Food to Prevent Vascular Calcification in Chronic Kidney Disease
Abstract
1. Introduction
2. Vascular Calcification in CKD
- Vascular calcification (VC) is a phenomenon involving the deposition of calcium and phosphorus within the layers of the arteries. Medial calcification, which presents as rail-train deposits along the vasculature, is particularly prevalent in patients suffering from CKD, but it is associated with aging and diabetes mellitus, too. It mainly affects the aorta and peripheral arteries. The deposition of mineral content within the media is preceded by phenotypic changes in vascular smooth muscle cells (VSMCs) and leads to arterial stiffness, significantly contributing to heart failure and increased cardiovascular morbidity. The accumulation of uremic toxins, the imbalance of calcium and phosphate, and a lack of calcification inhibitors have been implicated in the pathogenesis of calcification.
- Intimal calcification displays a patchy distribution pattern and preferentially affects the coronary and carotid arteries. It is part of the atherosclerosis process. In patients with dyslipidemia and hypertension and smokers, atherosclerotic plaques occur as a consequence of inflammation and endothelial damage. It is common to find both types of calcifications in CKD patients. Accumulation of mineral content in atherosclerotic plaques may increase the risk of ischemic events such as stroke, ischemic coronary syndromes, or ischemic arteriopathy of the lower limbs [18,19].
- Other ectopic extraskeletal calcifications may occur. Valvular calcification is highly prevalent in CKD patients, contributing to chronic heart failure; calcifications in the joints can cause pain and functional impotence, and calcifications in the subcutaneous tissue can lead to resistant pruritus.
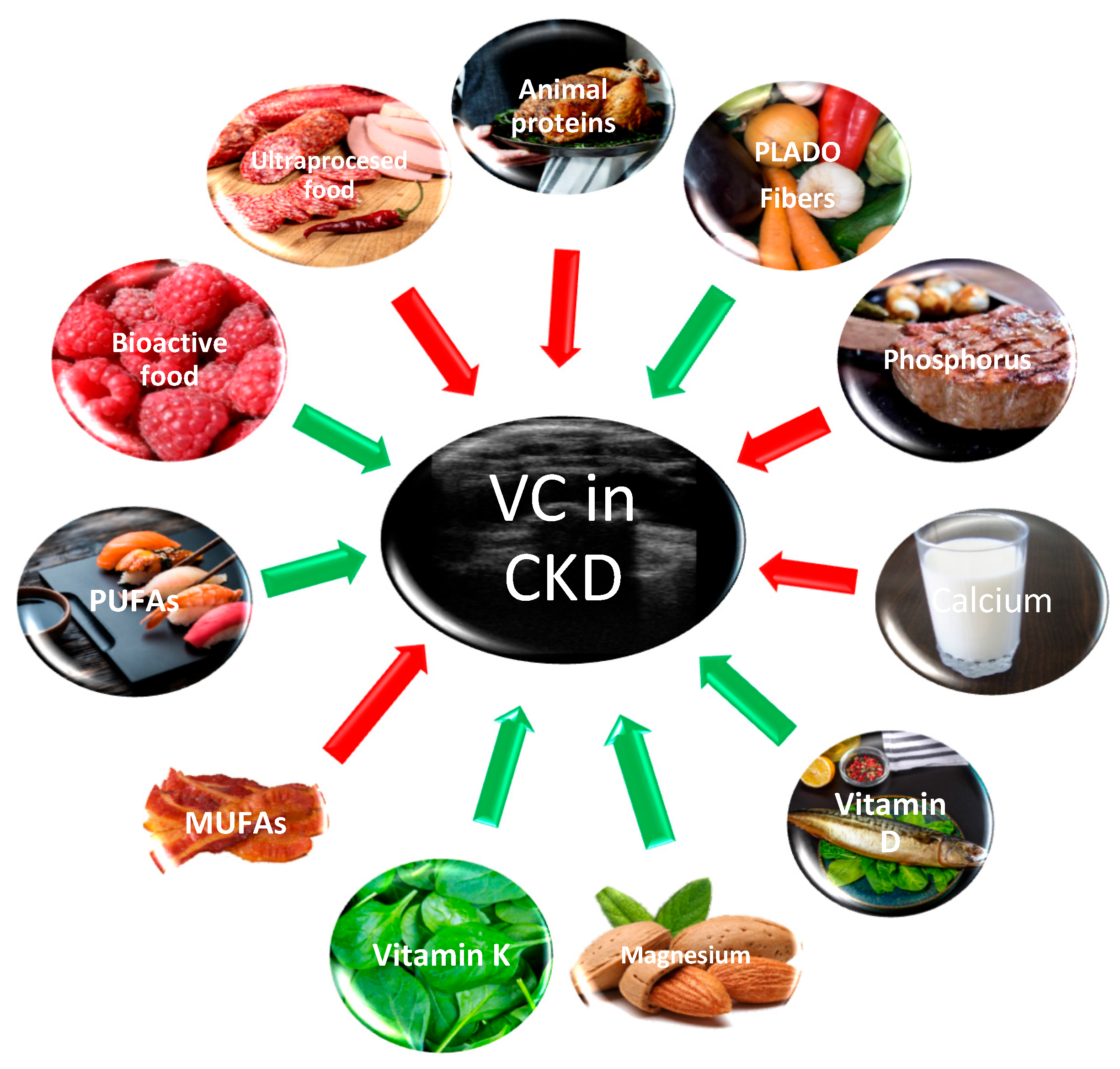
3. Food for CKD Patients
4. Phosphorus, Vitamin D, and Calcium and Vascular Calcification in CKD
5. Magnesium and Vascular Calcification in CKD
6. Vitamin K and Vascular Calcification in CKD
7. Lipids and Vascular Calcification in CKD
8. Uremic Toxins, Microbiota, Fibers, and Vascular Calcification in CKD
9. Protein Intake and Vascular Calcification in CKD
10. Bioactive and Senolytic Food and Vascular Calcification in CKD
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.-U.; Kasiske, B.L. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009, 76, S1–S130. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO). CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andía, J.B.; Martín-Carro, B.; Martín-Vírgala, J.; Rodríguez-Carrio, J.; Bande-Fernández, J.J.; Alonso-Montes, C.; Carrillo-López, N. Chronic kidney disease—Mineral and bone disorders: Pathogenesis and management. Calcif. Tissue Int. 2021, 108, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Owolabi, M.O. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, D.; Rusu, C.; Potra, A.; Bondor, C.; Ticala, M.; Tirinescu, D.; Coman, A.; Orasan, O.; Moldovan, I.; Orasan, R.; et al. Arterial calcifications and osteoprotegerin in chronic hemodialysis patients: Impact on 6-year survival. Int. Urol. Nephrol. 2022, 54, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern. Med. 2019, 180, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Song, Q.; Wang, X. Reversion from pre–diabetes mellitus to normoglycemia and risk of cardiovascular disease and all-cause mortality in a chinese population: A prospective cohort study. J. Am. Heart Assoc. 2021, 10, e019045. [Google Scholar] [CrossRef]
- Kawarazaki, W.; Fujita, T. Kidney and epigenetic mechanisms of salt-sensitive hypertension. Nat. Rev. Nephrol. 2021, 17, 350–363. [Google Scholar] [CrossRef]
- Wen, L.; Yang, H.L.; Lin, L.; Ma, L.; Fu, P. The Emerging role of epigenetic methylation in kidney disease. Curr. Med. Chem. 2022, 29, 3732–3747. [Google Scholar] [CrossRef]
- Chou, Y.H.; Chen, Y.M. Aging and renal disease: Old questions for new challenges. Aging Dis. 2021, 12, 515–528. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Mattix-Kramer, H.J.; Moore, L.W. Culinary medicine as a core component of the medical nutrition therapy for kidney health and disease. J. Ren. Nutr. 2021, 31, 1–4. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef]
