Chronodisruption and Gut Microbiota: Triggering Glycemic Imbalance in People with Type 2 Diabetes
Abstract
1. Introduction
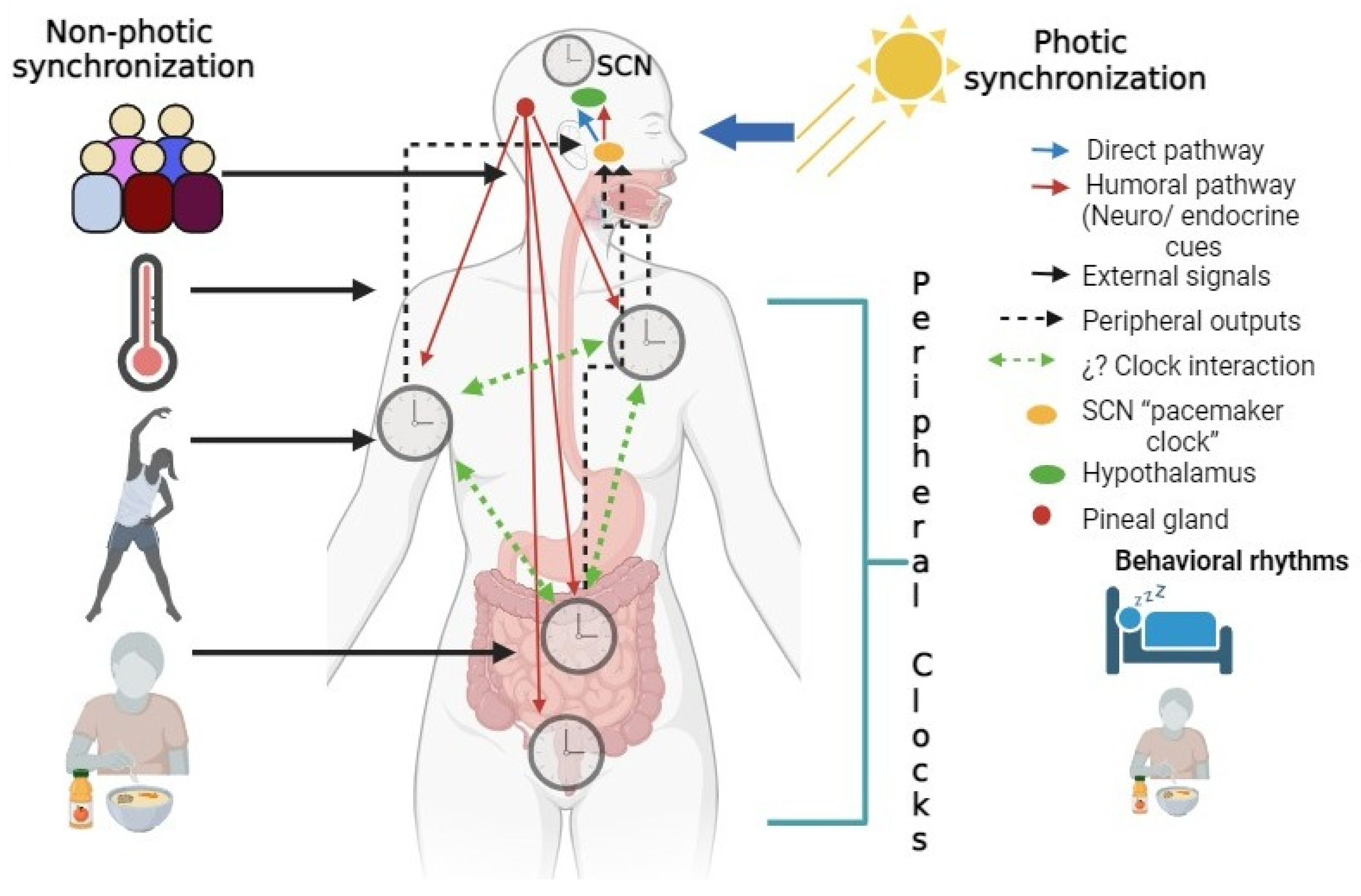
2. Circadian Rhythms
3. Chronodisruption
4. Sleep as a Modulator of Circadian Rhythms
5. Circadian Rhythms in the Enteral System
6. The Gut–Brain Axis (GBA)
7. Microbiota and Circadian Rhythms
8. Alterations of Gut Microbiota Diversity Associated with Chronodisruption
9. Gut Microbiota Dysbiosis Arises from Sleep Deprivation
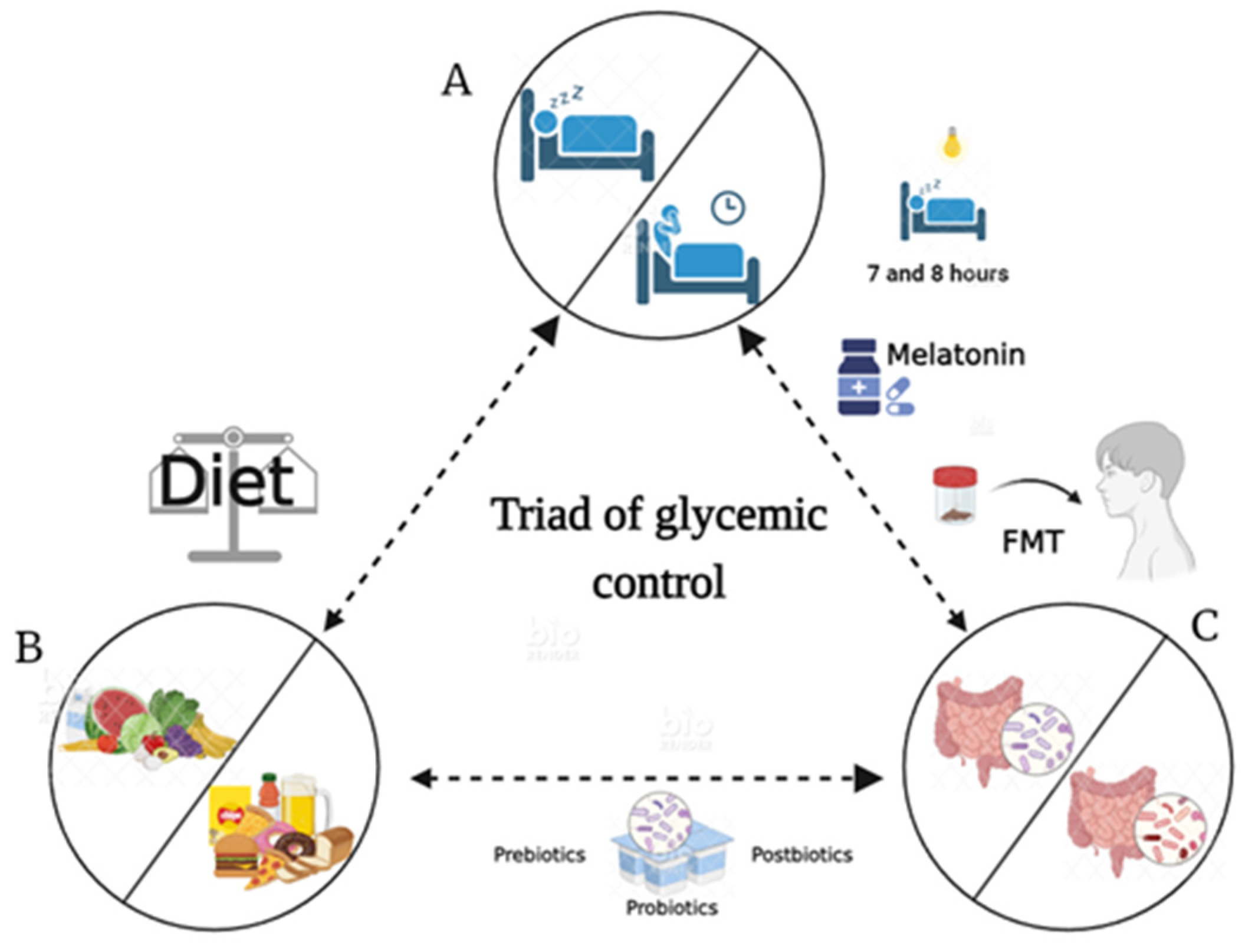
10. Chronotherapy, Therapeutic Effects, and Beneficial Effects by Microbiota
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gahagan, S. Development of Eating Behavior: Biology and Context. J. Dev. Behav. Pediatr. 2012, 33, 261–271. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian Rhythms, Sleep, and Metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G. Sleep, Circadian Rhythms and Health. Interface Focus 2020, 10, 20190098. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Woźniak, A.; Taub, P.R. Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients 2021, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Lenn, N.J. A Retinohypothalamic Projection in the Rat. J. Comp. Neurol. 1972, 146, 1–14. [Google Scholar] [CrossRef]
- Stephan, F.K.; Zucker, I. Circadian Rhythms in Drinking Behavior and Locomotor Activity of Rats Are Eliminated by Hypothalamic Lesions. Proc. Natl. Acad. Sci. USA 1972, 69, 1583–1586. [Google Scholar] [CrossRef]
- Hofman, M.A.; Fliers, E.; Goudsmit, E.; Swaab, D.F. Morphometric Analysis of the Suprachiasmatic and Paraventricular Nuclei in the Human Brain: Sex Differences and Age-Dependent Changes. J. Anat. 1988, 160, 127–143. [Google Scholar]
- Buijs, R.M.; Guzmán Ruiz, M.A.; Méndez Hernández, R.; Rodríguez Cortés, B. The Suprachiasmatic Nucleus; a Responsive Clock Regulating Homeostasis by Daily Changing the Setpoints of Physiological Parameters. Auton. Neurosci. 2019, 218, 43–50. [Google Scholar] [CrossRef] [PubMed]
- King, D.P.; Takahashi, J.S. Molecular Genetics of Circadian Rhythms in Mammals. Annu. Rev. Neurosci. 2000, 23, 713–742. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, A. Clock Genes in Mammalian Peripheral Tissues. Cell Tissue Res. 2002, 309, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Bozek, K.; Relógio, A.; Kielbasa, S.M.; Heine, M.; Dame, C.; Kramer, A.; Herzel, H. Regulation of Clock-Controlled Genes in Mammals. PLoS ONE 2009, 4, e4882. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, J.A.; Olsson, A.H.; Nagorny, C.L.; Malmgren, S.; Dekker-Nitert, M.; Ling, C.; Mulder, H. Regulation of Core Clock Genes in Human Islets. Metabolism 2012, 61, 978–985. [Google Scholar] [CrossRef]
- Shi, S.; Ansari, T.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian Disruption Leads to Insulin Resistance and Obesity. Curr. Biol. 2013, 23, 372–381. [Google Scholar] [CrossRef]
