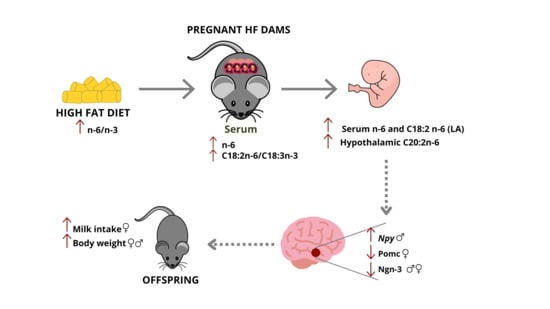
Sex-Dependent Variations in Hypothalamic Fatty Acid Profile and Neuropeptides in Offspring Exposed to Maternal Obesity and High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animal Study
2.3. Serum Analysis
2.4. Fetal Sexing
2.5. Quantitative Real-Time PCR (qPCR)
2.6. Chromatography Coupled to Mass Spectrometry (GC-MS)
2.7. Western Blot
2.8. Immunofluorescence
2.9. Cell Culture
2.10. Statistical Analysis
3. Results
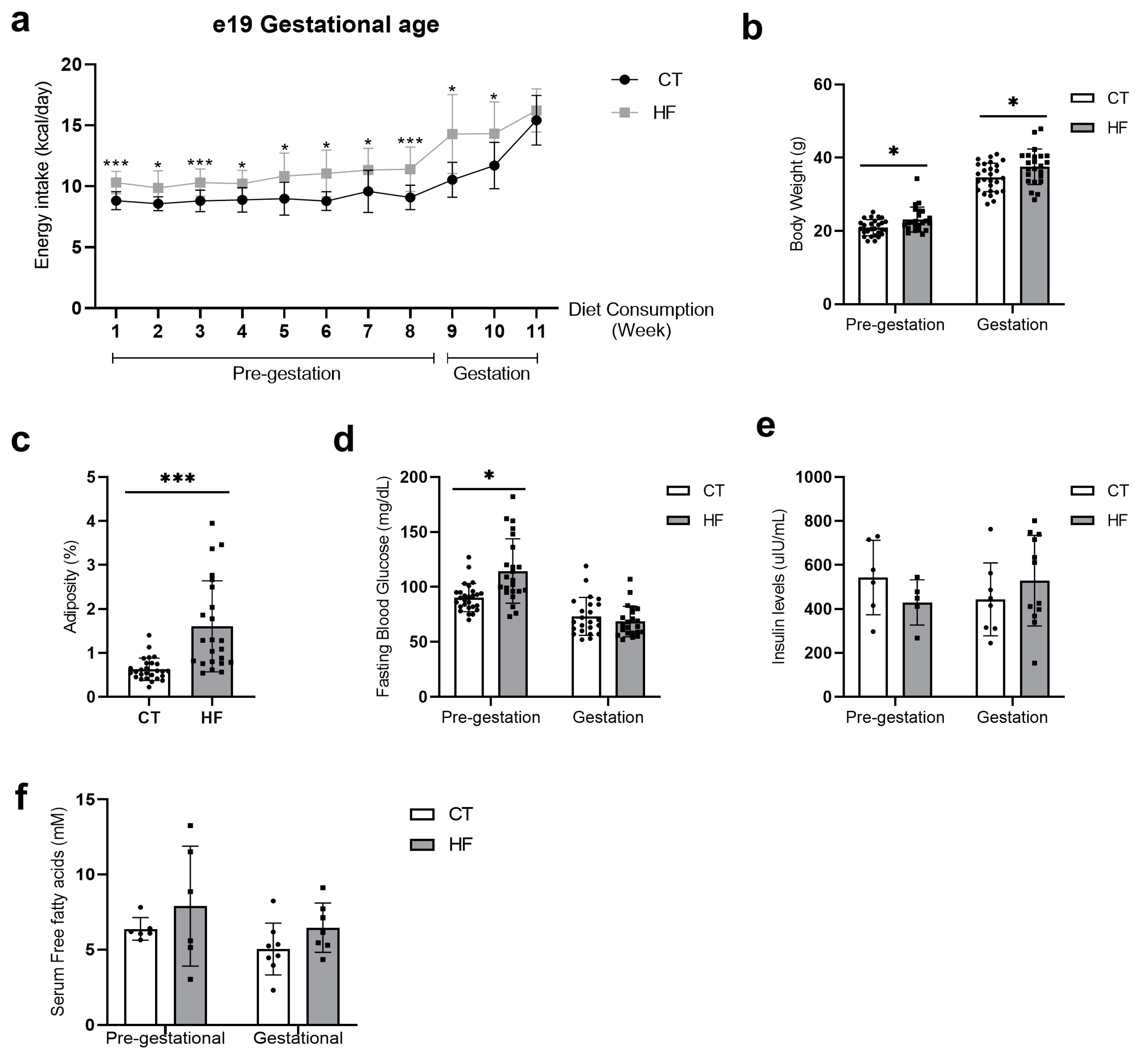
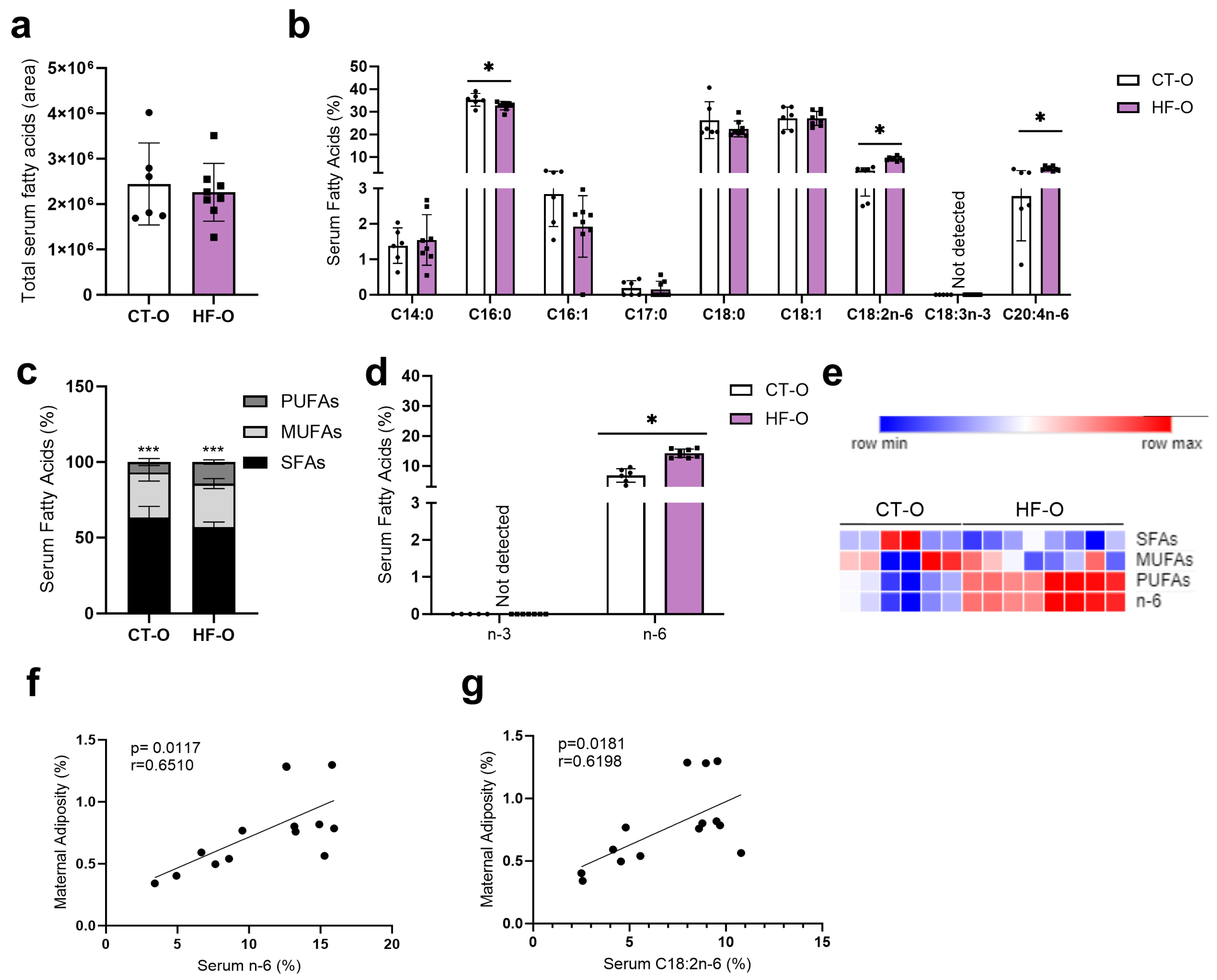
3.1. Prepregnancy and Pregnancy HF Diet Consumption Promotes Increased Maternal Adiposity and Alters Serum Fatty Acid Profile
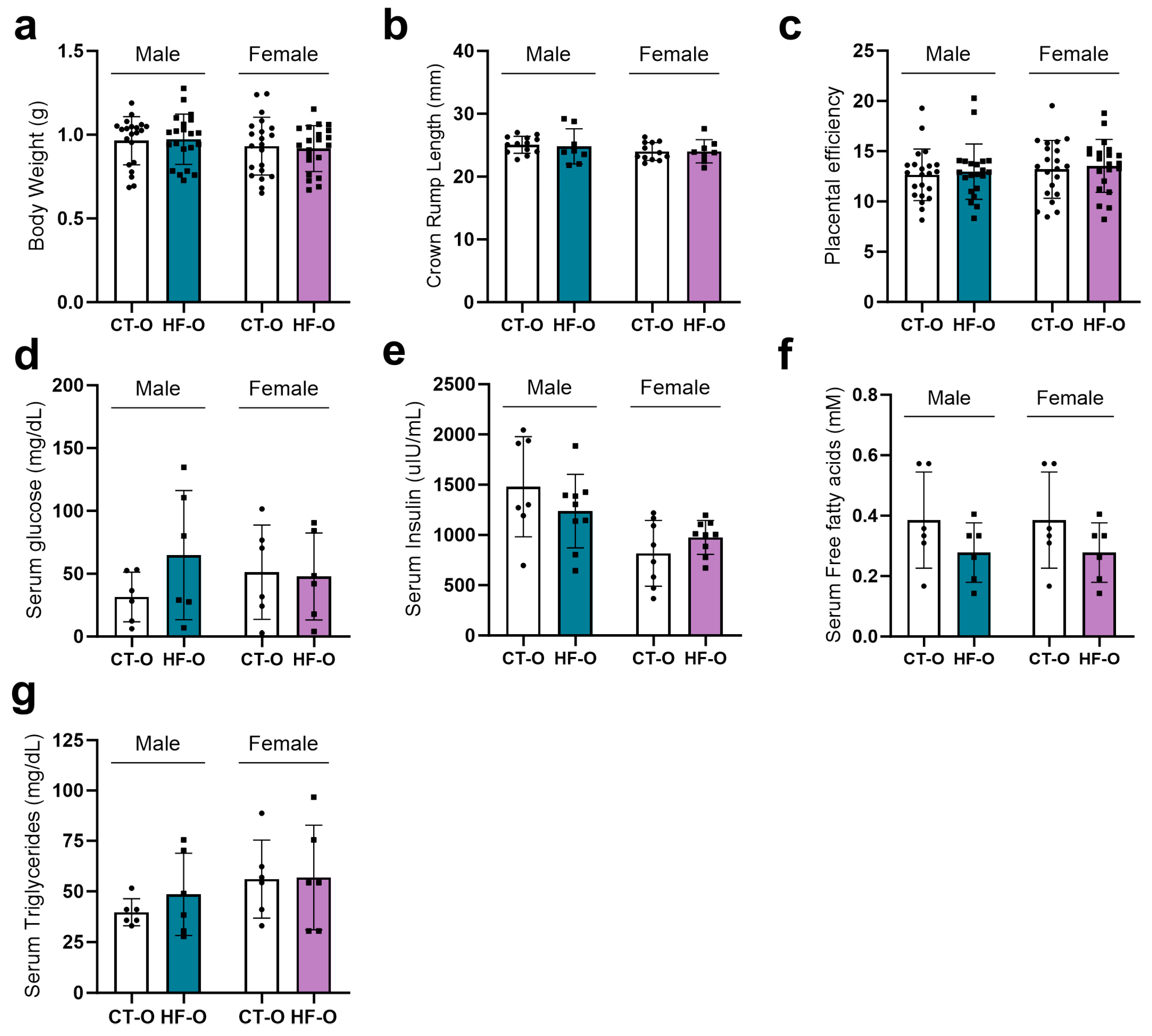
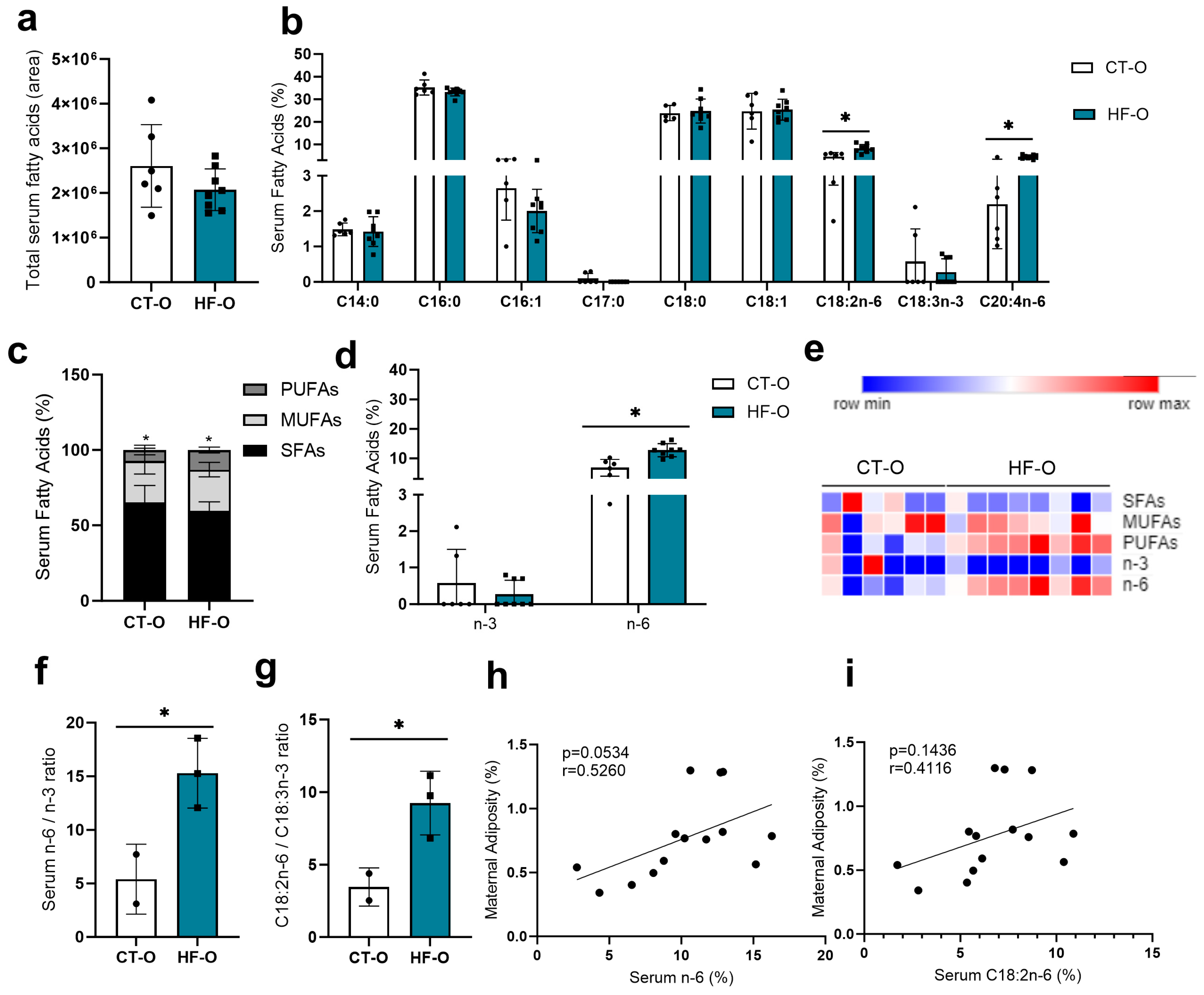
3.2. Sex-Specific Alteration in Serum Fatty Acid Profile in Fetuses of the Maternal HF Diet
3.3. Hypothalamic Fatty Acid C20:2n-6 Is Associated with Maternal Adiposity
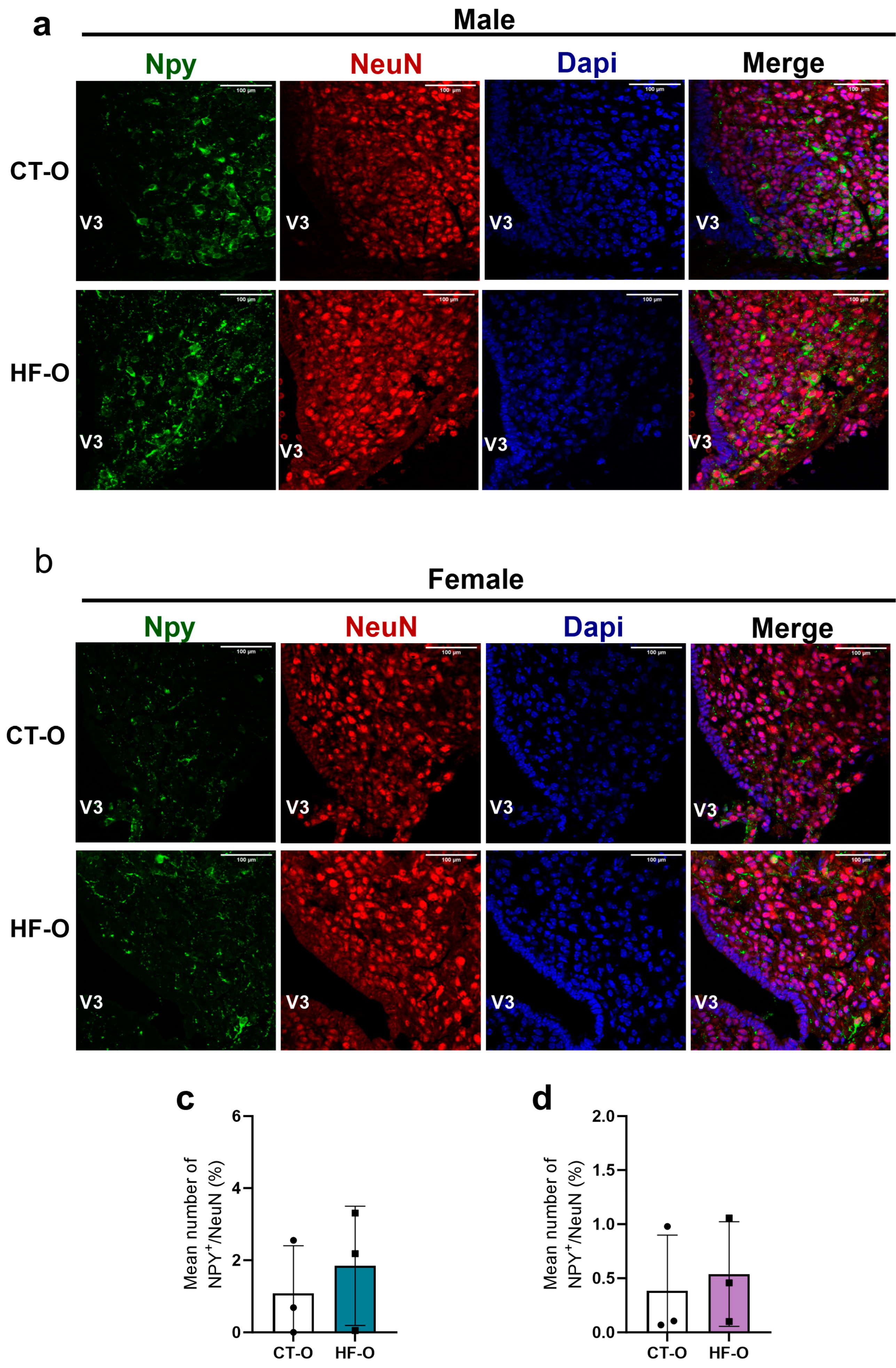
3.4. Sex-Specific Changes in Expression of Hypothalamic Neuropeptides (NPY, POMC) and the bHLH Gene in HF-O Fetuses
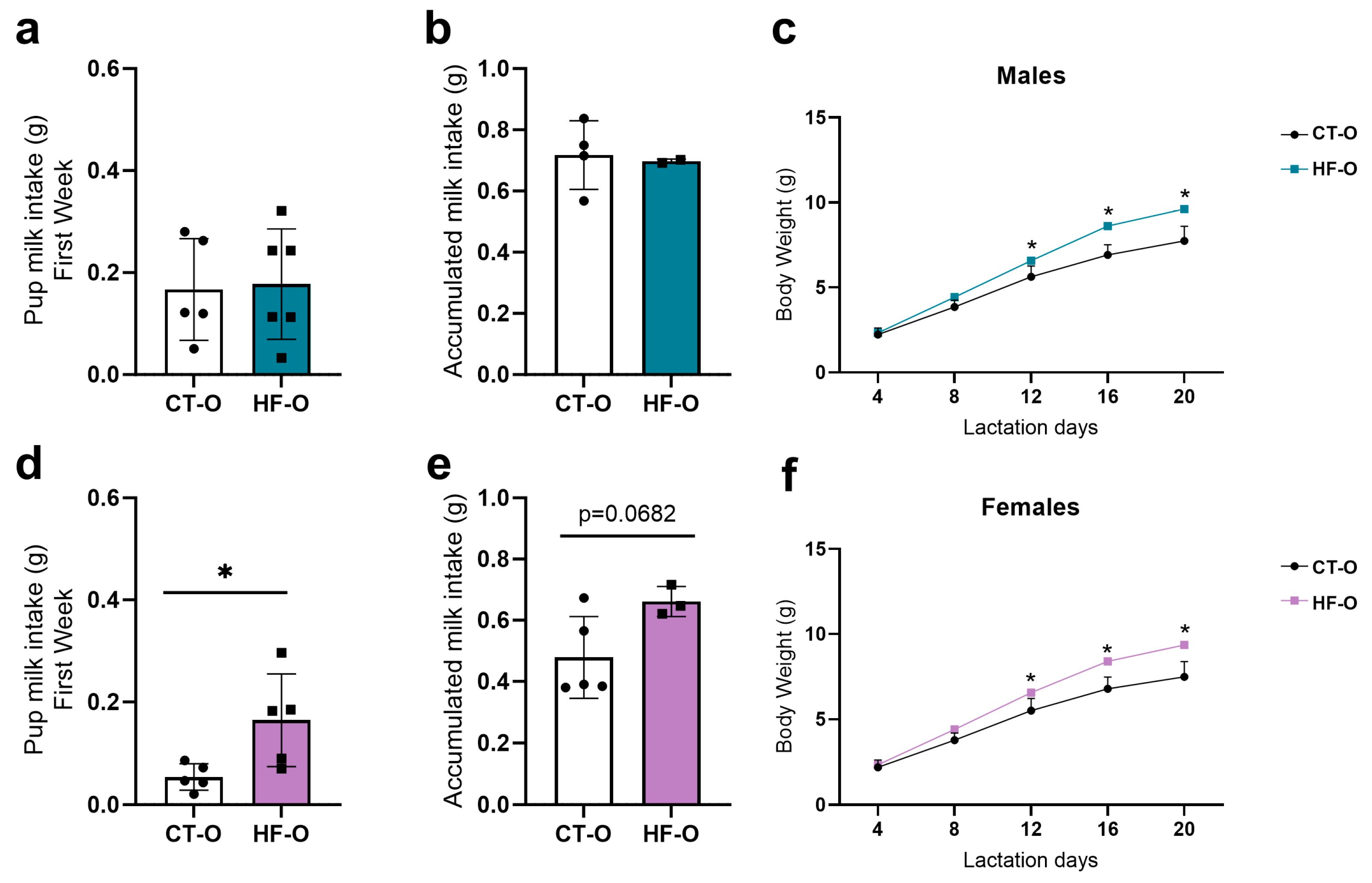
3.5. Fetal Changes in the Expression of Hypothalamic Neuropeptides and bHLH Factors Impact Milk Intake and Increase Weight Gain in HF-O
3.6. In Vitro Treatment of Hypothalamic Neuronal Cell Line with Linoleic Acid (LA) in Reduces Expression of Pomc and Ngn3 Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bentham, J.; Di Cesare, M.; Bilano, V.; Bixby, H.; Zhou, B.; Stevens, G.A.; Riley, L.M.; Taddei, C.; Hajifathalian, K.; Lu, Y.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Ministério da Saúde Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico Estimativas Sobre Frequência e Distribuição Sociodemográfica de Fatores de Risco e Proteção 2021. 2022. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/vigitel/vigitel-brasil-2021-estimativas-sobre-frequencia-e-distribuicao-sociodemografica-de-risk-and-protection-factors-for-chronic-diseases (accessed on 22 September 2023).