- Alvarenga, L.; Reis, D.C.; Kemp, J.A.; Teixeira, K.T.R.; Fouque, D.; Mafra, D. Using the concept of food as medicine to mitigate inflammation in patients undergoing peritoneal dialysis. In Therapeutic Apheresis and Dialysis; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Hu, E.A.; Steffen, L.M.; Grams, M.E.; Crews, D.C.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Dietary patterns and risk of incident chronic kidney disease: The atherosclerosis risk in communities study. Am. J. Clin. Nutr. 2019, 110, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, X.; Ghosh, S.; Xie, C.; Chen, J.; Huang, H. Role of gut microbiota-derived metabolites on vascular calcification in CKD. J. Cell. Mol. Med. 2021, 25, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Ionescu, R.F.; Enache, R.M.; Cretoiu, S.M.; Voinea, S.C. Gut microbiome, functional food, atherosclerosis, and vascular calcifications—Is there a missing link? Microorganisms 2021, 9, 1913. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and oxidative stress in chronic kidney disease and dialysis patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef]
- Hutcheson, J.D. Cardiovascular calcification heterogeneity in chronic kidney disease. Circ. Res. 2023, 132, 993–1012. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.D.S.; Roderick, P.J. Kidney disease in the Global Burden of Disease Study 2017. Nat. Rev. Nephrol. 2019, 15, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, J.; Yu, D.; Liu, M. Plant or animal-based or PLADO diets: Which should chronic kidney disease patients choose? J. Ren. Nutr. 2023, 33, 228–235. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Murray, C.J. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Duan, M.J.; Dekker, L.H.; Carrero, J.J.; Avesani, C.M.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J. Ultraprocessed food consumption and kidney function decline in a population-based cohort in the Netherlands. Am. J. Clin. Nutr. 2022, 116, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Moreira, A.S.B.; Canella, D.S.; Rodrigues, J.; Santin, F.; Wanderley, B.; Lourenço, R.A.; Avesani, C.M. Elderly patients on hemodialysis have worse dietary quality and higher consumption of ultraprocessed food than elderly without chronic kidney disease. Nutrition 2017, 41, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Tyson, C.C.; Lin, P.-H.; Corsino, L.; Batch, B.C.; Allen, J.; Sapp, S.; Barnhart, H.; Nwankwo, C.; Burroughs, J.; Svetkey, L.P. Short-term effects of the DASH diet in adults with moderate chronic kidney disease: A pilot feeding study. Clin. Kidney J. 2016, 9, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Chatziioannou, A.; Cunliffe, V.T.; Flanagan, J.M.; Hanson, M.; Kyrtopoulos, S.; Kirsch-Volders, M. Epigenetic memory in response to environmental stressors. FASEB J. 2017, 31, 2241. [Google Scholar] [CrossRef]
- Neytchev, O.; Witasp, A.; Nordfors, L.; Qureshi, A.R.T.; Wennberg, L.; Erlandsson, H.; Stenvinkel, P. FC 123 Renal transplantation mitigates increased biological (epigenetic) age in chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36 (Suppl. S1), gfab147-002. [Google Scholar] [CrossRef]
- Witasp, A.; Luttropp, K.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Wennberg, L.; Ekström, T.J.; Shiels, P.G.; Stenvinkel, P.; Nordfors, L. Longitudinal genome-wide DNA methylation changes in response to kidney failure replacement therapy. Sci. Rep. 2022, 12, 470. [Google Scholar] [CrossRef]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutierrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, D.; Rusu, C.; Potra, A.; Moldovan, I.; Patiu, I.M.; Gherman-Caprioara, M.; Kacso, I.M. Osteoprotegerin and uremic osteoporosis in chronic hemodialysis patients. Int. Urol. Nephrol. 