Impact of Dietary Isoflavones in Standard Chow on Reproductive Development in Juvenile and Adult Female Mice with Different Metabolic Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
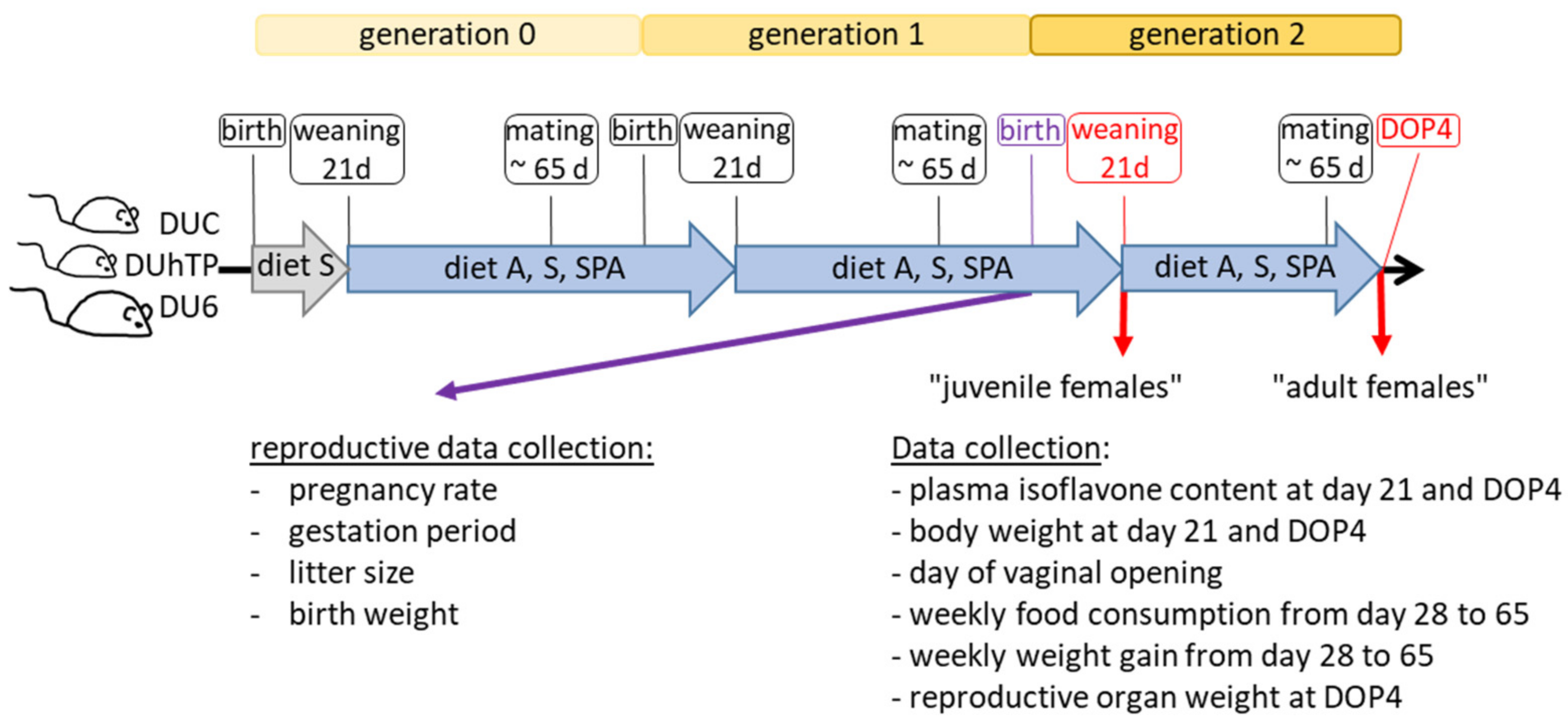
2.2. Study Design and Sampling
2.3. Quantitation of IFs in the Diets and Plasma Samples
2.4. RNA Isolation and Gene Expression Analysis
2.5. Quantification of 17 β-Estradiol by ELISA
2.6. Statistics
3. Results
3.1. IF Profile in Three Different Commercial Mouse Diets
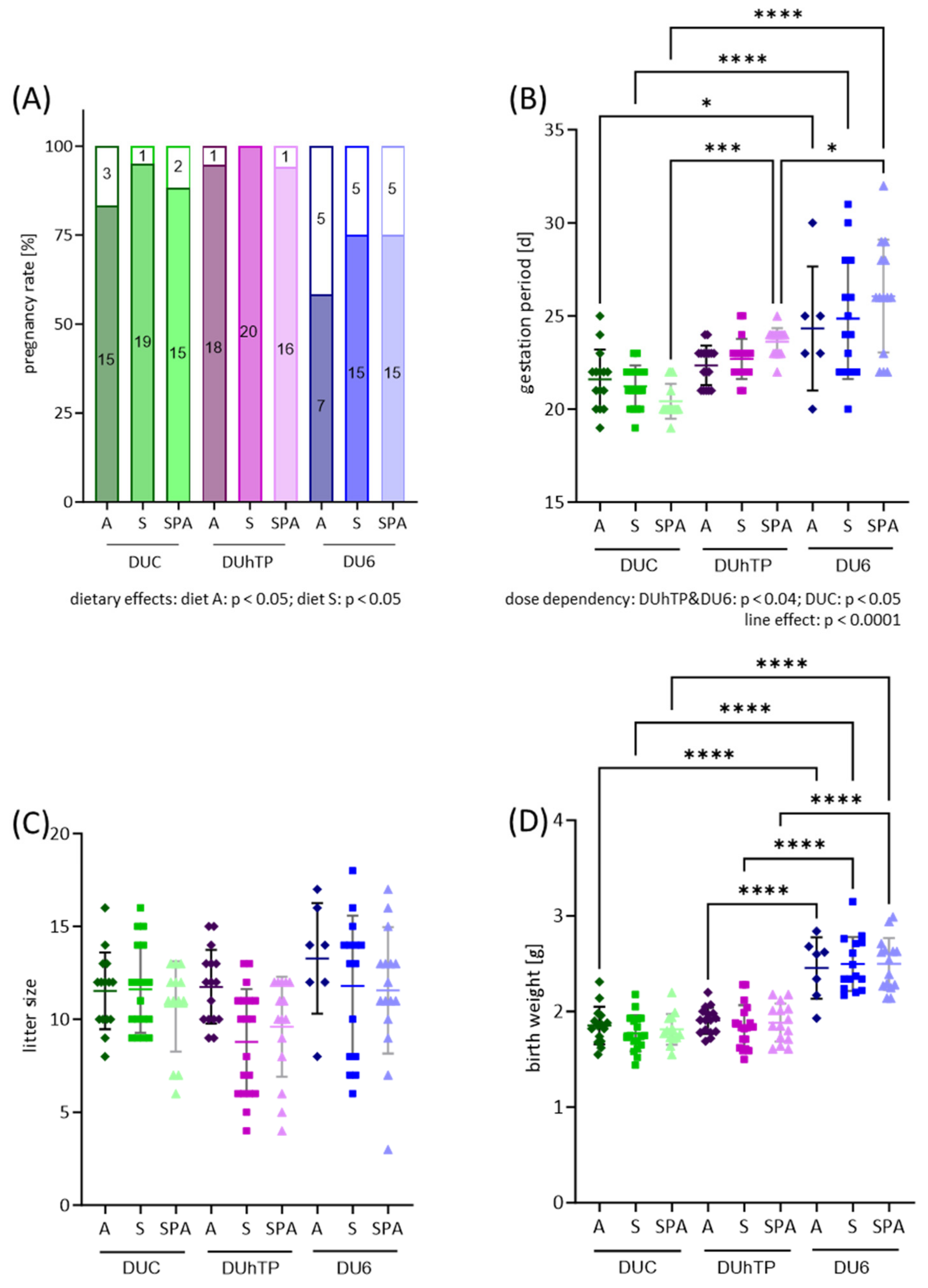
3.2. Effect of Different Diets on Reproduction of the 1st Generation
3.3. Body Weight and IF Concentrations in Plasma from Juvenile Female Mice
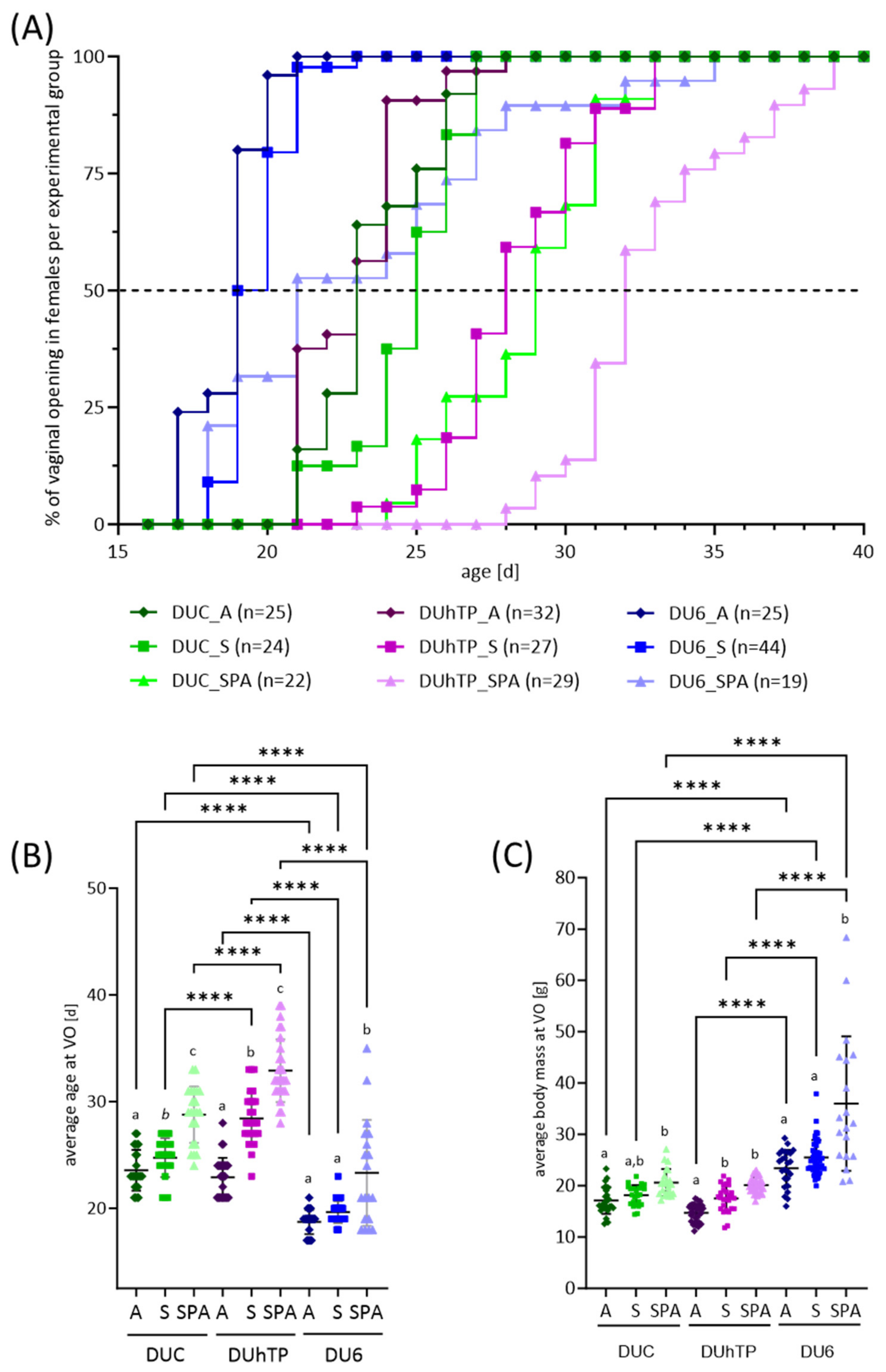
3.4. Effects of Genetic Background and Diet on Reproductive Development in Juvenile Female Mice
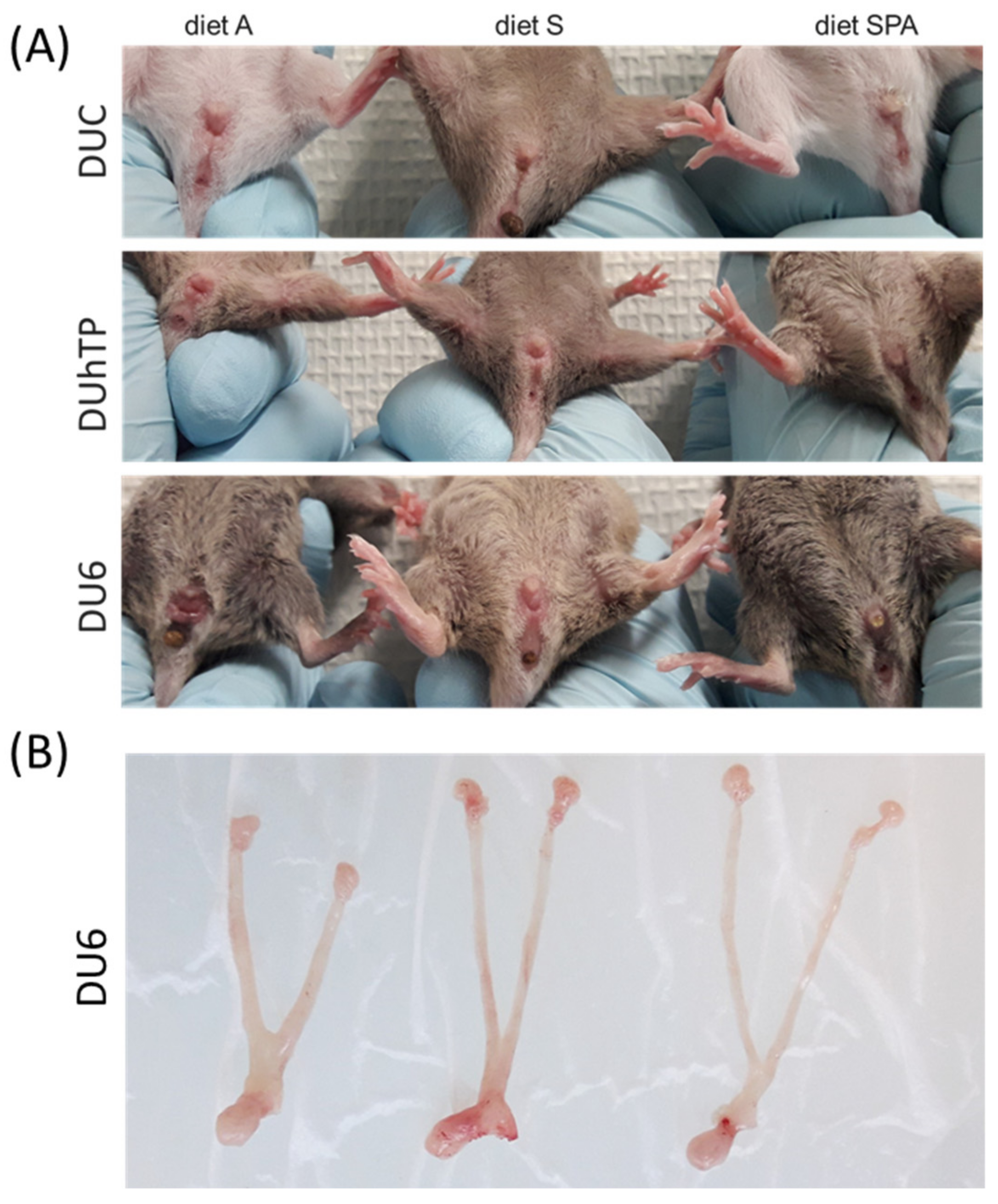
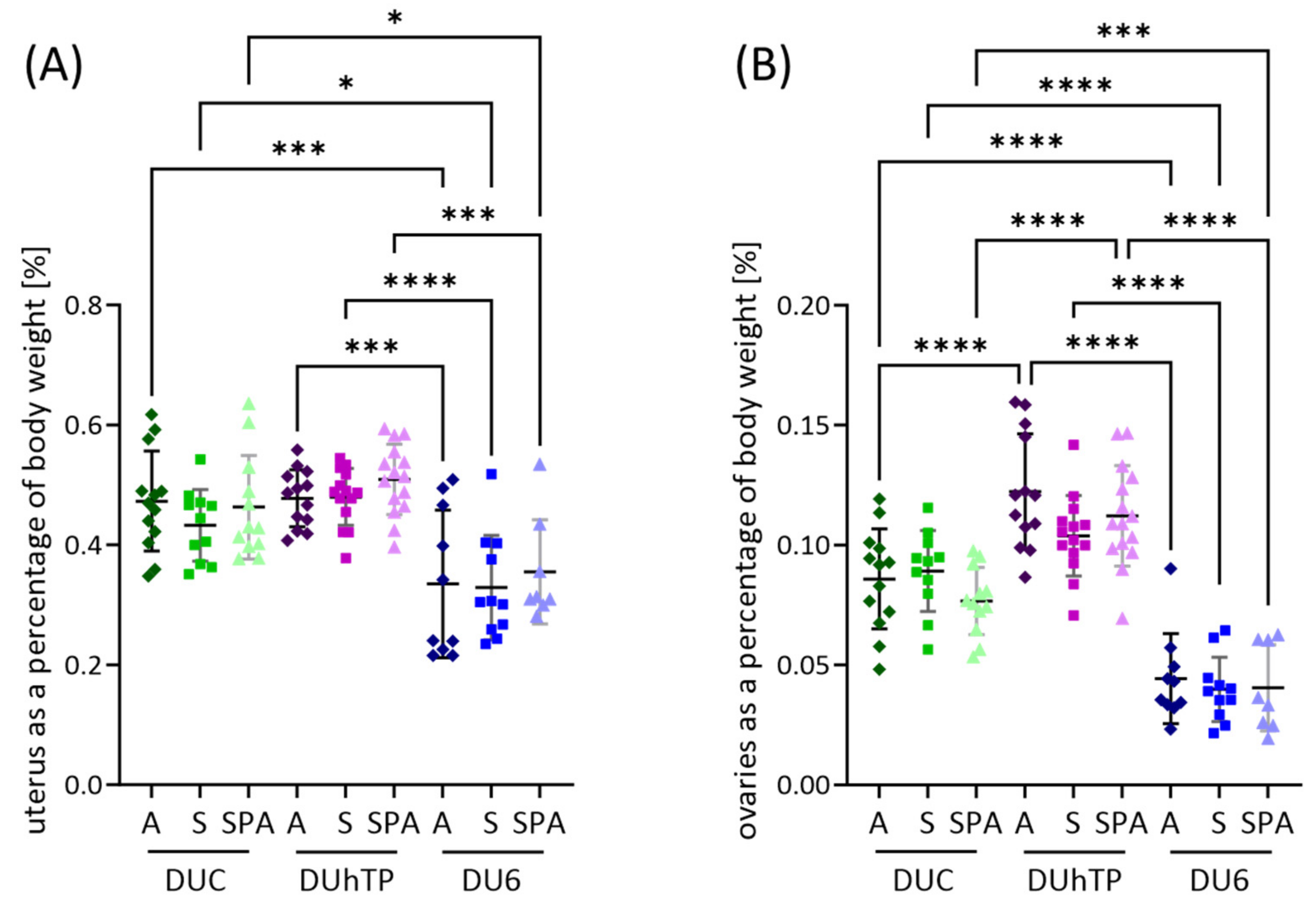
3.5. Effects of Genetic Background and Diet on Body Weight, Reproductive Organ Weights, and Feed Intake in Adult Female Mice
3.6. IF Concentrations in Plasma from Adult Female Mice
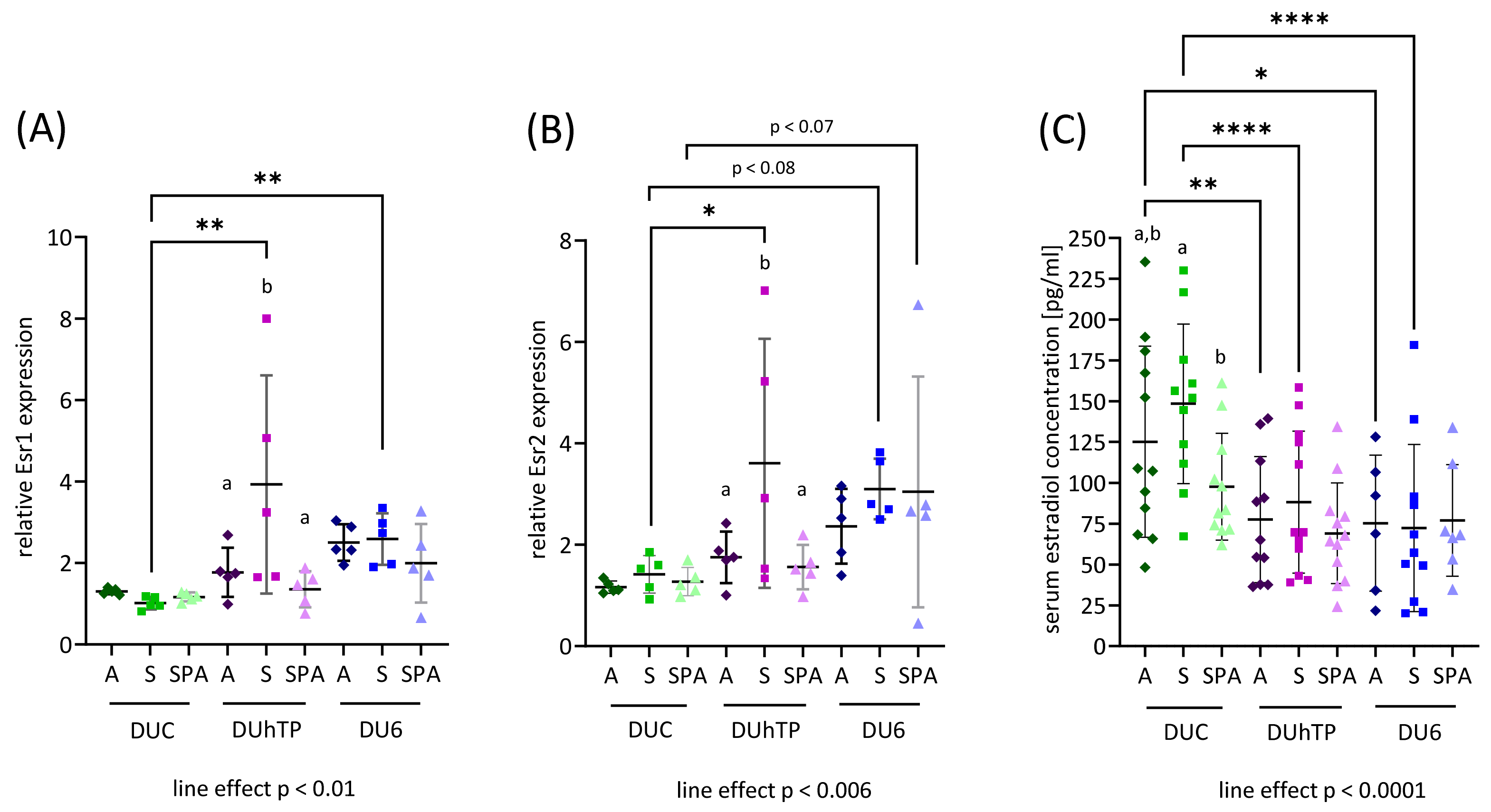
3.7. Effects of Genetics and Diet on the Expression of Estrogen Receptors in the Ovaries
4. Discussion
4.1. Commercially Available Chow is Isoflavone-Rich and Causes Therapeutic Plasma IFs
4.2. Dietary Effects on Reproductive Performance and Interactions with Genotype
4.3. IF-Containing Diets Accelerate Reproductive Development
4.4. Reproductive Organ Weights, Estrogen Receptor Expression, and Serum Estradiol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Křížová, L.; Křešťáková, V.; Dadáková, K.; Kašparovský, T. Production of Bovine Equol-Enriched Milk: A Review. Animals 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Kasparovska, J.; Krizova, L.; Lochman, J.; Dadakova, K.; Kasparovsky, T. Soybean-Derived Isoflavone Determination in Rumen Fluid and Milk by LC–MS-(TOF). J. Chromatogr. Sci. 2016, 54, 997–1003. [Google Scholar] [CrossRef]
