Implications of Protein and Sarcopenia in the Prognosis, Treatment, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
1. Introduction
Diagnosis, Staging, and Management of MASLD
2. Sarcopenia
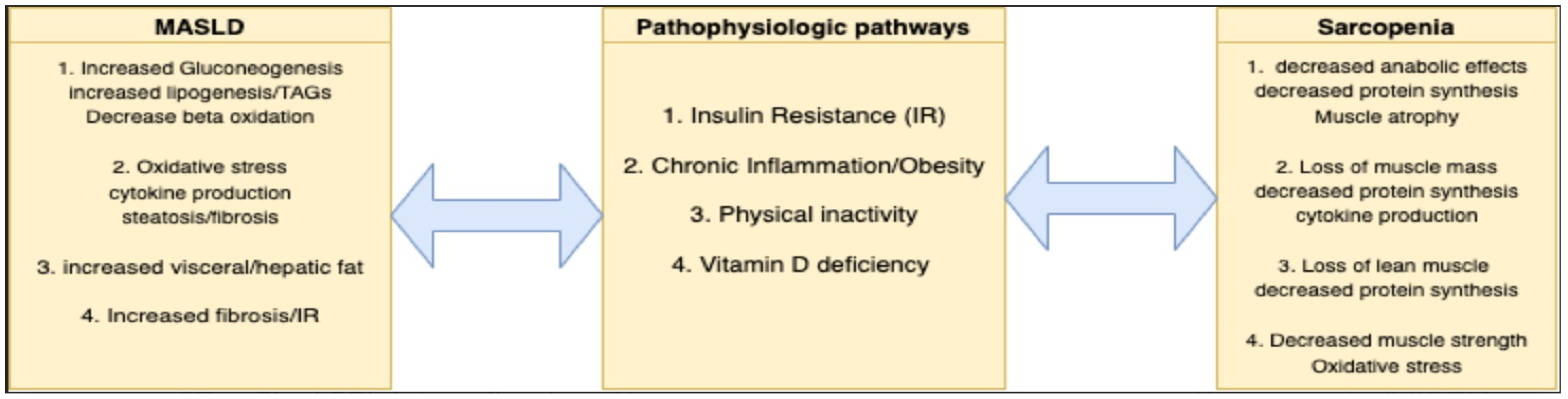
3. Pathophysiological Considerations of Sarcopenia in MASLD
3.1. Insulin Resistance and Lipogenesis
3.2. Obesity and Inflammation
3.3. Physical Inactivity
3.4. Vitamin D Deficiency
4. Evaluation of Sarcopenia in Patients with MASLD
Special Consideration in Advanced Liver Disease and Cirrhosis
5. Management of Sarcopenia in Patients with MASLD
5.1. Nutritional Interventions
5.2. Exercise and Physical Activity
5.3. Special Considerations in Advanced Liver Disease and Cirrhosis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef]
- Huang, T.; Behary, J.; Zekry, A. Non-alcoholic fatty liver disease: A review of epidemiology, risk factors, diagnosis and management. Intern. Med. J. 2020, 50, 1038–1047. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- E Powell, E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. S5), 990S–991S. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef]
- Guarino, M.; Cossiga, V.; Becchetti, C.; Invernizzi, F.; Lapenna, L.; Lavezzo, B.; Lenci, I.; Merli, M.; Pasulo, L.; Zanetto, A.; et al. Sarcopenia in chronic advanced liver diseases: A sex-oriented analysis of the literature. Dig. Liver Dis. 2022, 54, 997–1006. [Google Scholar] [CrossRef]
- Marušić, M.; Paić, M.; Knobloch, M.; Pršo, A.-M.L. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, e6613827. [Google Scholar] [CrossRef]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Parlati, L.; Régnier, M.; Guillou, H.; Postic, C. New targets for NAFLD. JHEP Rep. 2021, 3, 100346. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, K.M. Sarcopenia and fatty liver disease. Hepatol. Int. 2019, 13, 674–687. [Google Scholar] [CrossRef]
- Zhai, Y.; Xiao, Q. The Common Mechanisms of Sarcopenia and NAFLD. BioMed Res. Int. 2017, 2017, e6297651. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK572122/ (accessed on 15 October 2023).