- Chu, S.Y.; Kim, S.Y.; Bish, C.L. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern. Child Health J. 2009, 13, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.; Barros, F.C.; Buffarini, R.; Horta, B.L.; Menezes, A.M.B.; Barros, A.J.D.; Domingues, M.R.; Victora, C.G.; Pelotas Cohorts Study Group. Infant nutrition and growth: Trends and inequalities in four population-based birth cohorts in Pelotas, Brazil, 1982–2015. Int. J. Epidemiol. 2019, 48, i80. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The fetal and infant origins of adult disease. BMJ Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237.e1–237.e13. [Google Scholar] [CrossRef] [PubMed]
- Poher, A.L.; Arsenijevic, D.; Asrih, M.; Dulloo, A.G.; Jornayvaz, F.R.; Rohner-Jeanrenaud, F.; Veyrat-Durebex, C. Preserving of Postnatal Leptin Signaling in Obesity-Resistant Lou/C Rats following a Perinatal High-Fat Diet. PLoS ONE 2016, 11, e0162517. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sjölander, A.; Långström, N.; Rodriguez, A.; Serlachius, E.; D’Onofrio, B.M.; Lichtenstein, P.; Larsson, H. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: A population-based cohort study using a sibling-comparison design. Int. J. Epidemiol. 2014, 43, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tylavsky, F.A.; Han, J.C.; Kocak, M.; Fowke, J.H.; Davis, R.L.; Lewinn, K.; Bush, N.R.; Zhao, Q. Maternal metabolic factors during pregnancy predict early childhood growth trajectories and obesity risk: The CANDLE Study. Int. J. Obes. 2019, 43, 1914–1922. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Sandboge, S.; Salonen, M.K.; Kajantie, E.; Osmond, C. Long-term consequences of maternal overweight in pregnancy on offspring later health: Findings from the Helsinki Birth Cohort Study. Ann. Med. 2014, 46, 434–438. [Google Scholar] [CrossRef]
- De Fante, T.; Simino, L.A.; Reginato, A.; Payolla, T.B.; Vitoréli, D.C.G.; De Souza, M.; Torsoni, M.A.; Milanski, M.; Torsoni, A.S. Diet-Induced Maternal Obesity Alters Insulin Signalling in Male Mice Offspring Rechallenged with a High-Fat Diet in Adulthood. PLoS ONE 2016, 11, e0160184. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53. [Google Scholar] [CrossRef]
- Wallace, J.G.; Bellissimo, C.J.; Yeo, E.; Fei Xia, Y.; Petrik, J.J.; Surette, M.G.; Bowdish, D.M.; Sloboda, D.M. Obesity during pregnancy results in maternal intestinal inflammation, placental hypoxia, and alters fetal glucose metabolism at mid-gestation. Sci. Rep. 2019, 9, 17621. [Google Scholar] [CrossRef] [PubMed]
