. Psychiatry 2018, 23, 1345–1355. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Sun, X.; He, Y.; Li, Y.; Sun, C. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int. J. Mol. Sci. 2014, 15, 6298–6313. [Google Scholar] [CrossRef]
- Kintaka, Y.; Wada, N.; Shioda, S.; Nakamura, S.; Yamazaki, Y.; Mochizuki, K. Excessive folic acid supplementation in pregnant mice impairs insulin secretion and induces the expression of genes associated with fatty liver in their offspring. Heliyon 2020, 6, e03597. [Google Scholar] [CrossRef]
- Mussai, E.X.; Lofft, Z.A.; Vanderkruk, B.; Boonpattrawong, N.; Miller, J.W.; Smith, A.; Bottiglieri, T.; Devlin, A.M. Folic acid supplementation in a mouse model of diabetes in pregnancy alters insulin sensitivity in female mice and beta cell mass in offspring. FASEB J. 2023, 37, e23200. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Shu, Y.P.; Yang, X.Y.; Huang, W.; Chen, J.; Yu, N.N.; Zhao, M. Effects of folic acid supplementation in pregnant mice on glucose metabolism disorders in male offspring induced by lipopolysaccharide exposure during pregnancy. Sci. Rep. 2023, 13, 7984. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Martel, J.; Karahan, G.; Angle, C.; Behan, N.A.; Chan, D.; MacFarlane, A.J.; Trasler, J.M. Moderate maternal folic acid supplementation ameliorates adverse embryonic and epigenetic outcomes associated with assisted reproduction in a mouse model. Hum. Reprod. 2019, 34, 851–862. [Google Scholar] [CrossRef]
- Chan, D.; Ly, L.; Rebolledo, E.M.D.; Martel, J.; Landry, M.; Scott-Boyer, M.P.; Droit, A.; Trasler, J.M. Transgenerational impact of grand-paternal lifetime exposures to both folic acid deficiency and supplementation on genome-wide DNA methylation in male germ cells. Andrology 2023, 11, 927–942. [Google Scholar] [CrossRef] [PubMed]
| Reference | Country | Sample Size | Research Type | Assessment of Folic Acid Use (Dosage and Stage) | Measurements | Folate Level (nmol/L) | Key Findings |
|---|---|---|---|---|---|---|---|
| McNulty et al., 2013 [31] | United Kingdom | 59 women in the folic acid group, 60 women in the placebo group | Randomized controlled trial (RCT) | All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. | Serum and red blood cell folate, serum vitamin B-12, and plasma homocysteine were analyzed. | Serum folate: 45.7 ± 21.3 in placebo group and 47.0 ± 21.0 in treatment group. Red blood cell folate: 1106 ± 746 in placebo group and 1203 ± 639 in treatment group. | Continued FA supplementation during the second and third trimesters significantly increased the levels of maternal and cord red blood folate, and decrease the level of plasma homocysteine. |
| Cochrane et al., 2023 [32] | Canada | 60 mother–child pairs | Randomized control trials (RCT) | Pregnant women were enrolled at 8–21 weeks of gestation and randomized to 0.6 mg/day folic acid or an equimolar dose (0.625 mg) of (6S)-5-methyltetrahydrofolic acid [(6S)-5-MTHF]. | Folate and cord blood unmetabolized folic acid (UMFA) in human milk collected 1 week postpartum were quantified via LC–MS/MS. | Total folate in human milk: 47 ± 20 in (6S)-5-MTHF group and 61 ± 28 in folic acid group. UMFA in human milk: 0.6 in (6S)-5-MTHF group and 12 in folic acid group. | Compared to natural folate, folic acid supplementation showed similar levels of total folate, but significantly higher levels of UMFA in the milk. |
| Timmermans et al., 2009 [33] | Netherlands | 6353 pregnancies | Prospective birth cohort | Self-reported questionnaires on folic acid use. | Fetal growth measured in mid- and late pregnancy by ultrasound; birth weight, small for gestational age (SGA), and preterm birth were recorded at birth. | NA | Periconceptional folic acid use was associated with increased fetal growth resulting in higher placental and birth weight, and decreased risks of low birth weight and small for gestational age (SGA). |
| Eryilmaz et al., 2018 [34] | United States | 292 youths 8–18 years of age | Retrospective clinical cohort | None, partial, or full prenatal folic acid fortification exposure. | Cortical thickness was measured by brain MRI scans. | NA | Prenatal exposure of folic acid was associated with cortical thickness increase in the bilateral, frontal, and temporal regions, as well as delayed age-associated cortical thinning in the temporal and parietal regions. |
| Yusuf et al., 2019 [35] | United States | 345 smoking pregnant women | Randomized control trials (RCT) | Participants were randomly assigned to receive either 0.8 mg folic acid/d or 4 mg folic acid/d. | Fetal growth was assessed by intrauterine ultrasound. | NA | Infants of mother who received high dose folic acid showed no difference in brain weight, but were 0.33 percentage points lower in brain/body weight ratio compared to standard dose group. |
| Henry et al., 2018 [36] | United Kingdom | 22 mother–child pairs in the folic acid group and 17 mother–child pairs in the placebo group | Randomized controlled trial (RCT) | All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. | Emotional intelligence and resilience were assessed in children at 6–7 years. | NA | Children of folic-acid-treated mothers had higher scores in emotional intelligence and resilience at 6–7 years old. |
