The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation
Abstract
1. Introduction
2. The Relationship between Vitamin D Insufficiency and Asthma
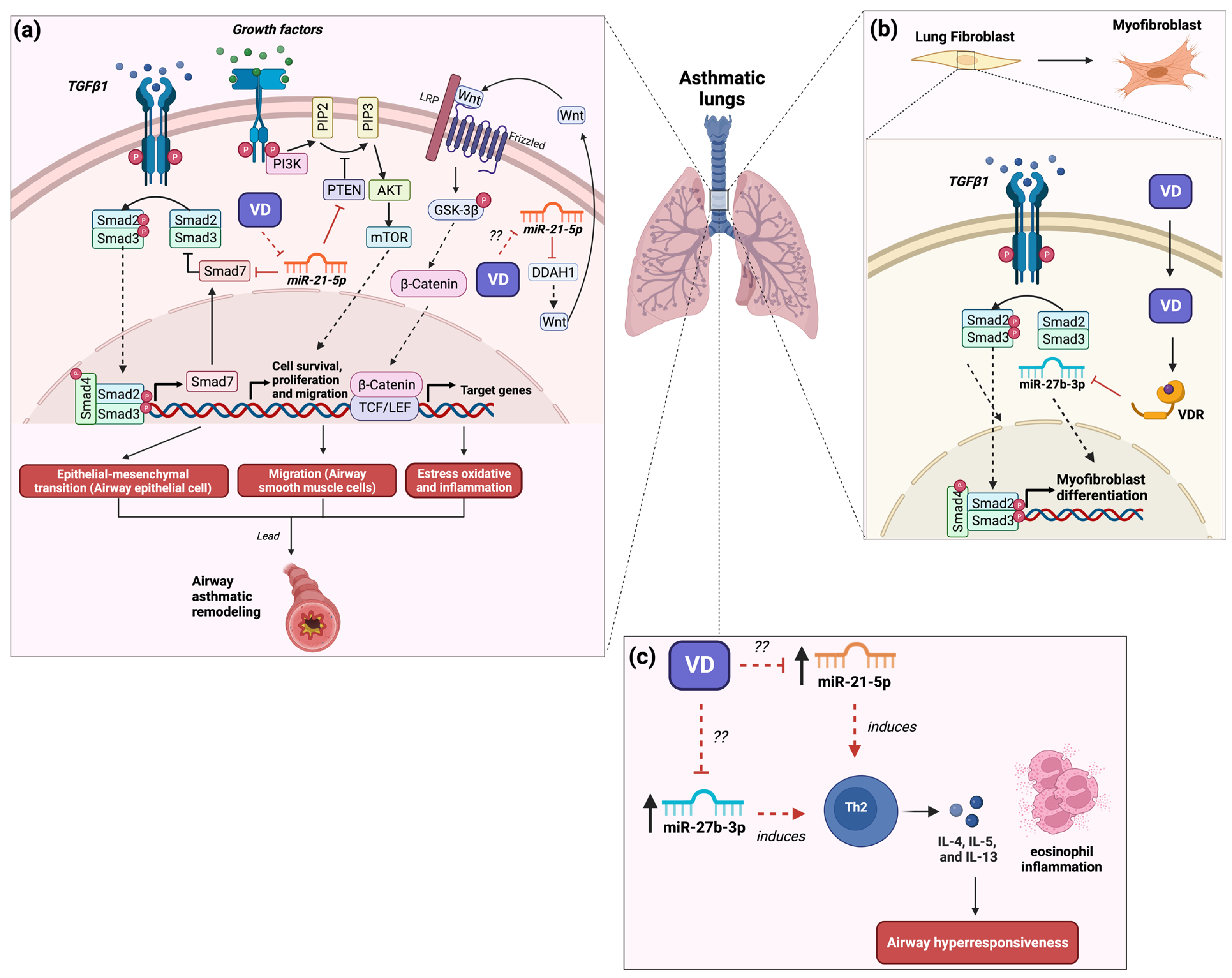
3. The Role of MicroRNA-21 in Asthma and Its Modulation by Vitamin D3
4. The Role of MicroRNA-27b in Asthma and Its Modulation by Vitamin D3
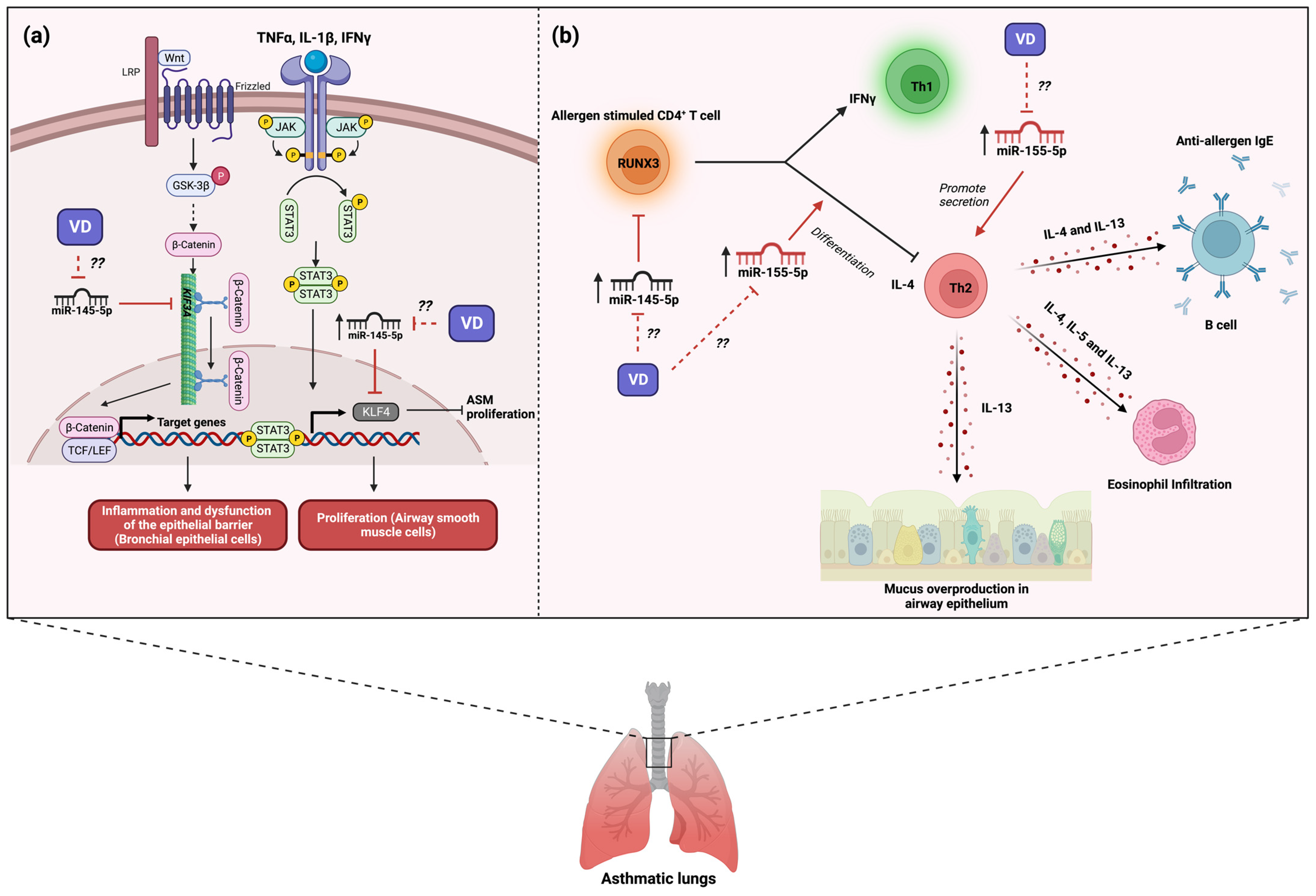
5. The Role of MicroRNA-145 in Asthma and Its Modulation by Vitamin D3
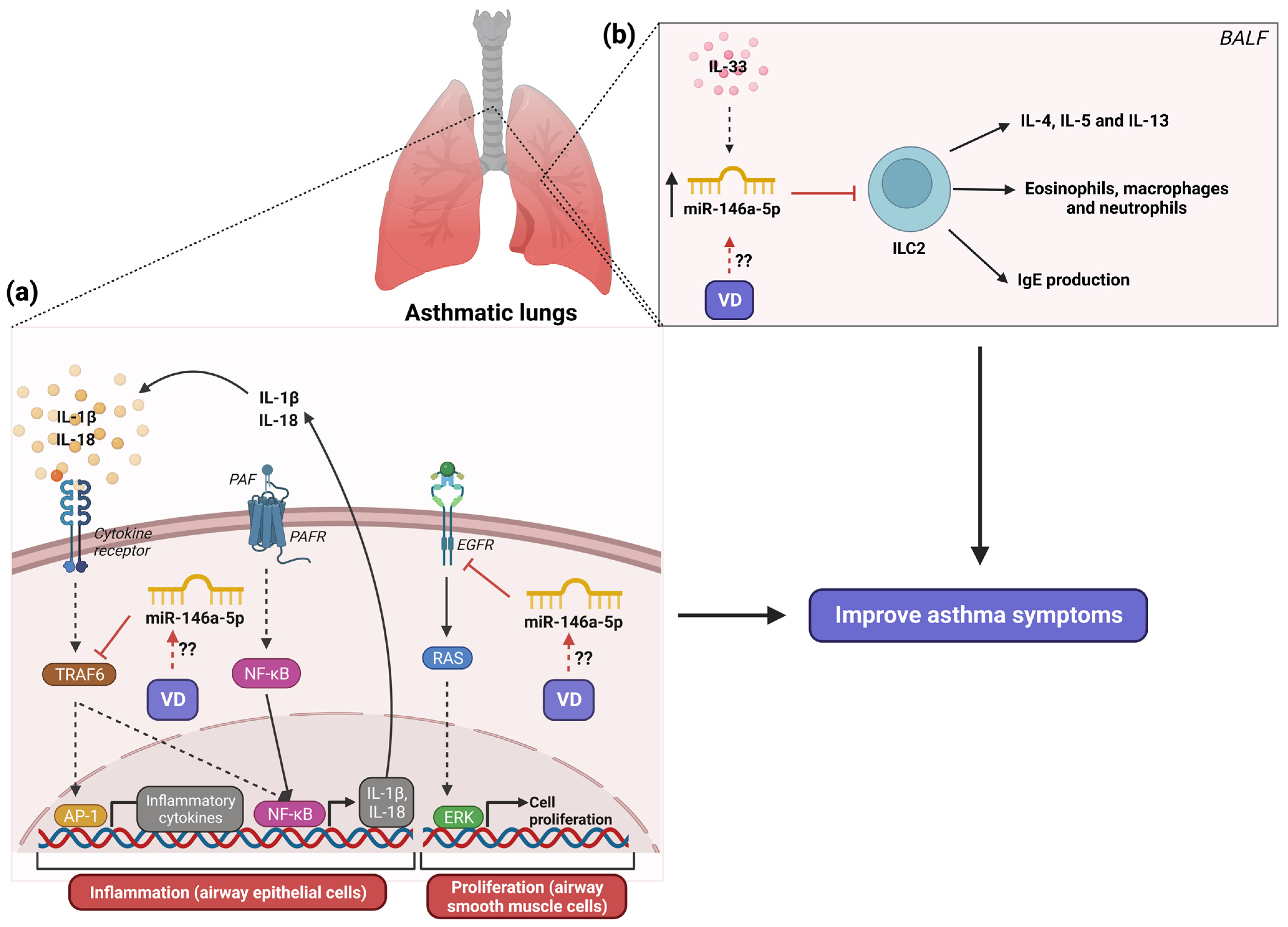
6. The Role of MicroRNA-146a in Asthma and Its Modulation by Vitamin D3
7. The Role of MicroRNA-155 in Asthma and Its Modulation by Vitamin D3
8. The Role of Miscellaneous MicroRNAs and Vitamin D3 in Asthma
9. Future Directions
- More studies that assess the vitamin D3 effects on the regulation of miRNAs related to inflammation in asthmatic patients are needed;
- The impact of genetic variants on vitamin D receptors on the regulation of microRNAs associated with asthma should be assessed;
- The effect of the intake of vitamin D3-enriched foods vs. the supplementation of vitamin D3 on the regulation of miRNA expression in asthmatic patients should be assessed.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, Risk Factors, and Management of Asthma in China: A National Cross-Sectional Study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Worldwide Asthma Epidemiology: Insights from the Global Health Data Exchange Database. Int. Forum. Allergy Rhinol. 2020, 10, 75–80. [Google Scholar] [CrossRef]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma Epidemiology and Risk Factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Gogna, M.; Cepeda, A.; Yañez, A.; Solé, D.; Cooper, P.; Avila, L.; Soto-Quiros, M.; Castro-Rodriguez, J.A.; Celedón, J.C. Asthma in Latin America. Thorax 2015, 70, 898. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyathad, T.; Sözener, Z.C.; Satitsuksanoa, P.; Akdis, C.A. Immunologic Mechanisms in Asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef] [PubMed]
- Porsbjerg, C.; Melén, E.; Lehtimäki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Banno, A.; Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. Bidirectional Interaction of Airway Epithelial Remodeling and Inflammation in Asthma. Clin. Sci. 2020, 134, 1063–1079. [Google Scholar] [CrossRef]
- Busse, W.W.; Kraft, M.; Rabe, K.F.; Deniz, Y.; Rowe, P.J.; Ruddy, M.; Castro, M. Understanding the Key Issues in the Treatment of Uncontrolled Persistent Asthma with Type 2 Inflammation. Eur. Respir. J. 2021, 58, 2003393. [Google Scholar] [CrossRef]
- Maspero, J.; Adir, Y.; Al-Ahmad, M.; Celis-Preciado, C.A.; Colodenco, F.D.; Giavina-Bianchi, P.; Lababidi, H.; Ledanois, O.; Mahoub, B.; Perng, D.-W.; et al. Type 2 Inflammation in Asthma and Other Airway Diseases. ERJ Open Res. 2022, 8, 00576-2021. [Google Scholar] [CrossRef]
- Fu, L.; Fei, J.; Tan, Z.-X.; Chen, Y.-H.; Hu, B.; Xiang, H.-X.; Zhao, H.; Xu, D.-X. Low Vitamin D Status Is Associated with Inflammation in Patients with Chronic Obstructive Pulmonary Disease. J. Immunol. 2021, 206, 515–523. [Google Scholar] [CrossRef]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jing, D.; Liang, H.; Li, D.; Chang, Q.; Shen, M.; Pan, P.; Liu, H.; Zhang, Y. Vitamin D Status and Asthma, Lung Function, and Hospitalization among British Adults. Front. Nutr. 2022, 9, 954768. [Google Scholar] [CrossRef] [PubMed]
- Doumat, G.; Mehta, G.D.; Mansbach, J.M.; Hasegawa, K.; Camargo, C.A. Association between Early Childhood Vitamin D Status and Age 6-Year Lung Function among Children with a History of Severe Bronchiolitis in Infancy. Nutrients 2023, 15, 2379. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.G.; Mahalingam, S.; Soumya, V.C. Vitamin D and Its Association with Severity and Control of Childhood Bronchial Asthma. Indian. J. Public Health 2023, 67, 3–7. [Google Scholar] [PubMed]