- Nørskov, N.P.; Givens, I.; Purup, S.; Stergiadis, S. Concentrations of phytoestrogens in conventional, organic and free-range retail milk in England. Food Chem. 2019, 295, 1–9. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen Content of Foods of Animal Origin: Dairy Products, Eggs, Meat, Fish, and Seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Soukup, S.T.; Stoll, D.A.; Danylec, N.; Schoepf, A.; Kulling, S.E.; Huch, M. Metabolism of daidzein and genistein by gut bacteria of the class coriobacteriia. Foods 2021, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Soukup, S.T.; Engelbert, A.K.; Watzl, B.; Bub, A.; Kulling, S.E. Microbial Metabolism of the Soy Isoflavones Daidzein and Genistein in Postmenopausal Women: Human Intervention Study Reveals New Metabotypes. Nutrients 2023, 15, 2352. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Pan, M.-H.; Ho, C.-T. Anti-obesity molecular mechanism of soy isoflavones: Weaving the way to new therapeutic routes. Food Funct. 2017, 8, 3831–3846. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Rogozin, E.A.; Cho, Y.-Y.; Heo, Y.-S.; Bode, A.M.; Lee, H.J.; Dong, Z. Equol, a metabolite of the soybean isoflavone daidzein, inhibits neoplastic cell transformation by targeting the MEK/ERK/p90RSK/activator protein-1 pathway. J. Biol. Chem. 2007, 282, 32856–32866. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Kulling, S.E.; Schwartz, H.; Rowland, I.; Ruefer, C.E.; Rimbach, G.; Cassidy, A.; Magee, P.; Millar, J.; Hall, W.L.; et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol. Nutr. Food Res. 2009, 53, S266–S309. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Pilšáková, L.; Riečanský, I.; Jagla, F. The physiological actions of isoflavone phytoestrogens. Physiol. Res. 2010, 59, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, J.; Ball, M.; Murkies, A.; Dennerstein, L. Dietary phytoestrogen intake in mid-life Australian-born women: Relationship to health variables. Climacteric 2000, 3, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake and body composition in postmenopausal women. Menopause 2003, 10, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Montani, C.; Romani, A.; Vignolini, P.; Pampaloni, B.; Tanini, A.; Brandi, M.; Alonso-Magdalena, P.; Nadal, A.; Ottobrini, L.; et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 2006, 147, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Christopher, R.; Cederroth, S.N. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar]
- Yang, G.; Shu, X.; Jin, F.; Elasy, T.; Li, H.; Li, Q.; Huang, F.; Zhang, X.; Gao, Y.; Zheng, W. Soyfood consumption and risk of glycosuria: A cross-sectional study within the Shanghai Women’s Health Study. Eur. J. Clin. Nutr. 2004, 58, 615. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Greany, K.A.; Nettleton, J.A.; Wangen, K.E.; Thomas, W.; Kurzer, M.S. Probiotic consumption does not enhance the cholesterol-lowering effect of soy in postmenopausal women. J. Nutr. 2004, 134, 3277–3283. [Google Scholar] [CrossRef]
- Jayagopal, V.; Albertazzi, P.; Kilpatrick, E.S.; Howarth, E.M.; Jennings, P.E.; Hepburn, D.A.; Atkin, S.L. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002, 25, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Gadbury, G.; Schwartz, L.G.; Murugesan, R.; Kraker, J.L.; Heshka, S.; Fontaine, K.R.; Heymsfield, S.B. A novel soy-based meal replacement formula for weight loss among obese individuals: A randomized controlled clinical trial. Eur. J. Clin. Nutr. 2003, 57, 514. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J. Nutr. Biochem. 2005, 16, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Iqbal, M.; Steinle, J.; Oitker, J.; Higginbotham, D.; Peterson, R.; Banz, W. Soy protein influences the development of the metabolic syndrome in male obese ZDFxSHHF rats. Horm. Metab. Res. 2005, 37, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Rasbach, K.A.; Schnellmann, R.G. Isoflavones promote mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 2008, 325, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, E.C.; Ward, W.E. Early exposure to soy isoflavones and effects on reproductive health: A review of human and animal studies. Nutrients 2010, 2, 1156–1187. [Google Scholar] [CrossRef] [PubMed]
