Dieta de la Milpa: A Culturally-Concordant Plant-Based Dietary Pattern for Hispanic/Latine People with Chronic Kidney Disease
Abstract
1. Introduction
2. Components of the Dieta de la Milpa Dietary Pattern
3. Potential Benefits for People with CKD (Figure 2)
3.1. Dietary Patterns vs. Nutrient Intake

3.2. Dietary Acid Load
3.3. Dietary Protein Sources
3.4. Phosphorus Management
3.5. Potassium Management
3.6. The Gut Microbiome
3.7. Inflammation
3.8. Cultural Appropriateness
4. Key Research Questions to Evaluate the Measurement, Feasibility, and Effectiveness of the Diet a de la Milpa
4.1. The Development or Adaptation of a Metric to Evaluate the Adherence to the Dieta de la Milpa
4.2. Evaluation of the Feasibility and Acceptability of the Dieta de la Milpa in People with CKD
4.3. Evaluation of the Effectiveness of the Dieta de la Milpa in CKD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Lora, C.M.; Lash, J.P.; Ricardo, A.C. CKD and ESRD in US Hispanics. Am. J. Kidney Dis. 2019, 73, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Luxardo, R.; Kramer, A.; González-Bedat, M.C.; Massy, Z.A.; Jager, K.J.; Rosa-Diez, G.; Noordzij, M. The epidemiology of renal replacement therapy in two different parts of the world: The Latin American Dialysis and Transplant Registry versus the European Renal Association-European Dialysis and Transplant Association Registry. Rev. Panam. Salud Publica 2018, 42, e87. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Sola, L.; Correa-Rotter, R.; Granado, R.C.-D.; Douthat, W.G.; Bellorin-Font, E. Kidney disease in Latin America: Current status, challenges, and the role of the ISN in the development of nephrology in the region. Kidney Int. 2018, 94, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Beto, J.A.; Ramirez, W.E.; Bansal, V.K. Medical nutrition therapy in adults with chronic kidney disease: Integrating evidence and consensus into practice for the generalist registered dietitian nutritionist. J. Acad. Nutr. Diet. 2014, 114, 1077–1087. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Shinaberger, C.S.; Greenland, S.; Kopple, J.D.; Van Wyck, D.; Mehrotra, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am. J. Clin. Nutr. 2008, 88, 1511–1518. [Google Scholar] [CrossRef]
- Biruete, A.; Jeong, J.H.; Barnes, J.L.; Wilund, K.R. Modified Nutritional Recommendations to Improve Dietary Patterns and Outcomes in Hemodialysis Patients. J. Ren. Nutr. 2017, 27, 62–70. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Tortorici, A.R.; Chen, J.L.; Kamgar, M.; Lau, W.L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary restrictions in dialysis patients: Is there anything left to eat? Semin. Dial. 2015, 28, 159–168. [Google Scholar] [CrossRef]
- Kant, A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef]
- Campbell, K.L.; Carrero, J.J. Diet for the Management of Patients With Chronic Kidney Disease; It Is Not the Quantity, but the Quality That Matters. J. Ren. Nutr. 2016, 26, 279–281. [Google Scholar] [CrossRef]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 725–735. [Google Scholar] [CrossRef]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.-J.; Campbell, K.L.; Strippoli, G.F.M. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef]