- Simino, L.A.D.P.; Panzarin, C.; Torsoni, M.A.; Ignácio-Souza, L.M.; Milanski, M.; Torsoni, A.S. Maternal resistance to diet-induced obesity partially protects newborn and post-weaning male mice offspring from metabolic disturbances. J. Dev. Orig. Health Dis. 2021, 12, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, D.; Abrahante Llorens, J.E.; Buckley, L.; Tkac, I.; Rao, R.; Paulsen, M.E. Female and male C57BL/6J offspring exposed to maternal obesogenic diet develop altered hypothalamic energy metabolism in adulthood. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E448–E466. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Ferrini, M.G.; Han, G.; Narwani, K.; Ross, M.G. Maternal High Fat Diet Programs Male Mice Offspring Hyperphagia and Obesity: Mechanism of Increased Appetite Neurons via Altered Neurogenic Factors and Nutrient Sensor AMPK. Nutrients 2020, 12, 3326. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Central and peripheral control of food intake. Endocr. Regul. 2017, 51, 52–70. [Google Scholar] [CrossRef]
- Toda, C.; Santoro, A.; Kim, J.D.; Diano, S. POMC Neurons: From Birth to Death. Annu. Rev. Physiol. 2017, 79, 209. [Google Scholar] [CrossRef]
- Dearden, L.; Buller, S.; Furigo, I.C.; Fernandez-Twinn, D.S.; Ozanne, S.E. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol. Metab. 2020, 42, 101079. [Google Scholar] [CrossRef]
- McNay, D.E.G.; Pelling, M.; Claxton, S.; Guillemot, F.; Ang, S.L. Mash1 Is Required for Generic and Subtype Differentiation of Hypothalamic Neuroendocrine Cells. Mol. Endocrinol. 2006, 20, 1623–1632. [Google Scholar] [CrossRef]
- Pelling, M.; Anthwal, N.; McNay, D.; Gradwohl, G.; Leiter, A.B.; Guillemot, F.; Ang, S.L. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev. Biol. 2011, 349, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Lemes, S.F.; de Souza, A.C.P.; Payolla, T.B.; Versutti, M.D.; da Silva Ramalho, A.D.F.; Mendes-da-Silva, C.; Souza, C.M.; Milanski, M.; Torsoni, A.S.; Torsoni, M.A. Maternal Consumption of High-fat Diet in Mice Alters Hypothalamic Notch Pathway, NPY Cell Population and Food Intake in Offspring. Neuroscience 2018, 371, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Han, G.; Ross, M.G. Programmed Hyperphagia in Offspring of Obese Dams: Altered Expression of Hypothalamic Nutrient Sensors, Neurogenic Factors and Epigenetic Modulators. Appetite 2016, 99, 193. