Maternal Vitamin C Intake during Pregnancy Influences Long-Term Offspring Growth with Timing- and Sex-Specific Effects in Guinea Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animal Model
2.3. Delivery and Postnatal Care of Offspring
2.4. Oral Glucose Tolerance Tests
2.5. Cortisol Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Euthanasia and Tissue Collection
2.7. Statistical Analysis
3. Results
3.1. Maternal Physiology
3.1.1. Maternal Characteristics
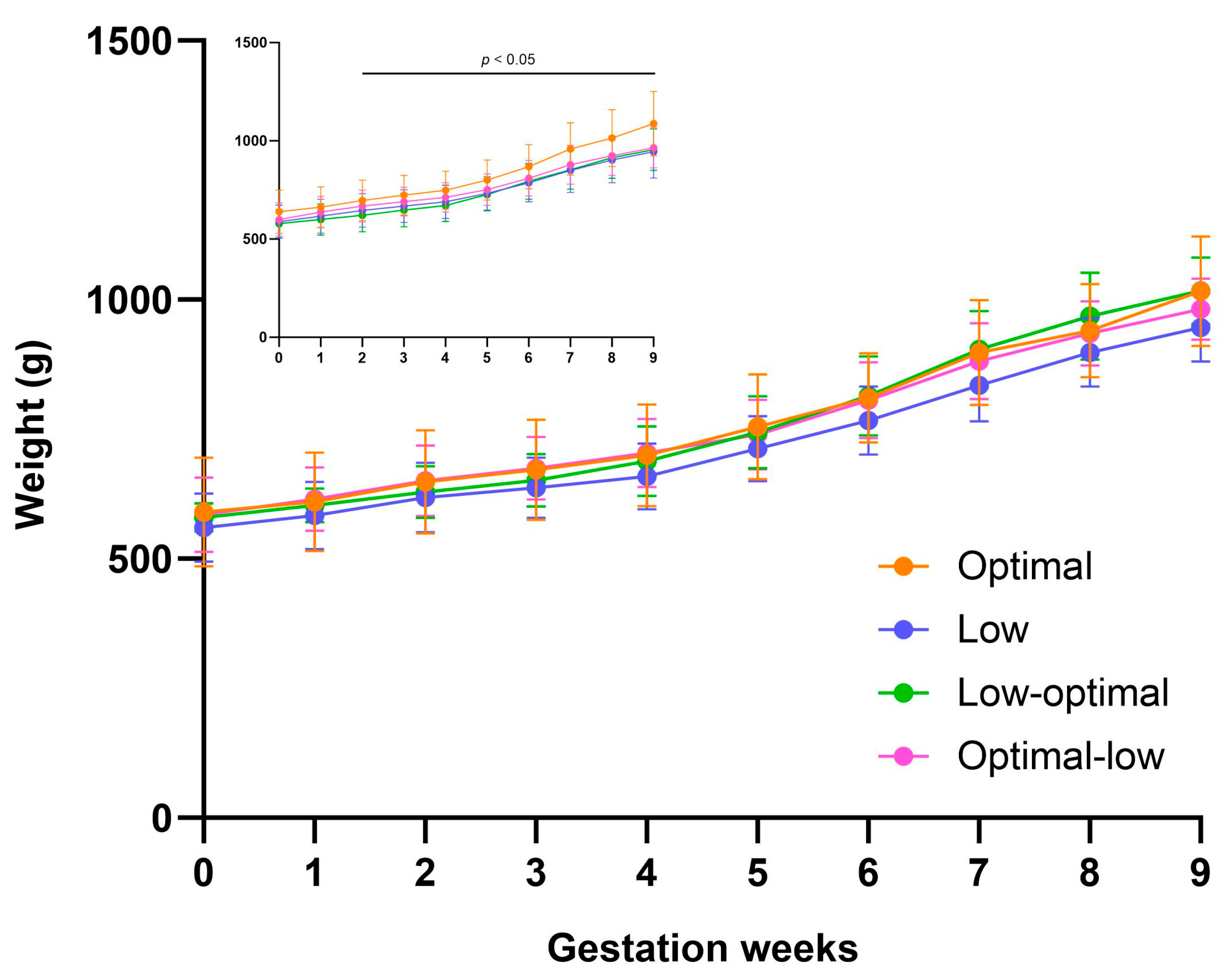
3.1.2. Pregnancy Weight Gain
3.1.3. Pregnancy Outcomes
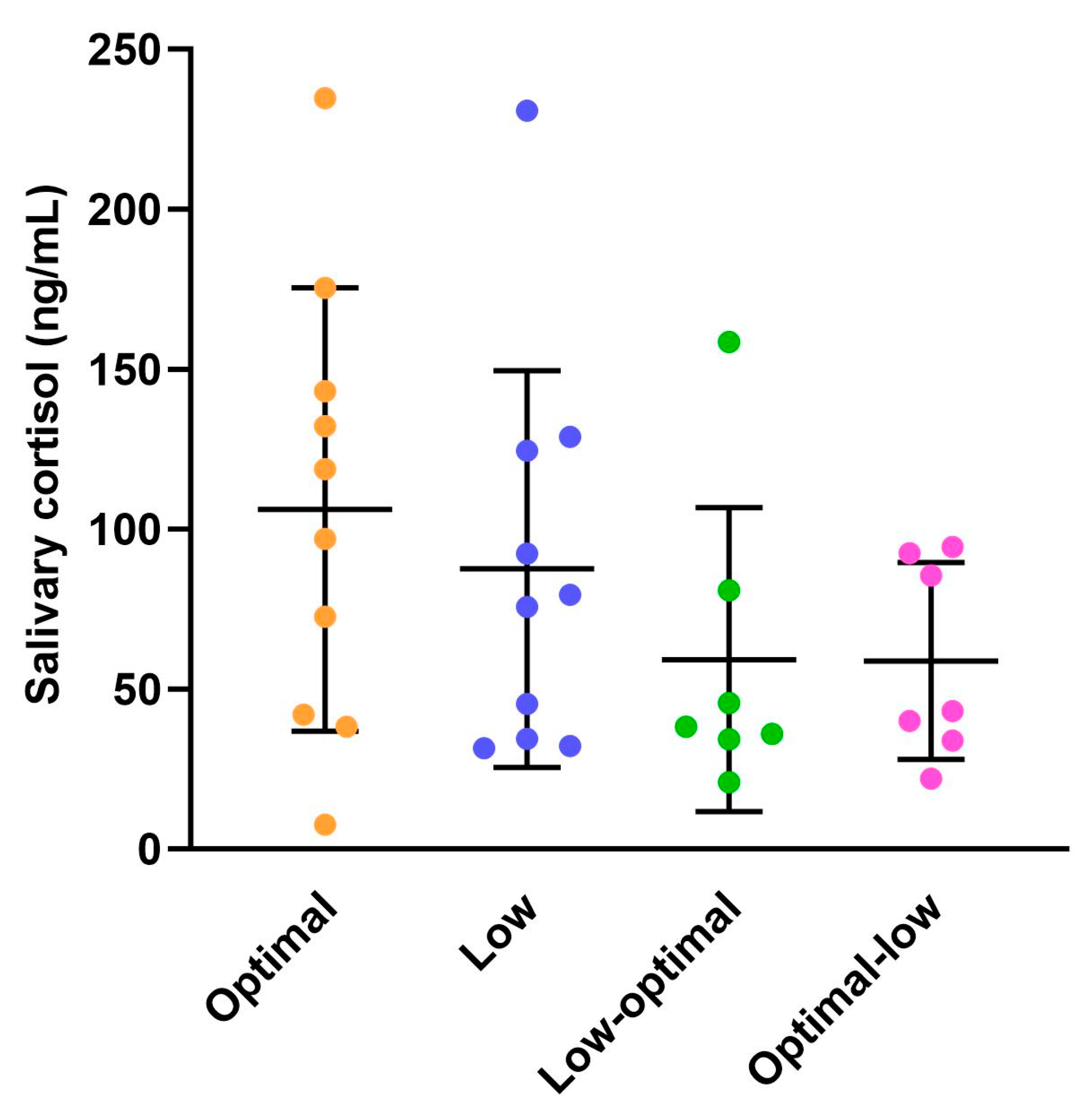
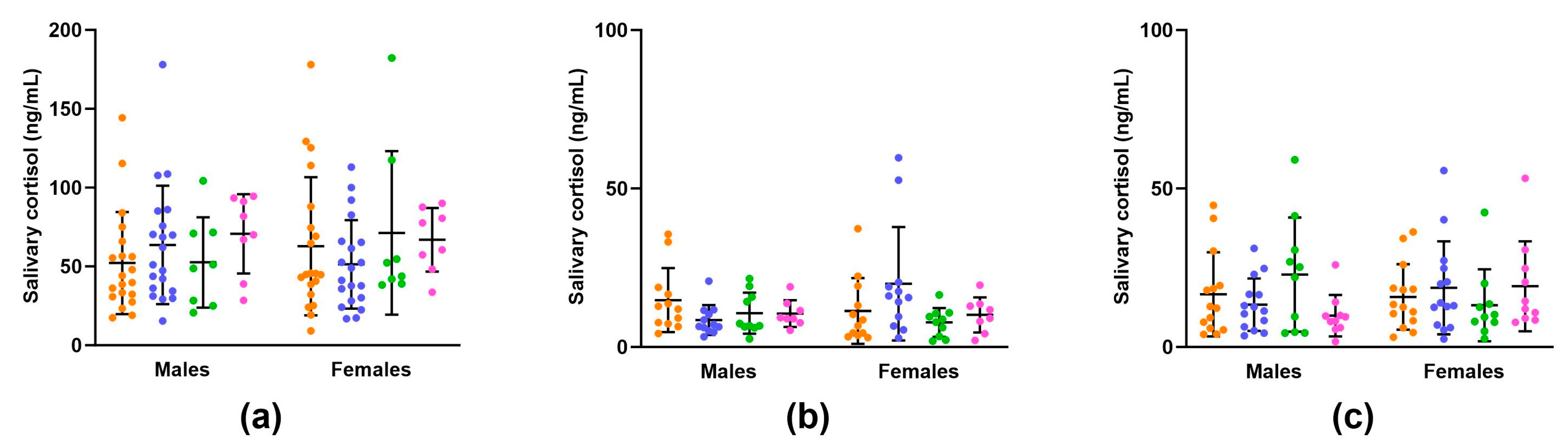
3.1.4. Maternal Salivary Cortisol Concentrations
3.2. Offspring Physiology
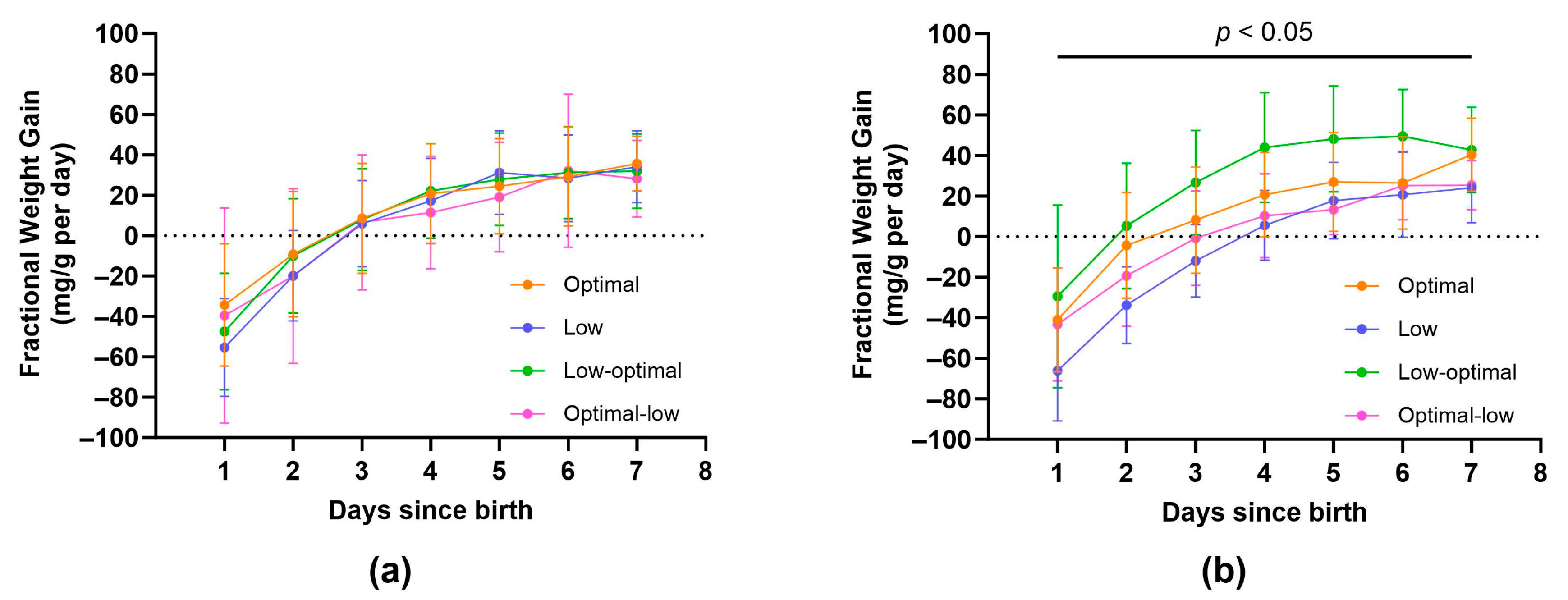
3.2.1. Neonate Characteristics
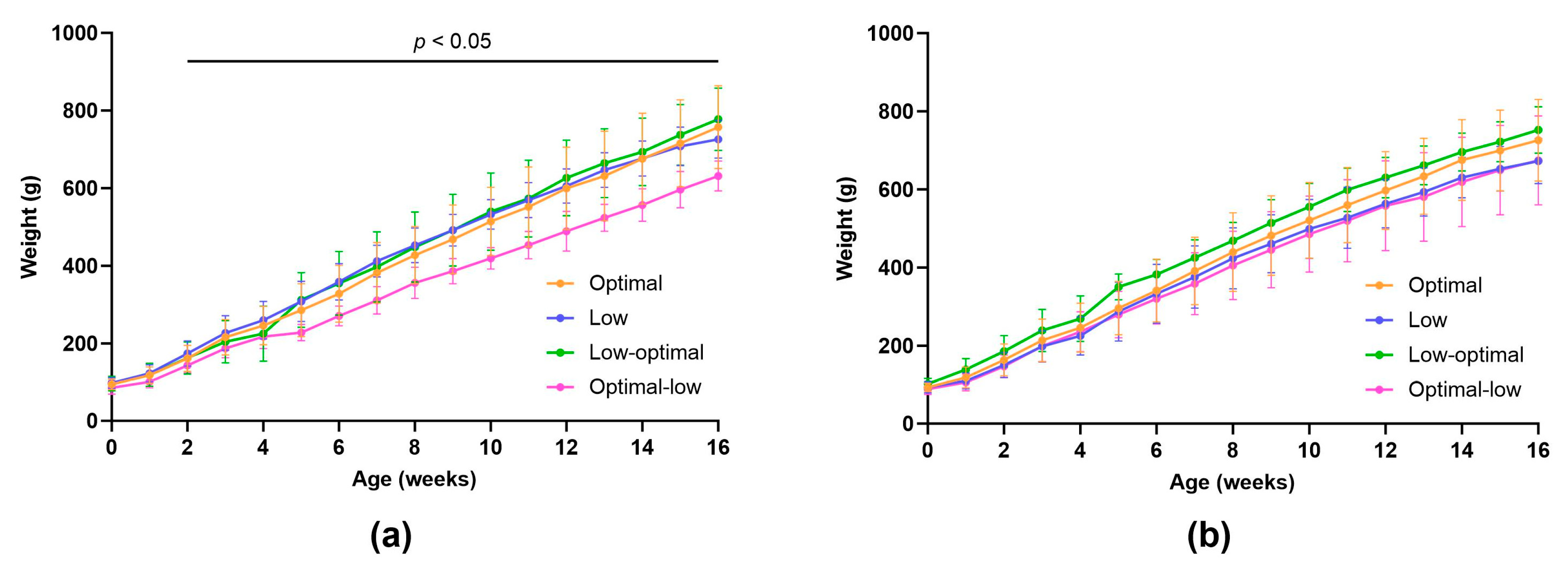
3.2.2. Weanling Characteristics
3.2.3. Juvenile and Adolescent Characteristics
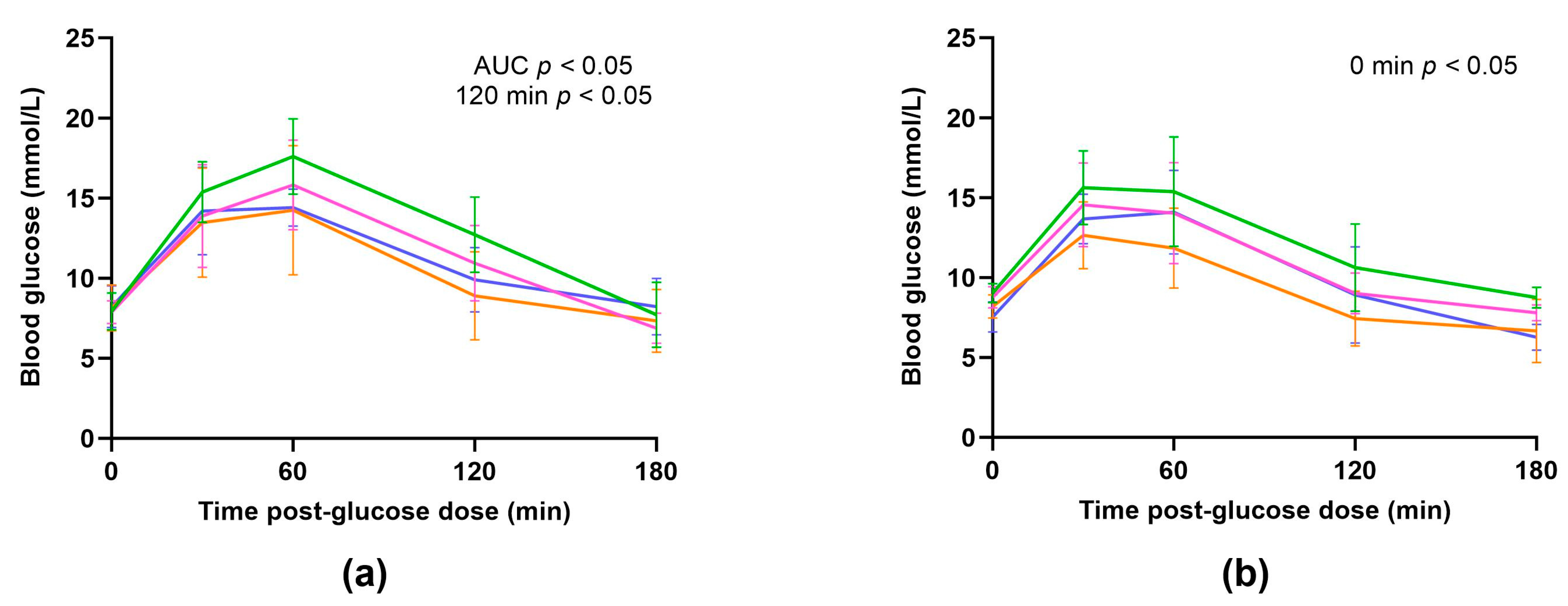
3.2.4. Oral Glucose Tolerance Tests
3.2.5. Offspring Salivary Cortisol Concentrations
3.2.6. Offspring Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, R.; Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Zhang, H.; Chen, X.; Chen, J.; et al. Prenatal famine exposure, adulthood obesity patterns and risk of type 2 diabetes. Int. J. Epidemiol. 2018, 47, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Lumey, L.H.; Khalangot, M.D.; Vaiserman, A.M. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.V.L.; Dyson, R.M.; Vanderboor, C.M.G.; Sarr, O.; Anderson, J.; Berry, M.J.; Regnault, T.R.H.; Peng, L.; Gray, C. Maternal Fructose Intake Causes Developmental Reprogramming of Hepatic Mitochondrial Catalytic Activity and Lipid Metabolism in Weanling and Young Adult Offspring. Int. J. Mol. Sci. 2022, 23, 999. [Google Scholar] [CrossRef]
- Bayol, S.A.; Simbi, B.H.; Fowkes, R.C.; Stickland, N.C. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic Fatty liver disease in rat offspring. Endocrinology 2010, 151, 1451–1461. [Google Scholar] [CrossRef]
- Roseboom, T.J.; van der Meulen, J.H.; Osmond, C.; Barker, D.J.; Ravelli, A.C.; Schroeder-Tanka, J.M.; van Montfrans, G.A.; Michels, R.P.; Bleker, O.P. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–1945. Heart 2000, 84, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Ekamper, P.; van Poppel, F.; Stein, A.D.; Bijwaard, G.E.; Lumey, L.H. Prenatal famine exposure and adult mortality from cancer, cardiovascular disease, and other causes through age 63 years. Am. J. Epidemiol. 2015, 181, 271–279. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van der Meulen, J.H.; Michels, R.P.; Osmond, C.; Barker, D.J.; Hales, C.N.; Bleker, O.P. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998, 351, 173–177. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van Der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999, 70, 811–816. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.R.; Page, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Rajan, D.P.; Huang, W.; Dutta, B.; Devoe, L.D.; Leibach, F.H.; Ganapathy, V.; Prasad, P.D. Human placental sodium-dependent vitamin C transporter (SVCT2): Molecular cloning and transport function. Biochem. Biophys. Res. Commun. 1999, 262, 762–768. [Google Scholar] [CrossRef]
- Sotiriou, S.; Gispert, S.; Cheng, J.; Wang, Y.; Chen, A.; Hoogstraten-Miller, S.; Miller, G.F.; Kwon, O.; Levine, M.; Guttentag, S.H.; et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 2002, 8, 514–517. [Google Scholar] [CrossRef]
- Jain, S.K.; Wise, R.; Yanamandra, K.; Dhanireddy, R.; Bocchini, J.A., Jr. The effect of maternal and cord-blood vitamin C, vitamin E and lipid peroxide levels on newborn birth weight. Mol. Cell. Biochem. 2008, 309, 217–221. [Google Scholar] [CrossRef]