- Malheiro, A.P.G.; Gianfrancesco, L.; Nogueira, R.J.N.; Grotta, M.B.; Morcillo, A.M.; Ribeiro, J.D.; Toro, A.A.D.C. Association between Serum Vitamin D Levels and Asthma Severity and Control in Children and Adolescents. Lung 2023, 201, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ying, Q.; Zhu, W.; Chen, J. Vitamin D and Asthma Occurrence in Children: A Systematic Review and Meta-Analysis. J. Pediatr. Nurs. 2022, 62, e60–e68. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between Vitamin D Status and Asthma Control: A Meta-Analysis of Randomized Trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Sengar, R.S. Biogenesis and Mechanisms of MicroRNA-Mediated Gene Regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wei, Y. Modulators of MicroRNA Function in the Immune System. Int. J. Mol. Sci. 2020, 21, 2357. [Google Scholar] [CrossRef]
- Li, J.; Tiwari, A.; Mirzakhani, H.; Wang, A.L.; Kho, A.T.; McGeachie, M.J.; Litonjua, A.A.; Weiss, S.T.; Tantisira, K.G. Circulating Microrna: Incident Asthma Prediction and Vitamin d Effect Modification. J. Pers. Med. 2021, 11, 307. [Google Scholar] [CrossRef]
- Beckett, E.L.; Veysey, M.; Yates, Z.; Lucock, M. Modulation of MicroRNA by Vitamin D in Cancer Studies. In Handbook of Nutrition, Diet, and Epigenetics; Springer: Berlin/Heidelberg, Germany, 2019; Volume 3, pp. 1747–1768. [Google Scholar]
- Kyyaly, M.A.; Vorobeva, E.V.; Kothalawala, D.M.; Fong, W.C.G.; He, P.; Sones, C.L.; Al-zahrani, M.; Sanchez-elsner, T.; Arshad, S.H.; Kurukulaaratchy, R.J. MicroRNAs: A Promising Tool for Asthma Diagnosis and Severity Assessment. A Systematic Review. J. Pers. Med. 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, R.P.; Wang, Y.; Howrylak, J.; Chinchilli, V.M.; Craig, T.J.; August, A.; Ishmael, F.T. Circulating MicroRNAs as Biomarkers in Patients with Allergic Rhinitis and Asthma. J. Allergy Clin. Immunol. 2016, 137, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Kho, A.T.; Sharma, S.; Davis, J.S.; Spina, J.; Howard, D.; McEnroy, K.; Moore, K.; Sylvia, J.; Qiu, W.; Weiss, S.T.; et al. Circulating MicroRNAs: Association with Lung Function in Asthma. PLoS ONE 2016, 11, e0157998. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y.; Wang, C.; Ma, Y.; He, S.; Kang, Y.; Yang, J. Increased MiR-223-3p in Leukocytes Positively Correlated with IL-17A in Plasma of Asthmatic Patients. Iran. J. Allergy Asthma Immunol. 2020, 19, 289–296. [Google Scholar] [CrossRef] [PubMed]
- de Candia, P.; Torri, A.; Pagani, M.; Abrignani, S. Serum MicroRNAs as Biomarkers of Human Lymphocyte Activation in Health and Disease. Front. Immunol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Rostami Hir, S.; Alizadeh, Z.; Mazinani, M.; Mahlooji Rad, M.; Fazlollahi, M.R.; Kazemnejad, A.; Zavaran Hosseini, A.; Moin, M. Exosomal MicroRNAs as Biomarkers in Allergic Asthma. Iran. J. Allergy Asthma Immunol. 2021, 20, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mera, S.; Martelo-Vidal, L.; Miguéns-Suárez, P.; Saavedra-Nieves, P.; Arias, P.; González-Fernández, C.; Mosteiro-Añón, M.; Corbacho-Abelaira, M.D.; Blanco-Aparicio, M.; Méndez-Brea, P.; et al. Serum Exosome Inflamma-MiRs Are Surrogate Biomarkers for Asthma Phenotype and Severity. Allergy 2023, 78, 141–155. [Google Scholar] [CrossRef]
- ElKashef, S.M.M.A.E.; Ahmad, S.E.A.; Soliman, Y.M.A.; Mostafa, M.S. Role of MicroRNA-21 and MicroRNA-155 as Biomarkers for Bronchial Asthma. Innate Immun. 2021, 27, 61. [Google Scholar] [CrossRef]
- Hammad Mahmoud Hammad, R.; Hamed, D.H.E.D.; Eldosoky, M.A.E.R.; Ahmad, A.A.E.S.; Osman, H.M.; Abd Elgalil, H.M.; Mahmoud Hassan, M.M. Plasma MicroRNA-21, MicroRNA-146a and IL-13 Expression in Asthmatic Children. Innate Immun. 2018, 24, 171–179. [Google Scholar] [CrossRef]