- Bennetts, H.; Uuderwood, E.; Shier, F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 1946, 22, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Feraco, A.; Storz, M.A.; Lombardo, M. The role of soy and soy isoflavones on women’s fertility and related outcomes: An update. J. Nutr. Sci. 2022, 11, e17. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.W.; Brooks, N.; Milburn, G.M.; Soames, A.; Stone, S.; Hall, M.; Ashby, J. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol. Sci. 2003, 71, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Marraudino, M.; Ponti, G.; Moussu, C.; Farinetti, A.; Macchi, E.; Accornero, P.; Gotti, S.; Collado, P.; Keller, M.; Panzica, G. Early postnatal genistein administration affects mice metabolism and reproduction in a sexually dimorphic way. Metabolites 2021, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Padilla-Banks, E.; Clark, G.; Newbold, R.R. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J. Chromatogr. B 2002, 777, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Padilla-Banks, E.; Goulding, E.H.; Lao, S.-P.C.; Newbold, R.R.; Williams, C.J. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol. Reprod. 2009, 80, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Padilla-Banks, E.; Newbold, R.R. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol. Reprod. 2005, 73, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Padilla-Banks, E.; Newbold, R.R. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod. Toxicol. 2007, 23, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Environmental estrogens and obesity. Mol. Cell. Endocrinol. 2009, 304, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hillisch, A.; Peters, O.; Kosemund, D.; Müller, G.; Walter, A.; Schneider, B.; Reddersen, G.; Elger, W.; Fritzemeier, K.