- Carriazo, S.; Ramos, A.M.; Sanz, A.B.; Sanchez-Niño, M.D.; Kanbay, M.; Ortiz, A. Chronodisruption: A Poorly Recognized Feature of CKD. Toxins 2020, 12, 151. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J. Defining Chronodisruption. J. Pineal Res. 2009, 46, 245–247. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Korkmaz, A.; Ma, S. Obesity and Metabolic Syndrome: Association with Chronodisruption, Sleep Deprivation, and Melatonin Suppression. Ann. Med. 2012, 44, 564–577. [Google Scholar] [CrossRef]
- Pallesen, S.; Bjorvatn, B.; Waage, S.; Harris, A.; Sagoe, D. Prevalence of Shift Work Disorder: A Systematic Review and Meta-Analysis. Front. Psychol. 2021, 12, 638252. [Google Scholar] [CrossRef]
- Karlsson, B.; Knutsson, A.; Lindahl, B. Is There an Association between Shift Work and Having a Metabolic Syndrome? Results from a Population Based Study of 27,485 People. Occup. Environ. Med. 2001, 58, 747–752. [Google Scholar] [CrossRef]
- Ralph, M.R.; Foster, R.G.; Davis, F.C.; Menaker, M. Transplanted Suprachiasmatic Nucleus Determines Circadian Period. Science 1990, 247, 975–978. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Gooley, J.J. Sleep and Circadian Rhythms in Humans. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Jewett, M.E.; Wyatt, J.K.; Ritz-De Cecco, A.; Khalsa, S.B.; Dijk, D.J.; Czeisler, C.A. Time Course of Sleep Inertia Dissipation in Human Performance and Alertness. J. Sleep. Res. 1999, 8, 1–8. [Google Scholar] [CrossRef]
- Borbély, A.A. A Two Process Model of Sleep Regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [PubMed]
- Daan, S.; Beersma, D.G.; Borbély, A.A. Timing of Human Sleep: Recovery Process Gated by a Circadian Pacemaker. Am. J. Physiol. 1984, 246, R161–R183. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Kilduff, T.S. The Neurobiology of Sleep and Wakefulness. Psychiatr. Clin. N. Am. 2015, 38, 615–644. [Google Scholar] [CrossRef]
- St-Onge, M.-P. Sleep-Obesity Relation: Underlying Mechanisms and Consequences for Treatment. Obes. Rev. 2017, 18 (Suppl. 1), 34–39. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Shockley, K.R.; Romer, M.A.; Galante, R.J.; Zimmerman, J.E.; Naidoo, N.; Baldwin, D.A.; Jensen, S.T.; Churchill, G.A.; Pack, A.I. Macromolecule Biosynthesis: A Key Function of Sleep. Physiol. Genom. 2007, 31, 441–457. [Google Scholar] [CrossRef]
- Pandey, A.; Oliver, R.; Kar, S.K. Differential Gene Expression in Brain and Liver Tissue of Wistar Rats after Rapid Eye Movement Sleep Deprivation. Clocks Sleep 2020, 2, 442–465. [Google Scholar] [CrossRef]
- Liew, C.-C.; Ma, J.; Tang, H.-C.; Zheng, R.; Dempsey, A.A. The Peripheral Blood Transcriptome Dynamically Reflects System Wide Biology: A Potential Diagnostic Tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef]
