Chrononutrition in the Prevention and Management of Metabolic Disorders: A Literature Review
Abstract
1. Introduction
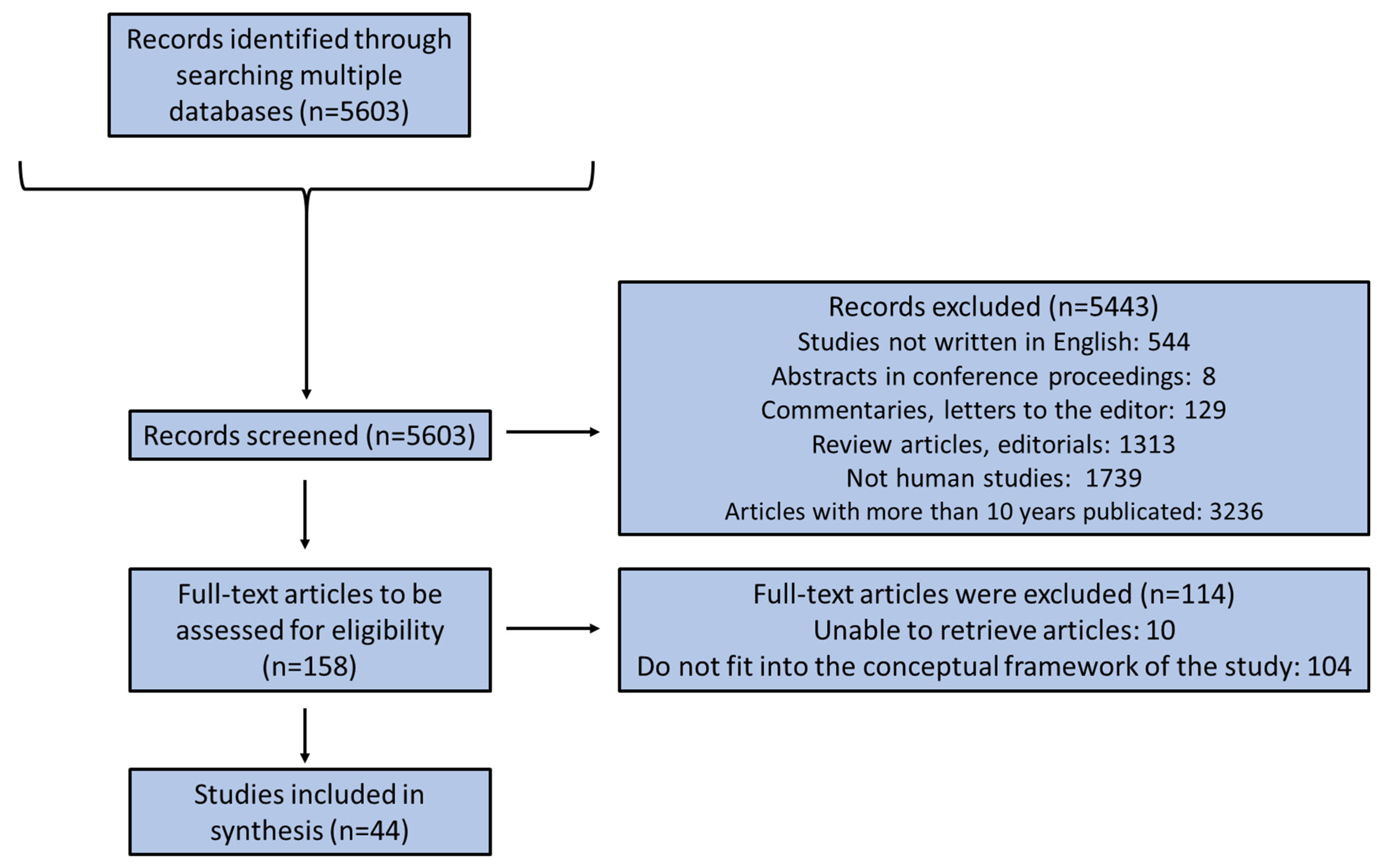
2. Methods
3. Results
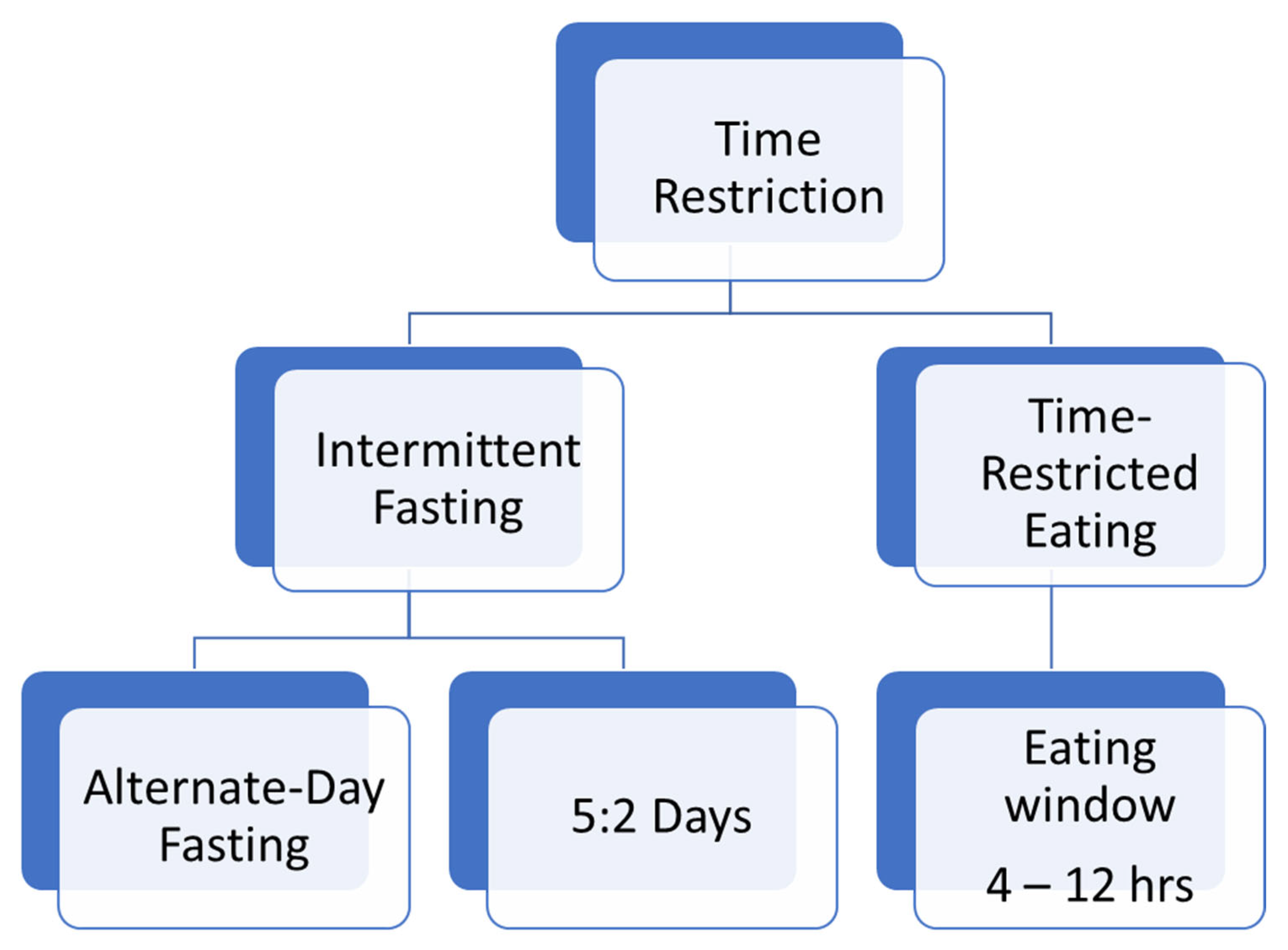
3.1. TRE or TRF and Obesity
3.2. TRE or TRF and Diabetes Mellitus
3.3. TRE or TRF and Cardiovascular Diseases
3.4. TRE or TRF and Non-Alcoholic Fatty Liver Disease (NAFLD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Codoñer-Franch, P.; Gombert, M.; Martínez-Raga, J.; Cenit, M.C. Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies. Int. J. Mol. Sci. 2023, 24, 7579. [Google Scholar] [CrossRef]
- Ahluwalia, M.K. Chrononutrition-When We Eat Is of the Essence in Tackling Obesity. Nutrients 2022, 14, 5080. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Perez-Diaz-Del-Campo, N.; Castelnuovo, G.; Caviglia, G.P.; Armandi, A.; Rosso, C.; Bugianesi, E. Role of Circadian Clock on the Pathogenesis and Lifestyle Management in Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 5053. [Google Scholar] [CrossRef]
- Lavallee, C.M.; Bruno, A.; Ma, C.; Raman, M. The Role of Intermittent Fasting in the Management of Nonalcoholic Fatty Liver Disease: A Narrative Review. Nutrients 2022, 14, 4655. [Google Scholar] [CrossRef] [PubMed]
- Tippairote, T.; Janssen, S.; Chunhabundit, R. Restoration of metabolic tempo through time-restricted eating (TRE) as the preventive measure for metabolic diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 2444–2453. [Google Scholar] [CrossRef]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef]
- Kamarul Zaman, M.; Teng, N.; Kasim, S.S.; Juliana, N.; Alshawsh, M.A. Effects of time-restricted eating with different eating duration on anthropometrics and cardiometabolic health: A systematic review and meta-analysis. World J. Cardiol. 2023, 15, 354–374. [Google Scholar] [CrossRef]
- Mishra, S.; Persons, P.A.; Lorenzo, A.M.; Chaliki, S.S.; Bersoux, S. Time-Restricted Eating and Its Metabolic Benefits. J. Clin. Med. 2023, 12, 7007. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Nadkarni, D.; Martin, L.; Newberry, C.; Kumar, S.; Kushner, T. Intermittent fasting improves hepatic end points in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Commun. 2023, 7, e0212. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.N.C.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-restricted Eating for the Prevention and Management of Metabolic Diseases. Endocr. Rev. 2022, 43, 405–436. [Google Scholar] [CrossRef]
- Schuppelius, B.; Peters, B.; Ottawa, A.; Pivovarova-Ramich, O. Time Restricted Eating: A Dietary Strategy to Prevent and Treat Metabolic Disturbances. Front. Endocrinol. 2021, 12, 683140. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Parr, E.B.; Kleckner, A.S. Cardiometabolic health impacts of time-restricted eating: Implications for type 2 diabetes, cancer and cardiovascular diseases. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 378–387. [Google Scholar] [CrossRef]
