Maternal Intake of Either Fructose or the Artificial Sweetener Acesulfame-K Results in Differential and Sex-Specific Alterations in Markers of Skin Inflammation and Wound Healing Responsiveness in Mouse Offspring: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
- (a)
- Control (CD; standard diet and drinking water);
- (b)
- Artificial sweetener (AS; standard diet and 12.5 mM Ace-K in drinking water);
- (c)
- Fructose (FR; standard diet and 34.7 mM fructose in drinking water).
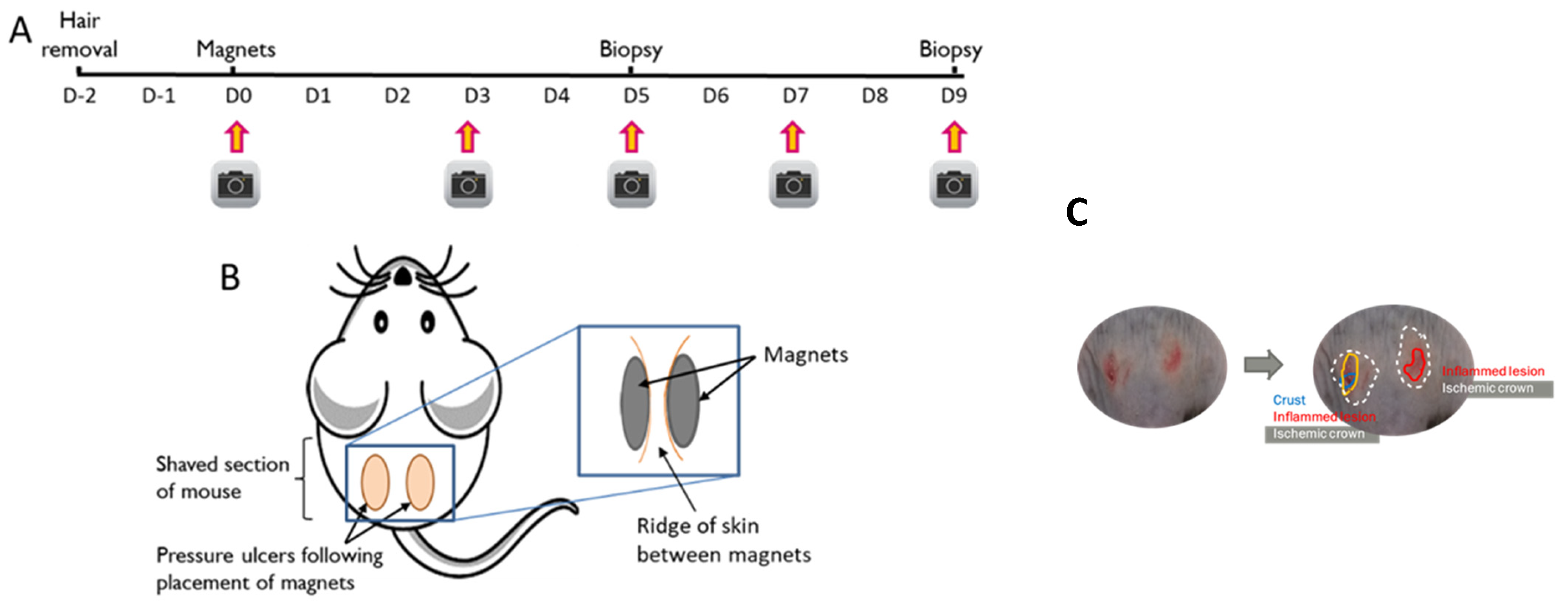
2.2. Pressure Injury Model
2.2.1. Preparation of Skin
2.2.2. Skin Compression
2.2.3. Characterisation of Wound Area
2.2.4. Histology and Immunostaining
2.2.5. Gene Expression Analysis
2.2.6. Statistical Analysis
3. Results
3.1. Physiological Data
3.2. Skin Characterisation Prior to Wound Induction
3.2.1. Healthy Skin Gene Expression
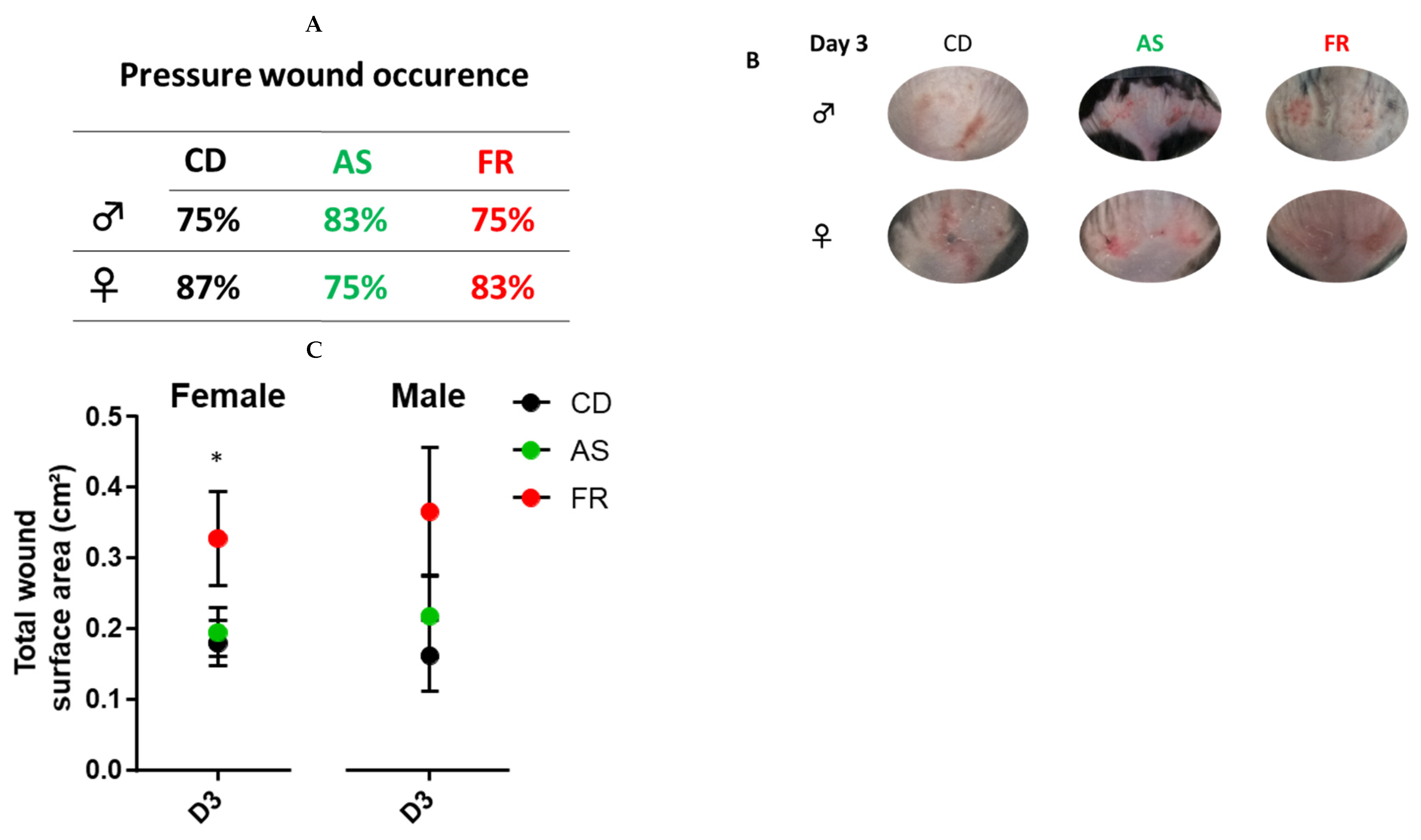
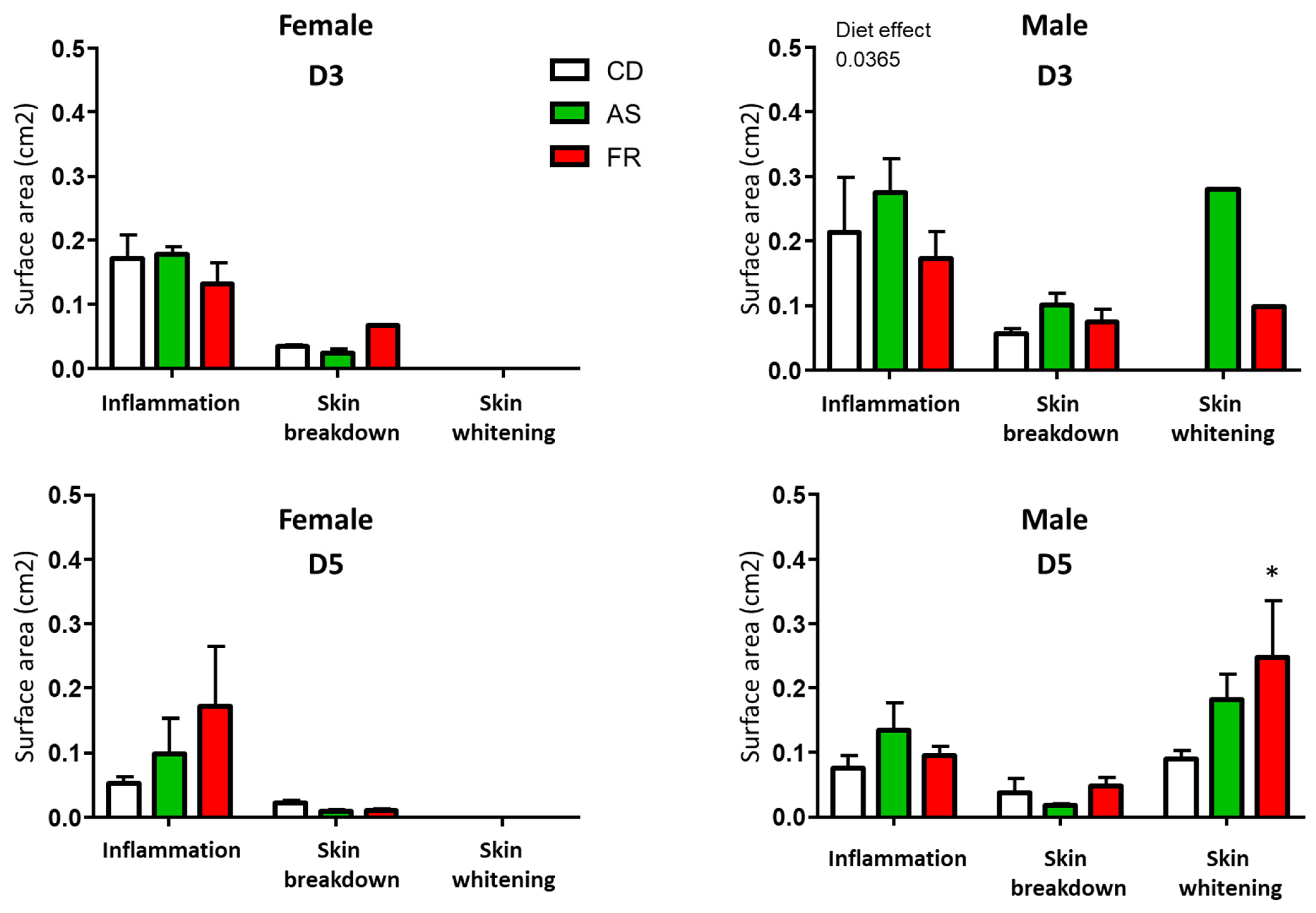
3.2.2. Wound Characterisation
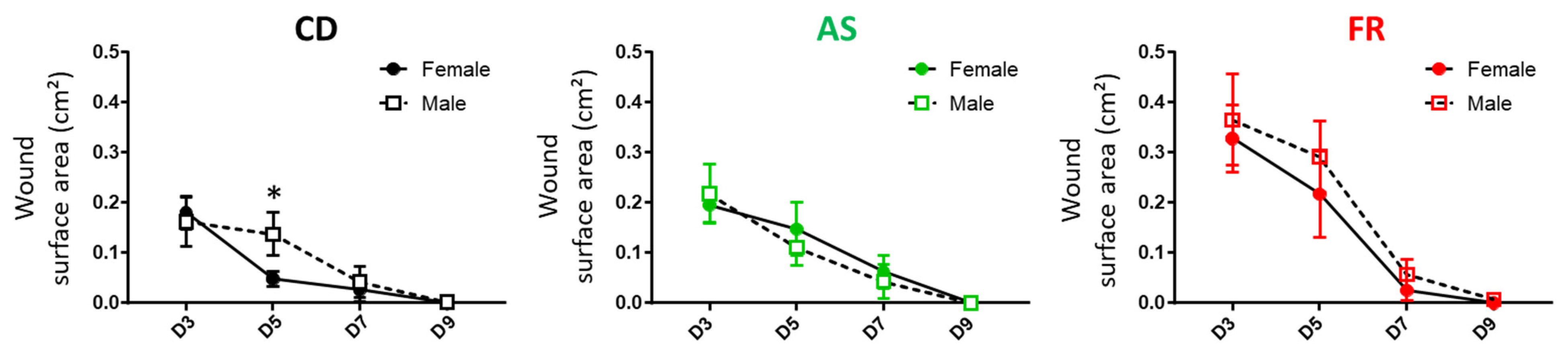
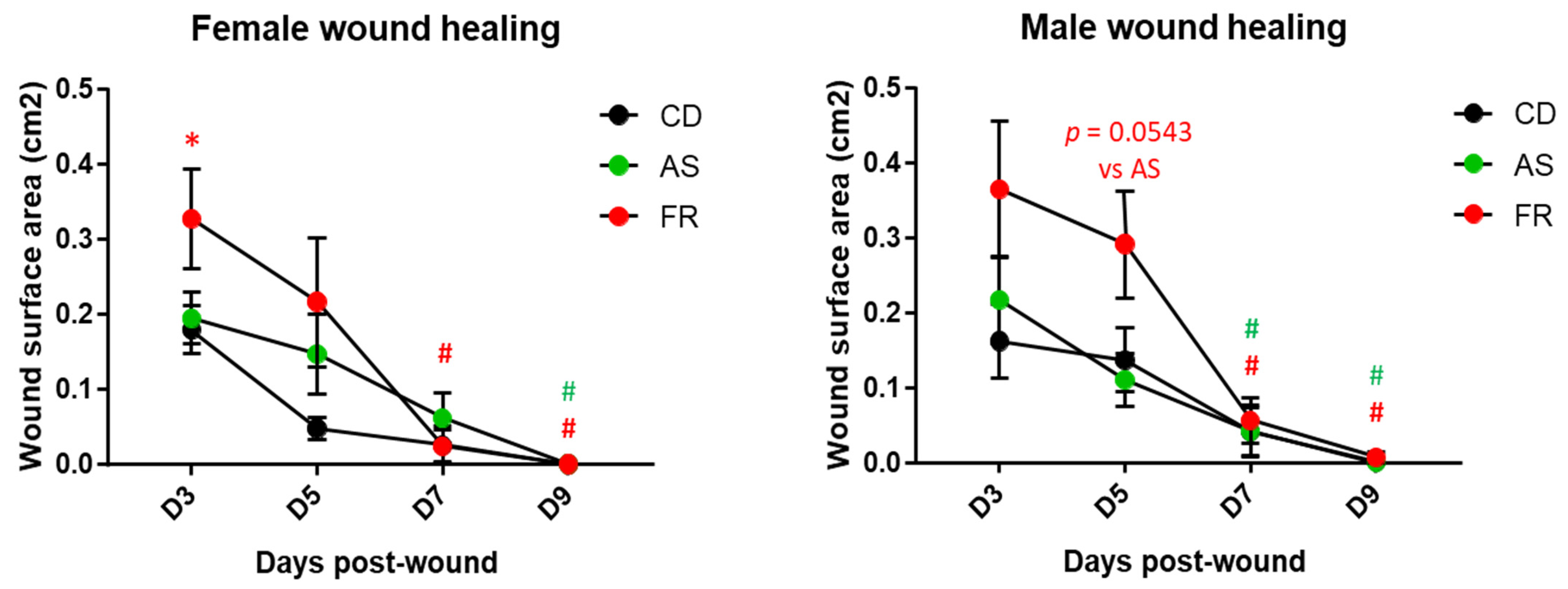
3.2.3. Wound Healing
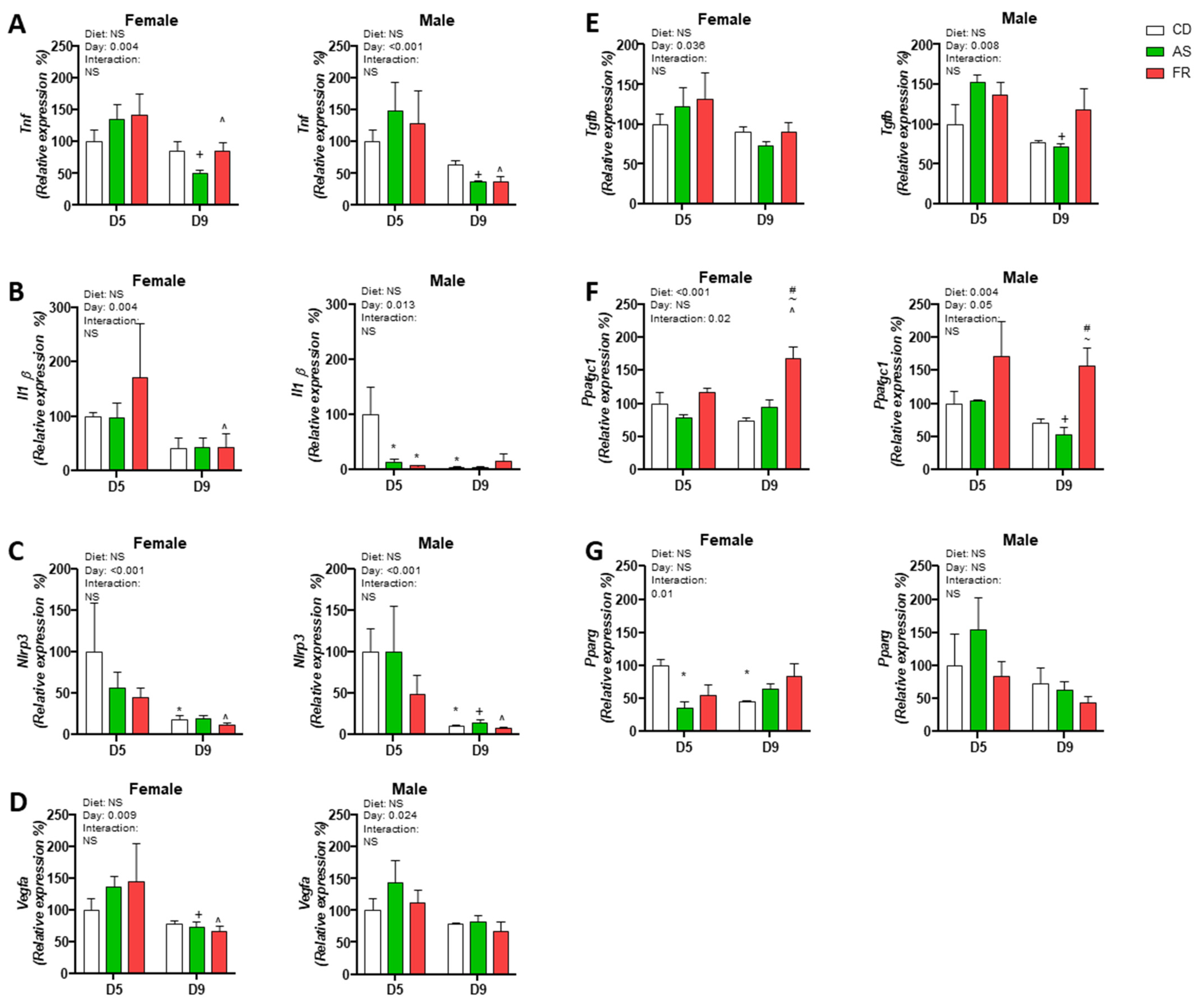
3.2.4. Pressure Injury Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Mouse Gene | ID |
|---|---|
| Il1b | Mm00434228_m1 |
| Nlrp3 | Mm00840904_m1 |
| Pparγ | Mm00440940_m1 |
| Ppargc1 | Mm01208835_m1 |
| Tgfb | Mm01178820 + m1 |
| Tnfα | Mm00443258_m1 |
| Vegfa | Mm00437306_m1 |
| Females | CD | AS | FR | Main Effect (p < 0.05) |
|---|---|---|---|---|
| Birth weight (g) | 1.38 ± 0.06 | 1.30 ± 0.03 | 1.36 ± 0.02 | NS |
| Weaning weight (g) | 8.06 ± 0.62 | 7.86 ± 0.56 | 7.74 ± 0.71 | NS |
