Plant Sterol-Enriched Palm Oil Intervention to Improve Lipid Profile and Inflammation Status in Hyperlipidemic Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- n—sample size per group
- —variance of either group (assumed to be 11% and equal for both groups)
- —minimal detectable difference between the two means (6%)
- —standard normal deviates at an level of significance and at a power, respectively. is 1.96 at a 5% level of significance for two-sided tests, while is 0.84 at 80% power.
2.2. Treatment Palm Oil
2.3. Data Collection and Analysis
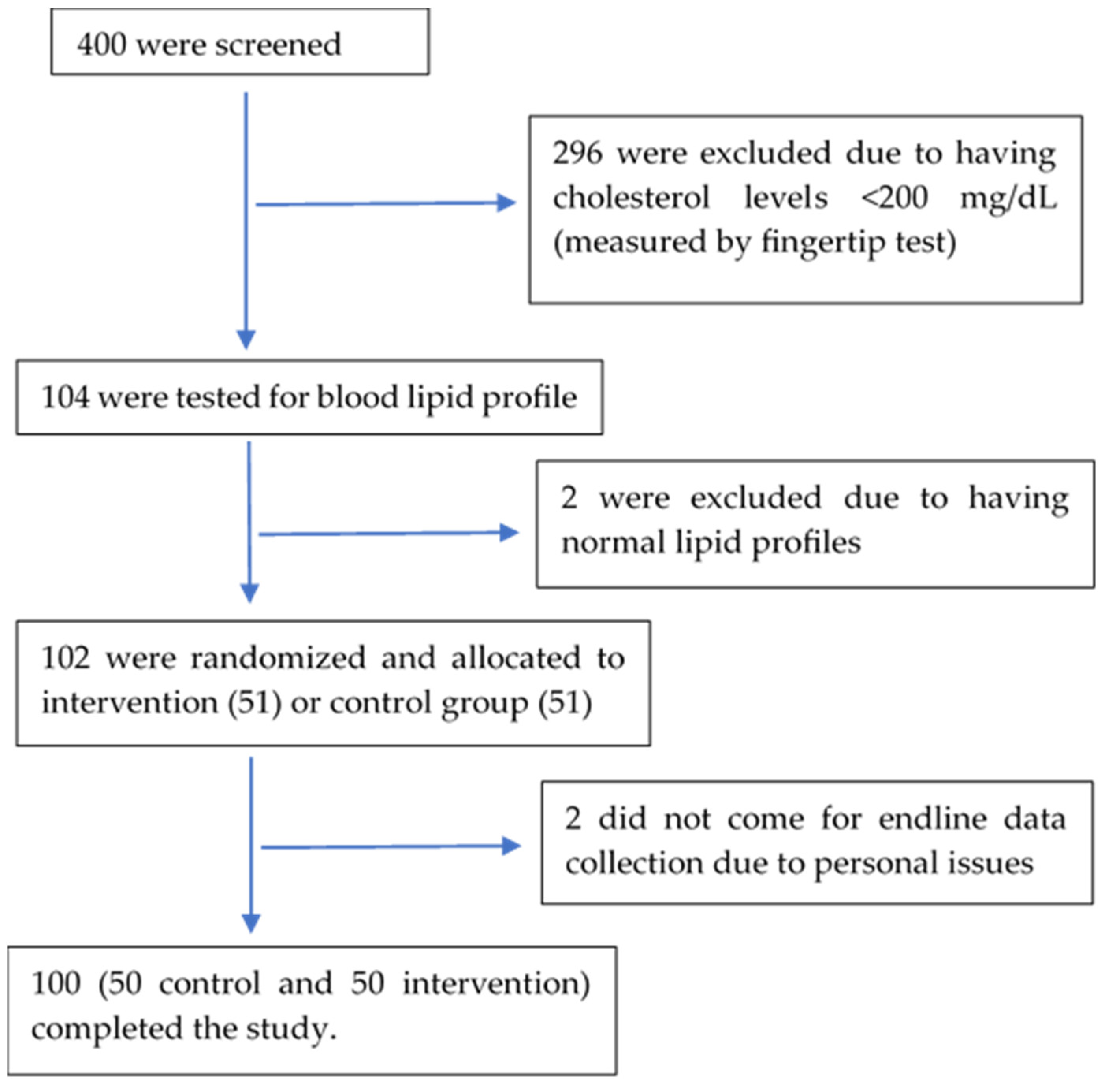
3. Results