2017, 49, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.E.; Rowsell, T.S.; Lansing, A.P.; Jeronimo, P.S.; Lee, L.H.; Svajger, B.A.; Adams, M.A. Vascular calcification maladaptively participates in acute phosphate homeostasis. Cardiovasc. Res. 2023, 119, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Lioufas, N.M.; Pascoe, E.M.; Hawley, C.M.; Elder, G.J.; Badve, S.V.; Block, G.A.; Johnson, D.W.; Toussaint, N.D. Systematic review and meta-analyses of the effects of phosphate-lowering agents in nondialysis CKD. J. Am. Soc. Nephrol. 2022, 33, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, N.D.; Pedagogos, E.; Lioufas, N.M.; Elder, G.J.; Pascoe, E.M.; Badve, S.V.; IMPROVE-CKD Trial Investigators. A randomized trial on the effect of phosphate reduction on vascular end points in CKD (IMPROVE-CKD). J. Am. Soc. Nephrol. 2020, 31, 2653–2666. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Fujii, H.; Kono, K.; Goto, S.; Watanabe, K.; Sakamoto, K.; Nishi, S. Clinical implication of consistently strict phosphate control for coronary and valvular calcification in incident patients undergoing hemodialysis. J. Atheroscler. Thromb. 2023, 30, 64159. [Google Scholar] [CrossRef]
- Machado, A.D.; Gómez, L.M.; Marchioni, D.M.L.; Dos Anjos, F.S.N.; Molina, M.D.C.B.; Lotufo, P.A.; Benseñor, I.J.M.; Titan, S.M.O. Association between dietary intake and coronary artery calcification in non-dialysis chronic kidney disease: The PROGREDIR Study. Nutrients 2018, 10, 372. [Google Scholar] [CrossRef]
- Ter Braake, A.D.; Smit, A.E.; Bos, C.; van Herwaarden, A.E.; Alkema, W.; van Essen, H.W.; Bravenboer, N.; Vervloet, M.G.; Hoenderop, J.G.J.; de Baaij, J.H.F. Magnesium prevents vascular calcification in Klotho deficiency. Kidney Int. 2020, 97, 487–501. [Google Scholar] [CrossRef]
- Talari, H.R.; Zakizade, M.; Soleimani, A.; Bahmani, F.; Ghaderi, A.; Mirhosseini, N.; Eslahi, M.; Babadi, M.; Mansournia, M.A.; Asemi, Z. Effects of magnesium supplementation on carotid intima-media thickness and metabolic profiles in diabetic haemodialysis patients: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2019, 121, 809–817. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Obi, Y.; Monden, C.; Oka, T.; Yamaguchi, S.; Matsui, I.; Hashimoto, N.; Matsumoto, A.; Shimada, K.; et al. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J. Am. Soc. Nephrol. 2019, 30, 1073–1085. [Google Scholar] [CrossRef]
- Bressendorff, I.; Hansen, D.; Schou, M.; Kragelund, C.; Svensson, M.; Hashemi, B.; Brandi, L. The effect of magnesium supplementation on vascular calcification in CKD: A randomized clinical trial (MAGiCAL-CKD). J. Am. Soc. Nephrol. 2023, 34, 886–894. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Y.; Li, H.; Wang, Y.; Niu, Z.; Zhou, W.; Wang, D. Associations of whole blood zinc levels with coronary artery calcification and future cardiovascular events in CKD patients. Biol. Trace Elem. Res. 2024, 202, 46–55. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.M.; Booth, S.L.; Fu, X.; Shobeiri, N.; Pang, J.J.; Adams, M.A.; Holden, R.M. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013, 83, 835–844. [Google Scholar] [CrossRef] [PubMed]
- El Shinnawy, H.; Elsaid, T.; Farid, S.; Shamseldin, A.; Ibrahim, S.; Elsharabasy, R. The effect of oral vitamin K2 versus K1 on vascular calcification in hemodialysis patients: A randomized controlled trial. Nephrol. Dial. Transplant. 2022, 37 (Suppl. S3), gfac080.026. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Xu, D. Inhibition of maintenance hemodialysis related vascular calcification by vitamin K in chronic kidney disease. Int. J. Clin. Exp. Med. 2017, 10, 15309–15315. [Google Scholar]
- Haroon, S.; Davenport, A.; Ling, L.H.; Tai, B.C.; Schurgers, L.; Chen, Z.; Wong, W.K. Randomized controlled clinical trial of the effect of treatment with vitamin K2 on vascular calcification in hemodialysis patients (Trevasc-HDK). Kidney Int. Rep. 2023, 8, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Levy-Schousboe, K.; Frimodt-Møller, M.; Hansen, D.; Peters, C.D.; Kjærgaard, K.D.; Jensen, J.D.; Strandhave, C.; Elming, H.; Larsen, C.T.; Sandstrøm, H.; et al. Vitamin K supplementation and arterial calcification in dialysis: Results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin. Kidney J. 2021, 14, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.M.; Booth, S.L.; Zimmerman, D.; Moist, L.; Norman, P.; Day, A.G.; Heyland, D.K. Inhibit progression of coronary artery calcification with vitamin k in hemodialysis patients (the iPACK-HD study): A randomized, placebo-controlled multi-centre, pilot trial. Nephrol. Dial. Transplant. 2022, 38, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Kanai, S.; Uto, K.; Honda, K.; Hagiwara, N.; Oda, H. Eicosapentaenoic acid reduces warfarin-induced arterial calcification in rats. Atherosclerosis 2011, 215, 43–51. [Google Scholar] [CrossRef]