| Cull weight (g) | 20.07 ± 0.39 | 19.52 ± 0.27 | 20.21 ± 0.35 | NS |
| Glucose (mmol/L) | 8.82 ± 0.22 | 8.44 ± 0.18 | 8.34 ± 0.29 | NS |
| HOMA-IR | 1.89 ± 0.18 | 1.52 ± 0.17 | 1.63 ± 0.17 | NS |
| Males | CD | AS | FR | Main Effect (p < 0.05) |
| Birth weight (g) | 1.43 ± 0.05 | 1.40 ± 0.03 | 1.36 ± 0.03 | NS |
| Weaning weight (g) | 8.30 ± 0.26 | 8.23 ± 0.42 | 7.65 ± 0.26 | NS |
| Cull weight (g) | 26.28 ± 0.45 | 26.01 ± 0.26 | 25.57 ± 0.75 | NS |
| Glucose (mmol/L) | 9.22 ± 0.19 | 8.35 ± 0.22 * | 9.49 ± 0.25 | 0.002 |
| HOMA-IR | 3.18 ± 0.33 | 3.12 ± 0.27 | 3.53 ± 0.32 | NS |
References
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. Br. J. Sports Med. 2016, 50, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Sloboda, D.M.; Li, M.; Patel, R.; Clayton, Z.E.; Yap, C.; Vickers, M.H. Early Life Exposure to Fructose and Offspring Phenotype: Implications for Long Term Metabolic Homeostasis. J. Obes. 2014, 2014, 203474. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.E.; Vickers, M.H.; Bernal, A.; Yap, C.; Sloboda, D.M. Early Life Exposure to Fructose Alters Maternal, Fetal and Neonatal Hepatic Gene Expression and Leads to Sex-Dependent Changes in Lipid Metabolism in Rat Offspring. PLoS ONE 2015, 10, e0141962. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Clayton, Z.E.; Yap, C.; Sloboda, D.M. Maternal Fructose Intake during Pregnancy and Lactation Alters Placental Growth and Leads to Sex-Specific Changes in Fetal and Neonatal Endocrine Function. Endocrinology 2011, 152, 1378–1387. [Google Scholar] [CrossRef]