- Möller-Levet, C.S.; Archer, S.N.; Bucca, G.; Laing, E.E.; Slak, A.; Kabiljo, R.; Lo, J.C.Y.; Santhi, N.; von Schantz, M.; Smith, C.P.; et al. Effects of Insufficient Sleep on Circadian Rhythmicity and Expression Amplitude of the Human Blood Transcriptome. Proc. Natl. Acad. Sci. USA 2013, 110, E1132–E1141. [Google Scholar] [CrossRef]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of Sleep Deprivation on the Human Metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef]
- Moore, J.G.; Englert, E. Circadian Rhythm of Gastric Acid Secretion in Man. Nature 1970, 226, 1261–1262. [Google Scholar] [CrossRef]
- Goo, R.H.; Moore, J.G.; Greenberg, E.; Alazraki, N.P. Circadian Variation in Gastric Emptying of Meals in Humans. Gastroenterology 1987, 93, 515–518. [Google Scholar] [CrossRef]
- Lindberg, G.; Iwarzon, M.; Hammarlund, B. 24-Hour Ambulatory Electrogastrography in Healthy Volunteers. Scand. J. Gastroenterol. 1996, 31, 658–664. [Google Scholar] [CrossRef]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted Feeding Uncouples Circadian Oscillators in Peripheral Tissues from the Central Pacemaker in the Suprachiasmatic Nucleus. Genes. Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A.; Hellmich, H.L.; Cornélissen, G.; Halberg, F.; Shahinian, V.B.; Bostwick, J.; Savidge, T.C.; Cassone, V.M. Clock Gene Expression in the Murine Gastrointestinal Tract: Endogenous Rhythmicity and Effects of a Feeding Regimen. Gastroenterology 2007, 133, 1250–1260. [Google Scholar] [CrossRef]
- Mendoza, J. Circadian Clocks: Setting Time by Food. J. Neuroendocrinol. 2007, 19, 127–137. [Google Scholar] [CrossRef]
- Palmieri, O.; Mazzoccoli, G.; Bossa, F.; Maglietta, R.; Palumbo, O.; Ancona, N.; Corritore, G.; Latiano, T.; Martino, G.; Rubino, R.; et al. Systematic Analysis of Circadian Genes Using Genome-Wide cDNA Microarrays in the Inflammatory Bowel Disease Transcriptome. Chronobiol. Int. 2015, 32, 903–916. [Google Scholar] [CrossRef]
- Giebfried, J.; Lorentz, A. Relationship between the Biological Clock and Inflammatory Bowel Disease. Clocks Sleep 2023, 5, 260–275. [Google Scholar] [CrossRef]
- Gombert, M.; Carrasco-Luna, J.; Pin-Arboledas, G.; Codoñer-Franch, P. The Connection of Circadian Rhythm to Inflammatory Bowel Disease. Transl. Res. 2019, 206, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Desmet, L.; Thijs, T.; Segers, A.; Verbeke, K.; Depoortere, I. Chronodisruption by Chronic Jetlag Impacts Metabolic and Gastrointestinal Homeostasis in Male Mice. Acta Physiol. 2021, 233, e13703. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, N.R.; Fierstein, J.S. Circadian Rhythms of Intestinal Sucrase and Glucose Transport: Cued by Time of Feeding. Am. J. Physiol. 1976, 230, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Pan, X. Clock Genes, Intestinal Transport and Plasma Lipid Homeostasis. Trends Endocrinol. Metab. 2009, 20, 177–185. [Google Scholar] [CrossRef]