| McNulty et al., 2019 [37] | United Kingdom | 37 mother–child pairs in the folic acid group and 33 mother–child pairs in the placebo group | Randomized controlled trial (RCT) | All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters | Cognitive performance was evaluated in children at 3 and 7 years. | NA | Children of folic-acid-treated mothers scored higher in cognition at 3 years old and had higher scores in word reasoning at 7 years old. |
| Caffrey et al., 2021 [38] | United Kingdom | 68 mother–child pairs (37 in FA group, 31 in placebo group) | Randomized controlled trial (RCT) | All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. | Cognitive performance was assessed by the Wechsler Intelligence Scale in children at 11 years old. | NA | Children of folic-acid-treated mothers scored higher in two Processing Speed tests, and showed more efficient semantic processing of language at 11 years old. |
| Julvez et al., 2009 [39] | Spain | 420 mother–child pairs | Prospective birth cohort | Interviewer-administered questionnaires at the end of the first trimester of pregnancy. | Psychological outcomes were assessed in children at age 4 years. | NA | Maternal use of folic acid supplements was positively associated with verbal, motor, verbal-executive function, social competence, and inattention symptoms. |
| Veena et al., 2010 [40] | India | 536 mother–child pairs | Prospective Mysore Parthenon birth cohort | Folate levels were measured from maternal plasma samples collected at 30 weeks of gestation. | Cognitive function was measured in children at 9–10 years. | NA | It showed a positive association between maternal plasma folate levels and children’s cognitive performance. |
| Roth et al., 2011 [41] | Norway | 38,954 mother–child pairs | Prospective mother–child cohort | Self-report 3-year follow-up questionnaires. | Children’s language competency at age 3 years measured by maternal report. | NA | Maternal use of folic acid in early pregnancy was associated with a reduced risk of severe language delay in children at the age of 3 years. |
| Chatzi et al., 2012 [42] | Greece | 553 mother–child pairs | Prospective mother–child cohort | Interviewer-administered questionnaires at 14–18 weeks of gestation. | Neurodevelopment was assessed in children at 18 months. | NA | Children of mothers with reported doses of 5 mg/d or more folic acid had a 5-unit increase in receptive communication and a 3.5-unit increase in expressive communication. |
| Villamor et al., 2012 [43] | United States | 1210 mother–child pairs | Prospective pre-birth cohort | Questionnaires on the use of food frequency during the first and second trimesters of pregnancy. | The cognition and visual-motor skills were assessed in children at age 3 years. | NA | For every 600 ug/day increase in total folate intake during the first trimester of pregnancy, there was a 1.6-point increase in the PPVT-III scores in the children at age 3 years. |
| Wu et al., 2012 [44] | Canada | 154 mother–child pairs | Prospective birth cohort | Folate levels were measured from maternal plasma samples collected at 16 and 36 weeks of gestation. | Neurodevelopment was assessed in children at 18 months of age. | NA | No association was found between maternal plasma folate concentrations and child cognitive development. |
| Boeke et al., 2013 [45] | United States | 895 mother–child pairs | Prospective pre-birth cohort | Semiquantitative food frequency questionnaire (FFQ) at each of the first- and second-trimester study visits. | Visual memory and verbal and non-verbal intelligence were assessed in children at the age of 7 years. | NA | No associations were found between maternal folate intake and the cognitive outcomes in children aged 7 years. |
| Huang et al., 2020 [46] | China | 180 mother–child pairs | Prospective birth cohort | Serum folate concentrations were measured in blood samples collected from pregnant women at early, middle and late stages of pregnancy. | Gross motor skills, fine motor skills, language, adaptive behavior, and social behavior were assessed in children at the age of 2 years. | NA | Maternal serum folate in late pregnancy was positively associated with children’s language development while maternal serum folate in early pregnancy was inversely related to fine motor development in the children at the age of 2 years. |
| Irvine et al., 2023 [47] | Canada | 309 mother–child pairs | Prospective birth cohort | Maternal RBC folate status assessed during the second trimester of pregnancy. | Neurodevelopment was assessed in children at 3–5 years old. | NA | Maternal folate status during the second trimester of pregnancy was associated with improved executive function development, but not associated with children’s intelligence, language, memory, or motor outcomes at 3–5 years of age. |
| Tamura et al., 2005 [48] | United States | 335 mother–child pairs | Retrospective birth cohort | Both plasma and whole-blood folate concentrations were measured from maternal blood samples collected at 19, 26, and 37 weeks of gestation. | Six tests were performed in children at a mean of 5.3 years to assess their neurodevelopment. | NA | No association was found between maternal plasma and erythrocyte folate concentrations and children’s cognitive development. |
