Vitamin D Status in Belgian Children: A Regional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Subjects
2.2. Laboratory Assessment and Reference Values
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Cohort
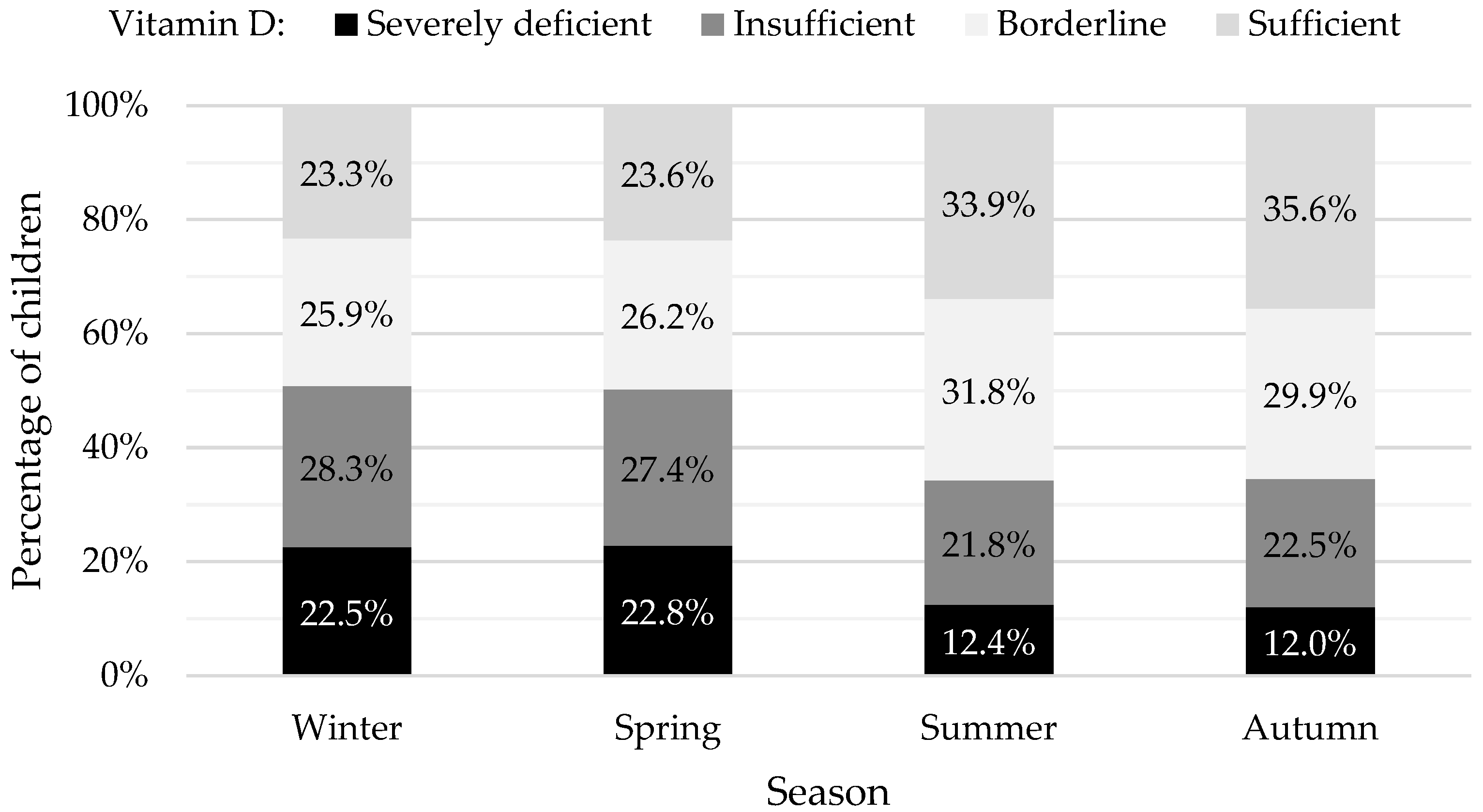
3.2. Vitamin D Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Antonucci, R.; Locci, C.; Clemente, M.G.; Chicconi, E.; Antonucci, L. Vitamin D deficiency in childhood: Old lessons and current challenges. J. Pediatr. Endocrinol. Metab. 2018, 31, 247–260. [Google Scholar] [CrossRef]
- Gröber, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Derm. Endocrinol. 2013, 5, 331–347. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef]
- Lawson, D.E.; Paul, A.A.; Black, A.E.; Cole, T.J.; Mandal, A.R.; Davie, M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br. Med. J. 1979, 2, 303–305. [Google Scholar] [CrossRef]
- Vissing Landgrebe, A.; Asp Vonsild Lund, M.; Lausten-Thomsen, U.; Frithioff-Bøjsøe, C.; Esmann Fonvig, C.; Lind Plesner, J.; Aas Holm, L.; Jespersen, T.; Hansen, T.; Christian Holm, J. Population-based pediatric reference values for serum parathyroid hormone, vitamin D, calcium, and phosphate in Danish/North-European white children and adolescents. Clin. Chim. Acta 2021, 523, 483–490. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Wharton, B.; Bishop, N. Rickets. Lancet 2003, 362, 1389–1400. [Google Scholar] [CrossRef]
- Shroff, R.; Wan, M.; Nagler, E.V.; Bakkaloglu, S.; Fischer, D.C.; Bishop, N.; Cozzolino, M.; Bacchetta, J.; Edefonti, A.; Stefanidis, C.J.; et al. Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol. Dial. Transpl. 2017, 32, 1098–1113. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Preziosi, P.; Maamer, M.; Arnaud, S.; Galan, P.; Hercberg, S.; Meunier, P.J. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997, 7, 439–443. [Google Scholar] [CrossRef]
- Gentile, C.; Chiarelli, F. Rickets in Children: An Update. Biomedicines 2021, 9, 738. [Google Scholar] [CrossRef]
- Ozkan, B. Nutritional rickets. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 137–143. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Ladang, A.; Rousselle, O.; Huyghebaert, L.; Bekaert, A.C.; Kovacs, S.; Le Goff, C.; Cavalier, E. Parathormone, bone alkaline phosphatase and 25-hydroxyvitamin D status in a large cohort of 1200 children and teenagers. Acta Clin. Belg. 2022, 77, 4–9. [Google Scholar] [CrossRef]
- De Ronne, N.; De Schepper, J. Vitamine D suppletie bij de zuigeling en het jonge kind: Aanbevelingen Kind en Gezin en Voedingscel Vlaamse Vereniging voor kindergeneeskunde. Farm. Tijdschr. Voor Belg. 2013, 91, 32–43. [Google Scholar]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Thamm, M.; Rieckmann, N.; Durazo-Arvizu, R.A.; Dowling, K.G.; Škrabáková, Z.; Cashman, K.D.; Sempos, C.T.; et al. Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Public Health 2018, 18, 845. [Google Scholar] [CrossRef]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Erba, P.; Saggese, G. Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Ital. J. Pediatr. 2014, 40, 54. [Google Scholar] [CrossRef]