- Joo, H.; Park, S.Y.; Park, S.Y.; Park, S.Y.; Kim, S.H.; Cho, Y.S.; Yoo, K.H.; Jung, K.S.; Rhee, C.K. Phenotype of Asthma-COPD Overlap in COPD and Severe Asthma Cohorts. J. Korean Med. Sci. 2022, 37, e236. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, N.; Cheng, Q.; Zhang, H.; Liu, F.; Shang, Y. Mir-21-5p in Macrophage-Derived Exosomes Targets Smad7 to Promote Epithelial Mesenchymal Transition of Airway Epithelial Cells. J. Asthma Allergy 2021, 14, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Shi, H.; Xu, J.; Zhang, D.; Wu, Y.; Zhou, S.; Sun, X. MiR-21 Modulates Human Airway Smooth Muscle Cell Proliferation and Migration in Asthma through Regulation of PTEN Expression. Exp. Lung Res. 2015, 41, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hur, J.; Kang, J.Y.; Rhee, C.K.; Lee, S.Y. MicroRNA-21 Inhibition Suppresses Alveolar M2 Macrophages in an Ovalbumin-Induced Allergic Asthma Mice Model. Allergy Asthma Immunol. Res. 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, Q.; Zhang, Y. MicroRNA-21 Released from Mast Cells-Derived Extracellular Vesicles Drives Asthma in Mice by Potentiating Airway Inflammation and Oxidative Stress. Am. J. Transl. Res. 2021, 13, 7475. [Google Scholar] [PubMed]
- Sheane, B.; Smyth, P.; Scott, K.; Aziz, R.; Buckley, M.; Lodge, E.; Kiely, N.; Kingston, M.; McGovern, E.; Healy, M.; et al. An Association between MicroRNA-21 Expression and Vitamin D Deficiency in Coronary Artery Disease. Microrna 2015, 4, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Grieco, G.E.; Cataldo, D.; Ceccarelli, E.; Nigi, L.; Catalano, G.; Brusco, N.; Mancarella, F.; Ventriglia, G.; Fondelli, C.; Guarino, E.; et al. Serum Levels of MiR-148a and MiR-21-5p Are Increased in Type 1 Diabetic Patients and Correlated with Markers of Bone Strength and Metabolism. Non-Coding RNA 2018, 4, 37. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, J.; Yu, Z. Budesonide Up-Regulates Vitamin D Receptor Expression in Human Bronchial Fibroblasts and Enhances the Inhibitory Effect of Calcitriol on Airway Remodeling. Allergol. Immunopathol. 2019, 47, 585–590. [Google Scholar] [CrossRef]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. MiR-21 Mediates Fibrogenic Activation of Pulmonary Fibroblasts and Lung Fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Prattichizzo, F.; Martino, E.; Anastasio, C.; Mele, L.; La Grotta, R.; Sardu, C.; Ceriello, A.; Marfella, R.; Paolisso, G.; et al. MiR-27b Attenuates Mitochondrial Oxidative Stress and Inflammation in Endothelial Cells. Redox Biol. 2023, 62, 102681. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, L.J.; Liu, H.; Song, Y.J.; Yang, Q.Q.; Liu, Y.; Qian, S.W.; Tang, Q.Q. Exosomal MiR-27b-3p Secreted by Visceral Adipocytes Contributes to Endothelial Inflammation and Atherogenesis. Cell Rep. 2023, 42, 111948. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhong, N.; Fang, Y.; Cai, Q.; Lu, M.; Lu, Q. MicroRNA 27b-3p Modulates SYK in Pediatric Asthma Induced by Dust Mites. Front. Pediatr. 2018, 6, 301. [Google Scholar] [CrossRef] [PubMed]
- Macglashan, D.; Moore, G.; Muchhal, U. Regulation of IgE-Mediated Signalling in Human Basophils by CD32b and Its Role in Syk down-Regulation: Basic Mechanisms in Allergic Disease. Clin. Exp. Allergy 2014, 44, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Coskunpinar, E.; Akcesme, B.; Tas, S.K.; Aynaci, A. Investigation of MiRNAs That Are Effective in the Pathogenesis of Asthma. J. Asthma 2023, 60, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, E.; Miglino, N.; Hashim, A.; Nassa, G.; Stellato, C.; Tamm, M.; Baty, F.; Brutsche, M.; Weisz, A.; Borger, P. Small RNA Profiling Reveals Deregulated Phosphatase and Tensin Homolog (PTEN)/Phosphoinositide 3-Kinase (PI3K)/Akt Pathway in Bronchial Smooth Muscle Cells from Asthmatic Patients. J. Allergy Clin. Immunol. 2016, 137, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, A.; Santolini, M.; Nakano, T.; Schiller, M.; Teranishi, M.; Gellert, P.; Ponomareva, Y.; Braun, T.; Uchida, S.; Weiss, S.T.; et al. A Systems Immunology Approach Identifies the Collective Impact of 5 MiRs in Th2 Inflammation. JCI Insight 2018, 3, e97503. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, A.; Shi, Y.; Ma, Y.; Du, Y. 1a,25-DihydroxyVitamin D3 Prevents the Differentiation of Human Lung Fibroblasts via MicroRNA-27b Targeting the Vitamin D Receptor. Int. J. Mol. Med. 2015, 36, 967–974. [Google Scholar] [CrossRef]