-H. Dissecting physiological roles of estrogen receptor α and β with potent selective ligands from structure-based design. Mol. Endocrinol. 2004, 18, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E.; et al. Polycystic ovary syndrome in insulin-resistant adolescents with obesity: The role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Heger, S.; Körner, A.; Meigen, C.; Gausche, R.; Keller, A.; Keller, E.; Kiess, W. Impact of weight status on the onset and parameters of puberty: Analysis of three representative cohorts from central Europe. J. Pediatr. Endocrinol. Metab. 2008, 21, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.T.; Arah, O.A.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansen, C.H. Childhood overweight and obesity and timing of puberty in boys and girls: Cohort and sibling-matched analyses. Int. J. Epidemiol. 2020, 49, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.R. Body weight and the initiation of puberty. Clin. Obstet. Gynecol. 1985, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Campisi, S.C.; Humayun, K.N.; Rizvi, A.; Lou, W.; Söder, O.; Bhutta, Z.A. Later puberty onset among chronically undernourished adolescents living in a Karachi slum, Pakistan. Acta Paediatr. 2020, 109, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, K. Menses requires energy: A review of how disordered eating, excessive exercise, and high stress lead to menstrual irregularities. Clin. Ther. 2020, 42, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kapczuk, K. Elite athletes and pubertal delay. Minerva Pediatr. 2017, 69, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, B.; Ackerman, K.E. Recommendations and nutritional considerations for female athletes: Health and performance. Sports Med. 2021, 51, 43–57. [Google Scholar] [CrossRef]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2017. Available online: https://pubmed.ncbi.nlm.nih.gov/29262142/ (accessed on 30 June 2024).