- Huybrechts, I.; Lin, Y.; De Keyzer, W.; Sioen, I.; Mouratidou, T.; Moreno, L.A.; Slimani, N.; Jenab, M.; Vandevijvere, S.; De Backer, G.; et al. Dietary sources and sociodemographic and economic factors affecting vitamin D and calcium intakes in Flemish preschoolers. Eur. J. Clin. Nutr. 2011, 65, 1039–1047. [Google Scholar] [CrossRef]
- Webb, A.R.; Kift, R.; Durkin, M.T.; O’Brien, S.J.; Vail, A.; Berry, J.L.; Rhodes, L.E. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br. J. Dermatol. 2010, 163, 1050–1055. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]
- Sioen, I.; Mouratidou, T.; Kaufman, J.M.; Bammann, K.; Michels, N.; Pigeot, I.; Vanaelst, B.; Vyncke, K.; De Henauw, S. Determinants of vitamin D status in young children: Results from the Belgian arm of the IDEFICS (Identification and Prevention of Dietary- and Lifestyle-Induced Health Effects in Children and Infants) Study. Public Health Nutr. 2012, 15, 1093–1099. [Google Scholar] [CrossRef][Green Version]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Gori, M.; Carlone, G.; Erba, P.; Massimetti, G.; Federico, G.; Saggese, G. Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: A cross-sectional study. Eur. J. Pediatr. 2013, 172, 1607–1617. [Google Scholar] [CrossRef]
- Oskarsson, V.; Eliasson, M.; Salomaa, V.; Reinikainen, J.; Männistö, S.; Palmieri, L.; Donfrancesco, C.; Sans, S.; Costanzo, S.; de Gaetano, G.; et al. Influence of geographical latitude on vitamin D status: Cross-sectional results from the BiomarCaRE consortium. Br. J. Nutr. 2022, 128, 2208–2218. [Google Scholar] [CrossRef]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.; Diepgen, T.L.; Green, A.C.; van der Pols, J.C.; Bernard, B.A.; Ly, F.; Bernerd, F.; et al. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019, 181, 916–931. [Google Scholar] [CrossRef]
- Hoge, A.; Donneau, A.F.; Streel, S.; Kolh, P.; Chapelle, J.P.; Albert, A.; Cavalier, E.; Guillaume, M. Vitamin D deficiency is common among adults in Wallonia (Belgium, 51°30′ North): Findings from the Nutrition, Environment and Cardio-Vascular Health study. Nutr. Res. 2015, 35, 716–725. [Google Scholar] [CrossRef]
- Raaijmakers, A.; Van Winckel, M.; Plaete, J.; Bovijn, L.; Van Overmeire, B.; Vandenplas, Y.; Stevens, G. Vitamine D voor kinderen in Vlaanderen. Tijdschr. Voor Geneeskd. En. Gezondheidszorg 2023, 5, 10–47671. [Google Scholar] [CrossRef]
- Bass, J.K.; Chan, G.M. Calcium nutrition and metabolism during infancy. Nutrition 2006, 22, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A. Vitamin D in Preterm and Full-Term Infants. Ann. Nutr. Metab. 2020, 76 (Suppl. S2), 6–14. [Google Scholar] [CrossRef] [PubMed]