- Kordkhayli, M.M.; Mansouri, F.; Talebi, F.; Noorbakhsh, F.; Saboor-Yaraghi, A.A. Influence of Vitamins A and D on the Expression of MicroRNA27-3p Isoforms and GATA3 in Experimental Autoimmune Encephalomyelitis. Iran. J. Allergy Asthma Immunol. 2022, 21, 429–440. [Google Scholar] [CrossRef]
- Ge, X.; Yuan, L.; Wei, J.; Nguyen, T.; Tang, C.; Liao, W.; Li, R.; Yang, F.; Zhang, F.; Zhao, B.; et al. Vitamin D/VDR Signaling Induces MiR-27a/b Expression in Oral Lichen Planus. Sci. Rep. 2020, 10, 301. [Google Scholar] [CrossRef]
- Quintanilha, B.J.; Reis, B.Z.; Corrêa, T.A.F.; Duarte, G.B.D.S.; Rogero, M.M. MicroRNAs and Inflammation Biomarkers in Obesity. In Precision Medicine for Investigators, Practitioners and Providers; Academic Press: Cambridge, MA, USA, 2020; pp. 179–185. [Google Scholar] [CrossRef]
- Kadkhoda, S.; Ghafouri-Fard, S. Function of MiRNA-145–5p in the Pathogenesis of Human Disorders. Pathol. Res. Pract. 2022, 231, 153780. [Google Scholar] [CrossRef]
- Lacedonia, D.; Palladino, G.P.; Foschino-Barbaro, M.P.; Scioscia, G.; Elisiana, G. Carpagnano Expression Profiling of MiRNA-145 and MiRNA-338 in Serum and Sputum of Patients with COPD, Asthma, and Asthma–COPD Overlap Syndrome Phenotype. Int. J. Chron. Obs. Pulmon. Dis. 2017, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.C.; Paciência, I.; Ferreira, A.C.; Martins, C.; Rufo, J.C.; Silva, D.; Cunha, P.; Farraia, M.; Moreira, P.; Delgado, L.; et al. Development and Validation of Exhaled Breath Condensate MicroRNAs to Identify and Endotype Asthma in Children. PLoS ONE 2019, 14, e0224983. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Li, J.; Kho, A.T.; Sun, M.; Lu, Q.; Weiss, S.T.; Tantisira, K.G.; McGeachie, M.J. COPD-Associated MiR-145-5p Is Downregulated in Early-Decline FEV1 Trajectories in Childhood Asthma. J. Allergy Clin. Immunol. 2021, 147, 2181–2190. [Google Scholar] [CrossRef]
- Collison, A.; Mattes, J.; Plank, M.; Foster, P.S. Inhibition of House Dust Mite-Induced Allergic Airways Disease by Antagonism of MicroRNA-145 Is Comparable to Glucocorticoid Treatment. J. Allergy Clin. Immunol. 2011, 128, 160–167.e4. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Du, Y.; Fu, Z.; Geng, G. MicroRNA-145-5p Promotes Asthma Pathogenesis by Inhibiting Kinesin Family Member 3A Expression in Mouse Airway Epithelial Cells. J. Int. Med. Res. 2019, 47, 3307. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dai, L.L.; Wang, X.; Jia, L.Q.; Jing, X.G.; Li, P.F.; Liu, M.; Wang, H.; An, L. MicroRNA-145 down-Regulates Mucin 5AC to Alleviate Airway Remodeling and Targets EGFR to Inhibit Cytokine Expression. Oncotarget 2017, 8, 46312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, X.; Wu, Y.; Fang, P.; Shi, H.; Xu, J.; Li, M. Effects of MiRNA-145 on Airway Smooth Muscle Cells Function. Mol. Cell Biochem. 2015, 409, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Zhang, Y.W.; Qian, X.F.; Bian, T. MiR-371, MiR-138, MiR-544, MiR-145, and MiR-214 Could Modulate Th1/Th2 Balance in Asthma through the Combinatorial Regulation of Runx3. Am. J. Transl. Res. 2017, 9, 3184. [Google Scholar]