- Kistler, B.M.; Moore, L.W.; Benner, D.; Biruete, A.; Boaz, M.; Brunori, G.; Chen, J.; Drechsler, C.; Guebre-Egziabher, F.; Hensley, M.K.; et al. The International Society of Renal Nutrition and Metabolism Commentary on the National Kidney Foundation and Academy of Nutrition and Dietetics KDOQI Clinical Practice Guideline for Nutrition in Chronic Kidney Disease. J. Ren. Nutr. 2021, 31, 116–120 e1. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Mattei, J. Mediterranean Diet and Cardiometabolic Diseases in Racial/Ethnic Minority Populations in the United States. Nutrients 2018, 10, 352. [Google Scholar] [CrossRef]
- Hiza, H.A.; Casavale, K.O.; Guenther, P.M.; Davis, C.A. Diet quality of Americans differs by age, sex, race/ethnicity, income, and education level. J. Acad. Nutr. Diet. 2013, 113, 297–306. [Google Scholar] [CrossRef]
- Cervantes, L.; Rizzolo, K.; Carr, A.L.; Steiner, J.F.; Chonchol, M.; Powe, N.; Cukor, D.; Hasnain-Wynia, R. Social and Cultural Challenges in Caring for Latinx Individuals With Kidney Failure in Urban Settings. JAMA Netw Open 2021, 4, e2125838. [Google Scholar] [CrossRef]
- Perez, L.; Biruete, A. Lack of Cultural and Language Concordant Nutrition Education for Hispanic/Latinx Individuals with Chronic Kidney Disease: A Call to Action. J. Am. Soc. Nephrol. 2022, 33, 1262–1264. [Google Scholar] [CrossRef]
- Almaguer-González, J.A.; García-Ramírez, J.H.; Padilla-Mirazo, M.; González-Ferral, M. Dieta de la Milpa: Modelo de Alimentación Mesoamericana Biocompatible; Secretaria de Salud: Ciudad de Mexico, Mexico, 2019. [Google Scholar]
- Sánchez-Velázquez, O.A.; Luna-Vital, D.A.; Morales-Hernandez, N.; Contreras, J.; Villaseñor-Tapia, E.C.; Fragoso-Medina, J.A.; Mojica, L. Nutritional, bioactive components and health properties of the milpa triad system seeds (corn, common bean and pumpkin). Front. Nutr. 2023, 10, 1169675. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Granados, C.; Panduro, A.; Gonzalez-Aldaco, K.; Sepulveda-Villegas, M.; Rivera-Iñiguez, I.; Roman, S. Tailoring Nutritional Advice for Mexicans Based on Prevalence Profiles of Diet-Related Adaptive Gene Polymorphisms. J. Pers. Med. 2017, 7, 16. [Google Scholar] [CrossRef]
- Gwirtz, J.A.; Garcia-Casal, M.N. Processing maize flour and corn meal food products. Ann. N. Y. Acad. Sci. 2014, 1312, 66–75. [Google Scholar] [CrossRef]
- Turco, V.L.; Potortì, A.G.; Rando, R.; Ravenda, P.; Dugo, G.; Di Bella, G. Functional properties and fatty acids profile of different beans varieties. Nat. Prod. Res. 2016, 30, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.F.; Mojica, L.; Berhow, M.A.; Brownstein, K.; Cervantes, E.L.; de Mejia, E.G. Black and pinto beans (Phaseolus vulgaris L.) unique mexican varieties exhibit antioxidant and anti-inflammatory potential. Food Res. Int. 2023, 169, 112816. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Biruete, A.; Kistler, B.M.; Khor, B.-H.; Ebrahim, Z.; Giannini, R.; Sussman-Dabach, E.J.; Avesani, C.M.; Chan, M.; Lambert, K.; et al. New insights into dietary approaches to potassium management in chronic kidney disease. J. Ren. Nutr. 2023, 33, S6–S12. [Google Scholar] [CrossRef]
- Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Tables of Composition of Mexican Foods and Food Products (Condensed Version; 2015); Departamento de Ciencia y Tecnología de los Alimentos: Ciudad de México, México, 2016. [Google Scholar]
- Instituto de Nutrición de Centro América y Panamá (INCAP) y Organización Panamericana de la Salud (OPS). Tabla de composicion de alimentos de Centroamérica; INCAP/OPS: Apartado, Guatemala, 2007. [Google Scholar]
- Perez-Lizaur, A.B.; Palacios-Gonzalez, B. Sistema Mexicano de Alimentos Equivalentes, 5th ed.; Fomento de Nutrición y SaludÑ: Mexico City, Mexico, 2022. [Google Scholar]
- Sussman, E.J.; Singh, B.; Clegg, D.; Palmer, B.F.; Kalantar-Zadeh, K. Let Them Eat Healthy: Can Emerging Potassium Binders Help Overcome Dietary Potassium Restrictions in Chronic Kidney Disease? J. Ren. Nutr. 2020, 30, 475–483. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Green, T.; Harrison, T.N.; Reynolds, K. Dietary approaches to prevent hypertension. Curr. Hypertens Rep. 2013, 15, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sang, Y.; Ballew, S.H.; Tin, A.; Chang, A.R.; Matsushita, K.; Coresh, J.; Kalantar-Zadeh, K.; Molnar, M.Z.; Grams, M.E. Race, Serum Potassium, and Associations with ESRD and Mortality. Am. J. Kidney Dis. 2017, 70, 244–251. [Google Scholar] [CrossRef]
- Biruete, A.; Gallant, K.M.H.; Lloyd, L.; Meade, A.; Moe, S.M.; St-Jules, D.E.; Kistler, B.M. ‘Phos’tering a clear message: The evolution of dietary phosphorus management in chronic kidney disease. J. Ren. Nutr. 2023, 33, S13–S20. [Google Scholar] [CrossRef]
- Avesani, C.M.; Cuppari, L.; Nerbass, F.B.; Lindholm, B.; Stenvinkel, P. Ultraprocessed foods and chronic kidney disease-double trouble. Clin. Kidney J. 2023, 16, 1723–1736. [Google Scholar] [CrossRef]
- K/DOQI, National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2000, 35 (Suppl. S2), S1–S140. [Google Scholar]
- Asghari, G.; Momenan, M.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary pattern and incidence of chronic kidney disease among adults: A population-based study. Nutr. Metab. 2018, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiménez-Moleón, J.J.; Lindholm, B.; Cederholm, T.; Ärnlöv, J.; Risérus, U.; Sjögren, P.; Carrero, J.J. Mediterranean diet, kidney function, and mortality in men with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Wai, S.N.; Kelly, J.T.; Johnson, D.W.; Campbell, K.L. Dietary Patterns and Clinical Outcomes in Chronic Kidney Disease: The CKD.QLD Nutrition Study. J. Ren. Nutr. 2017, 27, 175–182. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Tuot, D.S.; Pavkov, M.E.; Burrows, N.R.; Stack, A.G.; Saran, R.; Bragg-Gresham, J.; Powe, N.R.; Hsu, C.-Y.; et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int. 2019, 95, 1433–1442. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Di Iorio, B.R.; Chatrenet, A.; D’alessandro, C.; Nazha, M.; Capizzi, I.; Vigotti, F.N.; Fois, A.; Maxia, S.; Saulnier, P.; et al. Dietary satisfaction and quality of life in chronic kidney disease patients on low-protein diets: A multicentre study with long-term outcome data (TOrino-Pisa study). Nephrol. Dial. Transpl. 2019, 35, 790–802. [Google Scholar] [CrossRef]