- Kim, T.N.; Park, M.S.; Ryu, J.Y.; Choi, H.Y.; Hong, H.C.; Yoo, H.J.; Kang, H.J.; Song, W.; Park, S.W.; Baik, S.H.; et al. Impact of Visceral Fat on Skeletal Muscle Mass and Vice Versa in a Prospective Cohort Study: The Korean Sarcopenic Obesity Study (KSOS). PLoS ONE 2014, 9, e115407. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- De Fré, C.H.; De Fré, M.A.; Kwanten, W.J.; De Beeck, B.J.O.; Van Gaal, L.F.; Francque, S.M. Sarcopenia in patients with non-alcoholic fatty liver disease: Is it a clinically significant entity? Obes. Rev. 2019, 20, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Chong, M.S.; Lim, J.P.; Leung, B.P.; Ding, Y.Y.; Tay, L.; Ismail, N.H.; Yeo, A.; Yew, S. Monocyte chemoattractant protein-1: A proinflammatory cytokine elevated in sarcopenic obesity. Clin. Interv. Aging 2015, 10, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1062–1079. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, Y.J.; Lee, M.W.; Kim, S.M. Full-length adiponectin protects hepatocytes from palmitate-induced apoptosis via inhibition of c-Jun NH2 terminal kinase. FEBS J. 2009, 276, 2278–2284. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Tsiaousi, E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2010, 12, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Toulis, K.A.; Goulis, D.G.; Zavos, C.; Kountouras, J. Serum total adiponectin in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2011, 60, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.M.; Kountouras, J.; Zavos, C. Nonlinear Distribution of Adiponectin in Patients with Nonalcoholic Fatty Liver Disease Limits Its Use in Linear Regression Analysis. J. Clin. Gastroenterol. 2010, 44, 229–230. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Johnson, N.A.; Sachinwalla, T.; Walton, D.W.; Smith, K.; Armstrong, A.; Thompson, M.W.; George, J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009, 50, 1105–1112. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Andersson, D.P.; Kerr, A.G.; Dahlman, I.; Rydén, M.; Arner, P. Relationship Between a Sedentary Lifestyle and Adipose Insulin Resistance. Diabetes 2023, 72, 316–325. [Google Scholar] [CrossRef]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D and Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): An Update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef]
- Ma, M.; Long, Q.; Chen, F.; Zhang, T.; Wang, W. Active vitamin D impedes the progression of non-alcoholic fatty liver disease by inhibiting cell senescence in a rat model. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 513–523. [Google Scholar] [CrossRef]
- Roth, C.L.; Elfers, C.T.; Figlewicz, D.P.; Melhorn, S.J.; Morton, G.J.; Hoofnagle, A.; Yeh, M.M.; Nelson, J.E.; Kowdley, K.V. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and toll-like receptor activation. Hepatology 2012, 55, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Hosoyama, T.; Tomida, M.; Yamamoto, Y.; Nakamichi, Y.; Kato, S.; Kawai-Takaishi, M.; Ishizuka, S.; Nishita, Y.; Tange, C.; et al. Influence of vitamin D on sarcopenia pathophysiology: A longitudinal study in humans and basic research in knockout mice. J. Cachexia-Sarcopenia Muscle 2022, 13, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Kaur, N.; Gupta, P.; Saini, V.; Sherawat, S.; Gupta, S.; Dua, A.; Kumar, V.; Injeti, E.; Mittal, A. Cinnamaldehyde regulates H2O2-induced skeletal muscle atrophy by ameliorating the proteolytic and antioxidant defense systems. J. Cell. Physiol. 2019, 234, 6194–6208. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo De Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Glüer, C.-C.; Bosy-Westphal, A.; Müller, M.J. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef]