- Lee, B.E.; Hong, Y.C.; Lee, K.H.; Kim, Y.J.; Kim, W.K.; Chang, N.S.; Park, E.A.; Park, H.S.; Hann, H.J. Influence of maternal serum levels of vitamins C and E during the second trimester on birth weight and length. Eur. J. Clin. Nutr. 2004, 58, 1365–1371. [Google Scholar] [CrossRef]
- Jang, W.; Kim, H.; Lee, B.E.; Chang, N. Maternal fruit and vegetable or vitamin C consumption during pregnancy is associated with fetal growth and infant growth up to 6 months: Results from the Korean Mothers and Children’s Environmental Health (MOCEH) cohort study. Nutr. J. 2018, 17, 105. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kanai, T.; Sato, K.; Lee, J.; Jeong, K.S.; Shimokado, K.; Maruyama, N.; Ishigami, A. Insufficient ascorbic acid intake during gestation induces abnormal cardiac dilation in fetal and neonatal SMP30/GNL knockout mice. Pediatr. Res. 2013, 73, 578–584. [Google Scholar] [CrossRef]
- Schjoldager, J.G.; Paidi, M.D.; Lindblad, M.M.; Birck, M.M.; Kjærgaard, A.B.; Dantzer, V.; Lykkesfeldt, J.; Tveden-Nyborg, P. Maternal vitamin C deficiency during pregnancy results in transient fetal and placental growth retardation in guinea pigs. Eur. J. Nutr. 2015, 54, 667–676. [Google Scholar] [CrossRef]
- Habibzadeh, N.; Schorah, C.J.; Smithells, R.W. The effects of maternal folic acid and vitamin C nutrition in early pregnancy on reproductive performance in the guinea-pig. Br. J. Nutr. 1986, 55, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Tveden-Nyborg, P.; Vogt, L.; Schjoldager, J.G.; Jeannet, N.; Hasselholt, S.; Paidi, M.D.; Christen, S.; Lykkesfeldt, J. Maternal vitamin C deficiency during pregnancy persistently impairs hippocampal neurogenesis in offspring of guinea pigs. PLoS ONE 2012, 7, e48488. [Google Scholar] [CrossRef] [PubMed]
- Tveden-Nyborg, P.; Johansen, L.K.; Raida, Z.; Villumsen, C.K.; Larsen, J.O.; Lykkesfeldt, J. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am. J. Clin. Nutr. 2009, 90, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Paidi, M.D.; Schjoldager, J.G.; Lykkesfeldt, J.; Tveden-Nyborg, P. Prenatal vitamin C deficiency results in differential levels of oxidative stress during late gestation in foetal guinea pig brains. Redox Biol. 2014, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Trueba, G.P.; Poulsen, H.E.; Christen, S. Vitamin C deficiency in weanling guinea pigs: Differential expression of oxidative stress and DNA repair in liver and brain. Br. J. Nutr. 2007, 98, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.E.; Dawes, S.M.; Meredith, M.E.; Babaev, V.R.; Li, L.; May, J.M. Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic. Biol. Med. 2010, 49, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Liggins, G.C. The role of cortisol in preparing the fetus for birth. Reprod. Fertil. Dev. 1994, 6, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chadio, S.E.; Kotsampasi, B.; Papadomichelakis, G.; Deligeorgis, S.; Kalogiannis, D.; Menegatos, I.; Zervas, G. Impact of maternal undernutrition on the hypothalamic-pituitary-adrenal axis responsiveness in sheep at different ages postnatal. J. Endocrinol. 2007, 192, 495–503. [Google Scholar] [CrossRef]
- Kapoor, A.; Dunn, E.; Kostaki, A.; Andrews, M.H.; Matthews, S.G. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J. Physiol. 2006, 572 Pt 1, 31–44. [Google Scholar] [CrossRef]
- Kapoor, A.; Matthews, S.G. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J. Physiol. 2005, 566 Pt 3, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.C.; Palliser, H.K.; Dyson, R.M.; Hirst, J.J.; Berry, M.J. Long-term effects of preterm birth on behavior and neurosteroid sensitivity in the guinea pig. Pediatr. Res. 2016, 80, 275–283. [Google Scholar] [CrossRef]
- Bruehl, H.; Rueger, M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Javier, E.; Arentoft, A.; Wolf, O.T.; Convit, A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 2439–2445. [Google Scholar] [CrossRef]