- Raphael, K.L. Metabolic Acidosis in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 263–275. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.; Leal-Escobar, G.; Garza-García, C.; Rodríguez-Castellanos, F. Dietary Acid Load: Mechanisms and evidence of its health repercussions. Nefrologia 2019, 39, 343–354. [Google Scholar] [CrossRef]
- Betz, M.V.; Penniston, K.L. Primary Contributors to Dietary Acid Load in Patients with Urolithiasis. J. Ren. Nutr. 2023, 33, 53–58. [Google Scholar] [CrossRef]
- Brown, L.; Luciano, A.; Pendergast, J.; Khairallah, P.; Anderson, C.A.; Sondheimer, J.; Hamm, L.L.; Ricardo, A.C.; Rahman, M.; Miller, E.R.; et al. Predictors of Net Acid Excretion in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2019, 74, 203–212. [Google Scholar] [CrossRef]
- Scialla, J.J.; Asplin, J.; Dobre, M.; Chang, A.R.; Lash, J.; Hsu, C.-Y.; Kallem, R.R.; Hamm, L.L.; Feldman, H.I.; Chen, J.; et al. Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int. 2017, 91, 204–215. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef]
- Goraya, N.; Munoz-Maldonado, Y.; Simoni, J.; Wesson, D.E. Fruit and Vegetable Treatment of Chronic Kidney Disease-Related Metabolic Acidosis Reduces Cardiovascular Risk Better than Sodium Bicarbonate. Am. J. Nephrol. 2019, 49, 438–448. [Google Scholar] [CrossRef]
- Goraya, N.; Munoz-Maldonado, Y.; Simoni, J.; Wesson, D.E. Treatment of Chronic Kidney Disease-Related Metabolic Acidosis with Fruits and Vegetables Compared to NaHCO. J. Ren. Nutr. 2021, 31, 239–247. [Google Scholar] [CrossRef]
- Joshi, S.; Shah, S.; Kalantar-Zadeh, K. Adequacy of Plant-Based Proteins in Chronic Kidney Disease. J. Ren. Nutr. 2019, 29, 112–117. [Google Scholar] [CrossRef]
- Burstad, K.M.; Cladis, D.P.; Wiese, G.N.; Butler, M.; Gallant, K.M.H. Effects of Plant-Based Protein Consumption on Kidney Function and Mineral Bone Disorder Outcomes in Adults With Stage 3-5 Chronic Kidney Disease: A Systematic Review. J. Ren. Nutr. 2023, 33, 717–730. [Google Scholar] [CrossRef]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. 2017, 27, 233–242. [Google Scholar] [CrossRef]
- Chauveau, P.; Koppe, L.; Combe, C.; Lasseur, C.; Trolonge, S.; Aparicio, M. Vegetarian diets and chronic kidney disease. Nephrol. Dial. Transpl. 2019, 34, 199–207. [Google Scholar] [CrossRef]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Tallman, D.A.; Khor, B.-H.; Karupaiah, T.; Khosla, P.; Chan, M.; Kopple, J.D. Nutritional Adequacy of Essential Nutrients in Low Protein Animal-Based and Plant-Based Diets in the United States for Chronic Kidney Disease Patients. J. Ren. Nutr. 2023, 33, 249–260. [Google Scholar] [CrossRef]
- Moe, S.M.; Drüeke, T.; Lameire, N.; Eknoyan, G. Chronic kidney disease-mineral-bone disorder: A new paradigm. Adv. Chronic Kidney Dis. 2007, 14, 3–12. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder: Synopsis of the Kidney Disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann. Intern. Med. 2018, 168, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Calvo, M.S. Hidden sources of phosphorus in the typical American diet: Does it matter in nephrology? Semin. Dial. 2003, 16, 186–188. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Armstrong, C.L.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2014, 40, 582–591. [Google Scholar] [CrossRef]