- Ito, T.; Bai, T.; Tanaka, T.; Yoshida, K.; Ueyama, T.; Miyajima, M.; Negishi, T.; Kawasaki, T.; Takamatsu, H.; Kikutani, H.; et al. Semaphorin 4D induces vaginal epithelial cell apoptosis to control mouse postnatal vaginal tissue remodeling. Mol. Med. Rep. 2015, 11, 829–836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009, 48, A.4I.1–A.4I.8. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, T.M.; Silveira, M.A.; Zampieri, T.T.; Frazao, R.; Donato, J., Jr. Fatness rather than leptin sensitivity determines the timing of puberty in female mice. Mol. Cell. Endocrinol. 2016, 423, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tortoriello, D.V.; McMinn, J.; Chua, S.C. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology 2004, 145, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Palma-Vera, S.E.; Reyer, H.; Langhammer, M.; Reinsch, N.; Derezanin, L.; Fickel, J.; Qanbari, S.; Weitzel, J.M.; Franzenburg, S.; Hemmrich-Stanisak, G.; et al. Genomic characterization of the world’s longest selection experiment in mouse reveals the complexity of polygenic traits. BMC Biol. 2022, 20, 52. [Google Scholar]
- Rodrigo, N.; Saad, S.; Pollock, C.; Glastras, S.J. Diet modification before or during pregnancy on maternal and foetal outcomes in rodent models of maternal obesity. Nutrients 2022, 14, 2154. [Google Scholar] [CrossRef] [PubMed]
- Brenmoehl, J.; Walz, C.; Spitschak, M.; Wirthgen, E.; Walz, M.; Langhammer, M.; Tuchscherer, A.; Naumann, R.; Hoeflich, A. Partial phenotype conversion and differential trait response to conditions of husbandry in mice. J. Comp. Physiol. B 2018, 188, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Brenmoehl, J.; Ohde, D.; Albrecht, E.; Walz, C.; Tuchscherer, A.; Hoeflich, A. Browning of subcutaneous fat and higher surface temperature in response to phenotype selection for advanced endurance exercise performance in male DUhTP mice. J. Comp. Physiol. B 2017, 187, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Molzberger, A.F.; Soukup, S.T.; Kulling, S.E.; Diel, P. Proliferative and estrogenic sensitivity of the mammary gland are modulated by isoflavones during distinct periods of adolescence. Arch. Toxicol. 2013, 87, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Mallien, A.S.; Soukup, S.T.; Pfeiffer, N.; Brandwein, C.; Kulling, S.E.; Chourbaji, S.; Gass, P. Effects of soy in laboratory rodent diets on the basal, affective, and cognitive behavior of C57BL/6 mice. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Soukup, S.T.; Al-Maharik, N.; Botting, N.; Kulling, S.E. Quantification of soy isoflavones and their conjugative metabolites in plasma and urine: An automated and validated UHPLC-MS/MS method for use in large-scale studies. Anal. Bioanal. Chem. 2014, 406, 6007–6020. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.; Cordón, R.; Folch, J.M. DAG expression: High-throughput gene expression analysis of real-time PCR data using standard curves for relative quantification. PLoS ONE 2013, 8, e80385. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, A.; Fitzner, B.; Walz, C.; Hecker, M.; Tuchscherer, A.; Bastian, M.; Brenmoehl, J.; Schröder, I.; Willenberg, H.S.; Reincke, M.; et al. Systemic Effects by Intrathecal Administration of Triamcinolone Acetonide in Patients With Multiple Sclerosis. Front. Endocrinol. 2020, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D. Weight and age at puberty in female and male mice of strains selected for large and small body size. Genet. Res. 1984, 44, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.H.; Janning, P.; Diel, P.; Bolt, H.M. Estrogenic isoflavones in rodent diets. Toxicol. Lett. 2002, 128, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen Content of Cereals and Cereal-Based Foods Consumed in the UK. Nutr. Cancer 2009, 61, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Cimafranca, M.A.; Davila, J.; Ekman, G.C.; Andrews, R.N.; Neese, S.L.; Peretz, J.; Woodling, K.A.; Helferich, W.G.; Sarkar, J.; Flaws, J.A.; et al. Acute and chronic effects of oral genistein administration in neonatal mice. Biol. Reprod. 2010, 83, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Setchell, K.D. Animal models impacted by phytoestrogens in commercial chow: Implications for pathways influenced by hormones. Lab. Investig. 2001, 81, 735–747. [Google Scholar] [CrossRef]
- Soukup, S.T.; Helppi, J.; Müller, D.R.; Zierau, O.; Watzl, B.; Vollmer, G.; Diel, P.; Bub, A.; Kulling, S.E. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: A cross-species and sex comparison. Arch. Toxicol. 2016, 90, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Renne, U.; Langhammer, M.; Brenmoehl, J.; Walz, C.; Zeissler, A.; Tuchscherer, A.; Piechotta, M.; Wiesner, R.J.; Bielohuby, M.; Hoeflich, A.; et al. Lifelong obesity in a polygenic mouse model prevents age- and diet-induced glucose intolerance-obesity is no road to late-onset diabetes in mice. PLoS ONE 2013, 8, e79788. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.-M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, S.M.; Nguyen, M.L.T.; Marks, D.L. Increased maternal fat consumption during pregnancy alters body composition in neonatal mice. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E1243–E1253. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Marei, W.; Moorkens, K.; Bols, P.; De Neubourg, D.; Leroy, J. Obese outbred mice only partially benefit from diet normalization or calorie restriction as preconception care interventions to improve metabolic health and oocyte quality. Hum. Reprod. 2022, 37, 2867–2884. [Google Scholar] [CrossRef] [PubMed]
- Currer, J.M.; Corrow, D.; Strobel, M.; Flurkey, K. Breeding strategies and techniques. In Handbook on Genetically Standardized Mice; The Jackson Laboratory: Bar Harbor, ME, USA, 2009; Volume 241. [Google Scholar]