- Wirtz, P.H.; von Kanel, R.; Emini, L.; Ruedisueli, K.; Groessbauer, S.; Maercker, A.; Ehlert, U. Evidence for altered hypothalamus-pituitary-adrenal axis functioning in systemic hypertension: Blunted cortisol response to awakening and lower negative feedback sensitivity. Psychoneuroendocrinology 2007, 32, 430–436. [Google Scholar] [CrossRef]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Walker, E.F.; Trotman, H.D.; Pearce, B.D.; Addington, J.; Cadenhead, K.S.; Cornblatt, B.A.; Heinssen, R.; Mathalon, D.H.; Perkins, D.O.; Seidman, L.J.; et al. Cortisol levels and risk for psychosis: Initial findings from the North American prodrome longitudinal study. Biol. Psychiatry 2013, 74, 410–417. [Google Scholar] [CrossRef]
- Xiong, F.; Zhang, L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front. Neuroendocrinol. 2013, 34, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2020, 14, 601939. [Google Scholar] [CrossRef] [PubMed]
- Coker, S.J.; Smith-Diaz, C.C.; Dyson, R.M.; Vissers, M.C.M.; Berry, M.J. The Epigenetic Role of Vitamin C in Neurodevelopment. Int. J. Mol. Sci. 2022, 23, 1208. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Coker, S.J.; Dyson, R.M.; Smith-Díaz, C.C.; Vissers, M.C.M.; Berry, M.J. Effects of Low Vitamin C Intake on Fertility Parameters and Pregnancy Outcomes in Guinea Pigs. Nutrients 2023, 15, 4107. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.V.L.; Dyson, R.M.; Berry, M.J.; Gray, C. Fructose Consumption During Pregnancy Influences Milk Lipid Composition and Offspring Lipid Profiles in Guinea Pigs. Front. Endocrinol. 2020, 11, 550. [Google Scholar] [CrossRef]
- Morrison, J.L.; Botting, K.J.; Darby, J.R.T.; David, A.L.; Dyson, R.M.; Gatford, K.L.; Gray, C.; Herrera, E.A.; Hirst, J.J.; Kim, B.; et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol. 2018, 596, 5535–5569. [Google Scholar] [CrossRef]
- Martinho, F. Dystocia caused by ectopic pregnancy in a guinea pig (Cavia porcellus). Vet. Clin. Exot. Anim. Pract. 2006, 9, 713–716. [Google Scholar] [CrossRef]
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marcal, V.M.; Lobo, T.F.; Peixoto, A.B.; Araujo Junior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef]
- Mitchell, M.L. Fetal brain to liver weight ratio as a measure of intrauterine growth retardation: Analysis of 182 stillborn autopsies. Mod. Pathol. 2001, 14, 14–19. [Google Scholar] [CrossRef][Green Version]
- Longo, S.; Borghesi, A.; Tzialla, C.; Stronati, M. IUGR and infections. Early Hum. Dev. 2014, 90 (Suppl. S1), S42–S44. [Google Scholar] [CrossRef]
- Nowakowska, B.A.; Pankiewicz, K.; Nowacka, U.; Niemiec, M.; Kozlowski, S.; Issat, T. Genetic Background of Fetal Growth Restriction. Int. J. Mol. Sci. 2021, 23, 36. [Google Scholar] [CrossRef]
- Lumey, L.H. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944–1945. Paediatr. Perinat. Epidemiol. 1992, 6, 240–253. [Google Scholar] [CrossRef]
- Odegard, R.A.; Vatten, L.J.; Nilsen, S.T.; Salvesen, K.A.; Austgulen, R. Preeclampsia and fetal growth. Obstet. Gynecol. 2000, 96, 950–955. [Google Scholar] [PubMed]
- Ibanez, L.; Suarez, L.; Lopez-Bermejo, A.; Diaz, M.; Valls, C.; de Zegher, F. Early development of visceral fat excess after spontaneous catch-up growth in children with low birth weight. J. Clin. Endocrinol. Metab. 2008, 93, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Korada, M.; Wood, C.L.; Pearce, M.S.; Swamy, R.; Cheetham, T.D. Catch-up growth and metabolic outcomes in adolescents born preterm. Arch. Dis. Child. 2016, 101, 1026–1031. [Google Scholar] [CrossRef]
- Singhal, A. Should We Promote Catch-Up Growth or Growth Acceleration in Low-Birthweight Infants? Nestle Nutr. Inst. Workshop Ser. 2015, 81, 51–60. [Google Scholar] [PubMed]
- Mace, K.; Shahkhalili, Y.; Aprikian, O.; Stan, S. Dietary fat and fat types as early determinants of childhood obesity: A reappraisal. Int. J. Obes. 2006, 30 (Suppl. S4), S50–S57. [Google Scholar] [CrossRef]