- Nakamura, K.; Miura, D.; Saito, Y.; Yunoki, K.; Koyama, Y.; Satoh, M.; Ito, H. Eicosapentaenoic acid prevents arterial calcification in klotho mutant mice. PLoS ONE 2017, 12, e0181009. [Google Scholar] [CrossRef]
- Son, Y.K.; Lee, S.M.; Kim, S.E.; Kim, K.H.; Lee, S.Y.; Bae, H.R.; Han, J.Y.; Park, Y.; An, W.S. Association between vascular calcification scores on plain radiographs and fatty acid contents of erythrocyte membrane in hemodialysis patients. J. Ren. Nutr. 2012, 22, 58–66. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, A.; Li, L.; Ye, Y.; Liang, Q.; Dong, Q.; Yan, J. Downregulation of HDAC9 by the ketone metabolite β-hydroxybutyrate suppresses vascular calcification. J. Pathol. 2022, 258, 213–226. [Google Scholar] [CrossRef]
- Merino-Ribas, A.; Araujo, R.; Pereira, L.; Campos, J.; Barreiros, L.; Segundo, M.A.; Silva, N.; Costa, C.F.F.A.; Quelhas-Santos, J.; Trindade, F.; et al. Vascular calcification and the gut and blood microbiome in chronic kidney disease patients on peritoneal dialysis: A pilot study. Biomolecules 2022, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Z.; Fan, Y.; Feng, L.; Zhong, X.; Li, W.; Guo, T.; Ning, X.; Li, Z.; Ou, C. Lactobacillus rhamnosus GG aggravates vascular calcification in chronic kidney disease: A potential role for extracellular vesicles. Life Sci. 2023, 331, 122001. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, P.; Buades, J.M.; Berga, F.; Gelabert, M.M.; Molina, M.; Íñigo, M.V.; Grases, F. Protective effect of myo-inositol hexaphosphate (phytate) on abdominal aortic calcification in patients with chronic kidney disease. J. Ren. Nutr. 2016, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Bellasi, A.; Bushinsky, D.; Bover, J.; Rodriguez, M.; Ketteler, M.; Chertow, G.M. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: Results of a randomized phase 2b study. Circulation 2020, 141, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, G.; Tang, N.; Lu, P.; Jiang, L.; Lv, J.; Lei, D. Identification of dietary components in association with abdominal aortic calcification. Food Funct. 2023, 14, 8383–8395. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Du, Y.; Li, G.; Wang, L.; Zhou, F. Resveratrol ameliorated vascular calcification by regulating Sirt-1 and Nrf2. Transplant. Proc. 2016, 48, 3378–3386. [Google Scholar] [CrossRef]
- Chang, X.Y.; Cui, L.; Wang, X.Z.; Zhang, L.; Zhu, D.; Zhou, X.R.; Hao, L.R. Quercetin attenuates vascular calcification through suppressed oxidative stress in adenine-induced chronic renal failure rats. BioMed Res. Int. 2017, 2017, 5716204. [Google Scholar] [CrossRef]
- Calvo, M.S.; Dunford, E.K.; Uribarri, J. Industrial use of phosphate food additives: A mechanism linking ultra-processed food intake to cardiorenal disease risk? Nutrients 2023, 15, 3510. [Google Scholar] [CrossRef]
- Su, G.; Saglimbene, V.; Wong, G.; Bernier-Jean, A.; Carrero, J.J.; Natale, P.; Strippoli, G.F. Dietary phosphorus, its sources, and mortality in adults on haemodialysis: The DIET-HD Study. Nutrients 2022, 14, 4064. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.I.; Oh, J.; Razzaque, M.S. Common Dietary Sources of Natural and Artificial Phosphate in Food. In Phosphate Metabolism; Springer: Cham, Switzerland, 2022; pp. 99–105. [Google Scholar]
- Byrne, F.N.; Gillman, B.A.; Kiely, M.; Palmer, B.; Shiely, F.; Kearney, P.M.; Earlie, J.; Bowles, M.B.; Keohane, F.M.; Connolly, P.P.; et al. Pilot randomized controlled trial of a standard versus a modified low-phosphorus diet in hemodialysis patients. Kidney Int. Rep. 2020, 5, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, R.N.; Armstrong, C.L.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2014, 40, 582. [Google Scholar] [CrossRef]