- Moyersoen, I.; Lachat, C.; Cuypers, K.; Ridder, K.; Devleesschauwer, B.; Tafforeau, J.; Vandevijvere, S.; Vansteenland, M.; De Meulenaer, B.; Van Camp, J.; et al. Do Current Fortification and Supplementation Programs Assure Adequate Intake of Fat-Soluble Vitamins in Belgian Infants, Toddlers, Pregnant Women, and Lactating Women? Nutrients 2018, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Moyersoen, I.; Devleesschauwer, B.; Dekkers, A.; Verkaik-Kloosterman, J.; De Ridder, K.; Vandevijvere, S.; Tafforeau, J.; Van Oyen, H.; Lachat, C.; Van Camp, J. A Novel Approach to Optimize Vitamin D Intake in Belgium through Fortification Based on Representative Food Consumption Data. J. Nutr. 2019, 149, 1852–1862. [Google Scholar] [CrossRef] [PubMed]

| Total (n) | Female (n) | Proportion (%) | Male (n) | Proportion (%) | ||
|---|---|---|---|---|---|---|
| Age in years | <1 | 2354 (15.8%) | 1079 | 45.8% | 1275 | 54.2% |
| 1–6 | 5070 (34.1%) | 2348 | 46.3% | 2722 | 53.7% | |
| 7–12 | 4121 (27.7%) | 2141 | 52.0% | 1980 | 48.0% | |
| 13–18 | 3342 (22.4%) | 2016 | 60.3% | 1326 | 39.7% | |
| Total | 14,887 (100.0%) | 7584 | 50.9% | 7303 | 49.1% |
| Age | |||||||
|---|---|---|---|---|---|---|---|
| Years | Total | ||||||
| <1 | 1–6 | 7–12 | 13–18 | ||||
| Vitamin D status | Severely deficient <12 ng/mL | Count (n) | 526 | 423 | 853 | 833 | 2635 |
| Percentage | 22.3% | 8.3% | 20.7% | 24.9% | 17.7% | ||
| Insufficient 12–20 ng/mL | Count (n) | 547 | 875 | 1343 | 981 | 3746 | |
| Percentage | 23.2% | 17.3% | 32.6% | 29.4% | 25.2% | ||
| Borderline 20–30 ng/mL | Count (n) | 521 | 1483 | 1250 | 961 | 4215 | |
| Percentage | 22.1% | 29.3% | 30.3% | 28.8% | 28.3% | ||
| Sufficient >30 ng/mL | Count (n) | 760 | 2289 | 675 | 567 | 4291 | |
| Percentage | 32.3% | 45.1% | 16.4% | 17.0% | 28.8% | ||
| Total | Count (n) | 2354 | 5070 | 4121 | 3342 | 14,887 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van de Walle, L.; Vandenplas, Y.; Toelen, J.; Raaijmakers, A. Vitamin D Status in Belgian Children: A Regional Study. Nutrients 2024, 16, 657. https://doi.org/10.3390/nu16050657
Van de Walle L, Vandenplas Y, Toelen J, Raaijmakers A. Vitamin D Status in Belgian Children: A Regional Study. Nutrients. 2024; 16(5):657. https://doi.org/10.3390/nu16050657
Chicago/Turabian StyleVan de Walle, Louise, Yvan Vandenplas, Jaan Toelen, and Anke Raaijmakers. 2024. "Vitamin D Status in Belgian Children: A Regional Study" Nutrients 16, no. 5: 657. https://doi.org/10.3390/nu16050657
APA StyleVan de Walle, L., Vandenplas, Y., Toelen, J., & Raaijmakers, A. (2024). Vitamin D Status in Belgian Children: A Regional Study. Nutrients, 16(5), 657. https://doi.org/10.3390/nu16050657