- Sun, J.C.; Tan, Z.T.; He, C.J.; Hu, H.L.; Zhai, C.L.; Qian, G. Time-restricted eating with calorie restriction on weight loss and cardiometabolic risk: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 1014–1025. [Google Scholar] [CrossRef]
- Silva, A.I.; Direito, M.; Pinto-Ribeiro, F.; Ludovico, P.; Sampaio-Marques, B. Effects of Intermittent Fasting on Regulation of Metabolic Homeostasis: A Systematic Review and Meta-Analysis in Health and Metabolic-Related Disorders. J. Clin. Med. 2023, 12, 3699. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, K.; Sujka, M. Effect of Various Types of Intermittent Fasting (IF) on Weight Loss and Improvement of Diabetic Parameters in Human. Curr. Nutr. Rep. 2021, 10, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Naous, E.; Achkar, A.; Mitri, J. Intermittent Fasting and Its Effects on Weight, Glycemia, Lipids, and Blood Pressure: A Narrative Review. Nutrients 2023, 15, 3661. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Chair, S.Y.; Cai, H.; Cao, X.; Qin, Y.; Cheng, H.Y.; Ng, M.T. Intermittent Fasting in Weight Loss and Cardiometabolic Risk Reduction: A Randomized Controlled Trial. J. Nurs. Res. 2022, 30, e185. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qin, Y.L.; Shi, Z.Y.; Chen, J.H.; Zeng, M.J.; Zhou, W.; Chen, R.Q.; Chen, Z.Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutr. Health 2020, 26, 79–85. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.D.; Falqueto, H.; Mânica, A.; Zanini, D.; de Oliveira, T.; de Sá, C.A.; Cardoso, A.M.; Manfredi, L.H. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J. Transl. Med. 2021, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, V.; Cienfuegos, S.; Lin, S.; Ezpeleta, M.; Ready, K.; Corapi, S.; Wu, J.; Lopez, J.; Gabel, K.; Tussing-Humphreys, L.; et al. Effect of Time-Restricted Eating on Weight Loss in Adults with Type 2 Diabetes: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2339337. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res. Clin. Pract. 2019, 151, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Kunduraci, Y.E.; Ozbek, H. Does the Energy Restriction Intermittent Fasting Diet Alleviate Metabolic Syndrome Biomarkers? A Randomized Controlled Trial. Nutrients 2020, 12, 3213. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, A.; Tripolt, N.J.; Pferschy, P.N.; Kojzar, H.; Aziz, F.; Müller, A.; Schauer, M.; Oulhaj, A.; Aberer, F.; Sourij, C.; et al. Efficacy and Safety of Intermittent Fasting in People With Insulin-Treated Type 2 Diabetes (INTERFAST-2)-A Randomized Controlled Trial. Diabetes Care 2023, 46, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Peeke, P.M.; Greenway, F.L.; Billes, S.K.; Zhang, D.; Fujioka, K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: Results of a randomized, controlled, virtual clinical trial. Nutr. Diabetes 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Khalfallah, M.; Elnagar, B.; Soliman, S.S.; Eissa, A.; Allaithy, A. The Value of Intermittent Fasting and Low Carbohydrate Diet in Prediabetic Patients for the Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2023, 120, e20220606. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e363. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience 2020, 42, 667–686. [Google Scholar] [CrossRef]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults with Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef]
- Feehan, J.; Mack, A.; Tuck, C.; Tchongue, J.; Holt, D.Q.; Sievert, W.; Moore, G.T.; de Courten, B.; Hodge, A. Time-Restricted Fasting Improves Liver Steatosis in Non-Alcoholic Fatty Liver Disease-A Single Blinded Crossover Trial. Nutrients 2023, 15, 4870. [Google Scholar] [CrossRef]
- Badran, H.; Elsabaawy, M.; Sakr, A.; Eltahawy, M.; Elsayed, M.; Elsabaawy, D.M.; Abdelkreem, M. Impact of intermittent fasting on laboratory, radiological, and anthropometric parameters in NAFLD patients. Clin. Exp. Hepatol. 2022, 8, 118–124. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Gabel, K.; Cienfuegos, S.; Kalam, F.; Lin, S.; Pavlou, V.; Song, Z.; Haus, J.M.; Koppe, S.; Alexandria, S.J.; et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell Metab. 2023, 35, 56–70.e53. [Google Scholar] [CrossRef]
- Wei, X.; Lin, B.; Huang, Y.; Yang, S.; Huang, C.; Shi, L.; Liu, D.; Zhang, P.; Lin, J.; Xu, B.; et al. Effects of Time-Restricted Eating on Nonalcoholic Fatty Liver Disease: The TREATY-FLD Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e233513. [Google Scholar] [CrossRef]
- Katsi, V.; Papakonstantinou, I.P.; Soulaidopoulos, S.; Katsiki, N.; Tsioufis, K. Chrononutrition in Cardiometabolic Health. J. Clin. Med. 2022, 11, 296. [Google Scholar] [CrossRef]
- Gabel, K.; Varady, K.A. Current research: Effect of time restricted eating on weight and cardiometabolic health. J. Physiol. 2022, 600, 1313–1326. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Baharlooie, M.; Karimi, B.; Mokhtari, K.; Rosenkranz, S.K.; Santos, H.O. Effects of intermittent fasting combined with physical exercise on cardiometabolic outcomes: Systematic review and meta-analysis of clinical studies. Nutr. Rev. 2023, 15, nuad155. [Google Scholar] [CrossRef] [PubMed]
- Ezpeleta, M.; Cienfuegos, S.; Lin, S.; Pavlou, V.; Gabel, K.; Tussing-Humphreys, L.; Varady, K.A. Time-restricted eating: Watching the clock to treat obesity. Cell Metab. 2023, 36, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.L.; Katsilambros, N.L.; Koliaki, C.C. Intermittent Energy Restriction, Weight Loss and Cardiometabolic Risk: A Critical Appraisal of Evidence in Humans. Healthcare 2021, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Schroor, M.M.; Joris, P.J.; Plat, J.; Mensink, R.P. Effects of Intermittent Energy Restriction Compared with Those of Continuous Energy Restriction on Body Composition and Cardiometabolic Risk Markers—A Systematic Review and Meta-Analysis of Randomized Controlled Trials in Adults. Adv. Nutr. 2024, 15, 100130. [Google Scholar] [CrossRef] [PubMed]