- Farhat, G.; Dewison, F.; Stevenson, L. Knowledge and Perceptions of Non-Nutritive Sweeteners within the UK Adult Population. Nutrients 2021, 13, 444. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, P.; Wang, Y.; Cui, W.; Li, D. The relationship between the use of artificial sweeteners and cancer: A meta-analysis of case–control studies. Food Sci. Nutr. 2021, 9, 4589–4597. [Google Scholar] [CrossRef]
- Singh, N.; Lubana, S.S.; Arora, S.; Sachmechi, I. A Study of Artificial Sweeteners and Thyroid Cancer Risk. J. Clin. Med. Res. 2020, 12, 492–498. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef]
- Azad, M.B.; Sharma, A.K.; de Souza, R.J.; Dolinsky, V.W.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Lefebvre, D.L.; Sears, M.R.; et al. Association between Artificially Sweetened Beverage Consumption during Pregnancy and Infant Body Mass Index. JAMA Pediatr. 2016, 170, 662–670. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; Sears, M.R.; Subbarao, P.; Sycuro, L.K.; Azad, M.B.; Arrieta, M.-C. Maternal consumption of artificially sweetened beverages during pregnancy is associated with infant gut microbiota and metabolic modifications and increased infant body mass index. Gut Microbes 2021, 13, 1857513. [Google Scholar] [CrossRef]
- Zhu, Y.; Olsen, S.F.; Mendola, P.; Halldorsson, T.; Rawal, S.; Hinkle, S.N.; Yeung, E.H.; Chavarro, J.; Grunnet, L.G.; Granström, C.; et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. Leuk. Res. 2017, 46, 1499–1508. [Google Scholar] [CrossRef]
- Cai, C.; Sivak, A.; Davenport, M.H. Effects of prenatal artificial sweeteners consumption on birth outcomes: A systematic review and meta-analysis. Public Health Nutr. 2021, 24, 5024–5033. [Google Scholar] [CrossRef]