- Fan, L.; Wang, X.; Fan, L.; Chen, Q.; Zhang, H.; Pan, H.; Xu, A.; Wang, H.; Yu, Y. MicroRNA-145 Influences the Balance of Th1/Th2 via Regulating RUNX3 in Asthma Patients. Exp. Lung Res. 2016, 42, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Aladel, A.; Khatoon, F.; Khan, M.I.; Alsheweir, A.; Almutairi, M.G.; Almutairi, S.O.; Almutairi, F.K.; Osmonaliev, K.; Beg, M.M.A. Evaluation of MiRNA-143 and MiRNA-145 Expression and Their Association with Vitamin-D Status Among Obese and Non-Obese Type-2 Diabetic Patients. J. Multidiscip. Healthc. 2022, 15, 2979. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Gao, L.; Yang, Y.; Tong, D.; Guo, B.; Liu, L.; Li, Z.; Song, T.; Huang, C. MiR-145 Mediates the Antiproliferative and Gene Regulatory Effects of Vitamin D3 by Directly Targeting E2F3 in Gastric Cancer Cells. Oncotarget 2015, 6, 7675–7685. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-lópez, N.; Panizo, S.; Arcidiacono, M.V.; de la Fuente, S.; Martínez-arias, L.; Ottaviano, E.; Ulloa, C.; Ruiz-torres, M.P.; Rodríguez, I.; Cannata-Andía, J.B.; et al. Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic MicroRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia. Nutrients 2022, 14, 2589. [Google Scholar] [CrossRef]
- Mortazavi-Jahromi, S.S.; Aslani, M.; Mirshafiey, A. A Comprehensive Review on MiR-146a Molecular Mechanisms in a Wide Spectrum of Immune and Non-Immune Inflammatory Diseases. Immunol. Lett. 2020, 227, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.A.; Roff, A.N.; Panganiban, R.P.; Douglas, S.; Ishmael, F.T. MicroRNA-146a Is Induced by Inflammatory Stimuli in Airway Epithelial Cells and Augments the Anti-Inflammatory Effects of Glucocorticoids. PLoS ONE 2018, 13, e0205434. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.; Ekerljung, L.; Malmhäll, C.; Miron, N.; Rådinger, M. Circulating MicroRNAs Correlate to Clinical Parameters in Individuals with Allergic and Non-Allergic Asthma. Respir. Res. 2020, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Eldosoky, M.A.; Hammad, R.; Rushdi, A.; Ibrahim, H.F.; Tawfeik, A.M.; Mora, A.; Fahmy, S.F.; El-Ashmawy, H.; Ali, E.; Hamed, D.H.; et al. MicroRNA-146a-5p and MicroRNA-210-3p Correlate with T Regulatory Cells Frequency and Predict Asthma Severity in Egyptian Pediatric Population. J. Asthma Allergy 2023, 16, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.F.; Boldin, M.P.; Chaudhry, A.; Lin, L.L.; Taganov, K.D.; Hanada, T.; Yoshimura, A.; Baltimore, D.; Rudensky, A.Y. Function of MiR-146a in Controlling Treg Cell-Mediated Regulation of Th1 Responses. Cell 2010, 142, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Elnady, H.G.; Sherif, L.S.; Kholoussi, N.M.; Azzam, M.A.; Foda, A.R.; Helwa, I.; Sabry, R.N.; Eissa, E.; Fahmy, R.F. Aberrant Expression of Immune-Related MicroRNAs in Pediatric Patients with Asthma. Int. J. Mol. Cell Med. 2020, 9, 246. [Google Scholar] [CrossRef]
- Han, S.; Ma, C.; Bao, L.; Lv, L.; Huang, M. MiR-146a Mimics Attenuate Allergic Airway Inflammation by Impacted Group 2 Innate Lymphoid Cells in an Ovalbumin-Induced Asthma Mouse Model. Int. Arch. Allergy Immunol. 2018, 177, 302–310. [Google Scholar] [CrossRef]
- Laanesoo, A.; Urgard, E.; Periyasamy, K.; Laan, M.; Bochkov, Y.A.; Aab, A.; Magilnick, N.; Pooga, M.; Gern, J.E.; Johnston, S.L.; et al. Dual Role of the MiR-146 Family in Rhinovirus-Induced Airway Inflammation and Allergic Asthma Exacerbation. Clin. Transl. Med. 2021, 11, e427. [Google Scholar] [CrossRef]
- Fang, S.B.; Zhang, H.Y.; Wang, C.; He, B.X.; Liu, X.Q.; Meng, X.C.; Peng, Y.Q.; Xu, Z.B.; Fan, X.L.; Wu, Z.J.; et al. Small Extracellular Vesicles Derived from Human Mesenchymal Stromal Cells Prevent Group 2 Innate Lymphoid Cell-Dominant Allergic Airway Inflammation through Delivery of MiR-146a-5p. J. Extracell. Vesicles 2020, 9, 1723260. [Google Scholar] [CrossRef] [PubMed]