- Henley, C.; Colloton, M.; Cattley, R.C.; Shatzen, E.; Towler, D.A.; Lacey, D.; Martin, D. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2005, 20, 1370–1377. [Google Scholar] [CrossRef]
- Lee, S.M.; An, W.S. Supplementary nutrients for prevention of vascular calcification in patients with chronic kidney disease. Korean J. Intern. Med. 2019, 34, 459–469. [Google Scholar] [CrossRef]
- Ter Braake, A.D.; Shanahan, C.M.; de Baaij, J.H.F. Magnesium counteracts vascular calcification: Passive interference or active modulation? Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Smith, E.R.; Tiong, M.K.; Ruderman, I.; Toussaint, N.D. Interventions to attenuate vascular calcification progression in chronic kidney disease: A systematic review of clinical trials. J. Am. Soc. Nephrol. 2022, 33, 1011–1032. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Fusaro, M.; Ciceri, P.; Gasperoni, L.; Cianciolo, G. The role of vitamin K in vascular calcification. Adv. Chronic Kidney Dis. 2019, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Deshmukh, P.; Bhatt, A.; Sinha, A.H.; Chawla, P. Vitamin E Supplementation and Cardiovascular Health: A Comprehensive Review. Cureus 2023, 15, e48142. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega−3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Sun, W.; Byon, C.H.; Kim, D.H.; Choi, H.I.; Park, J.S.; Joo, S.Y.; Kim, S.W. Renoprotective effects of maslinic acid on experimental renal fibrosis in unilateral ureteral obstruction model via targeting MyD88. Front. Pharmacol. 2021, 12, 708575. [Google Scholar] [CrossRef]
- Panahi, Y.; Dashti-Khavidaki, S.; Farnood, F.; Noshad, H.; Lotfi, M.; Gharekhani, A. Therapeutic effects of omega-3 fatty acids on chronic kidney disease-associated pruritus: A literature review. Adv. Pharm. Bull. 2016, 6, 509–514. [Google Scholar] [CrossRef]
- Kitagawa, M.; Sugiyama, H.; Morinaga, H.; Inoue, T.; Takiue, K.; Ogawa, A.; Makino, H. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE 2013, 8, e56695. [Google Scholar] [CrossRef]
- Garg, P.K.; Guan, W.; Nomura, S.; Weir, N.L.; Karger, A.B.; Duprez, D.; Tsai, M.Y. Associations of plasma omega-3 and omega-6 PUFA levels with arterial elasticity: The multi-ethnic study of atherosclerosis. Eur. J. Clin. Nutr. 2022, 76, 1770–1775. [Google Scholar] [CrossRef]
- O’Neill, B.; Raggi, P. The ketogenic diet pros and cons. Atherosclerosis 2019, 292, 119–126. [Google Scholar] [CrossRef]
- Carriazo, S.; Perez-Gomez, M.V.; Cordido, A.; García-González, M.A.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Dietary care for ADPKD patients: Current status and future directions. Nutrients 2019, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.A.; Kruger, S.L.; Broderick, C.; Amarlkhagva, T.; Agrawal, S.; Dodam, J.R.; Mrug, M.; Lyons, L.A.; Weimbs, T. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab. 2019, 30, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Ramprasath, T.; Zou, M.H. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp. Mol. Med. 2020, 52, 548–555. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Matsson, M.P. Effects of intermittent fasting on health, aging and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Dube, P.; DeRiso, A.; Patel, M.; Battepati, D.; Khatib-Shahidi, B.; Sharma, H.; Gupta, R.; Malhotra, D.; Dworkin, L.; Haller, S.; et al. Vascular calcification in chronic kidney disease: Diversity in the vessel wall. Biomedicines 2021, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Ravid, J.D.; Kamel, M.H.; Chitalia, V.C. Uraemic solutes as therapeutic targets in CKD-associated cardiovascular disease. Nat. Rev. Nephrol. 2021, 17, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.G.; Ormanji, M.S.; Heilberg, I.P.; Bakker, S.J.L.; de Borst, M.H. Interplay between gut microbiota, bone health and vascular calcification in chronic kidney disease. Eur. J. Clin. Investig. 