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Stiles, L.; Torsoni, A.S.; Torsoni, M.A.; Shirihai, O.S.; Ross, M.G. TNFα-Induced Oxidative Stress and Mitochondrial Dysfunction Alter Hypothalamic Neurogenesis and Promote Appetite Versus Satiety Neuropeptide Expression in Mice. Brain Sci. 2022, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Essential fatty acid transfer and fetal development. Placenta 2005, 26, S70–S75. [Google Scholar] [CrossRef]
- Cetin, I.; Parisi, F.; Berti, C.; Mandó, C.; Desoye, G. Placental fatty acid transport in maternal obesity. J. Dev. Orig. Health Dis. 2012, 3, 409–414. [Google Scholar] [CrossRef]
- Song, L.; Wang, N.; Peng, Y.; Sun, B.; Cui, W. Placental lipid transport and content in response to maternal overweight and gestational diabetes mellitus in human term placenta. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 692–702. [Google Scholar] [CrossRef]
- Costa, S.M.R.; Isganaitis, E.; Matthews, T.J.; Hughes, K.; Daher, G.; Dreyfuss, J.M.; da Silva, G.A.P.; Patti, M.E. Maternal obesity programs mitochondrial and lipid metabolism gene expression in infant umbilical vein endothelial cells. Int. J. Obes. 2016, 40, 1627. [Google Scholar] [CrossRef]
- Reginato, A.; Siqueira, B.P.; Miyamoto, J.É.; Portovedo, M.; Costa, S.d.O.; de Fante, T.; Rodrigues, H.G.; Ignácio-Souza, L.M.; Torsoni, M.A.; Torsoni, A.S.; et al. Acute effects of fatty acids on autophagy in NPY neurones. J. Neuroendocr. 2020, 32, e12900. [Google Scholar] [CrossRef]
- Dalvi, P.S.; Chalmers, J.A.; Luo, V.; Han, D.Y.; Wellhauser, L.; Liu, Y.; Tran, D.Q.; Castel, J.; Luquet, S.; Wheeler, M.B.; et al. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int. J. Obes. 2017, 41, 149–158. [Google Scholar] [CrossRef]
- Wang, X.; Ge, A.; Cheng, M.; Guo, F.; Zhao, M.; Zhou, X.; Liu, L.; Yang, N. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp. Diabetes Res. 2012, 2012, 847246. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Zhang, Q.; Wang, J.; Zhan, J.; Xian, X.; Du, Z.; Wang, X.; Hao, A. Palmitic acid affects proliferation and differentiation of neural stem cells in vitro. J. Neurosci. Res. 2014, 92, 574–586. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Curi, R.; Maechler, P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J. Cell. Physiol. 2010, 222, 187–194. [Google Scholar] [CrossRef]