- Norman, R.J.; Clark, A.M. Obesity and reproductive disorders: A review. Reprod. Fertil. Dev. 1998, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Thagaard, I.N.; Krebs, L.; Holm, J.-C.; Christiansen, M.; Møller, H.; Lange, T.; Larsen, T. The effect of obesity on early fetal growth and pregnancy duration: A cohort study. J. Matern.-Fetal Neonatal Med. 2018, 31, 2941–2946. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Witters, I.; Van den Bergh, B.R.; Jans, G.; Devlieger, R. Obesity in pregnancy: Altered onset and progression of labour. Midwifery 2013, 29, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Heslehurst, N.; Vieira, R.; Hayes, L.; Crowe, L.; Jones, D.; Robalino, S.; Slack, E.; Rankin, J. Maternal body mass index and post-term birth: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Slack, E.; Best, K.E.; Rankin, J.; Heslehurst, N. Maternal obesity classes, preterm and post-term birth: A retrospective analysis of 479,864 births in England. BMC Pregnancy Childbirth 2019, 19, 434. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Iqbal, S.; Anwar, Z.; Ahdi, S.G.; Ali, S.H.; Pirzada, S. Pre-pregnancy maternal BMI as predictor of neonatal birth weight. PLoS ONE 2020, 15, e0240748. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, E.M. Maternal obesity influences birth weight more than gestational diabetes. BMC Pregnancy Childbirth 2021, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G. Endocrinology of parturition. J. Clin. Endocrinol. Metab. 2000, 85, 4421–4425. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, E.C.; Chen, J.; Ward, W.E. Early life exposure to isoflavones adversely affects reproductive health in first but not second generation female CD-1 mice. J. Nutr. 2011, 141, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Araki, K.; Khatib, K.; Martinou, J.-C.; Vassalli, P. Mouse vaginal opening is an apoptosis-dependent process which can be prevented by the overexpression of Bcl2. Dev. Biol. 1997, 184, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.; Schillo, K.; Hinshelwood, M.; Hauser, E. Body composition at vaginal opening in mice as influenced by food intake and photoperiod: Tests of critical body weight and composition hypotheses for puberty onset. Biol. Reprod. 1983, 29, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, C.; Zhang, Q.; Pan, Y.-H.; Dang, S.; Zhang, W. ADAMTS18 regulates vaginal opening through influencing the fusion of Mullerian duct and apoptosis of vaginal epithelial cells in mice. Reprod. Biol. 2021, 21, 100537. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, Y.; Danbara, N.; Tsujita-Kyutoku, M.; Yuri, T.; Uehara, N.; Tsubura, A. Effects of prepubertal exposure to xenoestrogen on development of estrogen target organs in female CD-1 mice. In Vivo 2005, 19, 487–494. [Google Scholar] [PubMed]
- Zhao, Y.; Tan, Y.S.; Strynar, M.J.; Perez, G.; Haslam, S.Z.; Yang, C. Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice. Reprod. Toxicol. 2012, 33, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tan, Y.S.; Harkema, J.R.; Haslam, S.Z. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod. Toxicol. 2009, 27, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Brenmoehl, J.; Ohde, D.; Walz, C.; Schultz, J.; Tuchscherer, A.; Rieder, F.; Renne, U.; Hoeflich, A. Dynamics of Fat Mass in DUhTP Mice Selected for Running Performance—Fat Mobilization in a Walk. Obes. Facts 2015, 8, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De Matteis, R.; Cinti, S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1244–E1253. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Hamm, M.L.; Bhat, G.K.; Thompson, W.E.; Mann, D.R. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol. Reprod. 2004, 71, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Dushay, J.; Flier, S.N.; Prabakaran, D.; Flier, J.S. Leptin accelerates the onset of puberty in normal female mice. J. Clin. Investig. 1997, 99, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.C.; Beckers, K.F.; Crissman, K.R.; Landry, C.A.; Flanagan, J.P.; Awad, R.M.; Piero, F.D.; Liu, C.-C.; Sones, J.L. Sexually dimorphic pubertal development and adipose tissue kisspeptin dysregulation in the obese and preeclamptic-like BPH/5 mouse model offspring. Front. Physiol. 2023, 14, 1070426. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.J.; Abed, F.S.; Dhefer, I.H. Does kisspeptin act as a neuropeptide or as an adipokine in obese people? J. Taibah Univ. Med. Sci. 2022, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- De Roux, N.; Genin, E.; Carel, J.-C.; Matsuda, F.; Chaussain, J.-L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.; Hedrick, J.A.; Vassileva, G.; Markowitz, L.; Abbondanzo, S.; Golovko, A.; Yang, S.; Monsma, F.J.; Gustafson, E.L. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biophys. Res. Commun. 2003, 312, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. Obstet. Gynecol. Surv. 2004, 59, 351–353. [Google Scholar] [CrossRef]
- Lapatto, R.; Pallais, J.C.; Zhang, D.; Chan, Y.-M.; Mahan, A.; Cerrato, F.; Le, W.W.; Hoffman, G.E.; Seminara, S.B. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 2007, 148, 4927–4936. [Google Scholar] [CrossRef] [PubMed]
- d’Anglemont de Tassigny, X.; Fagg, L.A.; Dixon, J.P.; Day, K.; Leitch, H.G.; Hendrick, A.G.; Zahn, D.; Franceschini, I.; Caraty, A.; Carlton, M.B.; et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc. Natl. Acad. Sci. USA 2007, 104, 10714–10719. [Google Scholar] [CrossRef] [PubMed]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The importance of leptin to reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-Å. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen receptor β: An overview and update. Nucl. Recept. Signal. 2008, 6, nrs-06003. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.-A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Lindzey, J.; Grandien, K.; Gustafsson, J.-A.k.; Korach, K.S. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 1997, 138, 4613–4621. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Bekele, R.; vanden Berg, J.H.; Kuswanti, Y.; Thapa, O.; Soltani, S.; van Leeuwen, F.R.; Rietjens, I.M.; Murk, A.J. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicol. In Vitro 2015, 29, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Beekmann, K.; de Haan, L.H.; Actis-Goretta, L.; Houtman, R.; van Bladeren, P.J.; Rietjens, I.M. The effect of glucuronidation on isoflavone induced estrogen receptor (ER) α and ERβ mediated coregulator interactions. J. Steroid Biochem. Mol. Biol. 2015, 154, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen–estrogen receptor signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Vinketova, K.; Koleva, V.; Puncheva, E.; Nashar, S.; Oreshkova, T. A sustained decrease in serum CD83 in pregnant women. J. Reprod. Immunol. 2022, 154, 103762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fishman, M.C.; Huang, P.L. Estrogen Mediates the Protective Effects of Pregnancy and Chorionic Gonadotropin in a Mouse Model of Vascular Injury. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2059–2065. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alonso, A.; Fernández, R.; Ordóñez, P.; Moreno, M.; Patterson, A.M.; González, C. Regulation of estrogen receptor alpha by estradiol in pregnant and estradiol treated rats. Steroids 2006, 71, 1052–1061. [Google Scholar] [CrossRef]