| Wehby and Murray, 2008 [49] | United States | 6774 mother–child pairs | Retrospective birth cohort | The 1988 National Maternal Infant Health Survey (NMIHS) and its 1991 Follow-up Survey data. | 16 Denver developmental screening items were measured in children at about 3 years of age. | NA | Folic acid use was associated with improved gross-motor development, but had marginally significant poorer performance for the personal–social domain. |
| Surén et al., 2013 [14] | Norway | 85,176 children | Prospective birth cohort | The information of mothers’ supplement intake before conception and in early pregnancy was obtained through questionnaire report at week 18 of gestation. | Cases of ASD were diagnosed and confirmed by the health specialists. | NA | Prenatal folic acid supplementation around the time of conception was associated with a lower risk of ASD incidence. |
| Raghavan et al., 2018 [23] | United States | 1257 mother–child pairs | Prospective birth cohort | A standard questionnaire was used to collect maternal data including supplement intake. Maternal plasma folate was measured from maternal blood samples collected 24–72 h post-delivery. | Children were diagnosed with ASD by the health specialists. | NA | It showed a “U shaped” relationship between maternal multivitamin supplementation frequency and ASD risk: moderate self-reported supplementation during pregnancy was associated with decreased risk of ASD, while low and high supplementation was associated with increased risk of ASD. |
| Raghavan et al., 2020 [50] | United States | 567 mother–child pairs | Prospective birth cohort | A standard questionnaire was used to collect maternal data. Plasma and RBC folate levels were measured from umbilical cord blood samples collected at the time of delivery. | Children were diagnosed with ASD by the health specialists. | NA | Higher concentration of cord blood unmetabolized folic acid (UMFA) was associated with increased risk of ASD. |
| Steegers-Theunissen et al., 2009 [51] | Netherlands | 120 mother–child pairs (86 mothers had used and 34 had not used folic acid periconceptionally) | Cross-sectional study | Questionnaire data via the mother on periconceptional folic acid use. | DNA methylation of IGF2 and folate levels in serum and red blood cells were measured using a mass spectrometry-based method in children between 12 and 18 months of age. | Serum folate in mothers: 15.3 in no FA group and 17.8 in yes FA group; Serum folate in children: 31.5 in no FA group and 32.1 in yes FA group; RBC folate in mothers: 687 in no FA group and 720 in yes FA group; RBC folate in children: 973 in no FA group and 1064 in yes FA group. | Children of mothers with periconceptional folic acid use had a 4.5% higher methylation of the IGF2 DMR and decreased birth weight. |
| Hoyo et al., 2011 [52] | United States | 438 pregnancies | Prospective cohort study | Preconception and prenatal FA supplementation was assessed from the self-administered questionnaire. | The methylation levels of two IGF2 DMRs measured via pro-sequencing in umbilical cord blood leukocytes. | NA | The methylation level of the IGF2/H19 imprinted region decreased with increasing FA intake before and during pregnancy. |
| Haggarty et al., 2013 [53] | United Kingdom | 913 mother–child pairs | Prospective cohort study | Folate levels were measured in maternal blood samples collected at 19 weeks of gestation and from cord blood samples. | DNA methylation level of 3 maternally methylated imprinted genes and 1 retrotransposon was measured in cord blood samples. | Maternal RBC folate: 456 Cord RBC folate: 657. | Folic acid supplement after 12 weeks of gestation was associated with a higher level of methylation in IGF2 and reduced methylation in both PEG3 and LINE-1. |
| Ondicova M et al., 2022 [54] | United Kingdom | 86 cord blood DNA samples | Randomized controlled trial (RCT) | All women took 400 μg/d of FA in the first trimester, and received 400 μg/d folic acid or placebo during the second and third trimesters. | Methylation profiles of cord blood were measured by the EPIC array and validated using pyrosequencing. | NA | FA supplementation resulted in significant methylation changes at specific classes of neurodevelopmental genes in the cord blood. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, Z.; Lin, Y.; Xie, H. Risk of Excess Maternal Folic Acid Supplementation in Offspring. Nutrients 2024, 16, 755. https://doi.org/10.3390/nu16050755
Xu X, Zhang Z, Lin Y, Xie H. Risk of Excess Maternal Folic Acid Supplementation in Offspring. Nutrients. 2024; 16(5):755. https://doi.org/10.3390/nu16050755
Chicago/Turabian StyleXu, Xiguang, Ziyu Zhang, Yu Lin, and Hehuang Xie. 2024. "Risk of Excess Maternal Folic Acid Supplementation in Offspring" Nutrients 16, no. 5: 755. https://doi.org/10.3390/nu16050755
APA StyleXu, X., Zhang, Z., Lin, Y., & Xie, H. (2024). Risk of Excess Maternal Folic Acid Supplementation in Offspring. Nutrients, 16(5), 755. https://doi.org/10.3390/nu16050755