2021, 51, e13588. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Stenvinkel, P.; Shanahan, C.; Pacifici, R. Inflammation and gut dysbiosis as drivers of CKD–MBD. Nat. Rev. Nephrol. 2023, 19, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.N.; Ni, S.H.; Li, Y.; Liu, X.; Deng, J.P.; Ouyang, X.L.; Kuang, X.Y. Association between dietary inflammatory index with all-cause and cardiovascular disease mortality among older US adults: A longitudinal cohort study among a nationally representative sample. Arch. Gerontol. Geriatr. 2024, 118, 105279. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.; Powe, N.R.; Meyer, T.W.; Hwang, S.; Hai, X.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Hostetter, T.H. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J. Am. Soc. Nephrol. 2017, 28, 321–331. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Lee, H.S.; Park, G.E.; Lee, J.W. Association between dietary fiber intake and all-cause and cardiovascular mortality in middle aged and elderly adults with chronic kidney disease. Front. Nutr. 2022, 9, 863391. [Google Scholar] [CrossRef]
- Wang, A.Y.-M.; Sea, M.M.-M.; Ng, K.; Wang, M.; Chan, I.H.-S.; Lam, C.W.-K.; Sanderson, J.E.; Woo, J. Dietary fiber intake, myocardial injury, and major adverse cardiovascular events among end-stage kidney disease patients: A prospective cohort study. Kidney Int. Rep. 2019, 4, 814–823. [Google Scholar] [CrossRef]
- Wang, K.; Qian, D.; Hu, Y.; Cheng, Y.; Ge, S.; Yao, Y. Nut consumption and effects on chronic kidney disease and mortality in the United States. Am. J. Nephrol. 2022, 53, 503–512. [Google Scholar] [CrossRef]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef]
- Filipska, I.; Winiarska, A.; Knysak, M.; Stompór, T. Contribution of gut microbiota-derived uremic toxins to the cardiovascular system mineralization. Toxins 2021, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Black, A.P.; Anjos, J.S.; Cardozo, L.; Carmo, F.L.; Dolenga, C.J.; Nakao, L.S.; de Carvalho Ferreira, D.; Rosado, A.; Eduardo, J.C.C.; Mafra, D.; et al. Does low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J. Ren. Nutr. 2018, 28, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kandouz, S.; Shendi, A.M.; Zheng, Y.; Sandeman, S.R.; Davenport, A. Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodial. Int. 2016, 20, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. Vegetarian diet in chronic kidney disease—A friend or foe. Nutrients 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-dominant low-protein diet for conservative management of chronic kidney disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Mafra, D.; Moraes, C.; Lobo, J.; Boaventura, G.T.; Farage, N.E.; Silva, W.S.; Cozzolino, S.F.; Malm, O. Brazil nut (Bertholletia excelsa, H.B.K.) improves oxidative stress and inflammation biomarkers in hemodialysis patients. Biol. Trace Elem. Res. 2014, 158, 105–112. [Google Scholar] [CrossRef]
- Narasaki, Y.; Rhee, C.M.; Kalantar-Zadeh, K. Going nuts to protect kidneys and to live longer with kidney disease. Am. J. Nephrol. 2022, 53, 423–426. [Google Scholar] [CrossRef]
- Saglimbene, V.M.; Wong, G.; Craig, J.C.; Ruospo, M.; Palmer, S.C.; Campbell, K.; Garcia-Larsen, V.; Natale, P.; Teixeira-Pinto, A.; Carrero, J.-J.; et al. The association of Mediterranean and DASH diets with mortality in adults on hemodialysis: The DIET-HD multinational cohort study. J. Am. Soc. Nephrol. 2018, 29, 1741–1751. [Google Scholar] [CrossRef]
- Su, G.; Saglimbene, V.; Wong, G.; Natale, P.; Ruospo, M.; Craig, J.C.; Strippoli, G.F. Healthy lifestyle and mortality among adults receiving hemodialysis: The DIET-HD study. Am. J. Kidney Dis. 2022, 79, 688–698. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Anderson, C.A.; Yang, W.; Zhang, X.; Fischer, M.J.; Dember, L.M.; Fink, J.C.; Frydrych, A.; Jensvold, N.G.; Lustigova, E.; et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2015, 65, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Mehta, O. Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Headley, S.A.; Germain, M.J. Impact of dietary potassium restrictions in CKD on clinical outcomes: Benefits of a plant-based diet. Kidney Med. 2020, 2, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Kaimori, J.Y.; Isaka, Y. Plant-dominant low protein diet: A potential alternative dietary practice for patients with chronic kidney disease. Nutrients 2023, 15, 1002. [Google Scholar] [CrossRef] [PubMed]
- Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-based diets for chronic kidney disease? It is time to reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Narasaki, Y.; Rhee, C.M. Dietary Therapy for Managing Hyperphosphatemia. Clin. J. Am. Soc. Nephrol. 2020, 16, 9–11. [Google Scholar] [CrossRef]
- Narasaki, Y.; Okuda, Y.; Kalantar, S.S.; You, A.S.; Novoa, A.; Nguyen, T.; Streja, E.; Nakata, T.; Colman, S.; Kalantar-Zadeh, K.; et al. Dietary potassium intake and mortality in a prospective hemodialysis cohort. J. Ren. Nutr. 2021, 31, 411–420. [Google Scholar] [CrossRef]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-based diets for kidney disease: A guide for clinicians. Am. J. Kidney Dis. 2021, 77, 287–296. [Google Scholar] [CrossRef]
- Tchkonia, T.; Kirkland, J.L. Aging, cell senescence, and chronic disease: Emerging therapeutic strategies. JAMA 2018, 320, 1319–1320. [Google Scholar] [CrossRef]
- Panchal, S.K.; John, O.D.; Mathai, M.L.; Brown, L. Anthocyanins in chronic diseases: The power of purple. Nutrients 2022, 14, 2161. [Google Scholar] [CrossRef]
- Marx, W.; Kelly, J.; Marshall, S.; Nakos, S.; Campbell, K.; Itsiopoulos, C. The effect of Polyphenol-rich interventions on cardiovascular risk factors in haemodialysis: A systematic review and meta-analysis. Nutrients 2017, 9, 1345. [Google Scholar] [CrossRef]
- Ribeiro, M.; Fanton, S.; Paiva, B.R.; Baptista, B.G.; Alvarenga, L.; Ribeiro-Alves, M.; Cardozo, L.F.; Mafra, D. Dark chocolate (70% cocoa) attenuates the inflammatory marker TNF-a in patients on hemodialysis. Clin. Nutr. ESPEN 2023, 53, 189–195. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; Melo, M.F.; Mota, J.C.; Mafra, D.; Guimarães, J.T.; Stockler-Pinto, M.B. Blueberry, cranberry, raspberry, and strawberry as modulators of the gut microbiota: Target for treatment of gut dysbiosis in chronic kidney disease? From current evidence to future possibilities. Nutr. Rev. 2024, 82, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, P.; Cozzolino, M. The emerging role of iron in heart failure and vascular calcification in CKD. Clin. Kidney J. 2021, 14, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, X.; Ma, L.; Yang, Z.; Ma, C. Association between dietary selenium intake and severe abdominal aortic calcification in the United States: A cross-sectional study. Food Funct. 2024, 15, 1575–1582. [Google Scholar] [CrossRef]
- Cardozo, L.F.; Borges, N.A.; Ribeiro, M.; Wang, A.Y.M.; Mafra, D. Protect the kidneys and save the heart using the concept of food as medicine. J. Ren. Nutr. 2023, 33, S110–S117. [Google Scholar] [CrossRef]
| Article | Design | Results |
|---|---|---|
| Shimizu 2023 [35] | Japanese study 64 incident HD patients Phosphate levels CAC by CT scans | Consistently strict phosphate control may slow the progression of coronary and valvular calcifications |
| Machado 2018 [36] | PROGREDIR study 373 non-dialysis CKD patients Food questionnaire Coronary artery calcification (CAC) by CT scans | Increased intake of food rich in phosphorus, calcium, and magnesium is associated with CAC |
| Ter Braake 2020 [37] | Klotho-deficient mice High dietary Mg | Mg prevents VC in Klotho deficiency |
| Talari 2019 [38] | RCT 54 HD patients with diabetes Mg oxide or placebo | Decrease in intima–media thickness after Mg supplementation |
| Sakaguchi 2019 [39] | RCT of 96 non-dialysis CKD patients Mg oxide versus carbon adsorbent CAC by CT scans Follow-up 2 years | CAC score was significantly smaller in the Mg oxide group |
| Bressendorf 2023 [40] | Magical-CKD study 150 CKD patients Supplementation with Mg for 12 months CAC by CT scans | No effect on CAC |
| Zhang 2023 [41] | 170 CKD patients and 62 healthy controls Blood zinc levels CAC by CT scans | Low zinc with moderate–severe CAC and CDV events |