- Kivihall, A.; Aab, A.; Soja, J.; Sładek, K.; Sanak, M.; Altraja, A.; Jakiela, B.; Bochenek, G.; Rebane, A. Reduced Expression of MiR-146a in Human Bronchial Epithelial Cells Alters Neutrophil Migration. Clin. Transl. Allergy 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Fussbroich, D.; Kohnle, C.; Schwenger, T.; Driessler, C.; Dücker, R.P.; Eickmeier, O.; Gottwald, G.; Jerkic, S.P.; Zielen, S.; Kreyenberg, H.; et al. A Combination of LCPUFAs Regulates the Expression of MiRNA-146a-5p in a Murine Asthma Model and Human Alveolar Cells. Prostaglandins Other Lipid Mediat. 2020, 147, 106378. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wufuer, D.; Wang, J.; Ding, J. MicroRNA MiR-146a-5p Inhibits the Inflammatory Response and Injury of Airway Epithelial Cells via Targeting TNF Receptor-Associated Factor 6. Bioengineered 2021, 12, 1916–1926. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, Q.; Zhao, G.; Jiang, J.; Li, Y.; Guo, Z. MiR-146a-5p Attenuates Allergic Airway Inflammation by Inhibiting the NLRP3 Inflammasome Activation in Macrophages. Int. Arch. Allergy Immunol. 2022, 183, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, Y.; Liu, Y.; Song, G.; Lv, G.; Wang, Y.; Wang, Y.; Li, X.; Yang, L. MicroRNA-146a Expression Inhibits the Proliferation and Promotes the Apoptosis of Bronchial Smooth Muscle Cells in Asthma by Directly Targeting the Epidermal Growth Factor Receptor. Exp. Ther. Med. 2016, 12, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, X.; Li, L.; Yang, J.; Guo, Y. 1α,25-Dihydroxyvitamin D3 Inhibits Activation of Hepatic Stellate Cells by Up-Regulating MiR-146a Expression. Chin. J. Gastroenterol. 2017, 22, 653–657. [Google Scholar] [CrossRef]
- Al-Rawaf, H.A.; Gabr, S.A.; Alghadir, A.H. Molecular Changes in Diabetic Wound Healing Following Administration of Vitamin D and Ginger Supplements: Biochemical and Molecular Experimental Study. Evid. Based Complement. Altern. Med. 2019, 2019, 4352470. [Google Scholar] [CrossRef]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.F. Vitamin D Limits Inflammation-Linked MicroRNA Expression in Adipocytes in Vitro and in Vivo: A New Mechanism for the Regulation of Inflammation by Vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef]
- Castillo, J.A.; Urcuqui-Inchima, S. Vitamin D Modulates Inflammatory Response of DENV-2-Infected Macrophages by Inhibiting the Expression of Inflammatory-Liked MiRNAs. Pathog. Glob. Health 2023, 117, 167–180. [Google Scholar] [CrossRef]
- Saati-Zarei, A.; Damirchi, A.; Tousi, S.M.T.R.; Babaei, P. Myocardial Angiogenesis Induced by Concurrent Vitamin D Supplementation and Aerobic-Resistance Training Is Mediated by Inhibiting MiRNA-15a, and MiRNA-146a and Upregulating VEGF/PI3K/ENOS Signaling Pathway. Pflugers Arch. 2023, 475, 541–555. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, W.; Jing, W. Indoor Air Pollution Aggravates Asthma in Chinese Children and Induces the Changes in Serum Level of MiR-155. Int. J. Environ. Health Res. 2018, 29, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Karam, R.A.; Abd Elrahman, D.M. Differential Expression of MiR-155 and Let-7a in the Plasma of Childhood Asthma: Potential Biomarkers for Diagnosis and Severity. Clin. Biochem. 2019, 68, 30–36. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.; Cheng, S.; Xu, Y.; Xuefei, Q.; Cao, Y.; Xie, J.; Wang, C.Y.; Xu, Y.; Xiong, W. Small Interfering RNA Directed against MicroRNA-155 Delivered by a Lentiviral Vector Attenuates Asthmatic Features in a Mouse Model of Allergic Asthma. Exp. Ther. Med. 2017, 14, 4391. [Google Scholar] [CrossRef] [PubMed]