3.1. Baseline Characteristics of Subjects
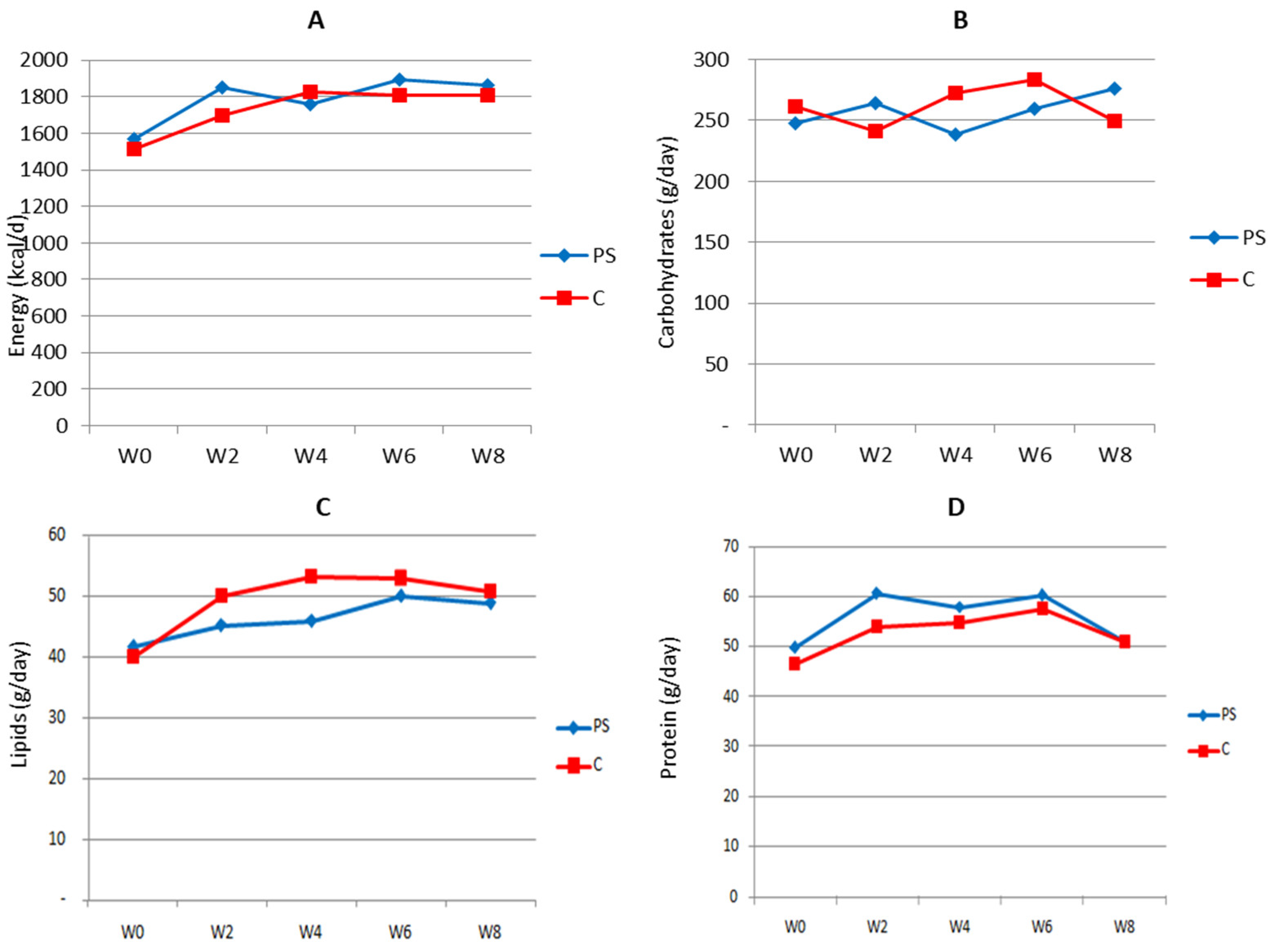
3.2. Energy and Nutrient Intake
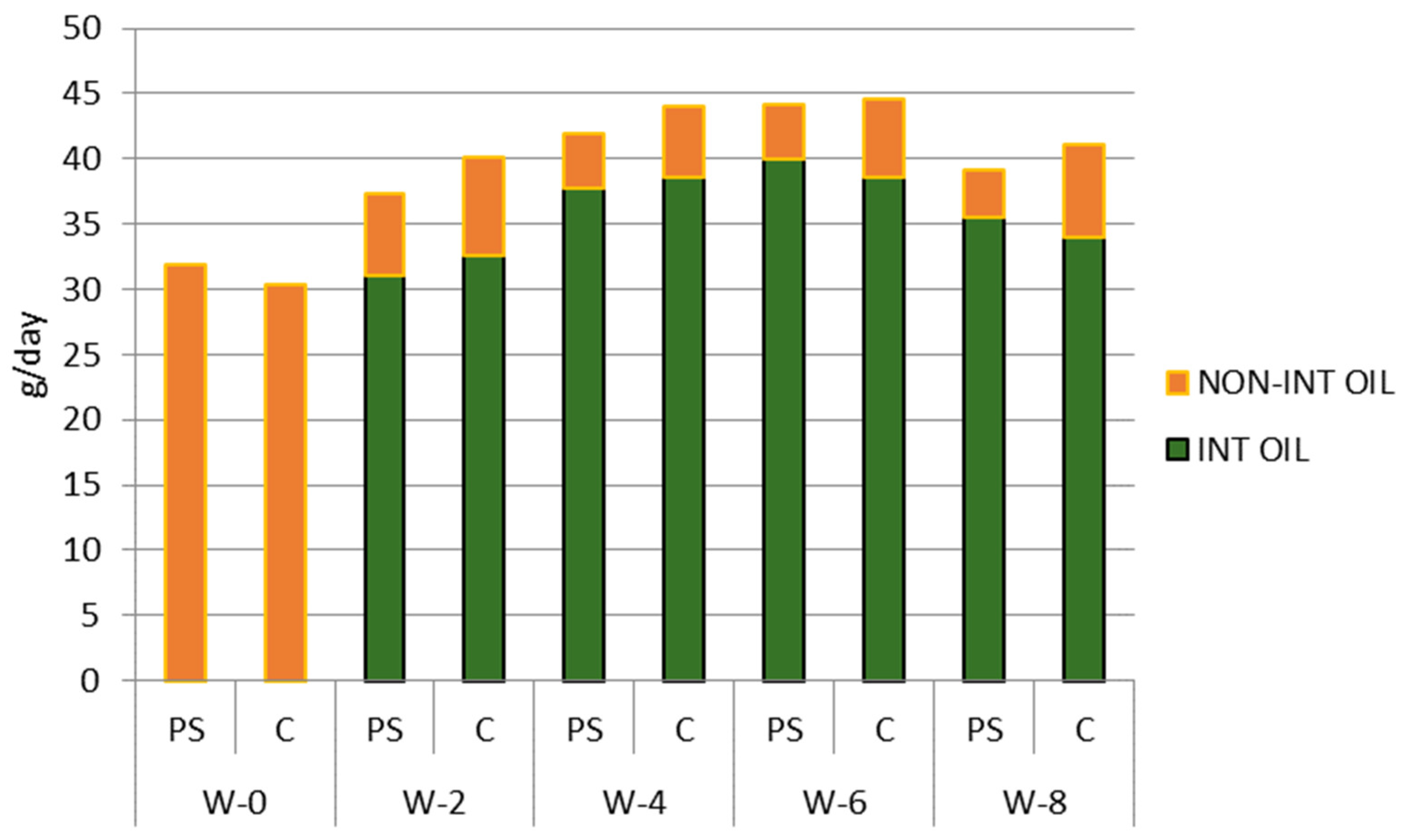
3.3. Palm Oil and Plant Sterol Consumption
3.4. Effect of Intervention on Lipid Profile and CRP Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roser, M.; Ritchie, H. Burden of Disease. Available online: https://www.ourworldindata.org/burden-of-disease (accessed on 21 August 2024).
- Ardiana, M.; Harsoyo, P.M.; Hermawan, H.O.; Sufiyah, I.M.; Firmanda, D.R.; Desita, S.R.; Paramitha, A.D.; Hariftyani, A.S.; Shabrina, F.A.; Triastuti, F. Higher Cardiovascular Risks and Atherogenic Index of Plasma Found in Police Officers of Developing Country in Surabaya, East Java, Indonesia. Clin. Epidemiol. Glob. Health 2022, 17, 101132. [Google Scholar] [CrossRef]
- Di Cesare, M.; McGhie, D.V.; Perel, P.; Mwangi, J.; Taylor, S.; Pervan, B.; Kabudula, C.; Narula, J.; Bixby, H.; Pineiro, D.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Ministry of Health Republic Indonesia. Hasil Utama Riset Kesehatan Dasar (Key Results of Basic Health Research) 2018. Available online: https://layanandata.kemkes.go.id/katalog-data/riskesdas/ketersediaan-data/riskesdas-2018 (accessed on 21 August 2024).