- Helder, J.v.D.; Verreijen, A.M.; van Dronkelaar, C.; Memelink, R.G.; Engberink, M.F.; Engelbert, R.H.H.; Weijs, P.J.M.; Tieland, M. Bio-Electrical Impedance Analysis: A Valid Assessment Tool for Diagnosis of Low Appendicular Lean Mass in Older Adults? Front. Nutr. 2022, 9, 874980. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Chapman, B.; Hoermann, R.; Angus, P.W.; Testro, A.; Scodellaro, T.; Gow, P.J. Handgrip Strength Adds More Prognostic Value to the Model for End-Stage Liver Disease Score Than Imaging-Based Measures of Muscle Mass in Men with Cirrhosis. Liver Transplant. 2019, 25, 1480–1487. [Google Scholar] [CrossRef]
- Wang, S.; Whitlock, R.; Xu, C.; Taneja, S.; Singh, S.; Abraldes, J.G.; Burak, K.W.; Bailey, R.J.; Lai, J.C.; Tandon, P. Fraility is associated with increased risk of cirrhosis disease progression and death. Hepatology 2021, 75, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, K.J.M.; McKenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front. Nutr. 2021, 8, 615849. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef]
- Beasley, J.M.; Wertheim, B.C.; LaCroix, A.Z.; Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Kritchevsky, S.; Shikany, J.M.; Eaton, C.; Chen, Z.; et al. Biomarker-Calibrated Protein Intake and Physical Function in the Women’s Health Initiative. J. Am. Geriatr. Soc. 2013, 61, 1863–1871. [Google Scholar] [CrossRef]
- McLean, R.R.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; Sahni, S. Dietary Protein Intake Is Protective Against Loss of Grip Strength Among Older Adults in the Framingham Offspring Cohort. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Santiago, E.C.S.; Roriz, A.K.C.; Ramos, L.B.; Ferreira, A.J.F.; Oliveira, C.C.; Gomes-Neto, M. Comparison of calorie and nutrient intake among elderly with and without sarcopenia: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, Y.-H.; Huh, J.H.; Kang, D.R.; Rhee, Y.; Lim, S.-K. Early-stage chronic kidney disease, insulin resistance, and osteoporosis as risk factors of sarcopenia in aged population: The Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008–2009. Osteoporos. Int. 2014, 25, 2189–2198. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic Resistance of Muscle Protein Synthesis with Aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-H.; Wu, S.-J.; Wang, S.-T.; Chang, Y.-F.; Chang, C.-S.; Kuan, T.-S.; Chuang, H.-Y.; Chang, C.-M.; Chou, W.; Wu, C.-H. Effects of enriched branched-chain amino acid supplementation on sarcopenia. Aging 2020, 12, 15091–15103. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.H.; Lips, P. Longitudinal Aging Study Amsterdam. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Shea, M.K.; A Fielding, R.; Dawson-Hughes, B. The effect of vitamin D supplementation on lower-extremity power and function in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 369–379. [Google Scholar] [CrossRef]
- Seo, M.-W.; Jung, S.-W.; Kim, S.-W.; Lee, J.-M.; Jung, H.C.; Song, J.-K. Effects of 16 Weeks of Resistance Training on Muscle Quality and Muscle Growth Factors in Older Adult Women with Sarcopenia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6762. [Google Scholar] [CrossRef]