- Carberry, A.E.; Colditz, P.B.; Lingwood, B.E. Body composition from birth to 4.5 months in infants born to non-obese women. Pediatr. Res. 2010, 68, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Gungor, D.E.; Paul, I.M.; Birch, L.L.; Bartok, C.J. Risky vs. rapid growth in infancy: Refining pediatric screening for childhood overweight. Arch. Pediatr. Adolesc. Med. 2010, 164, 1091–1097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [PubMed]
- Zhang, D.L.; Du, Q.; Djemli, A.; Julien, P.; Fraser, W.D.; Luo, Z.C. Early and Late Postnatal Accelerated Growth Have Distinct Effects on Metabolic Health in Normal Birth Weight Infants. Front. Endocrinol. 2017, 8, 340. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Monaghan, P. Compensation for a bad start: Grow now, pay later? Trends Ecol. Evol. 2001, 16, 254–260. [Google Scholar] [CrossRef]
- Buckberry, S.; Bianco-Miotto, T.; Bent, S.J.; Dekker, G.A.; Roberts, C.T. Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Mol. Hum. Reprod. 2014, 20, 810–819. [Google Scholar] [CrossRef]
- Broere-Brown, Z.A.; Baan, E.; Schalekamp-Timmermans, S.; Verburg, B.O.; Jaddoe, V.W.; Steegers, E.A. Sex-specific differences in fetal and infant growth patterns: A prospective population-based cohort study. Biol. Sex Differ. 2016, 7, 65. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Kajantie, E.; Osmond, C.; Thornburg, K.; Barker, D.J. Boys live dangerously in the womb. Am. J. Hum. Biol. 2010, 22, 330–335. [Google Scholar] [CrossRef]
- Kraemer, S. The fragile male. BMJ 2000, 321, 1609–1612. [Google Scholar] [CrossRef]
- Renaville, R.; Hammadi, M.; Portetelle, D. Role of the somatotropic axis in the mammalian metabolism. Domest. Anim. Endocrinol. 2002, 23, 351–360. [Google Scholar] [CrossRef]
- Hoffman, M.L.; Reed, S.A.; Pillai, S.M.; Jones, A.K.; McFadden, K.K.; Zinn, S.A.; Govoni, K.E. Physiology and endocrinology symposium: The effects of poor maternal nutrition during gestation on offspring postnatal growth and metabolism. J. Anim. Sci. 2017, 95, 2222–2232. [Google Scholar] [CrossRef]
- Bauer, M.K.; Breier, B.H.; Harding, J.E.; Veldhuis, J.D.; Gluckman, P.D. The fetal somatotropic axis during long term maternal undernutrition in sheep: Evidence for nutritional regulation in utero. Endocrinology 1995, 136, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, C.; Nissen, P.M.; Kuhn, G.; Vestergaard, M.; Ender, K.; Oksbjerg, N. Effects of maternal nutrition and porcine growth hormone (pGH) treatment during gestation on endocrine and metabolic factors in sows, fetuses and pigs, skeletal muscle development, and postnatal growth. Domest. Anim. Endocrinol. 2004, 27, 267–285. [Google Scholar] [CrossRef]
- Hoffman, M.L.; Rokosa, M.A.; Zinn, S.A.; Hoagland, T.A.; Govoni, K.E. Poor maternal nutrition during gestation in sheep reduces circulating concentrations of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in offspring. Domest. Anim. Endocrinol. 2014, 49, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.L.; Peck, K.N.; Forella, M.E.; Fox, A.R.; Govoni, K.E.; Zinn, S.A. The effects of poor maternal nutrition during gestation on postnatal growth and development of lambs. J. Anim. Sci. 2016, 94, 789–799. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Zhang, L.; Tuersunjiang, N.; Ma, Y.; Long, N.M.; Uthlaut, A.B.; Smith, D.T.; Nathanielsz, P.W.; Ford, S.P. Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R795–R804. [Google Scholar] [CrossRef] [PubMed]
- Freake, H.C.; Govoni, K.E.; Guda, K.; Huang, C.; Zinn, S.A. Actions and interactions of thyroid hormone and zinc status in growing rats. J. Nutr. 2001, 131, 1135–1141. [Google Scholar] [CrossRef]
- Rausch, M.I.; Tripp, M.W.; Govoni, K.E.; Zang, W.; Webert, W.J.; Crooker, B.A.; Hoagland, T.A.; Zinn, S.A. The influence of level of feeding on growth and serum insulin-like growth factor I and insulin-like growth factor-binding proteins in growing beef cattle supplemented with somatotropin. J. Anim. Sci. 2002, 80, 94–100. [Google Scholar] [CrossRef]