| Analytes | Diet A (n = 4) | Diet S (n = 4) | Diet SPA (n = 3) | |||
|---|---|---|---|---|---|---|
| Content [mg/g Diet] | Content (%) | Content [mg/g Diet] | Content (%) | Content [mg/g Diet] | Content (%) | |
| Sum of Daidzein in Aglycone Equivalents | 0.267 | 45.1 A | 0.157 | 39.9 B | n.d. | - |
| Sum of Genistein in Aglycone Equivalents | 0.287 | 48.2 | 0.195 | 50.2 | n.d. | - |
| Sum of Glycitein in Aglycone Equivalents | 0.041 | 6.8 | 0.039 | 10.0 | n.d. | - |
| Overall Isoflavone Aglycone Equivalents | 0.594 a | 100.0 | 0.392 b | 100.0 | n.d. | - |
| Juvenile Females | Line | DUC | DUhTP | DU6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet Count | A n = 8 | S n = 8 | SPA n = 6 | A n = 8 | S n = 8 | SPA n = 6 | A n = 8 | S n = 8 | SPA n = 8 | |
| Total Isoflavones | mean | 2475.3 a | 1939.8 a | 4.8 b | 4086.1 #a | 2291.1 b | 1.5 c | 5822.8 #§a | 4300.6 #§b | n.d. c |
| SD | 1142.5 | 746.1 | 8.8 | 666.4 | 510.7 | 2.3 | 1573.2 | 1464.1 | ||
| Total Daidzein Equivalents | mean | 952.3 a | 656.5 a | 2.4 b | 1689.3 a | 745.8 b | 1.5 c | 2542.9 #a | 1241.8 #§b | n.d. c |
| SD | 809.2 | 309.9 | 4.1 | 365.0 | 163.3 | 2.3 | 1219.0 | 291.8 | ||
| Daidzein (free Aglycone) | mean | 183.0 a | 102.7 a | n.d. b | 215.7 a | 73.7 b | n.d. c | 574.1 #§a | 229.5 #§b | n.d. c |
| SD | 154.1 | 54.2 | 74.9 | 22.5 | 195.3 | 41.9 | ||||
| Total Genistein Equivalents | mean | 718.2 a | 488.5 a,b | 2.0 b | 1353.9 #a | 564.0 b | n.d. c | 2550.5 #§a | 1183.1 #§b | n.d. c |
| SD | 560.7 | 243.3 | 4.9 | 546.0 | 148.3 | 787.0 | 507.1 | |||
| Genistein (free Aglycone) | mean | 74.5 a | 40.8 a,b | n.d. b | 102.0 a | 28.4 b | n.d. b | 307.5 #§a | 127.8 #§b | n.d. c |
| SD | 56.2 | 23.5 | 65.0 | 10.4 | 83.3 | 74.3 | ||||
| Sum of Equol, Equol-7-G and Equol-4′-S | mean | 804.9 a | 794.9 a | 1.5 b | 1042.9 a | 981.3 a | 0.3 b | 1036.9 a | 2003.6 #§b | n.d. c |
| SD | 640.2 | 281.9 | 3.1 | 237.0 | 300.2 | 0.8 | 729.5 | 1179.6 | ||
| Adult Females | Line | DUC | DUhTP | DU6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet Count | A n = 13 | S n = 11 | SPA n = 12 | A n = 13 | S n = 14 | SPA n = 15 | A n = 10 | S n = 11 | SPA n = 8 | |
| Body Mass | mean | 33.40 | 33.88 | 32.47 | 31.77 | 28.85 # | 29.75 | 89.95 #§ | 89.10 #§ | 86.04 #§ |
| SD | 3.57 | 2.51 | 3.58 | 2.45 | 1.77 | 2.25 | 8.21 | 4.96 | 10.58 | |
| Uterus | mean | 0.157 | 0.146 | 0.149 | 0.152 | 0.139 | 0.151 | 0.297 #§ | 0.293 #§ | 0.307 #§ |
| SD | 0.024 | 0.015 | 0.029 | 0.020 | 0.016 | 0.018 | 0.098 | 0.080 | 0.092 | |
| Ovaries | mean | 0.029 | 0.030 | 0.025 | 0.039 | 0.030 | 0.033 | 0.039 | 0.035 | 0.035 |
| SD | 0.008 | 0.007 | 0.005 | 0.010 | 0.005 | 0.007 | 0.013 | 0.012 | 0.017 | |
| Adult Females | Line | DUC | DUhTP | DU6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet Count | A n = 10 | S n = 10 | SPA n = 10 | A n = 10 | S n = 10 | SPA n = 10 | A n = 8 | S n = 10 | SPA n = 10 | |
| Feed Intake [g/d] | mean | 4.62 | 4.49 | 4.66 | 3.94 #a | 3.91 #a | 4.21 #b | 11.04 #§a | 9.98 #§b | 10.50 #§a,b |
| SD | 0.36 | 0.23 | 0.30 | 0.22 | 0.22 | 0.17 | 0.63 | 0.94 | 1.00 | |
| Calorie Intake [kcal/g BM/d] | mean | 0.57 a | 0.57 a | 0.62 b | 0.58 a | 0.59 a | 0.65 b | 0.51 #§ | 0.49 #§ | 0.53 #§ |
| SD | 0.03 | 0.04 | 0.04 | 0.03 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | |
| Adult Females | Line | DUC | DUhTP | DU6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet Count | A n = 13 | S n = 11 | SPA n = 11 | A n = 13 | S n = 14 | SPA n = 11 | A n = 10 | S n = 11 | SPA n = 6 | |
| Total Isoflavone | mean | 3541.7 a | 3075.2 a | 10.6 b | 3619.2 a | 2322.9 a | 4.5 b | 6738.4 #§a | 3448.4 b | 0.6 c |
| SD | 875.1 | 1066.2 | 15.7 | 752.0 | 1165.4 | 6.3 | 2918.1 | 1259.6 | 1.0 | |
| Total Daidzein Equivalents | mean | 846.6 a | 830.8 a | 4.6 b | 1342.1 #a | 703.5 b | 2.4 c | 1548.4 #a | 852.8 b | 0.0 c |
| SD | 248.7 | 395.0 | 7.8 | 515.7 | 413.1 | 2.3 | 550.5 | 272.9 | 0.0 | |
| Daidzein (free Aglycone) | mean | 119.6 a | 107.2 a | 0.0 b | 78.0 a | 36.6 a | 0.4 a | 316.0 #§a | 170.2 §b | 0.0 c |
| SD | 46.4 | 63.0 | 0.0 | 37.0 | 18.6 | 1.4 | 127.1 | 63.2 | 0.0 | |
| Total Genistein Equivalents | mean | 841.1 a | 997.0 a | 2.7 b | 1096.1 a | 655.6 a,b | 1.0 b | 2566.0 #§a | 966.2 b | 0.0 c |
| SD | 226.1 | 479.6 | 4.9 | 354.3 | 390.3 | 2.3 | 1698.0 | 468.0 | 0.0 | |
| Genistein (free Aglycone) | mean | 35.0 a | 35.4 a | 0.1 b | 24.3 a | 13.2 a | 0.5 a | 127.1 #§a | 55.8 §b | 0.3 c |
| SD | 11.1 | 19.3 | 0.5 | 10.4 | 6.9 | 1.6 | 44.7 | 27.9 | 0.7 | |
| Sum of Equol, Equol-7-G, and Equol-4′-S | mean | 1602.6 a | 1104.8 a | 3.2 b | 1078.6 a | 914.0 a | 0.2 b | 2181.2 §a | 1403.5 b | 0.4 c |
| SD | 600.0 | 499.5 | 5.4 | 317.0 | 382.9 | 0.7 | 1068.4 | 794.7 | 0.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, Z.; Soukup, S.T.; Lubs, A.; Ohde, D.; Walz, C.; Schoen, J.; Willenberg, H.S.; Hoeflich, A.; Brenmoehl, J. Impact of Dietary Isoflavones in Standard Chow on Reproductive Development in Juvenile and Adult Female Mice with Different Metabolic Phenotypes. Nutrients 2024, 16, 2697. https://doi.org/10.3390/nu16162697
Meyer Z, Soukup ST, Lubs A, Ohde D, Walz C, Schoen J, Willenberg HS, Hoeflich A, Brenmoehl J. Impact of Dietary Isoflavones in Standard Chow on Reproductive Development in Juvenile and Adult Female Mice with Different Metabolic Phenotypes. Nutrients. 2024; 16(16):2697. https://doi.org/10.3390/nu16162697
Chicago/Turabian StyleMeyer, Zianka, Sebastian T. Soukup, Anna Lubs, Daniela Ohde, Christina Walz, Jennifer Schoen, Holger S. Willenberg, Andreas Hoeflich, and Julia Brenmoehl. 2024. "Impact of Dietary Isoflavones in Standard Chow on Reproductive Development in Juvenile and Adult Female Mice with Different Metabolic Phenotypes" Nutrients 16, no. 16: 2697. https://doi.org/10.3390/nu16162697
APA StyleMeyer, Z., Soukup, S. T., Lubs, A., Ohde, D., Walz, C., Schoen, J., Willenberg, H. S., Hoeflich, A., & Brenmoehl, J. (2024). Impact of Dietary Isoflavones in Standard Chow on Reproductive Development in Juvenile and Adult Female Mice with Different Metabolic Phenotypes. Nutrients, 16(16), 2697. https://doi.org/10.3390/nu16162697