- Bridge-Comer, P.E.; Vickers, M.H.; Morton-Jones, J.; Spada, A.; Rong, J.; Reynolds, C.M. Impact of Maternal Intake of Artificial Sweetener, Acesulfame-K, on Metabolic and Reproductive Health Outcomes in Male and Female Mouse Offspring. Front. Nutr. 2021, 8, 745203. [Google Scholar] [CrossRef]
- Plows, J.F.; Morton-Jones, J.; Bridge-Comer, P.; Ponnampalam, A.; Stanley, J.L.; Vickers, M.H.; Reynolds, C.M. Consumption of the Artificial Sweetener Acesulfame Potassium throughout Pregnancy Induces Glucose Intolerance and Adipose Tissue Dysfunction in Mice. J. Nutr. 2020, 150, 1773–1781. [Google Scholar] [CrossRef]
- Bridge-Comer, P.E.; Vickers, M.H.; Morton-Jones, J.; Spada, A.; Rong, J.; Reynolds, C.M. Maternal intake of fructose or artificial sweetener during pregnancy and lactation has persistent effects on metabolic and reproductive health of dams post-weaning. J. Dev. Orig. Health Dis. 2022, 13, 642–649. [Google Scholar] [CrossRef]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The applied anatomy of human skin: A model for regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Leis, K.; Mazur, E.; Jabłońska, M.J.; Kolan, M.; Gałązka, P. Endocrine systems of the skin. Adv. Dermatol. Allergol. 2019, 36, 519–523. [Google Scholar] [CrossRef]
- Ezure, T.; Amano, S. Increased subcutaneous adipose tissue impairs dermal function in diet-induced obese mice. Exp. Dermatol. 2010, 19, 878–882. [Google Scholar] [CrossRef]
- Ibuki, A.; Akase, T.; Nagase, T.; Minematsu, T.; Nakagami, G.; Horii, M.; Sagara, H.; Komeda, T.; Kobayashi, M.; Shimada, T.; et al. Skin fragility in obese diabetic mice: Possible involvement of elevated oxidative stress and upregulation of matrix metalloproteinases. Exp. Dermatol. 2012, 21, 178–183. [Google Scholar] [CrossRef]