- Claahsen-van der Grinten, H.L.; Speiser, P.W.; Ahmed, S.F.; Arlt, W.; Auchus, R.J.; Falhammar, H.; Fluck, C.E.; Guasti, L.; Huebner, A.; Kortmann, B.B.M.; et al. Congenital Adrenal Hyperplasia-Current Insights in Pathophysiology, Diagnostics, and Management. Endocr. Rev. 2022, 43, 91–159. [Google Scholar] [CrossRef]
- Sohaib, S.A.; Hanson, J.A.; Newell-Price, J.D.; Trainer, P.J.; Monson, J.P.; Grossman, A.B.; Besser, G.M.; Reznek, R.H. CT appearance of the adrenal glands in adrenocorticotrophic hormone-dependent Cushing’s syndrome. AJR Am. J. Roentgenol. 1999, 172, 997–1002. [Google Scholar] [CrossRef]
- Vining, R.F.; McGinley, R.A.; Symons, R.G. Hormones in saliva: Mode of entry and consequent implications for clinical interpretation. Clin. Chem. 1983, 29, 1752–1756. [Google Scholar] [CrossRef]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef]
- Falhammar, H.; Frisen, L.; Hirschberg, A.L.; Norrby, C.; Almqvist, C.; Nordenskjold, A.; Nordenstrom, A. Increased Cardiovascular and Metabolic Morbidity in Patients with 21-Hydroxylase Deficiency: A Swedish Population-Based National Cohort Study. J. Clin. Endocrinol. Metab. 2015, 100, 3520–3528. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Fraga, N.R.; Minaeian, N.; Geffner, M.E. Components of Metabolic Syndrome in Youth with Classical Congenital Adrenal Hyperplasia. Front. Endocrinol. 2022, 13, 848274. [Google Scholar] [CrossRef] [PubMed]
- Scaroni, C.; Zilio, M.; Foti, M.; Boscaro, M. Glucose Metabolism Abnormalities in Cushing Syndrome: From Molecular Basis to Clinical Management. Endocr. Rev. 2017, 38, 189–219. [Google Scholar] [CrossRef]
- Horton, D.M.; Saint, D.A.; Owens, J.A.; Gatford, K.L.; Kind, K.L. Use of the hyperinsulinemic euglycemic clamp to assess insulin sensitivity in guinea pigs: Dose response, partitioned glucose metabolism, and species comparisons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R19–R28. [Google Scholar] [CrossRef] [PubMed]
- Podell, B.K.; Ackart, D.F.; Richardson, M.A.; DiLisio, J.E.; Pulford, B.; Basaraba, R.J. A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis. Model. Mech. 2017, 10, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Kind, K.L.; Clifton, P.M.; Grant, P.A.; Owens, P.C.; Sohlstrom, A.; Roberts, C.T.; Robinson, J.S.; Owens, J.A. Effect of maternal feed restriction during pregnancy on glucose tolerance in the adult guinea pig. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R140–R152. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Hales, C.N. Lifespan: Catch-up growth and obesity in male mice. Nature 2004, 427, 411–412. [Google Scholar] [CrossRef]
- Jimenez-Chillaron, J.C.; Patti, M.E. To catch up or not to catch up: Is this the question? Lessons from animal models. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 23–29. [Google Scholar] [CrossRef]
- Ong, K.K.; Loos, R.J. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006, 95, 904–908. [Google Scholar] [CrossRef]
- Demerath, E.W.; Reed, D.; Choh, A.C.; Soloway, L.; Lee, M.; Czerwinski, S.A.; Chumlea, W.C.; Siervogel, R.M.; Towne, B. Rapid postnatal weight gain and visceral adiposity in adulthood: The Fels Longitudinal Study. Obesity 2009, 17, 2060–2066. [Google Scholar] [CrossRef]
- Van Hulst, A.; Barnett, T.A.; Paradis, G.; Roy-Gagnon, M.H.; Gomez-Lopez, L.; Henderson, M. Birth Weight, Postnatal Weight Gain, and Childhood Adiposity in Relation to Lipid Profile and Blood Pressure during Early Adolescence. J. Am. Heart. Assoc. 2017, 6, e006302. [Google Scholar] [CrossRef]