| McCabe 2013 [42] | Rats with adenine-induced chronic renal failure and warfarin-induced VC | High dietary vitamin K1 increased vitamin K tissue concentrations and blunted the development of VC |
| El Shinnawy 2022 [43] | RCT on 120 HD patients given supplements of vitamin K2, vitamin K1, and placebo Matrix Gla protein levels | Matrix Gla protein levels showed a significant increase in the vitamin K2 group compared with vitamin K1 and placebo groups |
| Li 2017 [44] | 100 HD patients Used vitamin-K-enriched dialysate | Decreased VC scores as the effect of vitamin K |
| Haroon 2023 [45] | Trevasc-HDK RCT on 138 HD patients; CAC scores Vitamin K2 supplementation | No effect on VC |
| Levy-Schousboe 2021 [46] | RenaKvit RCT on 48 dialysis patients Vitamin K or placebo for 2 years Abdominal aortic calcification | No difference in VC |
| Holden 2022 [47] | iPACK-HD RCT on 86 HD patients Vitamin K1 for 12 months Coronary artery calcium score | No difference in progression of coronary artery calcification |
| Kanai 2011 [48] | Warfarin-induced medial arterial calcification in a rat model | Decreased medial arterial calcification after omega-3 fatty acid supplementation |
| Nakamura 2017 [49] | Eicosapentaenoic acid in Klotho mutant mice | Eicosapentaenoic acid limit VC |
| Son 2012 [50] | Cross-sectional study 31 HD patients Erythrocyte membrane content of monounsaturated fatty acids Plain radiographs for VC | Monounsaturated fatty acid erythrocyte content is significantly higher in HD patients with arterial medial calcification of the feet than in those without calcifications |
| Lan 2022 [51] | Cell culture Animal studies Ketone body β-hydroxybutyrate and VC in CKD model | Ketogenic diet through β-hydroxybutyrate suppresses VC in CKD through downregulation of HDAC9 |
| Merino-Ribas 2022 [52] | Cross-sectional study 44 CKD patients on peritoneal dialysis (PD) Gut and blood microbiomes VC on plain radiographs | Differences in microbiota between PD patients with and without VC |
| Wei 2023 [53] | CKD Rats with 1,25-dihydroxyvitamin D3 induced VC. Lactobacillus rhamnosus | Lactobacillus rhamnosus GG supplements worsened the VC in CKD |
| Sanchis 2016 [54] | 69 non-dialysis CKD patients Food questionnaire evaluated the phytate (Myo-inositol hexaphosphate) intake. VC on plain radiographs | Increased phytate intake was associated with less abdominal aorta calcification |
| Raggi 2020 [55] | RCT 274 HD patients Myo-inositol hexaphosphate Cardiovascular calcification on CT scan 52 weeks | Slowed progression of cardiovascular calcification with myo-inositol hexaphosphate |
| Li 2023 [56] | Data from NHANES 1862 participants Information on 35 dietary components VC on plain radiographs | Low contents of proteins, fiber and vitamin A and high contents of lipids and caffeine were associated with abdominal aorta calcification. High adherence to the plant-based pattern was associated with a lower risk of VC |
| Zhang 2016 [57] | Resveratrol | Resveratrol is a scavenger for many free radicals and ameliorates VC in CKD |
| Chang 2017 [58] | Rats with adenin-induced chronic renal failure. Quercetin | Quercetin exerted a protective effect on VC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, D.; Rusu, C.; Potra, A.; Tirinescu, D.; Ticala, M.; Kacso, I. Food to Prevent Vascular Calcification in Chronic Kidney Disease. Nutrients 2024, 16, 617. https://doi.org/10.3390/nu16050617
Moldovan D, Rusu C, Potra A, Tirinescu D, Ticala M, Kacso I. Food to Prevent Vascular Calcification in Chronic Kidney Disease. Nutrients. 2024; 16(5):617. https://doi.org/10.3390/nu16050617
Chicago/Turabian StyleMoldovan, Diana, Crina Rusu, Alina Potra, Dacian Tirinescu, Maria Ticala, and Ina Kacso. 2024. "Food to Prevent Vascular Calcification in Chronic Kidney Disease" Nutrients 16, no. 5: 617. https://doi.org/10.3390/nu16050617
APA StyleMoldovan, D., Rusu, C., Potra, A., Tirinescu, D., Ticala, M., & Kacso, I. (2024). Food to Prevent Vascular Calcification in Chronic Kidney Disease. Nutrients, 16(5), 617. https://doi.org/10.3390/nu16050617