- Malmhäll, C.; Alawieh, S.; Lu, Y.; Sjöstrand, M.; Bossios, A.; Eldh, M.; Rådinger, M. MicroRNA-155 Is Essential for TH2-Mediated Allergen-Induced Eosinophilic Inflammation in the Lung. J. Allergy Clin. Immunol. 2014, 133, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, Y.; Do, D.C.; Ke, X.; Zhang, S.; Lambert, K.; Kumar, S.; Hu, C.; Zhou, Y.; Ishmael, F.T.; et al. MiR-155 Modulates Cockroach Allergen and Oxidative Stress–Induced Cyclooxygenase-2 in Asthma. J. Immunol. 2018, 201, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Chia, N.; Kumar, R.K.; Foster, P.S.; Herbert, C. Enhanced Pro-Inflammatory Response of Macrophages to Interleukin-33 in an Allergic Environment. Int. Arch. Allergy Immunol. 2018, 176, 74–82. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D Promotes Negative Feedback Regulation of TLR Signaling via Targeting MicroRNA-155–SOCS1 in Macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef]
- Arboleda, J.F.; Fernandez, G.J.; Urcuqui-Inchima, S. Vitamin D-Mediated Attenuation of MiR-155 in Human Macrophages Infected with Dengue Virus: Implications for the Cytokine Response. Infect. Genet. Evol. 2019, 69, 12–21. [Google Scholar] [CrossRef]
- Chen, X.-F.; Zhang, L.-J.; Zhang, J.; Dou, X.; Shao, Y.; Jia, X.-J.; Zhang, W.; Yu, B. MiR-151a Is Involved in the Pathogenesis of Atopic Dermatitis by Regulating Interleukin-12 Receptor Β2. Exp. Dermatol. 2018, 27, 427–432. [Google Scholar] [CrossRef]
- Shannon, J.; Ernst, P.; Yamauchi, Y.; Olivenstein, R.; Lemiere, C.; Foley, S.; Cicora, L.; Ludwig, M.; Hamid, Q.; Martin, J.G. Differences in Airway Cytokine Profile in Severe Asthma Compared to Moderate Asthma. Chest 2008, 133, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Lawrence, M.G.; Teague, W.G.; Braciale, T.J.; Patrie, J.T.; Borish, L. Bronchoalveolar Lavage Cytokine Patterns in Children with Severe Neutrophilic and Paucigranulocytic Asthma. J. Allergy Clin. Immunol. 2021, 147, 686–693.e3. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.A.; Muehling, L.M.; Eccles, J.D.; Capaldo, B.J.; Agrawal, R.; Shirley, D.A.; Patrie, J.T.; Workman, L.J.; Schuyler, A.J.; Lawrence, M.G.; et al. TH1 Signatures Are Present in the Lower Airways of Children with Severe Asthma, Regardless of Allergic Status. J. Allergy Clin. Immunol. 2018, 141, 2048–2060.e13. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Garcia, A.S.; Garrido-Martín, E.M.; Rupani, H.; Lau, L.C.K.; Martinez-Nunez, R.T.; Howarth, P.H.; Sanchez-Elsner, T. Small RNA Species and MicroRNA Profiles Are Altered in Severe Asthma Nanovesicles from Broncho Alveolar Lavage and Associate with Impaired Lung Function and Inflammation. Noncoding RNA 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.J.; Zeng, L.; Luo, X.Q.; Geng, X.R.; Xu, R.; Chen, K.; Yang, G.; Luo, X.; Liu, Z.Q.; Liu, Z.G.; et al. Vitamin D3 Inhibits Micro RNA-17-92 to Promote Specific Immunotherapy in Allergic Rhinitis. Sci. Rep. 2017, 7, 546. [Google Scholar] [CrossRef]
- Haj-Salem, I.; Fakhfakh, R.; Bérubé, J.C.; Jacques, E.; Plante, S.; Simard, M.J.; Bossé, Y.; Chakir, J. MicroRNA-19a Enhances Proliferation of Bronchial Epithelial Cells by Targeting TGFβR2 Gene in Severe Asthma. Allergy 2015, 70, 212–219. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, J.; Ma, Y.; Wang, W.; Yang, J. MicroRNA-19a Inhibition Directly and Indirectly Ameliorates Th2 Airway Inflammation in Asthma by Targeting RUNX3. Inflammation 2023, 46, 370–387. [Google Scholar] [CrossRef]
- Fernandez, G.J.; Castillo, J.A.; Giraldo, D.M.; Urcuqui-Inchima, S. Vitamin D Regulates the Expression of Immune and Stress Response Genes in Dengue Virus-Infected Macrophages by Inducing Specific MicroRNAs. Microrna 2021, 10, 240–249. [Google Scholar] [CrossRef]