- Fabricius-Bjerre, S.; Jensen, R.B.; Faerch, K.; Larsen, T.; Molgaard, C.; Michaelsen, K.F.; Vaag, A.; Greisen, G. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS ONE 2011, 6, e20595. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Lillycrop, K.A.; Burdge, G.C.; Gluckman, P.D.; Hanson, M.A. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr. Res. 2007, 61 Pt 2, 5R–10R. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Low, F.M. The role of developmental plasticity and epigenetics in human health. Birth Defects Res. Part C Embryo Today 2011, 93, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Tomas, E.; Lin, Y.S.; Dagher, Z.; Saha, A.; Luo, Z.; Ido, Y.; Ruderman, N.B. Hyperglycemia and insulin resistance: Possible mechanisms. Ann. N. Y. Acad. Sci. 2002, 967, 43–51. [Google Scholar] [CrossRef]
- Jellinger, P.S. Metabolic consequences of hyperglycemia and insulin resistance. Clin. Cornerstone 2007, 8 (Suppl. S7), S30–S42. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Chen, P.Y.; Ganguly, A.; Rubbi, L.; Orozco, L.D.; Morselli, M.; Ashraf, D.; Jaroszewicz, A.; Feng, S.; Jacobsen, S.E.; Nakano, A.; et al. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol. Genom. 2013, 45, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martinez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Mao, S.Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Optimal | (n) | Low | (n) | Low-Optimal | (n) | Optimal-Low | (n) |
|---|---|---|---|---|---|---|---|---|
| Weight (g) at enrolment | 451.9 ± 49.0 | 35 | 432.7 ± 48.1 | 38 | 439.7 ± 37.7 | 22 | 428.6 ± 46.0 | 22 |
| Weight (g) at mating | 632.7 ± 111.8 | 34 | 588.5 ± 85.9 | 36 | 579.2 ± 73.1 | 22 | 594.1 ± 85.8 | 22 |
| Age (weeks) at mating | 14.9 ± 4.0 | 34 | 16.1 ± 5.2 | 36 | 15.4 ± 4.7 | 22 | 15.2 ± 4.5 | 22 |
| Outcomes | Optimal | (n) | Low | (n) | Low-Optimal | (n) | Optimal-Low | (n) |
|---|---|---|---|---|---|---|---|---|
| Miscarriage (delivery before GA62) (n = total pregnancies) | 4 (11.8%) | 34 | 2 (5.6%) | 36 | 0 | 22 | 1 (4.5%) | 22 |
| Foetal reabsorption (n = total pregnancies) | 2 (5.9%) | 34 | 9 (25%) | 36 | 5 (22.7%) | 22 | 3 (13.6%) | 22 |
| Premature delivery (born GA62–66) (n = litters born GA62+) | 0 | 30 | 2 (5.9%) | 34 | 2 (9.1%) | 22 | 1 (4.8%) | 21 |
| Stillbirth # of pregnancies (n = litters born GA62+) | 5 (16.7%) | 30 | 9 (26.5%) | 34 | 1 (4.5%) | 22 | 2 (9.5%) | 21 |
| Cumulative adverse outcome (n = total pregnancies) | 11 (32.4%) | 34 | 22 (61.1%) | 36 | 7 (31.8%) | 22 | 6 (27.3%) * | 22 |
| Stillbirth # of pups (n = total pups) | 6 (5.9%) | 102 | 11 (13.1%) | 85 | 2 (3.9%) | 51 | 1 (1.6%) * | 61 |
| Litter size (n pups) (n = term litters (born GA66+)) | 3.4 ± 1.0 (2–6) | 30 | 2.6 ± 0.9 (1–4) | 32 | 2.2 ± 0.8 (1–4) * | 20 | 2.9 ± 1.0 (1–4) | 20 |
| GA of pups at delivery (n = term litters of 2–4 pups) † | 68.6 ± 1.0 | 27 | 68.9 ± 1.2 | 27 | 69.5 ± 1.3 | 17 | 69.2 ± 1.0 | 18 |
| Litter birth weight (g) (n = term litters of 2–4 pups) † | 95.3 ± 10.4 | 27 | 97.2 ± 10.0 | 27 | 105.5 ± 11.7 * | 17 | 87.6 ± 11.4 | 18 |
| Pup sexes (n = total liveborn pups) | 49♂ 47♀ | 96 | 37♂ 37♀ | 74 | 24♂ 25♀ | 49 | 30♂ 30♀ | 60 |
| Characteristics | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Optimal (n = 44) | Low (n = 36) | Low-Optimal (n = 24) | Optimal-Low (n = 30) | Optimal (n = 42) | Low (n = 35) | Low-Optimal (n = 25) | Optimal-Low (n = 30) | |
| Body weight (g) | 93.3 ± 14.8 | 96.3 ± 13.2 | 102.2 ± 13.3 * | 87.8 ± 16.0 | 91.4 ± 12.9 | 93.5 ± 14.8 | 104.2 ± 13.1 * | 86.5 ± 14.4 |
| Crown-rump (mm) | 128.8 ± 8.8 | 129.0 ± 9.0 | 135.5 ± 8.5 * | 126.1 ± 10.8 | 127.7 ± 8.7 | 126.0 ± 8.7 | 137.5 ± 8.5 * | 124.4 ± 8.9 |
| Hind limb (mm) | 38.5 ± 5.0 | 35.1 ± 3.7 | 37.0 ± 2.5 | 36.6 ± 3.8 | 36.7 ± 4.3 | 34.7 ± 3.6 | 36.6 ± 3.4 | 33.3 ± 4.5 * |
| Hock-toe (mm) | 38.2 ± 5.1 | 36.8 ± 3.1 | 35.9 ± 2.3 | 36.6 ± 3.4 | 37.3 ± 4.6 | 35.0 ± 3.6 | 37.4 ± 3.0 | 34.7 ± 3.0 |
| Ponderal Index (PI) | 20.2 ± 3.4 | 22.3 ± 2.9 | 20.0 ± 1.8 * | 20.5 ± 3.6 | 20.6 ± 3.3 | 23.2 ± 2.7 | 20.3 ± 2.2 * | 22.7 ± 4.7 |