- Gnocchi, D.; Pedrelli, M.; Hurt-Camejo, E.; Parini, P. Lipids around the Clock: Focus on Circadian Rhythms and Lipid Metabolism. Biology 2015, 4, 104–132. [Google Scholar] [CrossRef]
- Rhoads, D.B.; Rosenbaum, D.H.; Unsal, H.; Isselbacher, K.J.; Levitsky, L.L. Circadian Periodicity of Intestinal Na+/Glucose Cotransporter 1 mRNA Levels Is Transcriptionally Regulated. J. Biol. Chem. 1998, 273, 9510–9516. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Stearns, A.T.; Rounds, J.; Irani, J.; Giuffrida, M.; Rhoads, D.B.; Ashley, S.W.; Tavakkolizadeh, A. Diurnal Rhythmicity in Glucose Uptake Is Mediated by Temporal Periodicity in the Expression of the Sodium-Glucose Cotransporter (SGLT1). Surgery 2008, 143, 813–818. [Google Scholar] [CrossRef][Green Version]
- Vachon, C.; Savoie, L. Circadian Variation in Intestinal Protein Content in Rat Fed Ad Libitum. J. Am. Coll. Nutr. 1989, 8, 25–34. [Google Scholar] [CrossRef]
- Soták, M.; Polidarová, L.; Musílková, J.; Hock, M.; Sumová, A.; Pácha, J. Circadian Regulation of Electrolyte Absorption in the Rat Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1066–G1074. [Google Scholar] [CrossRef]
- Corpe, C.P.; Burant, C.F. Hexose Transporter Expression in Rat Small Intestine: Effect of Diet on Diurnal Variations. Am. J. Physiol. 1996, 271, G211–G216. [Google Scholar] [CrossRef]
- Pácha, J.; Sumová, A. Circadian Regulation of Epithelial Functions in the Intestine. Acta Physiol. 2013, 208, 11–24. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; pp. 195–219. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Yu, Y.-J.; Adeli, K. Role of Gut Microbiota in Neuroendocrine Regulation of Carbohydrate and Lipid Metabolism via the Microbiota-Gut-Brain-Liver Axis. Microorganisms 2020, 8, 527. [Google Scholar] [CrossRef]
- Smith, J.G.; Sato, T.; Shimaji, K.; Koronowski, K.B.; Petrus, P.; Cervantes, M.; Kinouchi, K.; Lutter, D.; Dyar, K.A.; Sassone-Corsi, P. Antibiotic-Induced Microbiome Depletion Remodels Daily Metabolic Cycles in the Brain. Life Sci. 2022, 303, 120601. [Google Scholar] [CrossRef]
- Zhang, S.L.; Lahens, N.F.; Yue, Z.; Arnold, D.M.; Pakstis, P.P.; Schwarz, J.E.; Sehgal, A. A Circadian Clock Regulates Efflux by the Blood-Brain Barrier in Mice and Human Cells. Nat. Commun. 2021, 12, 617. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Frank, J.; Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain–Gut–Microbiome Interactions and Intermittent Fasting in Obesity. Nutrients 2021, 13, 584. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain-Gut-Microbiome Interactions in Obesity and Food Addiction. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 655–672. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Münzberg, H.; Morrison, C.D. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology 2017, 152, 1728–1738. [Google Scholar] [CrossRef]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-Gut-Brain Axis: Relationships among the Vagus Nerve, Gut Microbiota, Obesity, and Diabetes. Acta Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef]