- Van den Burg, E.L.; van Peet, P.G.; Schoonakker, M.P.; van de Haar, D.E.; Numans, M.E.; Pijl, H. Metabolic impact of intermittent energy restriction and periodic fasting in patients with type 2 diabetes: A systematic review. Nutr. Rev. 2023, 81, 1329–1350. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Tomlinson, J.W.; Hodson, L.; Ray, D.W. Timing of energy intake and the therapeutic potential of intermittent fasting and time-restricted eating in NAFLD. Gut 2023, 72, 1607–1619. [Google Scholar] [CrossRef]
- Abdulan, I.M.; Popescu, G.; Maștaleru, A.; Oancea, A.; Costache, A.D.; Cojocaru, D.-C.; Cumpăt, C.-M.; Ciuntu, B.M.; Rusu, B.; Leon, M.M. Winter Holidays and Their Impact on Eating Behavior—A Systematic Review. Nutrients 2023, 15, 4201. [Google Scholar] [CrossRef]
- Tsitsou, S.; Zacharodimos, N.; Poulia, K.A.; Karatzi, K.; Dimitriadis, G.; Papakonstantinou, E. Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 4778. [Google Scholar] [CrossRef] [PubMed]
- Alkhulaifi, F.; Darkoh, C. Meal Timing, Meal Frequency and Metabolic Syndrome. Nutrients 2022, 14, 1719. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J. Neurochem. 2021, 157, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, O.; Mari, A.; Deacon, C.F.; Carr, R.D.; Winzell, M.S.; Vikman, J.; Ahrén, B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J. Clin. Endocrinol. Metab. 2009, 94, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Kekäläinen, T.; Hietavala, E.M.; Hakamäki, M.; Sipilä, S.; Laakkonen, E.K.; Kokko, K. Personality Traits and Changes in Health Behaviors and Depressive Symptoms during the COVID-19 Pandemic: A Longitudinal Analysis from Pre-pandemic to Onset and End of the Initial Emergency Conditions in Finland. Int. J. Environ. Res. Public Health 2021, 18, 7732. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdi, T.; Heraclides, A.; Papageorgiou, A.; Philippou, E. The Effect of Personality on Chrononutrition during the COVID-19 Lockdown in Qatar. Nutrients 2022, 14, 2725. [Google Scholar] [CrossRef] [PubMed]
- Bazzani, A.; Marantonio, S.; Andreozzi, G.; Lorenzoni, V.; Bruno, S.; Cruz-Sanabria, F.; d′Ascanio, P.; Turchetti, G.; Faraguna, U. Late chronotypes, late mealtimes. Chrononutrition and sleep habits during the COVID-19 lockdown in Italy. Appetite 2022, 172, 105951. [Google Scholar] [CrossRef] [PubMed]
- Saals, B.; Boss, H.M.; Pot, G.K. Young people and adolescents have more irregular meals during the COVID-19 pandemic: A nested case-control study on chrono-nutrition before and during the COVID-19 pandemic. Chronobiol. Int. 2022, 39, 991–1000. [Google Scholar] [CrossRef]