| Characteristics | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Optimal (n = 23) | Low (n = 19) | Low-Optimal (n = 6) | Optimal-Low (n = 12) | Optimal (n = 22) | Low (n = 18) | Low-Optimal (n = 9) | Optimal-Low (n = 9) | |
| Brain-to-body wgt | 2.67 ± 0.43 | 2.58 ± 0.29 | 2.65 ± 0.36 | 2.83 ± 0.35 | 2.71 ± 0.46 | 2.52 ± 0.30 | 2.42 ± 0.27 | 2.87 ± 0.42 |
| Liver-to-body wgt | 3.94 ± 0.74 | 3.79 ± 0.36 | 4.22 ± 0.37 | 3.34 ± 0.44 * | 4.26 ± 0.56 | 3.88 ± 0.52 | 4.01 ± 0.73 | 3.70 ± 0.53 |
| Brain-to-liver ratio | 0.71 ± 0.19 | 0.68 ± 0.11 | 0.63 ± 0.09 | 0.86 ± 0.17 * | 0.66 ± 0.16 | 0.67 ± 0.11 | 0.61 ± 0.08 | 0.80 ± 0.20 |
| Heart-to-body wgt | 0.41 ± 0.04 | 0.40 ± 0.05 | 0.44 ± 0.02 | 0.44 ± 0.05 | 0.43 ± 0.04 | 0.40 ± 0.03 | 0.43 ± 0.03 | 0.41 ± 0.05 |
| Kidney-to-body wgt | 0.43 ± 0.04 | 0.44 ± 0.04 | 0.44 ± 0.04 | 0.39 ± 0.04 * | 0.43 ± 0.04 | 0.44 ± 0.04 | 0.42 ± 0.05 | 0.46 ± 0.04 |
| Adrenal-to-body wgt | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.004 * | 0.01 ± 0.003 * | 0.02 ± 0.01 | 0.05 ± 0.07 | 0.02 ± 0.004 | 0.03 ± 0.012 |
| Testis-to-body wgt | 0.07 ± 0.05 | 0.07 ± 0.09 | 0.04 ± 0.004 * | 0.04 ± 0.004 * | N/A | N/A | N/A | N/A |
| Subcut. Fat-to-body wgt | 1.50 ± 0.5 | 1.40 ± 0.3 | 1.20 ± 0.5 | 1.50 ± 0.2 | 1.34 ± 0.4 | 1.30 ± 0.3 | 1.38 ± 0.3 | 1.50 ± 0.2 |
| Visc. Fat-to-body wgt | 1.06 ± 0.3 | 1.04 ± 0.4 | 0.84 ± 0.12 | 1.05 ± 0.18 | 0.73 ± 0.3 | 0.81 ± 0.2 | 0.65 ± 0.08 | 0.81 ± 0.18 |
| Characteristics | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Optimal (n = 21) | Low (n = 17) | Low-Optimal (n = 17) | Optimal-Low (n = 18) | Optimal (n = 20) | Low (n = 17) | Low-Optimal (n = 16) | Optimal-Low (n = 20) | |
| Crown-rump (CRL) | 171.9 ± 14.7 | 179.3 ± 17.8 | 169.0 ± 19.7 | 169.1 ± 11.7 | 171.5 ± 14.1 | 168.1 ± 14.1 | 174.5 ± 16.0 | 166.6 ± 15.9 |
| CRL increase from birth | 36.9 ± 16.3 | 47.7 ± 17.3 | 39.5 ± 14.8 | 44.5 ± 11.5 | 40.5 ± 13.6 | 42.2 ± 15.7 | 39.5 ± 13.0 | 44.2 ± 10.1 |
| Hind limb (HL) | 45.2 ± 6.3 | 45.3 ± 3.3 | 41.4 ± 2.8 | 44.0 ± 4.6 | 43.9 ± 4.0 | 43.6 ± 3.9 | 43.7 ± 6.1 | 41.8 ± 4.7 |
| HL increase from birth | 6.9 ± 5.9 | 9.6 ± 3.5 | 6.9 ± 3.6 | 6.2 ± 4.3 | 6.2 ± 3.6 | 9.4 ± 4.4 | 8.1 ± 4.0 | 9.0 ± 4.9 |
| Hock-toe (HT) | 40.3 ± 4.4 | 41.1 ± 5.3 | 39.4 ± 2.8 | 41.6 ± 5.1 | 40.1 ± 4.6 | 39.6 ± 3.5 | 40.7 ± 3.4 | 38.1 ± 3.3 |
| HT increase from birth | 3.8 ± 4.5 | 4.7 ± 4.6 | 3.6 ± 2.0 | 4.5 ± 3.2 | 2.5 ± 2.1 | 4.5 ± 2.6 | 3.9 ± 3.4 | 4.0 ± 2.0 |
| Ponderal Index (PI) | 20.5 ± 2.5 | 20.1 ± 2.6 | 21.2 ± 2.8 | 19.7 ± 3.0 | 21.5 ± 2.7 | 21.3 ± 2.5 | 22.5 ± 4.2 | 21.4 ± 3.6 |
| Characteristics | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Optimal (n = 13) | Low (n = 10) | Low-Optimal (n = 9) | Optimal-Low (n = 10) | Optimal (n = 11) | Low (n = 10) | Low-Optimal (n = 8) | Optimal-Low (n = 11) | |
| Brain-to-body wgt | 1.31 ± 0.29 | 1.23 ± 0.20 | 1.57 ± 0.48 | 1.31 ± 0.14 | 1.30 ± 0.26 | 1.42 ± 0.27 | 1.32 ± 0.24 | 1.32 ± 0.19 |
| Liver-to-body wgt | 2.99 ± 0.45 | 3.35 ± 0.42 | 3.04 ± 0.32 | 3.01 ± 0.64 | 3.12 ± 0.68 | 3.11 ± 0.52 | 3.10 ± 0.33 | 3.24 ± 0.30 |
| Heart-to-body wgt | 0.33 ± 0.02 | 0.32 ± 0.03 | 0.30 ± 0.02 | 0.30 ± 0.03 | 0.30 ± 0.02 | 0.30 ± 0.04 | 0.28 ± 0.01 | 0.29 ± 0.02 |
| Kidney-to-body wgt | 0.42 ± 0.05 | 0.46 ± 0.08 | 0.47 ± 0.11 | 0.45 ± 0.04 | 0.43 ± 0.04 | 0.46 ± 0.03 | 0.37 ± 0.17 | 0.44 ± 0.03 |
| Adrenal-to-body wgt | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 * | 0.02 ± 0.004 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Testis-to-body wgt | 0.14 ± 0.04 | 0.09 ± 0.03 | 0.09 ± 0.06 | 0.09 ± 0.02 | N/A | N/A | N/A | N/A |
| Subcut. Fat-to-body wgt | 0.64 ± 0.27 | 0.77 ± 0.28 | 0.60 ± 0.27 | 0.89 ± 0.23 | 0.83 ± 0.37 | 0.68 ± 0.25 | 0.82 ± 0.21 | 0.83 ± 0.24 |
| Visc. Fat-to-body wgt | 0.17 ± 0.10 | 0.28 ± 0.12 | 0.15 ± 0.15 | 0.19 ± 0.09 | 0.26 ± 0.20 | 0.16 ± 0.06 | 0.22 ± 0.11 | 0.15 ± 0.06 |