- Lim, J.; Sharma, S.; Colyer, T.; Lee, S. The Future of the Indonesian Healthcare Ecosystem: The Outlook to 2030. Oliver Wyman 2018, 1, 4–5. [Google Scholar]
- Uli, R.E.; Satyana, R.P.U.; Zomer, E.; Magliano, D.; Liew, D.; Ademi, Z. Health and Productivity Burden of Coronary Heart Disease in the Working Indonesian Population Using Life-Table Modelling. BMJ Open 2020, 10, e039221. [Google Scholar] [CrossRef]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-Communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Wirtz, P.H.; von Känel, R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr. Cardiol. Rep. 2017, 19, 111. [Google Scholar] [CrossRef]
- Hussain, M.A.; Al Mamun, A.; Peters, S.A.E.; Woodward, M.; Huxley, R.R. The Burden of Cardiovascular Disease Attributable to Major Modifiable Risk Factors in Indonesia. J. Epidemiol. 2016, 26, 515–521. [Google Scholar] [CrossRef]
- Zhang, Y.; Dron, J.S.; Bellows, B.K.; Khera, A.V.; Liu, J.; Balte, P.P.; Oelsner, E.C.; Amr, S.S.; Lebo, M.S.; Nagy, A.; et al. Familial Hypercholesterolemia Variant and Cardiovascular Risk in Individuals with Elevated Cholesterol. JAMA Cardiol. 2024, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Fan, H.; Zhang, S.; Chen, C.; You, Y.; Wang, C.; Li, J.; Luo, L.; Cheng, Y.; Zhou, M.; et al. Association of LDL-C/HDL-C Ratio with Coronary Heart Disease: A Meta-Analysis. Indian Heart J. 2024, 76, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement Fromthe European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A.; Flack, J.M. Effect of Long-Term Exposure to Lower Low-Density Lipoprotein Cholesterol Beginning Early in Life on the Risk of Coronary Heart Disease: A Mendelian Randomization Analysis. Ration. Pharmacother. Cardiol. 2013, 9, 2631–2639. [Google Scholar] [CrossRef]
- Badalyan, S.S.; Markosyan, S.V.; Tariq, H.; Malik, Z.; Asghar, A. Meta-Analysis of Lowering LDL Cholesterol and Its Impact on the Cardiovascular System. Pak. J. Med. Health Sci. 2023, 17, 8–12. [Google Scholar] [CrossRef]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant Sterols and Plant Stanols in the Management of Dyslipidaemia and Prevention of Cardiovascular Disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Rizzoli, E.; Grandi, E.; D’Addato, S.; Borghi, C. The Effect of Dietary Supplementation with Plant Sterols on Total and LDL-Cholesterol in Plasma Is Affected by Adherence to Mediterranean Diet: Insights from the DESCO Randomized Clinical Study. Nutrients 2023, 15, 4555. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.; Bustamante, A.; Echeverría, F.; Sambra, V.; Rincón-Cervera, M.Á.; Farías, C.; Valenzuela, R. Metabolic Benefits of Phytosterols: Chemical, Nutritional, and Functional Aspects. Food Rev. Int. 2023, 1–23. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Y.; McClements, D.J.; Xu, D.; Chen, S. Production, Characterization, Delivery, and Cholesterol-Lowering Mechanism of Phytosterols: A Review. J. Agric. Food Chem. 2022, 70, 2483–2494. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Öztaş, Y.E.; Sabuncuoğlu, S. Sterols in Inflammatory Diseases: Implications and Clinical Utility. In Implication of Oxysterols and Phytosterols in Aging and Human Diseases; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2024; Volume 1440. [Google Scholar]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory Effect of Phytosterols in Experimental Murine Colitis Model: Prevention, Induction, Remission Study. PLoS ONE 2014, 9, e108112. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-Cholesterol Lowering of Plant Sterols and Stanols—Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef]