- Mirra, D.; Cione, E.; Spaziano, G.; Esposito, R.; Sorgenti, M.; Granato, E.; Cerqua, I.; Muraca, L.; Iovino, P.; Gallelli, L.; et al. Circulating MicroRNAs Expression Profile in Lung Inflammation: A Preliminary Study. J. Clin. Med. 2022, 11, 5446. [Google Scholar] [CrossRef]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human Airway Epithelial Extracellular Vesicle MiRNA Signature Is Altered upon Asthma Development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Sun, S.; An, H. Inter-Correlation of LncRNA THRIL with MicroRNA-34a and MicroRNA-125b and Their Relationship with Childhood Asthma Risk, Severity, and Inflammation. Allergol. Immunopathol. 2023, 51, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Lu, C.C.; Weng, C.J.; Yen, G.C. Protective Effects of Diallyl Sulfide on Ovalbumin-Induced Pulmonary Inflammation of Allergic Asthma Mice by MicroRNA-144, -34a, and -34b/c-Modulated Nrf2 Activation. J. Agric. Food Chem. 2016, 64, 151–160. [Google Scholar] [CrossRef]
- Alharris, E.; Alghetaa, H.; Seth, R.; Chatterjee, S.; Singh, N.P.; Nagarkatti, M.; Nagarkatti, P. Resveratrol Attenuates Allergic Asthma and Associated Inflammation in the Lungs Through Regulation of MiRNA-34a That Targets FoxP3 in Mice. Front. Immunol. 2018, 9, 427663. [Google Scholar] [CrossRef]
- Cui, H.; Ge, J.; Xie, N.; Banerjee, S.; Zhou, Y.; Antony, V.B.; Thannickal, V.J.; Liu, G. MIR-34a Inhibits Lung Fibrosis by Inducing Lung Fibroblast Senescence. Am. J. Respir. Cell Mol. Biol. 2017, 56, 168–178. [Google Scholar] [CrossRef]
- Shetty, S.K.; Tiwari, N.; Marudamuthu, A.S.; Puthusseri, B.; Bhandary, Y.P.; Fu, J.; Levin, J.; Idell, S.; Shetty, S. P53 and MiR-34a Feedback Promotes Lung Epithelial Injury and Pulmonary Fibrosis. Am. J. Pathol. 2017, 187, 1016–1034. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liu, G.L.; Lu, L.; Ge, L.; Wang, J.Y. Circ_CSNK1E Modulates Airway Smooth Muscle Cells Proliferation and Migration via MiR-34a-5p/VAMP2 Axis in Asthma. Cell Signal 2022, 95, 110340. [Google Scholar] [CrossRef] [PubMed]
- Gleba, J.J.; Kłopotowska, D.; Banach, J.; Mielko, K.A.; Turlej, E.; Maciejewska, M.; Kutner, A.; Wietrzyk, J. Micro-RNAs in Response to Active Forms of Vitamin D3 in Human Leukemia and Lymphoma Cells. Int. J. Mol. Sci. 2022, 23, 5019. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Sun, S.; An, H. Long Non-Coding RNA Colorectal Neoplasia Differentially Expressed Correlates Negatively with MiR-33a and MiR-495 and Positively with Inflammatory Cytokines in Asthmatic Children. Clin. Respir. J. 2021, 15, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Chen, Y.; Chen, X.; Yuan, B. Mechanism of MiR-181a-5p in Regulatory T/T-Helper 17 Immune Imbalance and Asthma Development in Mice with Allergic Rhinitis. Int. Arch. Allergy Immunol. 2022, 183, 375–388. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Díazcouder, A.; Romero-Nava, R.; Del-Río-Navarro, B.E.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A.; Reyes-Noriega, N.; Rodríguez-Cortés, O.; Leija-Martínez, J.J.; Vélez-Reséndiz, J.M.; Villafaña, S.; et al. The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation. Nutrients 2024, 16, 341. https://doi.org/10.3390/nu16030341
Hernández-Díazcouder A, Romero-Nava R, Del-Río-Navarro BE, Sánchez-Muñoz F, Guzmán-Martín CA, Reyes-Noriega N, Rodríguez-Cortés O, Leija-Martínez JJ, Vélez-Reséndiz JM, Villafaña S, et al. The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation. Nutrients. 2024; 16(3):341. https://doi.org/10.3390/nu16030341
Chicago/Turabian StyleHernández-Díazcouder, Adrián, Rodrigo Romero-Nava, Blanca E. Del-Río-Navarro, Fausto Sánchez-Muñoz, Carlos A. Guzmán-Martín, Nayely Reyes-Noriega, Octavio Rodríguez-Cortés, José J. Leija-Martínez, Juan Manuel Vélez-Reséndiz, Santiago Villafaña, and et al. 2024. "The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation" Nutrients 16, no. 3: 341. https://doi.org/10.3390/nu16030341
APA StyleHernández-Díazcouder, A., Romero-Nava, R., Del-Río-Navarro, B. E., Sánchez-Muñoz, F., Guzmán-Martín, C. A., Reyes-Noriega, N., Rodríguez-Cortés, O., Leija-Martínez, J. J., Vélez-Reséndiz, J. M., Villafaña, S., Hong, E., & Huang, F. (2024). The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation. Nutrients, 16(3), 341. https://doi.org/10.3390/nu16030341