- Murta, L.; Seixas, D.; Harada, L.; Damiano, R.F.; Zanetti, M. Intermittent Fasting as a Potential Therapeutic Instrument for Major Depression Disorder: A Systematic Review of Clinical and Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 15551. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Intermittent fasting and time-restricted eating role in dietary interventions and precision nutrition. Front. Public Health 2022, 10, 1017254. [Google Scholar] [CrossRef]
- Chavanne, A.; Jacobi, D. Precision medicine in endocrinology: Unraveling metabolic health through time-restricted eating. Ann. Endocrinol. 2024, 85, 63–69. [Google Scholar] [CrossRef]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F. The Association between Chronotype and Dietary Pattern among Adults: A Scoping Review. Int. J. Environ. Res. Public Health 2019, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, A.C.; Vilas-Boas, E.A.; Panveloski-Costa, A.C.; Leite, J.S.M.; Lucena, C.F.; Riva, P.; Emilio, H.; Carpinelli, A.R. Intermittent Fasting for Twelve Weeks Leads to Increases in Fat Mass and Hyperinsulinemia in Young Female Wistar Rats. Nutrients 2020, 12, 1029. [Google Scholar] [CrossRef] [PubMed]

| Study Type | Author (Country) | Study Population | Basic Results | References |
|---|---|---|---|---|
| Randomized controlled trial | Catenacci et al. (USA) | N = 26, obese female and male (ADF group N = 34, 16/8 TRF group N = 33, control group N = 34) | No significant difference in weight loss between groups. | [18] |
| Randomized controlled trial | Chair et al. (China) | N = 101, overweight or obese female and male | The reductions in body weight, body mass index, and waist circumference in the ADF and 16/8 TRF groups were more significant than those in the control group across the study period. | [19] |
| Randomized controlled trial | Cai et al. (China) | N = 271, female and male with NAFLD (ADF group, TRF group) | Both groups decreased body weight after 12 weeks. | [20] |
| Single-arm, paired-sample clinical trial | Wilkinson et al. (USA) | N = 19, overweight or obese female and male | Body weight was decreased after TRE intervention. | [21] |
| Prospective study | Gabel et al. (USA) | N = 14, obese female and male | Body weight was decreased after TRE intervention. | [22] |
| Randomized controlled trial | Schübel et al. (Germany) | N = 150, overweight or obese female and male | ICR and CCR are effective methods for weight loss. | [23] |
| Randomized controlled trial | Sundfør et al. (Norway) | N = 112, overweight or obese female and male | Both intermittent and continuous energy restriction resulted in similar weight loss. | [24] |
| Randomized clinical trial | Lowe et al. (USA) | N = 116, overweight or obese female and male | There was a significant decrease in weight in the TRE but no significant change in the consistent meal timing (CMT) group. | [25] |
| Non-randomized clinical trial | Schroder et al. (Brazil) | N = 32, obese women | TRF reduced body weight (~4 kg). | [26] |