- Martianto, D.; Bararah, A.; Andarwulan, N.; Średnicka-Tober, D. Cross-Sectional Study of Plant Sterols Intake as a Basis for Designing Appropriate Plant Sterol-Enriched Food in Indonesia. Nutrients 2021, 13, 452. [Google Scholar] [CrossRef]
- Barkas, F.; Bathrellou, E.; Nomikos, T.; Panagiotakos, D.; Liberopoulos, E.; Kontogianni, M.D. Plant Sterols and Plant Stanols in Cholesterol Management and Cardiovascular Prevention. Nutrients 2023, 15, 2845. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Jones, P.J.H.; Abumweis, S.S. Plant Sterols: Factors Affecting Their Efficacy and Safety as Functional Food Ingredients. Lipids Health Dis. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Tandra, H.; Suroso, A.I.; Syaukat, Y.; Najib, M. The Determinants of Competitiveness in Global Palm Oil Trade. Economies 2022, 10, 132. [Google Scholar] [CrossRef]
- Matthäus, B. Use of Palm Oil for Frying in Comparison with Other High-Stability Oils. Eur. J. Lipid Sci. Technol. 2007, 109, 400–409. [Google Scholar] [CrossRef]
- Tullis, P. How the World Got Hooked on Palm Oil. The Guardian, 19 February 2019. [Google Scholar]
- Khatiwada, D.; Palmén, C.; Silveira, S. Evaluating the Palm Oil Demand in Indonesia: Production Trends, Yields, and Emerging Issues. Biofuels 2021, 12, 135–147. [Google Scholar] [CrossRef]
- Anjani, I.G.; Saputri, A.B.; Armeira, A.N.P.; Januarita, D. Analisis Konsumsi Dan Produksi Minyak Kelapa Sawit Di Indonesia Dengan Menerapkan Metode Moving Average. JURIKOM (J. Ris. Komput.) 2022, 9, 1014–1019. [Google Scholar] [CrossRef]
- Martianto, D.; Sumedi, E.; Soekatri, M.; Herawati, T. Marketing and Distribution Survey of Cooking Oil at Makassar City; Koalisi Fortifikasi Indonesia: Jakarta, Indonesia, 2007. [Google Scholar]
- Indonesian Food Composition Data. Available online: https://panganku.org/id-ID/beranda (accessed on 21 August 2024).
- Acuff, R.V.; Cai, D.J.; Dong, Z.P.; Bell, D. The Lipid Lowering Effect of Plant Sterol Ester Capsules in Hypercholesterolemic Subjects. Lipids Health Dis. 2007, 6, 11. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef]
- Carr, T.P.; Ash, M.M.; Brown, A.W. Cholesterol-Lowering Phytosterols: Factors Affecting Their Use and Efficacy. Nutr. Diet. Suppl. 2010, 2, 59–72. [Google Scholar] [CrossRef]
- Clifton, P. Plant Sterol and Stanols—Comparison and Contrasts. Sterols versus Stanols in Cholesterol-Lowering: Is There a Difference? Atheroscler. Suppl. 2002, 3, 5–9. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mitra, A. Health Effects of Palm Oil. J. Hum. Ecol. 2009, 26, 197–203. [Google Scholar] [CrossRef]
- Vega-López, S.; Ausman, L.M.; Jalbert, S.M.; Erkkilä, A.T.; Lichtenstein, A.H. Palm and Partially Hydrogenated Soybean Oils Adversely Alter Lipoprotein Profiles Compared with Soybean and Canola Oils in Moderately Hyperlipidemic Subjects. Am. J. Clin. Nutr. 2006, 84, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Hjerpsted, J.; Raff, M. Palm Olein Increases Plasma Cholesterol Moderately Compared with Olive Oil in Healthy Individuals. Am. J. Clin. Nutr. 2011, 94, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis—From Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Singh, T.P.; Morris, D.R.; Smith, S.; Moxon, J.V.; Golledge, J. Systematic Review and Meta-Analysis of the Association Between C-Reactive Protein and Major Cardiovascular Events in Patients with Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 220–233. [Google Scholar] [CrossRef]
- Gagliardi, A.C.M.; Maranho, R.C.; Sousa, H.P.D.; Schaefer, E.J.; Santos, R.D. Effects of Margarines and Butter Consumption on Lipid Profiles, Inflammation Markers and Lipid Transfer to HDL Particles in Free-Living Subjects with the Metabolic Syndrome. Eur. J. Clin. Nutr. 2010, 64, 1141–1149. [Google Scholar] [CrossRef]