- Niijima, A. Blood Glucose Levels Modulate Efferent Activity in the Vagal Supply to the Rat Liver. J. Physiol. 1985, 364, 105–112. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s Role in Health and Diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, E.-J.; Roh, S.W.; Bae, J.-W. Diversity and Abundance of Single-Stranded DNA Viruses in Human Feces. Appl. Environ. Microbiol. 2011, 77, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The Athletic Gut Microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, S.-Y.; Wang, L.; Zhou, F. The Gut Microbiome: Implications for Neurogenesis and Neurological Diseases. Neural Regen. Res. 2022, 17, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ishiura, M. Circadian Rhythms of Cyanobacteria: Monitoring the Biological Clocks of Individual Colonies by Bioluminescence. J. Bacteriol. 1994, 176, 1881–1885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siebieszuk, A.; Sejbuk, M.; Witkowska, A.M. Studying the Human Microbiota: Advances in Understanding the Fundamentals, Origin, and Evolution of Biological Timekeeping. Int. J. Mol. Sci. 2023, 24, 16169. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Aravind, L. PAS: A Multifunctional Domain Family Comes to Light. Curr. Biol. 1997, 7, R674–R677. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.B.; Mezouar, S.; Boumaza, A.; Fiammingo, O.; Coiffard, B.; Pontarotti, P.; Desnues, B.; Mege, J.-L. RadA, a Key Gene of the Circadian Rhythm of Escherichia Coli. Int. J. Mol. Sci. 2022, 23, 6136. [Google Scholar] [CrossRef]
- Maniscalco, M.; Nannen, J.; Sodi, V.; Silver, G.; Lowrey, P.L.; Bidle, K.A. Light-Dependent Expression of Four Cryptic Archaeal Circadian Gene Homologs. Front. Microbiol. 2014, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Paulose, J.K.; Wright, J.M.; Patel, A.G.; Cassone, V.M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS ONE 2016, 11, e0146643. [Google Scholar] [CrossRef]
- Beli, E.; Prabakaran, S.; Krishnan, P.; Evans-Molina, C.; Grant, M.B. Loss of Diurnal Oscillatory Rhythms in Gut Microbiota Correlates with Changes in Circulating Metabolites in Type 2 Diabetic Db/Db Mice. Nutrients 2019, 11, 2310. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xiao, X.-H. Gut Microbiota: Closely Tied to the Regulation of Circadian Clock in the Development of Type 2 Diabetes Mellitus. Chin. Med. J. 2020, 133, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Nobs, S.P.; Tuganbaev, T.; Elinav, E. Microbiome Diurnal Rhythmicity and Its Impact on Host Physiology and Disease Risk. EMBO Rep. 2019, 20, e47129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Yoon, S.