- Argyropoulos, A.J.; Robichaud, P.; Balimunkwe, R.M.; Fisher, G.J.; Hammerberg, C.; Yan, Y.; Quan, T. Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin. PLoS ONE 2016, 11, e0153806. [Google Scholar] [CrossRef] [PubMed]
- Okano, J.; Kojima, H.; Katagi, M.; Nakagawa, T.; Nakae, Y.; Terashima, T.; Kurakane, T.; Kubota, M.; Maegawa, H.; Udagawa, J. Hyperglycemia Induces Skin Barrier Dysfunctions with Impairment of Epidermal Integrity in Non-Wounded Skin of Type 1 Diabetic Mice. PLoS ONE 2016, 11, e0166215. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Balukoff, N.C.; Marjanovic, J.; Chen, V.Y.; Stone, R.C.; Tomic-Canic, M. Molecular Pathophysiology of Chronic Wounds: Current State and Future Directions. Cold Spring Harb. Perspect. Biol. 2023, 15, a041243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Narayanan, S.; Sunkari, V.G.; Eliasson, S.; Botusan, I.R.; Grünler, J.; Catrina, A.I.; Radtke, F.; Xu, C.; Zhao, A.; et al. Triggering of a Dll4–Notch1 loop impairs wound healing in diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 6985–6994. [Google Scholar] [CrossRef]
- Stadler, I.; Zhang, R.-Y.; Oskoui, P.; Whittaker, M.B.S.; Lanzafame, R.J. Development of a Simple, Noninvasive, Clinically Relevant Model of Pressure Ulcers in the Mouse. J. Investig. Surg. 2004, 17, 221–227. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Regnault, T.R.; Gentili, S.; Sarr, O.; Toop, C.R.; Sloboda, D.M. Fructose, pregnancy and later life impacts. Clin. Exp. Pharmacol. Physiol. 2013, 40, 824–837. [Google Scholar] [CrossRef]