- Cupisti, A.; Kalantar-Zadeh, K. Management of natural and added dietary phosphorus burden in kidney disease. Semin. Nephrol. 2013, 33, 180–190. [Google Scholar] [CrossRef]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Murali, S.B.; Bross, R.; Nissenson, A.R.; Kopple, J.D. Dietary potassium intake and mortality in long-term hemodialysis patients. Am. J. Kidney Dis. 2010, 56, 338–347. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Fouque, D. Etiology-based dietary approach for managing hyperkalemia in people with chronic kidney disease. Nutr. Rev. 2022, 80, 2198–2205. [Google Scholar] [CrossRef]
- MacLaughlin, H.L.; Friedman, A.N.; Ikizler, T.A. Nutrition in Kidney Disease: Core Curriculum 2022. Am. J. Kidney Dis. 2022, 79, 437–449. [Google Scholar] [CrossRef]
- Sherman, R.A.; Mehta, O. Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1370–1373. [Google Scholar] [CrossRef]
- Clase, C.M.; Carrero, J.-J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Evenepoel, P.; Meijers, B.K.; Bammens, B.R.; Verbeke, K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. Suppl. 2009, 76, S12–S19. [Google Scholar] [CrossRef]
- Luis, D.; Zlatkis, K.; Comenge, B.; García, Z.; Navarro, J.F.; Lorenzo, V.; Carrero, J.J. Dietary Quality and Adherence to Dietary Recommendations in Patients Undergoing Hemodialysis. J. Ren. Nutr. 2016, 26, 190–195. [Google Scholar] [CrossRef]
- Biruete, A.; Shin, A.; Kistler, B.M.; Moe, S.M. Feeling gutted in chronic kidney disease (CKD): Gastrointestinal disorders and therapies to improve gastrointestinal health in individuals CKD, including those undergoing dialysis. Semin. Dial. 2021. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Moore, L.W.; Tortorici, A.R.; Chou, J.A.; St-Jules, D.E.; Aoun, A.; Rojas-Bautista, V.; Tschida, A.K.; Rhee, C.M.; Shah, A.A.; et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016, 17, 90. [Google Scholar] [CrossRef]
- Alexander, C.; Swanson, K.S.; Fahey, G.C., Jr.; Garleb, K.A. Perspective: Physiologic Importance of Short-Chain Fatty Acids from Nondigestible Carbohydrate Fermentation. Adv. Nutr. 2019, 10, 576–589. [Google Scholar] [CrossRef]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Su, G.; Qin, X.; Yang, C.; Sabatino, A.; Kelly, J.T.; Avesani, C.M.; Carrero, J.J. Fiber intake and health in people with chronic kidney disease. Clin. Kidney J. 2022, 15, 213–225. [Google Scholar] [CrossRef]
- Avila-Nava, A.; Noriega, L.G.; Tovar, A.R.; Granados, O.; Perez-Cruz, C.; Pedraza-Chaverri, J.; Torres, N. Food combination based on a pre-hispanic Mexican diet decreases metabolic and cognitive abnormalities and gut microbiota dysbiosis caused by a sucrose-enriched high-fat diet in rats. Mol. Nutr. Food Res. 2017, 61, 1501023. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Flores-López, A.G.; Aguilar-López, M.; Sánchez-Tapia, M.; Medina-Vera, I.; Díaz, D.; Tovar, A.R.; Torres, N. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome. J. Am. Heart Assoc. 2019, 8, e012401. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Méndez-Trujillo, V.; Hernández-Delgado, N.C.; Bermúdez-Humarán, L.G.; Reyes-Pavón, D. Looking inside Mexican Traditional Food as Sources of Synbiotics for Developing Novel Functional Products. Fermentation 2022, 8, 123. [Google Scholar] [CrossRef]