- Patková, J.; Anděl, M.; Trnka, J. Palmitate-Induced Cell Death and Mitochondrial Respiratory Dysfunction in Myoblasts are Not Prevented by Mitochondria-Targeted Antioxidants. Cell. Physiol. Biochem. 2014, 33, 1439–1451. [Google Scholar] [CrossRef]
- Xavier, J.M.; Rodrigues, C.M.P.; Solá, S. Mitochondria: Major Regulators of Neural Development. Neuroscientist 2015, 22, 346–358. [Google Scholar] [CrossRef]
- Wang, W.; Osenbroch, P.; Skinnes, R.; Esbensen, Y.; Bjørås, M.; Eide, L. Mitochondrial DNA Integrity Is Essential for Mitochondrial Maturation during Differentiation of Neural Stem Cells. Stem Cells 2010, 28, 2195–2204. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Cheng, L.; Yu, Y.; Zhang, Q.; Szabo, A.; Wang, H.; Huang, X.F. Arachidonic acid impairs hypothalamic leptin signaling and hepatic energy homeostasis in mice. Mol. Cell. Endocrinol. 2015, 412, 12–18. [Google Scholar] [CrossRef]
- Hernandez, L.L.; Grayson, B.E.; Yadav, E.; Seeley, R.J.; Horseman, N.D. High Fat Diet Alters Lactation Outcomes: Possible Involvement of Inflammatory and Serotonergic Pathways. PLoS ONE 2012, 7, 32598. [Google Scholar] [CrossRef]
- Shirai, N.; Suzuki, H.; Wada, S. Direct methylation from mouse plasma and from liver and brain homogenates. Anal. Biochem. 2005, 343, 48–53. [Google Scholar] [CrossRef]
- Fante, T.; Simino, L.A.P.; Fontana, M.F.; Reginato, A.; Ramalheira, T.G.; Rodrigues, H.G.; Lisboa, P.C.; de Moura, E.G.; Ignácio-Souza, L.M.; Milanski, M.; et al. Maternal high-fat diet consumption programs male offspring to mitigate complications in liver regeneration. J. Dev. Orig. Health Dis. 2022, 13, 575–582. [Google Scholar] [CrossRef]
- Gali Ramamoorthy, T.; Allen, T.J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Layé, S. Perinatal Dietary Polyunsaturated Fatty Acids in Brain Development, Role in Neurodevelopmental Disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef]