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: Evidence for a secondhand sugar effect. Proc. Nutr. Soc. 2019, 78, 262–271. [Google Scholar] [CrossRef]
- Diegelmann, R.F. Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regen. 2003, 11, 490–495. [Google Scholar] [CrossRef]
- Zhang, Z.; Kruglikov, I.; Zhao, S.; Zi, Z.; Gliniak, C.M.; Li, N.; Wang, M.; Zhu, Q.; Kusminski, C.M.; Scherer, P.E. Dermal adipocytes contribute to the metabolic regulation of dermal fibroblasts. Exp. Dermatol. 2021, 30, 102–111. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Graham, T.; Kahn, B.B. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol. Metab. Syndr. 2012, 4, 42. [Google Scholar] [CrossRef]
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. TNF-α production in the skin. Arch. Dermatol. Res. 2009, 301, 87–91. [Google Scholar] [CrossRef]
- Kim, B.E.; Howell, M.D.; Guttman, E.; Gilleaudeau, P.M.; Cardinale, I.R.; Boguniewicz, M.; Krueger, J.G.; Leung, D.Y. TNF-α Downregulates Filaggrin and Loricrin through c-Jun N-terminal Kinase: Role for TNF-α Antagonists to Improve Skin Barrier. J. Investig. Dermatol. 2011, 131, 1272–1279. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Prolla, T. PGC-1α in aging and anti-aging interventions. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1059–1066. [Google Scholar] [CrossRef]