- Wei, M.; Meng, D.; Guo, H.; He, S.; Tian, Z.; Wang, Z.; Yang, G.; Wang, Z. Hybrid Exercise Program for Sarcopenia in Older Adults: The Effectiveness of Explainable Artificial Intelligence-Based Clinical Assistance in Assessing Skeletal Muscle Area. Int. J. Environ. Res. Public Health 2022, 19, 9952. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.H.; Hewitt, J.; Keogh, J.W.; Bermeo, S.; Duque, G.; Henwood, T.R. Impact of resistance training on sarcopenia in nursing care facilities: A pilot study. Geriatr. Nurs. 2016, 37, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-D.; Tsauo, J.-Y.; Lin, L.-F.; Huang, S.-W.; Ku, J.-W.; Chou, L.-C.; Liou, T.-H. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity. Medicine 2017, 96, e7115. [Google Scholar] [CrossRef]
- Lu, L.; Mao, L.; Feng, Y.; Ainsworth, B.E.; Liu, Y.; Chen, N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology; Geriatrics (BSGG). Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.I.; Mikhail, A.; Lan, L.; Di Carlo, A.; Hamilton, B.; Barnard, K.; Hettinga, B.P.; Hatcher, E.; Tarnopolsky, M.G.; Nederveen, J.P.; et al. A Five-Ingredient Nutritional Supplement and Home-Based Resistance Exercise Improve Lean Mass and Strength in Free-Living Elderly. Nutrients 2020, 12, 2391. [Google Scholar] [CrossRef]
- Bell, K.E.; Snijders, T.; Zulyniak, M.; Kumbhare, D.; Parise, G.; Chabowski, A.; Phillips, S.M. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: A randomized controlled trial. PLoS ONE 2017, 12, e0181387. [Google Scholar] [CrossRef]
- Palmer, L.B.; Kuftinec, G.; Pearlman, M.; Green, C.H. Nutrition in Cirrhosis. Curr. Gastroenterol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Tandon, P.; Montano-Loza, A.J.; Lai, J.C.; Dasarathy, S.; Merli, M. Sarcopenia and frailty in decompensated cirrhosis. J. Hepatol. 2021, 75 (Suppl. 1), S147–S162. [Google Scholar] [CrossRef]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.D.; McCullough, A.J.; Dasarathy, S. Late evening snack: Exploiting a period of anabolic opportunity in cirrhosis. J. Gastroenterol. Hepatol. 2012, 27, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Leoni, L.; Valoriani, F.; Barbieri, R.; Pambianco, M.; Vinciguerra, M.; Sicuro, C.; Colecchia, A.; Menozzi, R.; Ravaioli, F. Unlocking the Power of Late-Evening Snacks: Practical Ready-to-Prescribe Chart Menu for Patients with Cirrhosis. Nutrients 2023, 15, 3471. [Google Scholar] [CrossRef]
- Guo, Y.J.; Tian, Z.B.; Jiang, N.; Ding, X.L.; Mao, T.; Jing, X. Effects of Late Evening Snack on Cirrhotic Patients: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2018, 2018, 9189062. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M. Late Evening Snack with Branched-Chain Amino Acids Supplementation Improves Survival in Patients with Cirrhosis. J. Clin. Med. 2020, 9, 1013. [Google Scholar] [CrossRef]
- Campollo, O.; Sprengers, D.; Dam, G.; Vilstrup, H.; McIntyre, N. Protein tolerance to standard and high protein meals in patients with liver cirrhosis. World J. Hepatol. 2017, 9, 667–676. [Google Scholar] [CrossRef]
- Córdoba, J.; López-Hellín, J.; Planas, M.; Sabín, P.; Sanpedro, F.; Castro, F.; Esteban, R.; Guardia, J. Normal protein diet for episodic hepatic encephalopathy: Results of a randomized study. J. Hepatol. 2004, 41, 38–43. [Google Scholar] [CrossRef]