- Haggarty, P.; Ashton, J.; Joynson, M.; Abramovich, D.R.; Page, K. Effect of Maternal Polyunsaturated Fatty Acid Concentration on Transport by the Human Placenta. Biol Neonate 1999, 75, 350–359. [Google Scholar] [CrossRef]
- Vaidya, H.; Cheema, S.K. Breastmilk with a high omega-6 to omega-3 fatty acid ratio induced cellular events similar to insulin resistance and obesity in 3T3-L1 adipocytes. Pediatr. Obes. 2018, 13, 285–291. [Google Scholar] [CrossRef]
- Suarez-Trujillo, A.; Huff, K.; Ramires Ferreira, C.; Paschoal Sobreira, T.J.; Buhman, K.K.; Casey, T. High-fat-diet induced obesity increases the proportion of linoleic acyl residues in dam serum and milk and in suckling neonate circulation. Biol. Reprod. 2020, 103, 736–749. [Google Scholar] [CrossRef]
- Haggarty, P.; Page, K.; Abramovich, D.R.; Ashton, J.; Brown, D. Long-chain polyunsaturated fatty acid transport across the perfused human placenta. Placenta 1997, 18, 635–642. [Google Scholar] [CrossRef]
- Sanches, A.P.V.; de Oliveira, J.L.; Ferreira, M.S.; Lima, B.d.S.; Miyamoto, J.É.; Simino, L.A.d.P.; Torsoni, M.A.; Torsoni, A.S.; Milanski, M.; Ignácio-Souza, L. Obesity phenotype induced by high-fat diet leads to maternal-fetal constraint, placental inefficiency, and fetal growth restriction in mice. J. Nutr. Biochem. 2022, 104, 108977. [Google Scholar] [CrossRef]
- Gibson, R.A.; Muhlhausler, B.; Makrides, M.; Gibson, R. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 2011, 7, 17–26. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Byard, R.W.; Simmer, K.; Gibson, R.A. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 1994, 60, 189–194. [Google Scholar] [CrossRef]
- Haggarty, P. Fatty Acid Supply to the Human Fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef]
- Lauritzen, L.; Hansen, H.S.; Jorgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Swyer, P.R.; Chance, G.W. Intrauterine fatty acid accretion rates in human brain: Implications for fatty acid requirements. Early Hum. Dev. 1980, 4, 121–129. [Google Scholar] [CrossRef]