-H.; Jung, Y.; Kim, N.; Min, U.; Chun, J.; Choi, I. Emotional Well-Being and Gut Microbiome Profiles by Enterotype. Sci. Rep. 2020, 10, 20736. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Gérard, C.; Vidal, H. Impact of Gut Microbiota on Host Glycemic Control. Front. Endocrinol. 2019, 10, 29. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, A.; Hu, Y.; Rong, N.; Zhang, Q.; Long, W.; Yang, S.; Nan, S.; Zhang, L.; Zhou, K.; et al. Compound Dietary Fiber and High-Grade Protein Diet Improves Glycemic Control and Ameliorates Diabetes and Its Comorbidities through Remodeling the Gut Microbiota in Mice. Front. Nutr. 2022, 9, 959703. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the Intestinal Microbiota Is Regulated by Gender and the Host Circadian Clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Llabre, J.E.; Trujillo, R.; Sroga, G.E.; Figueiro, M.G.; Vashishth, D. Circadian Rhythm Disruption with High-Fat Diet Impairs Glycemic Control and Bone Quality. FASEB J. 2021, 35, e21786. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Tam, D.N.H.; Shah, J.; Moriyama, M.; Varney, J.; Huy, N.T. Effects of Sleep Intervention on Glucose Control: A Narrative Review of Clinical Evidence. Prim. Care Diabetes 2021, 15, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, Circadian Rhythm, and Gut Microbiota. Sleep. Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, J.; Pulgar, R.; Mandakovic, D.; Porras, O.; Flores, C.A.; Luco, D.; Trujillo, C.A.; Díaz-Esquivel, B.; Alvarez, C.; Acevedo, A.; et al. Nocturnal Light Pollution Induces Weight Gain in Mice and Reshapes the Structure, Functions, and Interactions of Their Colonic Microbiota. Int. J. Mol. Sci. 2022, 23, 1673. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fang, D.; Wang, Z.; Liu, Y. Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. Int. J. Mol. Sci. 2023, 24, 9603. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-F.; Huang, W.-C.; Wu, C.W.; Huang, C.-Y.; Yang, Y.-C.S.H.; Tung, Y.-T. Acute Sleep Deprivation Exacerbates Systemic Inflammation and Psychiatry Disorders through Gut Microbiota Dysbiosis and Disruption of Circadian Rhythms. Microbiol. Res. 2023, 268, 127292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.-H.; Li, S.-X.; He, Z.-M.; Zhu, W.-L.; Ji, Y.-B.; Wang, Z.; Zhu, X.-M.; Yuan, K.; Bao, Y.-P.; et al. Gut Microbiota Modulates the Inflammatory Response and Cognitive Impairment Induced by Sleep Deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Hamjane, N.; Mechita, M.B.; Nourouti, N.G.; Barakat, A. Gut Microbiota Dysbiosis-Associated Obesity and Its Involvement in Cardiovascular Diseases and Type 2 Diabetes. A Systematic Review. Microvasc. Res. 2024, 151, 104601. [Google Scholar] [CrossRef]
- Li, N.; Wang, N.; Ran, P. Gut Microbiota Dysbiosis and Chronic Obstructive Pulmonary Disease: A Question of Chicken and Egg. Am. J. Respir. Crit. Care Med. 2023, 208, 1238–1240. [Google Scholar] [CrossRef]