- Cork, M.J.; Danby, S. Skin barrier breakdown: A renaissance in emollient therapy. Br. J. Nurs. 2009, 18, 872–877. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Barros, J.F.; Waclawiak, I.; Pecli, C.; Borges, P.A.; Georgii, J.L.; Ramos-Junior, E.S.; Canetti, C.; Courau, T.; Klatzmann, D.; Kunkel, S.L.; et al. Role of Chemokine Receptor CCR4 and Regulatory T Cells in Wound Healing of Diabetic Mice. J. Investig. Dermatol. 2019, 139, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Sanmiguel, J.C.; Olaru, F.; Li, J.; Mohr, E.; Jensen, L.E. Interleukin-1 regulates keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase-1 (IRAK1) dependent and independent pathways. Cell. Signal. 2009, 21, 685–694. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T. Molecular Mechanisms of VEGF-A Action during Tissue Repair. J. Investig. Dermatol. Symp. Proc. 2006, 11, 79–86. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A.; Perez-Amodio, S.; Rubio, N.; Vila, O.F.; Navarro-Requena, C.; Castaño, O.; Sanchez-Ferrero, A.; Marti-Munoz, J.; Alsina-Giber, M.; et al. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Mastrofrancesco, A.; Camera, E.; Desreumaux, P.; Paus, R.; Picardo, M. The role of PPARγ-mediated signalling in skin biology and pathology: New targets and opportunities for clinical dermatology. Exp. Dermatol. 2015, 24, 245–251. [Google Scholar] [CrossRef]
- Michalik, L.; Desvergne, B.; Tan, N.S.; Basu-Modak, S.; Escher, P.; Rieusset, J.; Peters, J.; Kaya, G.; Gonzalez, F.J.; Zakany, J.; et al. Impaired skin wound healing in peroxisome proliferator–activated receptor (PPAR)α and PPARβ mutant mice. J. Cell Biol. 2001, 154, 799–814. [Google Scholar] [CrossRef]