- Remes-Troche, J.M.; Taboada-Liceaga, H.; Gill, S.; Amieva-Balmori, M.; Rossi, M.; Hernández-Ramírez, G.; García-Mazcorro, J.F.; Whelan, K. Nopal fiber (Opuntia ficus-indica) improves symptoms in irritable bowel syndrome in the short term: A randomized controlled trial. Neurogastroenterol. Motil. 2021, 33, e13986. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; He, X.; Chin, E.L.; Tovar, A.R.; Torres, N.; Slupsky, C.M.; Raybould, H.E. Nopal feeding reduces adiposity, intestinal inflammation and shifts the cecal microbiota and metabolism in high-fat fed rats. PLoS ONE 2017, 12, e0171672. [Google Scholar] [CrossRef]
- Wiese, G.N.; Biruete, A.; Moorthi, R.N.; Moe, S.M.; Lindemann, S.R.; Gallant, K.M.H. Plant-Based Diets, the Gut Microbiota, and Trimethylamine N-Oxide Production in Chronic Kidney Disease: Therapeutic Potential and Methodological Considerations. J. Ren. Nutr. 2020, 31, 121–131. [Google Scholar] [CrossRef]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef]
- Suliman, M.E.; Heimbürger, O.; Bárány, P.; Anderstam, B.; Pecoits-Filho, R.; Ayala, E.R.; Qureshi, A.R.; Fehrman-Ekholm, I.; Lindholm, B.; Stenvinkel, P. Plasma pentosidine is associated with inflammation and malnutrition in end-stage renal disease patients starting on dialysis therapy. J. Am. Soc. Nephrol. 2003, 14, 1614–1622. [Google Scholar] [CrossRef]
- Aveles, P.R.; Criminácio, C.R.; Gonçalves, S.; Bignelli, A.T.; Claro, L.M.; Siqueira, S.S.; Nakao, L.S.; Pecoits-Filho, R. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin. Pract. 2010, 116, c294–c299. [Google Scholar] [CrossRef]
- Ori, Y.; Bergman, M.; Bessler, H.; Zingerman, B.; Levy-Drummer, R.S.; Gafter, U.; Salman, H. Cytokine secretion and markers of inflammation in relation to acidosis among chronic hemodialysis patients. Blood Purif. 2013, 35, 181–186. [Google Scholar] [CrossRef]
- Carrero, J.J.; Kistler, B.M.; Stenvinkel, P. Inflammation in Chronic Kidney Disease, 4th ed.; Kopple, J.D., Massry, S.G., Kalantar-Zadeh, K., Fouque, D., Eds.; Nutritional Management of Renal Disease; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Woods, J.A.; Wilund, K.R.; Martin, S.A.; Kistler, B.M. Exercise, inflammation and aging. Aging Dis. 2012, 3, 130–140. [Google Scholar]
- Perales-Vázquez, G.D.C.; Mercado-Mercado, G.; Rosa, L.A.; Sáyago-Ayerdi, S.G. Bioaccesibilidad y cinética de liberación in vitro de compuestos fenólicos en algunas salsas de la cocina mexicana. Rev. Espec. Cienc. Químico-Biológicas 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Goody, C.M.; Drago, L. Using Cultural Competence Constructs to Understand Food Practices and Provide Diabetes Care and Education. Diabetes Spectr. 2009, 22, 43–47. [Google Scholar] [CrossRef][Green Version]
- Edwards, C.; Orellana, E.; Rawlings, K.; Rodriguez-Pla, M.; Venkatesan, A. Changes in Glycemic Control Following Use of a Spanish-Language, Culturally Adapted Diabetes Program: Retrospective Study. JMIR Form. Res. 2022, 6, e40278. [Google Scholar] [CrossRef]