- Devaraj, S.; Autret, B.C.; Jialal, I. Reduced-Calorie Orange Juice Beverage with Plant Sterols Lowers C-Reactive Protein Concentrations and Improves the Lipid Profile in Human Volunteers. Am. J. Clin. Nutr. 2006, 84, 756–761. [Google Scholar] [CrossRef]
- Pathak, A.; Agrawal, A. Evolution of C-Reactive Protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef]
- Megawati, G.; Indraswari, N.; Johansyah, A.A.; Kezia, C.; Herawati, D.M.D.; Gurnida, D.A.; Musfiroh, I. Comparison of Hs-CRP in Adult Obesity and Central Obesity in Indonesia Based on Omega-3 Fatty Acids Intake: Indonesian Family Life Survey 5 (IFLS 5) Study. Int. J. Environ. Res. Public Health 2023, 20, 6734. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Valk, I.; van Andel, V.; Friedrichs, S.; Lütjohann, D.; Hrncirik, K.; Trautwein, E.A. Thermal Stability of Plant Sterols and Formation of Their Oxidation Products in Vegetable Oils and Margarines upon Controlled Heating. Chem. Phys. Lipids 2017, 207, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Knol, D.; Trautwein, E.A. Phytosterol Oxidation Products (POP) in Foods with Added Phytosterols and Estimation of Their Daily Intake: A Literature Review. Eur. J. Lipid Sci. Technol. 2016, 118, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.T.; Vergnes, M.F.; Kaloustian, J.; El-Moselhy, T.F.; Amiot-Carlin, M.J.; Portugal, H. Effect of Storage and Heating on Phytosterol Concentrations in Vegetable Oils Determined by GC/MS. J. Sci. Food Agric. 2006, 86, 220–225. [Google Scholar] [CrossRef]
- Pederiva, C.; Biasucci, G.; Banderali, G.; Capra, M.E. Plant Sterols and Stanols for Pediatric Patients with Increased Cardiovascular Risk. Children 2024, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, O.; Böhm, M.; Laufs, U. Controversial Role of Plant Sterol Esters in the Management of Hypercholesterolaemia. Eur. Heart J. 2009, 30, 404–409. [Google Scholar] [CrossRef]
- Köhler, J.; Teupser, D.; Elsässer, A.; Weingärtner, O. Plant Sterol Enriched Functional Food and Atherosclerosis. Br. J. Pharmacol. 2017, 174, 1281–1289. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Luister, A.; Schött, H.F.; Husche, C.; Schäfers, H.J.; Böhm, M.; Plat, J.; Gräber, S.; Lütjohann, D.; Laufs, U.; Weingärtner, O. Increased Plant Sterol Deposition in Vascular Tissue Characterizes Patients with Severe Aortic Stenosis and Concomitant Coronary Artery Disease. Steroids 2015, 99, 272–280. [Google Scholar] [CrossRef]
- Weingärtner, O.; Lütjohann, D.; Ji, S.; Weisshoff, N.; List, F.; Sudhop, T.; von Bergmann, K.; Gertz, K.; König, J.; Schäfers, H.J.; et al. Vascular Effects of Diet Supplementation with Plant Sterols. J. Am. Coll. Cardiol. 2008, 51, 1553–1561. [Google Scholar] [CrossRef]
- Helske, S.; Miettinen, T.; Gylling, H.; Mäyränpää, M.; Lommi, J.; Turto, H.; Werkkala, K.; Kupari, M.; Kovanen, P.T. Accumulation of Cholesterol Precursors and Plant Sterols in Human Stenotic Aortic Valves. J. Lipid Res. 2008, 49, 1511–1518. [Google Scholar] [CrossRef]
- McDaniel, A.L.; Alger, H.M.; Sawyer, J.K.; Kelley, K.L.; Kock, N.D.; Brown, J.M.; Temel, R.E.; Rudel, L.L. Phytosterol Feeding Causes Toxicity in ABCG5/G8 Knockout Mice. Am. J. Pathol. 2013, 182, 1131–1138. [Google Scholar] [CrossRef]