- Vickers, M.H.; Breier, B.H.; Cutfield, W.S.; Hofman, P.L.; Gluckman, P.D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Metab. 2000, 279, E83–E87. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bridge-Comer, P.E.; Vickers, M.H.; Ferraro, S.; Pagnon, A.; Reynolds, C.M.; Sigaudo-Roussel, D. Maternal Intake of Either Fructose or the Artificial Sweetener Acesulfame-K Results in Differential and Sex-Specific Alterations in Markers of Skin Inflammation and Wound Healing Responsiveness in Mouse Offspring: A Pilot Study. Nutrients 2023, 15, 2534. https://doi.org/10.3390/nu15112534
Bridge-Comer PE, Vickers MH, Ferraro S, Pagnon A, Reynolds CM, Sigaudo-Roussel D. Maternal Intake of Either Fructose or the Artificial Sweetener Acesulfame-K Results in Differential and Sex-Specific Alterations in Markers of Skin Inflammation and Wound Healing Responsiveness in Mouse Offspring: A Pilot Study. Nutrients. 2023; 15(11):2534. https://doi.org/10.3390/nu15112534
Chicago/Turabian StyleBridge-Comer, Pania E., Mark H. Vickers, Sandra Ferraro, Aurélie Pagnon, Clare M. Reynolds, and Dominique Sigaudo-Roussel. 2023. "Maternal Intake of Either Fructose or the Artificial Sweetener Acesulfame-K Results in Differential and Sex-Specific Alterations in Markers of Skin Inflammation and Wound Healing Responsiveness in Mouse Offspring: A Pilot Study" Nutrients 15, no. 11: 2534. https://doi.org/10.3390/nu15112534
APA StyleBridge-Comer, P. E., Vickers, M. H., Ferraro, S., Pagnon, A., Reynolds, C. M., & Sigaudo-Roussel, D. (2023). Maternal Intake of Either Fructose or the Artificial Sweetener Acesulfame-K Results in Differential and Sex-Specific Alterations in Markers of Skin Inflammation and Wound Healing Responsiveness in Mouse Offspring: A Pilot Study. Nutrients, 15(11), 2534. https://doi.org/10.3390/nu15112534