- Percy, P.; Percy, A.; Vilbergsson, G.; Månsson, J.E. Polyunsaturated Fatty Acid Accretion in First- and Second-Trimester Human Fetal Brain: Lack of Correlation with Levels in Paired Placental Samples. Biochem. Mol. Med. 1996, 59, 38–43. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Dinel, A.L.; Aubert, A.; Fressange-Mazda, C.; Le Ruyet, P.; Layé, S. Impact of Lactobacillus fermentum and dairy lipids in the maternal diet on the fatty acid composition of pups’ brain and peripheral tissues. Prostaglandins Leukot. Essent. Fatty Acids 2016, 115, 24–34. [Google Scholar] [CrossRef]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- Champeil-Potokar, G.; Chaumontet, C.; Guesnet, P.; Lavialle, M.; Denis, I. Docosahexaenoic acid (22:6n-3) enrichment of membrane phospholipids increases gap junction coupling capacity in cultured astrocytes. Eur. J. Neurosci. 2006, 24, 3084–3090. [Google Scholar] [CrossRef]
- Rey, C.; Nadjar, A.; Joffre, F.; Amadieu, C.; Aubert, A.; Vaysse, C.; Pallet, V.; Layé, S.; Joffre, C. Maternal n-3 polyunsaturated fatty acid dietary supply modulates microglia lipid content in the offspring. Prostaglandins Leukot. Essent. Fat. Acids 2018, 133, 1–7. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Gehman, M.F.; Yeh, S.-M. Maternal dietary fish oil enriches docosahexaenoate levels in brain subcellular fractions of offspring. J. Neurosci. Res. 1993, 35, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Sá Vido, D.; Nejm, M.B.; Silva, N.R.; Maria, S.; Silva, A.; Cravo, S.L.; Luz, J. Maternal obesity and late effects on offspring metabolism. Arq. Bras. Endocrinol. Metabol. 2014, 58, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Nozumi, M.; Ito, Y.; Natsume, R.; Kawasaki, A.; Nakatsu, F.; Abe, M.; Uchino, H.; Matsushita, N.; Ikeda, K.; et al. Very-long-chain fatty acids are crucial to neuronal polarity by providing sphingolipids to lipid rafts. Cell Rep. 2023, 42, 113195. [Google Scholar] [CrossRef]
- Hejr, H.; Ghareghani, M.; Zibara, K.; Ghafari, M.; Sadri, F.; Salehpour, Z.; Hamedi, A.; Negintaji, K.; Azari, H.; Ghanbari, A. The ratio of 1/3 linoleic acid to alpha linolenic acid is optimal for oligodendrogenesis of embryonic neural stem cells. Neurosci. Lett. 2017, 651, 216–225. [Google Scholar] [CrossRef]
- Reginato, A.; de Fante, T.; Portovedo, M.; da Costa, N.F.; Payolla, T.B.; Miyamotto, J.É.; Simino, L.A.; Ignácio-Souza, L.M.; Torsoni, M.A.; Torsoni, A.S.; et al. Autophagy proteins are modulated in the liver and hypothalamus of the offspring of mice with diet-induced obesity. J. Nutr. Biochem. 2016, 34, 30–41. [Google Scholar] [CrossRef]