- Solca, C.; Tint, G.S.; Patel, S.B. Dietary Xenosterols Lead to Infertility and Loss of Abdominal Adipose Tissue in Sterolin-Deficient Mice. J. Lipid Res. 2013, 54, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Thompson, P.D. Phytosterols and Vascular Disease. Atherosclerosis 2006, 186, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Genser, B.; Silbernagel, G.; De Backer, G.; Bruckert, E.; Carmena, R.; Chapman, M.J.; Deanfield, J.; Descamps, O.S.; Rietzschel, E.R.; Dias, K.C.; et al. Plant Sterols and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Eur. Heart J. 2012, 33, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Chapman, M.J.; Genser, B.; Kleber, M.E.; Fauler, G.; Scharnagl, H.; Grammer, T.B.; Boehm, B.O.; Mäkelä, K.M.; Kähönen, M.; et al. High Intestinal Cholesterol Absorption Is Associated with Cardiovascular Disease and Risk Alleles in ABCG8 and ABO: Evidence from the LURIC and YFS Cohorts and from a Meta-Analysis. J. Am. Coll. Cardiol. 2013, 62, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B.; Fumeron, F. Potential Risks Associated with Increased Plasma Plant-Sterol Levels. Diabetes Metab. 2015, 41, 76–81. [Google Scholar] [CrossRef]
- Glueck, C.J.; Speirs, J.; Tracy, T.; Streicher, P.; Illig, E.; Vandegrift, J. Relationships of Serum Plant Sterols (Phytosterols) and Cholesterol in 595 Hypercholesterolemic Subjects, and Familial Aggregation of Phytosterols, Cholesterol, and Premature Coronary Heart Disease in Hyperphytosterolemic Probands and Their First-Degree Relatives. Metabolism 1991, 40, 842–848. [Google Scholar] [CrossRef]
- Rajaratnam, R.A.; Gylling, H.; Miettinen, T.A. Independent Association of Serum Squalene and Noncholesterol Sterols with Coronary Artery Disease in Postmenopausal Women. J. Am. Coll. Cardiol. 2000, 35, 1185–1191. [Google Scholar] [CrossRef]
- Assmann, G.; Cullen, P.; Erbey, J.; Ramey, D.R.; Kannenberg, F.; Schulte, H. Plasma Sitosterol Elevations Are Associated with an Increased Incidence of Coronary Events in Men: Results of a Nested Case-Control Analysis of the Prospective Cardiovascular Münster (PROCAM) Study. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 13–21. [Google Scholar] [CrossRef]
- Matthan, N.R.; Pencina, M.; LaRocque, J.M.; Jacques, P.F.; D’Agostino, R.B.; Schaefer, E.J.; Lichtenstein, A.H. Alterations in Cholesterol Absorption/Synthesis Markers Characterize Framingham Offspring Study Participants with CHD. J. Lipid Res. 2009, 50, 1927–1935. [Google Scholar] [CrossRef]
- Silbernagel, G.; Fauler, G.; Hoffmann, M.M.; Lütjohann, D.; Winkelmann, B.R.; Boehm, B.O.; März, W. The Associations of Cholesterol Metabolism and Plasma Plant Sterols with All-Cause and Cardiovascular Mortality. J. Lipid Res. 2010, 51, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, S.; Vissers, M.N.; Von Bergmann, K.; Elharchaoui, K.; Lütjohann, D.; Luben, R.; Wareham, N.J.; Kastelein, J.J.P.; Khaw, K.T.; Boekholdt, S.M. Plasma Levels of Plant Sterols and the Risk of Coronary Artery Disease: The Prospective EPIC-Norfolk Population Study. J. Lipid Res. 2007, 48, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wilund, K.R.; Yu, L.; Xu, F.; Vega, G.L.; Grundy, S.M.; Cohen, J.C.; Hobbs, H.H. No Association between Plasma Levels of Plant Sterols and Atherosclerosis in Mice and Men. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, O.; Pinsdorf, T.; Rogacev, K.S.; Blömer, L.; Grenner, Y.; Gräber, S.; Ulrich, C.; Girndt, M.; Böhm, M.; Fliser, D.; et al. The Relationships of Markers of Cholesterol Homeostasis with Carotid Intima-Media Thickness. PLoS ONE 2010, 5, e13467. [Google Scholar] [CrossRef]
- Escurriol, V.; Cofán, M.; Moreno-Iribas, C.; Larrañaga, N.; Martínez, C.; Navarro, C.; Rodríguez, L.; González, C.A.; Corella, D.; Ros, E. Phytosterol Plasma Concentrations and Coronary Heart Disease in the Prospective Spanish EPIC Cohort. J. Lipid Res. 2010, 51, 618–624. [Google Scholar] [CrossRef]