- Aoun, C.; Papazian, T.; Helou, K.; El Osta, N.; Khabbaz, L.R. Comparison of five international indices of adherence to the Mediterranean diet among healthy adults: Similarities and differences. Nutr. Res. Pract. 2019, 13, 333–343. [Google Scholar] [CrossRef]
- Folsom, A.R.; Parker, E.D.; Harnack, L.J. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am. J. Hypertens 2007, 20, 225–232. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; Tinker, L.F.; Allison, M.A.; Breymeyer, K.L.; Garcia, L.; Kroenke, C.H.; Lampe, J.W.; Shikany, J.M.; Van Horn, L.; Neuhouser, M.L. Development and Use of a Traditional Mexican Diet Score in Relation to Systemic Inflammation and Insulin Resistance among Women of Mexican Descent. J. Nutr. 2015, 145, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Torres, M.; Kratz, M.; Lampe, J.W.; Tapsoba, J.D.D.; Breymeyer, K.L.; Levy, L.; Villaseñor, A.; Wang, C.-Y.; Song, X.; Neuhouser, M.L. Metabolic responses to a traditional Mexican diet compared with a commonly consumed US diet in women of Mexican descent: A randomized crossover feeding trial. Am. J. Clin. Nutr. 2016, 103, 366–374. [Google Scholar] [CrossRef]
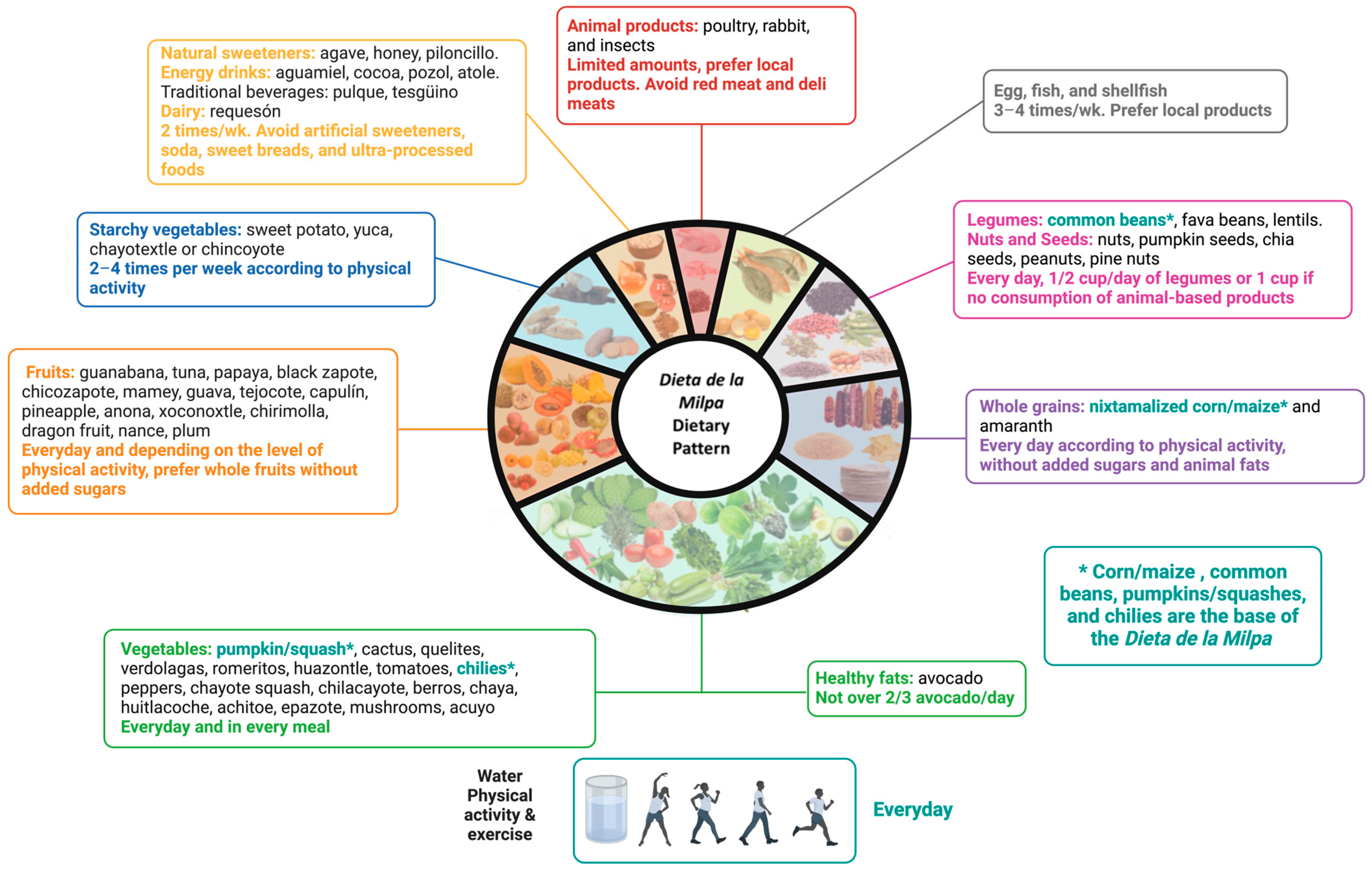
| Component | Examples [20] | Characteristics and Recommendations [20] | Considerations for People with CKD |
|---|---|---|---|
| (1) Vegetables | Nopal (cactus), tomatoes, green beans, quelites, quintile, verdolagas (purslane), romeritos, huazontle, green tomatoes, chilies, bell peppers, squash, chayote squash, chilacayote, colorines, izote flower, jicama, watercress, chaya, huitlacoche, achiote, epazote, vanilla, Mexican pepperleaf, mushrooms, and others. | Eat a combination of these daily in high quantities; make them the base of your plate. Nutrient-dense foods, high in dietary fiber, vitamins, and minerals. | Consider potassium content per portion and cooking methods to reduce potassium content [29]. Consider the use of potassium binders, as described by Sussman et al. [30] in order to liberalize the diet in those at risk of hyperkalemia. |
| (2) Starchy vegetables | Sweet potatoes, yuca, chayotextle. | 2–4 times per week considering physical activity. Combine them with legumes and vegetables | Consider potassium content per portion [29]. Consider carbohydrate content and its impact on insulin release, as this may limit the rise in serum potassium [29,31,32]. |
| (3) Fruits | Guanabana (soursop), tuna, papaya, black zapote, chicozapote, mamey, guava, tejocote, capulin, pineapple, anona, xoconostle, chirimoya (custard Apple), nance, berries, yellow plum, dragon fruit. | Consume daily according to physical activity. These foods are high in dietary fiber, vitamins, minerals, antioxidants and should not be consumed with added sugar. Whole fruit consumption is recommended rather than juice. | Consider potassium content per portion [29]. Consider carbohydrate content and its impact on insulin release, as this may limit the rise in serum potassium [29,31,32]. The objective is to maintain serum potassium < 5.5 mmol/L (ideal < 4 mmol/L) [32]. |
| (4) Legumes and (5) nuts | Common beans, lima beans, squash seeds (pepitas), chickpeas, lentils, chia seeds, peanuts, and pine nuts. | Consume daily ½ to 1 cup, prioritize daily consumption if animal-based proteins are not consumed. These foods are high in plant-based proteins, dietary fiber, iron, fat, and B-vitamins. | Consider cooking methods to reduce potassium and phosphorus in beans [29,33]. Consider portion control [29,33]. |