| Characteristics | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Optimal (n = 8) | Low (n = 7) | Low-Optimal (n = 8) | Optimal-Low (n = 8) | Optimal (n = 9) | Low (n = 7) | Low-Optimal (n = 8) | Optimal-Low (n = 9) | |
| Brain-to-body wgt | 0.49 ± 0.06 | 0.51 ± 0.04 | 0.52 ± 0.05 | 0.58 ± 0.03 * | 0.53 ± 0.08 | 0.56 ± 0.07 | 0.54 ± 0.05 | 0.57 ± 0.08 |
| Liver-to-body wgt | 3.06 ± 0.33 | 2.95 ± 0.30 | 2.91 ± 0.32 | 3.21 ± 0.23 | 3.30 ± 0.40 | 3.23 ± 0.50 | 3.35 ± 0.26 | 3.35 ± 0.52 |
| Heart-to-body wgt | 0.26 ± 0.01 | 0.27 ± 0.01 | 0.25 ± 0.03 | 0.26 ± 0.03 | 0.26 ± 0.02 | 0.27 ± 0.03 | 0.26 ± 0.02 | 0.27 ± 0.02 |
| Kidney-to-body wgt | 0.29 ± 0.03 | 0.30 ± 0.02 | 0.30 ± 0.03 | 0.30 ± 0.03 | 0.28 ± 0.03 | 0.30 ± 0.03 | 0.30 ± 0.03 | 0.32 ± 0.02 |
| Adrenal-to-body wgt | 0.02 ± 0.004 | 0.02 ± 0.004 | 0.02 ± 0.003 | 0.02 ± 0.003 | 0.03 ± 0.004 | 0.02 ± 0.002 | 0.02 ± 0.002 | 0.03 ± 0.004 |
| Testis-to-body wgt | 0.24 ± 0.05 | 0.16 ± 0.14 | 0.22 ± 0.04 | 0.24 ± 0.04 | N/A | N/A | N/A | N/A |
| Subcut. Fat-to-body wgt | 0.77 ± 0.27 | 0.91 ± 0.22 | 0.86 ± 0.13 | 0.58 ± 0.11 * | 1.03 ± 0.30 | 1.22 ± 0.44 | 1.25 ± 0.19 | 1.20 ± 0.16 |
| Visc. Fat-to-body wgt | 0.48 ± 0.17 | 0.53 ± 0.09 | 0.60 ± 0.11 | 0.43 ± 0.10 * | 0.59 ± 0.18 | 0.53 ± 0.17 | 0.73 ± 0.12 | 0.59 ± 0.12 |
| Characteristics | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Optimal (n = 8) | Low (n = 6) | Low-Optimal (n = 6) | Optimal-Low (n = 5) | Optimal (n = 8) | Low (n = 8) | Low-Optimal (n = 5) | Optimal-Low (n = 9) | |
| Absolute peak (mmol/L) | 14.7 ± 4.1 | 15.0 ± 2.0 | 17.6 ± 2.4 | 16.4 ± 2.1 | 13.3 ± 2.0 | 14.6 ± 2.1 | 16.1 ± 2.5 | 15.0 ± 2.7 |
| Delta-peak (mmol/L) | 6.6 ± 3.3 | 6.8 ± 1.9 | 9.7 ± 1.9 | 8.5 ± 2.2 | 5.1 ± 1.3 | 7.1 ± 2.6 | 7.1 ± 2.0 | 6.2 ± 2.6 |
| Time to peak (min) | 48.8 ± 15.5 | 55.0 ± 12.3 | 60.0 ± 0.0 | 54.0 ± 13.4 | 41.3 ± 15.5 | 52.5 ± 13.9 | 37.5 ± 15.0 | 40.0 ± 15.0 |
| AUC (mmol/L × min) | 1555 ± 608.1 | 1651 ± 1032 | 3101 ± 925.2 * | 2061 ± 902.4 | 749.4 ± 295.6 | 1695 ± 1642 | 1569 ± 1016 | 1129 ± 709.1 |
| Maternal Diet | Sex | Age | Euthanised or Spontaneous Death | Euthanasia or Autopsy Notes |
|---|---|---|---|---|
| Low | Male | Two months | Euthanised | Weight loss, loss of appetite, scruffy appearance. Abnormal maxillary incisors growing in on themselves. Animal likely in pain and struggling to eat. |
| Low-optimal | Male | 25 days | Spontaneous Death | Autopsy revealed enlarged heart and clotted blood in the heart and lungs. Assumed aspiration of foreign object. |
| Optimal-low | Female | Four days | Spontaneous Death | Runt of the litter, born small at 56.8 g, failed to regain birth weight during the first few days even with supplemental formula feeding. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coker, S.J.; Berry, M.J.; Vissers, M.C.M.; Dyson, R.M. Maternal Vitamin C Intake during Pregnancy Influences Long-Term Offspring Growth with Timing- and Sex-Specific Effects in Guinea Pigs. Nutrients 2024, 16, 369. https://doi.org/10.3390/nu16030369
Coker SJ, Berry MJ, Vissers MCM, Dyson RM. Maternal Vitamin C Intake during Pregnancy Influences Long-Term Offspring Growth with Timing- and Sex-Specific Effects in Guinea Pigs. Nutrients. 2024; 16(3):369. https://doi.org/10.3390/nu16030369
Chicago/Turabian StyleCoker, Sharna J., Mary J. Berry, Margreet C. M. Vissers, and Rebecca M. Dyson. 2024. "Maternal Vitamin C Intake during Pregnancy Influences Long-Term Offspring Growth with Timing- and Sex-Specific Effects in Guinea Pigs" Nutrients 16, no. 3: 369. https://doi.org/10.3390/nu16030369
APA StyleCoker, S. J., Berry, M. J., Vissers, M. C. M., & Dyson, R. M. (2024). Maternal Vitamin C Intake during Pregnancy Influences Long-Term Offspring Growth with Timing- and Sex-Specific Effects in Guinea Pigs. Nutrients, 16(3), 369. https://doi.org/10.3390/nu16030369