- Cabral, C.M.; Burns, D.L. Low-protein diets for hepatic encephalopathy debunked: Let them eat steak. Nutr. Clin. Pract. 2011, 26, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.P.; Marchesini, G.; Fabbri, A.; Rondelli, A.; Bugianesi, E.; Zoli, M.; Pisi, E. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J. Intern. Med. 1993, 233, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.; Ramos-Uribe, M.H.; Vargas, F.; Villalobos, A.; Ramos, C.; Márquez, M.A.; Ramos, G.G. Treatment of chronic portal?Systemic encephalopathy with vegetable and animal protein diets. A controlled crossover study. Dig. Dis. Sci. 1982, 27, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Jagdish, R.K. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin. Mol. Hepatol. 2019, 25, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2015, 9, CD001939. [Google Scholar] [CrossRef]
- Ruiz-Margain, A.; Macias-Rodriguez, R.U.; Rios-Torres, S.L.; Roman-Calleja, B.M.; Mendez-Guerrero, O.; Rodriguez-Cordova, P.; Torre, A. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev. Gastroenterol. Mex. Engl. 2018, 83, 9–15. [Google Scholar] [CrossRef]
- Buchard, B.; Boirie, Y.; Cassagnes, L.; Lamblin, G.; Coilly, A.; Abergel, A. Assessment of Malnutrition, Sarcopenia and Frailty in Patients with Cirrhosis: Which Tools Should We Use in Clinical Practice? Nutrients 2020, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A. A Multicenter Study to Define Sarcopenia in Patients With End-Stage Liver Disease. Liver Transplant. 2017, 23, 625–633. [Google Scholar] [CrossRef]
- Lai, J.C.; Feng, S.; Terrault, N.A.; Lizaola, B.; Hayssen, H.; Covinsky, K. Frailty Predicts Waitlist Mortality in Liver Transplant Candidates. Am. J. Transplant. 2014, 14, 1870–1879. [Google Scholar] [CrossRef]
- Kaido, T.; Ogawa, K.; Fujimoto, Y.; Ogura, Y.; Hata, K.; Ito, T.; Tomiyama, K.; Yagi, S.; Mori, A.; Uemoto, S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am. J. Transplant. 2013, 13, 1549–1556. [Google Scholar] [CrossRef]
- Englesbe, M.J.; Patel, S.P.; He, K.; Lynch, R.J.; Schaubel, D.E.; Harbaugh, C.; Holcombe, S.A.; Wang, S.C.; Segev, D.L.; Sonnenday, C.J. Sarcopenia and mortality after liver transplantation. J. Am. Coll. Surg. 2010, 211, 271–278. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Fujimoto, Y.; Ogawa, K.; Mori, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transplant. 2014, 20, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Takahashi, N.; Moynagh, M.R.; Narayanan, P.; Angirekula, M.; Mara, K.C.; Dierkhising, R.A.; Watt, K.D. The evolution and impact of sarcopenia pre- and post-liver transplantation. Aliment. Pharmacol. Ther. 2019, 49, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Richardson, B.; Bouquet, E.; Reid, E.; Mercer, E.; Goncalves, M.; Spann, A.; Annis, J.; Brittain, E.; Dreher, A.; et al. Cirrhosis-related sarcopenia may not resolve after liver transplantation. JHEP Rep. Innov. Hepatol. 2023, 5, 100881. [Google Scholar] [CrossRef]
- Carias, S.; Castellanos, A.L.; Vilchez, V.; Nair, R.; Cruz, A.C.D.; Watkins, J.; Barrett, T.; Trushar, P.; Esser, K.; Gedaly, R. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J. Gastroenterol. Hepatol. 2016, 31, 628–633. [Google Scholar] [CrossRef]
- Müller, M. Resting energy expenditure and nutritional state in patients with liver cirrhosis before and after liver transplantation. Clin. Nutr. 1994, 13, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S. Posttransplant Sarcopenia: An Underrecognized Early Consequence of Liver Transplantation. Dig. Dis. Sci. 2013, 58, 3103–3111. [Google Scholar] [CrossRef]