- Fassbender, K.; Lütjohann, D.; Dik, M.G.; Bremmer, M.; König, J.; Walter, S.; Liu, Y.; Letièmbre, M.; von Bergmann, K.; Jonker, C. Moderately Elevated Plant Sterol Levels Are Associated with Reduced Cardiovascular Risk-The LASA Study. Atherosclerosis 2008, 196, 283–288. [Google Scholar] [CrossRef]

| Variables | Indicators | Method |
|---|---|---|
| Subject characteristics | Age, medical history, education, occupation, family members, marital status | Interview/questionnaire |
| Blood pressure | Systole, diastole | Blood pressure monitor |
| Nutrient intake | Consumption of energy, carbohydrates, fat, protein | 2 × 24 h food recall |
| Nutritional status | BMI, body composition | Weight/height (W/H) measurement, bioimpedance analyzer |
| Blood lipid profile | HDL, LDL, triacylglycerols, cholesterol (total) | Enzymatic colorimetric method |
| Inflammation state | Serum hsCRP | Enzyme-linked immunosorbent assay (ELISA) |
| Variables | Intervention Group | Control Group | p-Value |
|---|---|---|---|
| Female, n (%) | 36 (75) | 40 (77) | - |
| Body mass index (kg/m2) | 28.67 ± 4.80 | 27.10 ± 5.00 | 0.36 |
| Body fat percentage (%) | 35.38 ± 6.10 | 33.50 ± 5.70 | 0.53 |
| Blood pressure—systole (mmHg) | 146.77 ± 27.10 | 141.50 ± 27.20 | 0.58 |
| Blood pressure—diastole (mmHg) | 90.48 ± 14.50 | 87.90 ± 12.40 | 0.47 |
| HDL (mg/dL) | 48.02 ± 9.00 | 46.40 ± 9.10 | 0.63 |
| LDL (mg/dL) | 141.84 ± 31.50 | 140.60 ± 30.60 | 0.65 |
| Cholesterol (total) (mg/dL) | 216.07 ± 36.20 | 216.50 ± 31.20 | 0.37 |
| Triacylglycerols (mg/dL) | 152.16 ± 81.30 | 151.60 ± 82.60 | 0.79 |
| CRP (mg/L) | 2.38 ± 2.40 | 2.90 ± 3.40 | 0.55 |
| Parameter | Group | Baseline | Endline | p-Value |
|---|---|---|---|---|
| Cholesterol (total) (mg/dL) | PS | 216.07 ± 36.20 a | 208.50 ± 40.66 b | 0.003 |
| C | 216.50 ± 31.20 | 226.69 ± 36.79 | ||
| Triglycerides (mg/dL) | PS | 152.16 ± 81.30 | 132.60 ± 69.14 | 0.357 |
| C | 151.60 ± 82.60 | 148.27 ± 72.11 | ||
| HDL cholesterol (mg/dL) | PS | 48.02 ± 9.00 | 49.10 ± 11.01 | 0.083 |
| C | 46.40 ± 9.10 | 48.25 ± 9.37 | ||
| LDL cholesterol (mg/dL) | PS | 141.84 ± 31.50 | 132.17 ± 35.49 | 0.027 |
| C | 140.60 ± 30.60 | 144.06 ± 33.16 | ||
| CRP (mg/L) | PS | 2.38 ± 2.40 a | 3.48 ± 3.77 b | 0.062 |
| C | 2.90 ± 3.40 | 3.00 ± 4.71 |
| PS | C | p-Value ** | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Endline | p-Value * | Baseline | Endline | p-Value * | ||
| TC/HDL | 4.73 | 4.56 | 0.000 | 4.80 | 4.82 | 0.786 | 0.024 |
| LDL/HDL | 3.08 | 2.88 | 0.001 | 3.13 | 3.10 | 0.547 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewi, M.; Martianto, D.; Andarwulan, N.; Kazimierczak, R.; Średnicka-Tober, D. Plant Sterol-Enriched Palm Oil Intervention to Improve Lipid Profile and Inflammation Status in Hyperlipidemic Individuals. Nutrients 2024, 16, 3370. https://doi.org/10.3390/nu16193370
Dewi M, Martianto D, Andarwulan N, Kazimierczak R, Średnicka-Tober D. Plant Sterol-Enriched Palm Oil Intervention to Improve Lipid Profile and Inflammation Status in Hyperlipidemic Individuals. Nutrients. 2024; 16(19):3370. https://doi.org/10.3390/nu16193370
Chicago/Turabian StyleDewi, Mira, Drajat Martianto, Nuri Andarwulan, Renata Kazimierczak, and Dominika Średnicka-Tober. 2024. "Plant Sterol-Enriched Palm Oil Intervention to Improve Lipid Profile and Inflammation Status in Hyperlipidemic Individuals" Nutrients 16, no. 19: 3370. https://doi.org/10.3390/nu16193370
APA StyleDewi, M., Martianto, D., Andarwulan, N., Kazimierczak, R., & Średnicka-Tober, D. (2024). Plant Sterol-Enriched Palm Oil Intervention to Improve Lipid Profile and Inflammation Status in Hyperlipidemic Individuals. Nutrients, 16(19), 3370. https://doi.org/10.3390/nu16193370