| (6) Healthy fats | Avocado. | Not more than ¾ of an avocado daily. High in dietary fiber, potassium, vitamin E, vitamin C, and monounsaturated fats. | Consider potassium content per portion [29]. |
| (7) Whole grains | Corn/maize, amaranth. | Daily according to physical activity; consider sources without added sugars and animal-based fats. High in energy, dietary fiber, calcium, iron, folic acid, phosphorus (phytate-bound), potassium. | Consider potassium content per portion [29]. Consider carbohydrate content and its impact on insulin release, as this may limit the rise in serum potassium [29,31,32]. |
| (8) Animal protein a. Eggs and seafood b. Poultry c. Insects | Eggs, catfish, trout, white fish, bass, mojarra, sierra, crab, mussels, oysters, acamayas, octopus, shrimp. Local chicken and turkey. Crickets, maguey worms, chinicuiles, chicatana ant, honey ant, jumiles. | With poultry and insects, 3–4 times per week. Moderate consumption of these foods is recommended. Combine with vegetables. Sources of protein and phosphorus. Insects are also sources of dietary fiber. | Consider protein depending on the stage of CKD [6]. Limited research on insects in CKD. |
| (9) Dairy | Requesón | ≤2 portions per week. Source of protein, phosphorus, calcium, and probiotic strains. | Consider sodium, potassium, and phosphorus content per portion [8,29,33]. |
| (10) Honey and sweeteners | Honey, agave nectar, piloncillo. | Not more than 2 teaspoons of piloncillo or honey in healthy individuals. | Consider sugar content [34]. |
| Water and fermented beverages | Water, pozol, aguamiel of maguey, chocolate, tesgüino. | Prefer water consumption. Limit consumption of fermented beverages high in sugar. | Consider sugar content [34]. |
| Characteristics | Advantages | Disadvantages | |
|---|---|---|---|
| Mediterranean Diet |
|
|
|
| DASH Diet |
|
|
|
| Dieta de la Milpa |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biruete, A.; Leal-Escobar, G.; Espinosa-Cuevas, Á.; Mojica, L.; Kistler, B.M. Dieta de la Milpa: A Culturally-Concordant Plant-Based Dietary Pattern for Hispanic/Latine People with Chronic Kidney Disease. Nutrients 2024, 16, 574. https://doi.org/10.3390/nu16050574
Biruete A, Leal-Escobar G, Espinosa-Cuevas Á, Mojica L, Kistler BM. Dieta de la Milpa: A Culturally-Concordant Plant-Based Dietary Pattern for Hispanic/Latine People with Chronic Kidney Disease. Nutrients. 2024; 16(5):574. https://doi.org/10.3390/nu16050574
Chicago/Turabian StyleBiruete, Annabel, Gabriela Leal-Escobar, Ángeles Espinosa-Cuevas, Luis Mojica, and Brandon M. Kistler. 2024. "Dieta de la Milpa: A Culturally-Concordant Plant-Based Dietary Pattern for Hispanic/Latine People with Chronic Kidney Disease" Nutrients 16, no. 5: 574. https://doi.org/10.3390/nu16050574
APA StyleBiruete, A., Leal-Escobar, G., Espinosa-Cuevas, Á., Mojica, L., & Kistler, B. M. (2024). Dieta de la Milpa: A Culturally-Concordant Plant-Based Dietary Pattern for Hispanic/Latine People with Chronic Kidney Disease. Nutrients, 16(5), 574. https://doi.org/10.3390/nu16050574