- Payolla, T.B.; Lemes, S.F.; de Fante, T.; Reginato, A.; Mendes da Silva, C.; de Oliveira Micheletti, T.; Rodrigues, H.G.; Torsoni, A.S.; Milanski, M.; Torsoni, M.A. High-fat diet during pregnancy and lactation impairs the cholinergic anti-inflammatory pathway in the liver and white adipose tissue of mouse offspring. Mol. Cell. Endocrinol. 2016, 422, 192–202. [Google Scholar] [CrossRef]






| Control Diet | HF Diet | |
|---|---|---|
| Carbohydrates (%) | 70 | 35 |
| Proteins (%) | 20 | 20 |
| Lipids (%) | 10 | 45 |
| Energy Density (kcal/g) | 3.82 | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baqueiro, M.d.N.; Simino, L.A.d.P.; Costa, J.P.; Panzarin, C.; Reginato, A.; Torsoni, M.A.; Ignácio-Souza, L.; Milanski, M.; Ross, M.G.; Coca, K.P.; et al. Sex-Dependent Variations in Hypothalamic Fatty Acid Profile and Neuropeptides in Offspring Exposed to Maternal Obesity and High-Fat Diet. Nutrients 2024, 16, 340. https://doi.org/10.3390/nu16030340
Baqueiro MdN, Simino LAdP, Costa JP, Panzarin C, Reginato A, Torsoni MA, Ignácio-Souza L, Milanski M, Ross MG, Coca KP, et al. Sex-Dependent Variations in Hypothalamic Fatty Acid Profile and Neuropeptides in Offspring Exposed to Maternal Obesity and High-Fat Diet. Nutrients. 2024; 16(3):340. https://doi.org/10.3390/nu16030340
Chicago/Turabian StyleBaqueiro, Mayara da Nóbrega, Laís Angélica de Paula Simino, João Paulo Costa, Carolina Panzarin, Andressa Reginato, Marcio Alberto Torsoni, Letícia Ignácio-Souza, Marciane Milanski, Michael G. Ross, Kelly Pereira Coca, and et al. 2024. "Sex-Dependent Variations in Hypothalamic Fatty Acid Profile and Neuropeptides in Offspring Exposed to Maternal Obesity and High-Fat Diet" Nutrients 16, no. 3: 340. https://doi.org/10.3390/nu16030340
APA StyleBaqueiro, M. d. N., Simino, L. A. d. P., Costa, J. P., Panzarin, C., Reginato, A., Torsoni, M. A., Ignácio-Souza, L., Milanski, M., Ross, M. G., Coca, K. P., Desai, M., & Torsoni, A. S. (2024). Sex-Dependent Variations in Hypothalamic Fatty Acid Profile and Neuropeptides in Offspring Exposed to Maternal Obesity and High-Fat Diet. Nutrients, 16(3), 340. https://doi.org/10.3390/nu16030340