| Study Type | Author (Country) | Study Population | Basic Results | References |
|---|---|---|---|---|
| Randomized controlled trial | Che et al. (China) | N = 104, overweight female and male with type 2 diabetes | Improvement in blood glucose and insulin sensitivity, quality of life, and reduction in the dosage of hypoglycemic drugs. | [27] |
| Randomized clinical trial | Pavlou et al. (USA) | N = 69, overweight female and male with type 2 diabetes | Greater weight loss and no differences in hemoglobin A1c (HbA1c); no difference between the TRE and CR groups compared with controls. | [28] |
| Single-arm, paired-sample trial | Wilkinson et al. (USA) | N = 19, overweight or obese female and male with metabolic syndrome | Reduction in HbA1c. | [21] |
| Randomized controlled trial | Chair et al. (China) | N = 101, overweight or obese female and male with prediabetes (ADF group N = 34, 16/8 TRF group N = 33, control group N = 34) | Significant reductions in blood glucose were observed. | [19] |
| Randomized controlled trial | Carter et al. (Australia) | N = 137, female and male with type 2 diabetes | No significant differences between groups in body composition, fasting glucose levels, lipid levels, or total medication effect score at 24 months. | [29] |
| Randomized controlled trial | Gabel et al. (USA) | N = 43, overweight or obese female and male with insulin resistance | Improvements in HOMA-IR due to ADF vs. daily CR. | [30] |
| Randomized controlled trial | Kunduraci et al. (Turkey) | N = 65, female and male with metabolic syndrome | No significant differences were observed in fasting plasma glucose, insulin, HbA1c, HOMA-IR in both groups. | [31] |
| Randomized controlled trial | Obermayer et al. (Austria) | Ν = 47, female and male with diabetes mellitus | The IF group showed a significant HbA1c reduction. | [32] |
| Controlled clinical trial | Peeke et al. (USA) | Ν = 60, obese female and male | The differences between groups were not significantly different. | [33] |
| Clinical trial | Khalfallah et al. (Egypt) | N = 485, prediabetic female and male | Combined with LCD, was associated with lower progression to diabetes mellitus and lower incidence of implications. | [34] |
| Study Type | Author (Country) | Study Population | Basic Results | References |
|---|---|---|---|---|
| Randomized controlled trial | Cienfuegos et al. (USA) | N = 59, obese female and male | Neither intervention had any effect on plasma lipid levels. | [35] |
| Randomized controlled trial | Sutton et al. (USA) | N = 8 men with prediabetes | Early time-restricted feeding (eTRF) improved insulin sensitivity, β cell responsiveness, blood pressure, oxidative stress, and appetite. | [36] |
| Clinical trial | Chow et al. (USA) | N = 46 obese female and male | Diastolic blood pressure, LDL cholesterol, HDL cholesterol, triglycerides, and homocysteine were not significantly different from controls after 12 weeks. | [37] |