- Sharma, G.; Biswas, S.S.; Mishra, J.; Navik, U.; Kandimalla, R.; Reddy, P.H.; Bhatti, G.K.; Bhatti, J.S. Gut Microbiota Dysbiosis and Huntington’s Disease: Exploring the Gut-Brain Axis and Novel Microbiota-Based Interventions. Life Sci. 2023, 328, 121882. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Cheng, L.; Wu, Y.; Wu, T.; Wen, J.; Huang, D.; Liao, Z.; Tan, C.; Luo, Y.; et al. Gut Microbiota Dysbiosis Characterized by Abnormal Elevation of Lactobacillus in Patients with Immune-Mediated Necrotizing Myopathy. Front. Cell Infect. Microbiol. 2023, 13, 1243512. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging Role of Gut Microbiota Dysbiosis in Neuroinflammation and Neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, T.; Cao, M.; Yuan, C.; Reiter, R.J.; Zhao, Z.; Zhao, Y.; Chen, L.; Fan, W.; Wang, X.; et al. Gut Microbiota Dysbiosis Induced by Decreasing Endogenous Melatonin Mediates the Pathogenesis of Alzheimer’s Disease and Obesity. Front. Immunol. 2022, 13, 900132. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. An Alternative Explanation for Alzheimer’s Disease and Parkinson’s Disease Initiation from Specific Antibiotics, Gut Microbiota Dysbiosis and Neurotoxins. Neurochem. Res. 2022, 47, 517–530. [Google Scholar] [CrossRef] [PubMed]
- García-Mena, J.; Corona-Cervantes, K.; Cuervo-Zanatta, D.; Benitez-Guerrero, T.; Vélez-Ixta, J.M.; Zavala-Torres, N.G.; Villalobos-Flores, L.E.; Hernández-Quiroz, F.; Perez-Cruz, C.; Murugesan, S.; et al. Gut Microbiota in a Population Highly Affected by Obesity and Type 2 Diabetes and Susceptibility to COVID-19. World J. Gastroenterol. 2021, 27, 7065–7079. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Chapter 24—Chronotherapy. In Handbook of Clinical Neurology; Swaab, D.F., Kreier, F., Lucassen, P.J., Salehi, A., Buijs, R.M., Eds.; The Human Hypothalamus: Anterior Region; Elsevier: Amsterdam, The Netherlands, 2021; Volume 179, pp. 357–370. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. Entrainment of the Human Circadian Clock. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; von Schantz, M. Associations between Chronotype, Morbidity and Mortality in the UK Biobank Cohort. Chronobiol. Int. 2018, 35, 1045–1053. [Google Scholar] [CrossRef]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The Relationship Between Breakfast Skipping, Chronotype, and Glycemic Control in Type 2 Diabetes. Chronobiol. Int. 2014, 31, 64–71. [Google Scholar] [CrossRef]
- Maghsoudipour, M.; Allison, M.A.; Patel, S.R.; Talavera, G.A.; Daviglus, M.; Zee, P.C.; Reid, K.J.; Makarem, N.; Malhotra, A. Associations of Chronotype and Sleep Patterns with Metabolic Syndrome in the Hispanic Community Health Study/Study of Latinos. Chronobiol. Int. 2022, 39, 1087–1099. [Google Scholar] [CrossRef]