- Choo, Y.J.; Cho, C.W.; Chang, M.C. Effects of supervised exercise on aerobic capacity and quality of life in patients with chronic liver disease and patients who underwent liver transplantation: A systematic review and meta-analysis. Int. J. Rehabil. Res. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Garcia, A.; Veneroso, C.; Soares, D.; Lima, A.; Correia, M. Effect of a physical exercise program on the functional capacity of liver transplant patients. Transplant. Proc. 2014, 46, 1807–1808. [Google Scholar] [CrossRef]
- De Smet, S.; O’donoghue, K.B.; Lormans, M.; Monbaliu, D.; Pengel, L. Does Exercise Training Improve Physical Fitness and Health in Adult Liver Transplant Recipients? A Systematic Review and Meta-analysis. Transplantation 2023, 107, e11–e26. [Google Scholar] [CrossRef]
- Perito, E.R.; Bucuvalas, J.; Lai, J.C. Functional status at listing predicts waitlist and posttransplant mortality in pediatric liver transplant candidates. Am. J. Transplant. 2019, 19, 1388–1396. [Google Scholar] [CrossRef]
| Testing Modality | Cut-Off Points |
|---|---|
| Grip strength | Females: <16 kg Males: <27 kg |
| Chair stand | >15 (s) for five chair raises |
| Testing Parameter | Cut-Off Points |
|---|---|
| ASM | Females: <15 kg Males: <20 kg |
| ASM/height2 | Females: <5.5 kg/m2 Males: <7 kg/m2 |
| Testing Modality | Cut-Off Points |
|---|---|
| 4 m gait speed |
|
| 400 m walk |
|
| Short physical performance battery (SPPB) |
|
| Timed up and go test (TUG) |
|
| Sarcopenia Component | Screening Modality | Assessment Criteria |
|---|---|---|
| Muscle mass | CT/MRI | Cross-sectional imaging of mid-thigh or L3 vertebra |
| DEXA | Assessment of appendicular skeletal mass | |
| BIA | Electrical analysis of fat and lean body mass | |
| Ultrasonography | Cross-sectional area/muscle thickness | |
| Anthropometry | Measurement of calf/midarm circumference | |
| Muscle strength | Handgrip strength | Measurement of strength with dynamometer |
| Chair stand | Time required to stand from a seated position | |
| Physical performance | 4 m gait speed | Evaluation of speed |
| 6 min walk | Evaluation of aerobic capacity |
| Testing Modality | Advantages | Limitations in Advanced Liver Disease |
|---|---|---|
| MRI | Highly accurate, low radiation | |
| CT | Highly accurate | |
| DEXA | Fast, low radiation, inexpensive | Fluid retention leads to underestimation of sarcopenia |
| BIA | Fast, no radiation, reproducible | Results affected by fluid retention and hydration status |
| Ultrasound | Fast, reproducible, no radiation | |
| Anthropometry | Fast, broadly available, inexpensive | Results affected by fluid retention |
|
Non-obese (BMI < 30 kg/m2): 35 kcal/kg/day. BMI 30–40 kg/m2: 25–35 kcal/kg/day. BMI > 40 kg/m2: 20–25 kcal/kg/day. |
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Buckholz, A.; Kumar, S.; Newberry, C. Implications of Protein and Sarcopenia in the Prognosis, Treatment, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients 2024, 16, 658. https://doi.org/10.3390/nu16050658
Singh A, Buckholz A, Kumar S, Newberry C. Implications of Protein and Sarcopenia in the Prognosis, Treatment, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients. 2024; 16(5):658. https://doi.org/10.3390/nu16050658
Chicago/Turabian StyleSingh, Avneet, Adam Buckholz, Sonal Kumar, and Carolyn Newberry. 2024. "Implications of Protein and Sarcopenia in the Prognosis, Treatment, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)" Nutrients 16, no. 5: 658. https://doi.org/10.3390/nu16050658
APA StyleSingh, A., Buckholz, A., Kumar, S., & Newberry, C. (2024). Implications of Protein and Sarcopenia in the Prognosis, Treatment, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients, 16(5), 658. https://doi.org/10.3390/nu16050658