| Randomized controlled trial | Martens et al. (USA) | N = 22, non-obese, healthy female and male | No significant differences in cardiometabolic risk factors. | [38] |
| Randomized controlled trial | Jamshed et al. (USA) | N = 90, obese female and male | Lower diastolic blood pressure and no differences in systolic blood pressure, heart rate or plasma lipid levels. | [39] |
| Randomized controlled trial | Cai et al. (China) | N = 271, female and male with NAFLD (ADF group vs. TRF group) | Intermittent fasting led to improvements in NAFLD due to fat loss. | [20] |
| Study Type | Author (Country) | Study Population | Basic Results | References |
|---|---|---|---|---|
| Single-blind randomized controlled trial | Feehan et al. (Australia) | N = 28, female and male with NAFLD | Intermittent fasting significantly decreased hepatic steatosis. | [40] |
| Clinical trial | Badran et al. (Egypt) | N = 98, NAFLD female and male | Intermittent fasting led to improvements in ultrasonographic, biochemical, and anthropometric parameters of NAFLD. | [41] |
| Randomized controlled trial | Cai et al. (China) | N = 271, female and male with NAFLD (ADF group vs. TRF group) | Intermittent fasting led to improvements in NAFLD due to fat loss. | [20] |
| Randomized controlled trial | Ezpeleta et al. (USA) | N = 80, obese female and male with NAFLD | Intrahepatic triglyceride (IHTG) content reduced in combination group (ADF + exercise). | [42] |
| Randomized controlled trial | Wei et al. (China) | N = 88, obese female and male with NAFLD | TRE did not produce additional benefits for reducing IHTG content, body fat, and metabolic risk factors compared with habitual meal timing. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mentzelou, M.; Papadopoulou, S.K.; Psara, E.; Voulgaridou, G.; Pavlidou, E.; Androutsos, O.; Giaginis, C. Chrononutrition in the Prevention and Management of Metabolic Disorders: A Literature Review. Nutrients 2024, 16, 722. https://doi.org/10.3390/nu16050722
Mentzelou M, Papadopoulou SK, Psara E, Voulgaridou G, Pavlidou E, Androutsos O, Giaginis C. Chrononutrition in the Prevention and Management of Metabolic Disorders: A Literature Review. Nutrients. 2024; 16(5):722. https://doi.org/10.3390/nu16050722
Chicago/Turabian StyleMentzelou, Maria, Sousana K. Papadopoulou, Evmorfia Psara, Gavriela Voulgaridou, Eleni Pavlidou, Odysseas Androutsos, and Constantinos Giaginis. 2024. "Chrononutrition in the Prevention and Management of Metabolic Disorders: A Literature Review" Nutrients 16, no. 5: 722. https://doi.org/10.3390/nu16050722
APA StyleMentzelou, M., Papadopoulou, S. K., Psara, E., Voulgaridou, G., Pavlidou, E., Androutsos, O., & Giaginis, C. (2024). Chrononutrition in the Prevention and Management of Metabolic Disorders: A Literature Review. Nutrients, 16(5), 722. https://doi.org/10.3390/nu16050722