- Dutil, C.; Chaput, J.-P. Inadequate Sleep as a Contributor to Type 2 Diabetes in Children and Adolescents. Nutr. Diabetes 2017, 7, e266. [Google Scholar] [CrossRef]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep Duration and Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Yapislar, H.; Haciosmanoglu, E.; Sarioglu, T.; Degirmencioglu, S.; Sogut, I.; Poteser, M.; Ekmekcioglu, C. Anti-Inflammatory Effects of Melatonin in Rats with Induced Type 2 Diabetes Mellitus. Life 2022, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Segal, E.; Elinav, E. A Day in the Life of the Meta-Organism: Diurnal Rhythms of the Intestinal Microbiome and Its Host. Gut Microbes 2015, 6, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Rao, M.C.; Chang, E.B. Gut Microbiota as a Transducer of Dietary Cues to Regulate Host Circadian Rhythms and Metabolism. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Risely, A.; Wilhelm, K.; Clutton-Brock, T.; Manser, M.B.; Sommer, S. Diurnal Oscillations in Gut Bacterial Load and Composition Eclipse Seasonal and Lifetime Dynamics in Wild Meerkats. Nat. Commun. 2021, 12, 6017. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [PubMed]
- Litichevskiy, L.; Thaiss, C.A. The Oscillating Gut Microbiome and Its Effects on Host Circadian Biology. Annu. Rev. Nutr. 2022, 42, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Khoruts, A.; Sadowsky, M.J. Understanding the Mechanisms of Faecal Microbiota Transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef]
- Vrieze, A.; Nood, E.V.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Su, L.; Hong, Z.; Zhou, T.; Jian, Y.; Xu, M.; Zhang, X.; Zhu, X.; Wang, J. Health Improvements of Type 2 Diabetic Patients through Diet and Diet plus Fecal Microbiota Transplantation. Sci. Rep. 2022, 12, 1152. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Cortés, M.L.; Meza-Alvarado, J.E.; García-Mena, J.; Hernández-Rodríguez, A. Chronodisruption and Gut Microbiota: Triggering Glycemic Imbalance in People with Type 2 Diabetes. Nutrients 2024, 16, 616. https://doi.org/10.3390/nu16050616
Moreno-Cortés ML, Meza-Alvarado JE, García-Mena J, Hernández-Rodríguez A. Chronodisruption and Gut Microbiota: Triggering Glycemic Imbalance in People with Type 2 Diabetes. Nutrients. 2024; 16(5):616. https://doi.org/10.3390/nu16050616
Chicago/Turabian StyleMoreno-Cortés, María Luisa, José Enrique Meza-Alvarado, Jaime García-Mena, and Azucena Hernández-Rodríguez. 2024. "Chronodisruption and Gut Microbiota: Triggering Glycemic Imbalance in People with Type 2 Diabetes" Nutrients 16, no. 5: 616. https://doi.org/10.3390/nu16050616
APA StyleMoreno-Cortés, M. L., Meza-Alvarado, J. E., García-Mena, J., & Hernández-Rodríguez, A. (2024). Chronodisruption and Gut Microbiota: Triggering Glycemic Imbalance in People with Type 2 Diabetes. Nutrients, 16(5), 616. https://doi.org/10.3390/nu16050616






