The Role of Antioxidants in the Therapy of Cardiovascular Diseases—A Literature Review
Abstract
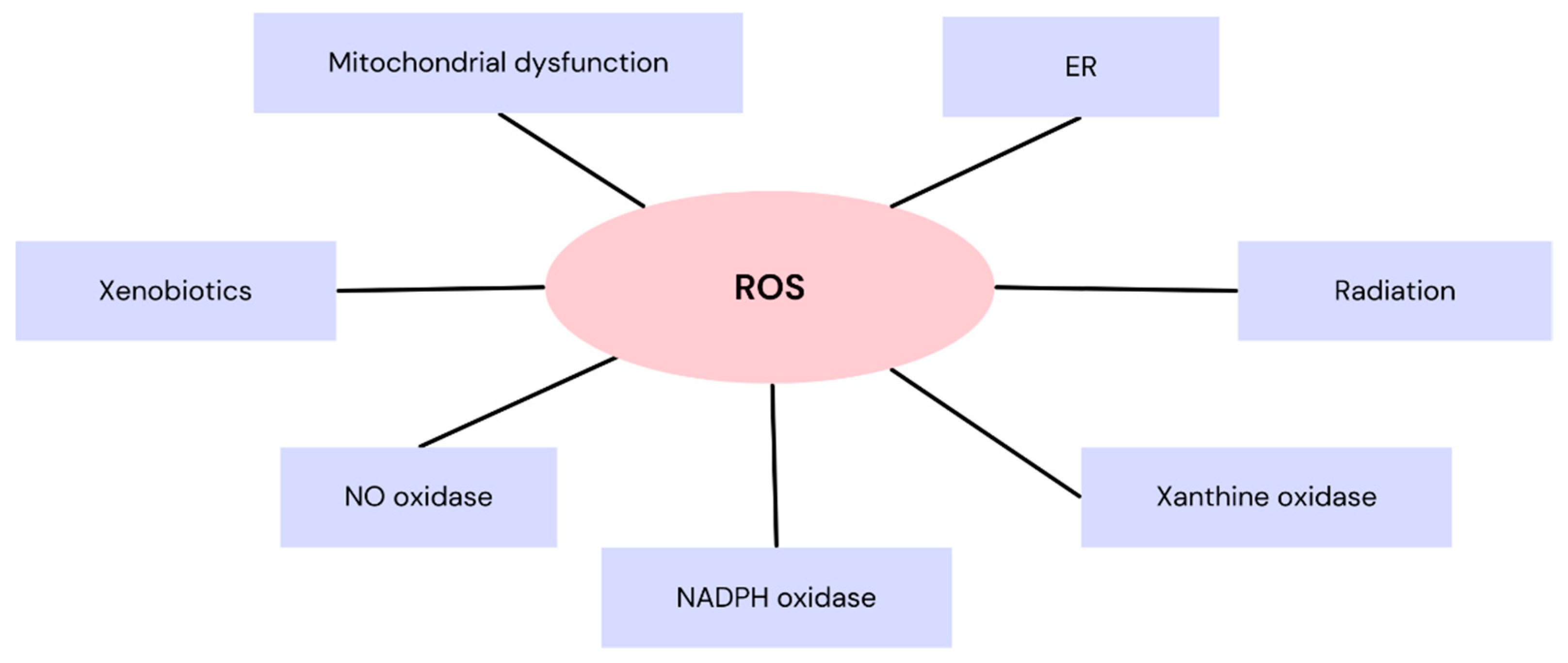
1. Introduction
2. Coenzyme Q10
2.1. Chemical Characteristics
2.2. Q10 Role’s in Heart Failure
2.3. Q10 Role’s in Coronary Artery Disease and Dyslipidemia
2.4. Q10 Role’s in Hypertension
3. Polyphenols
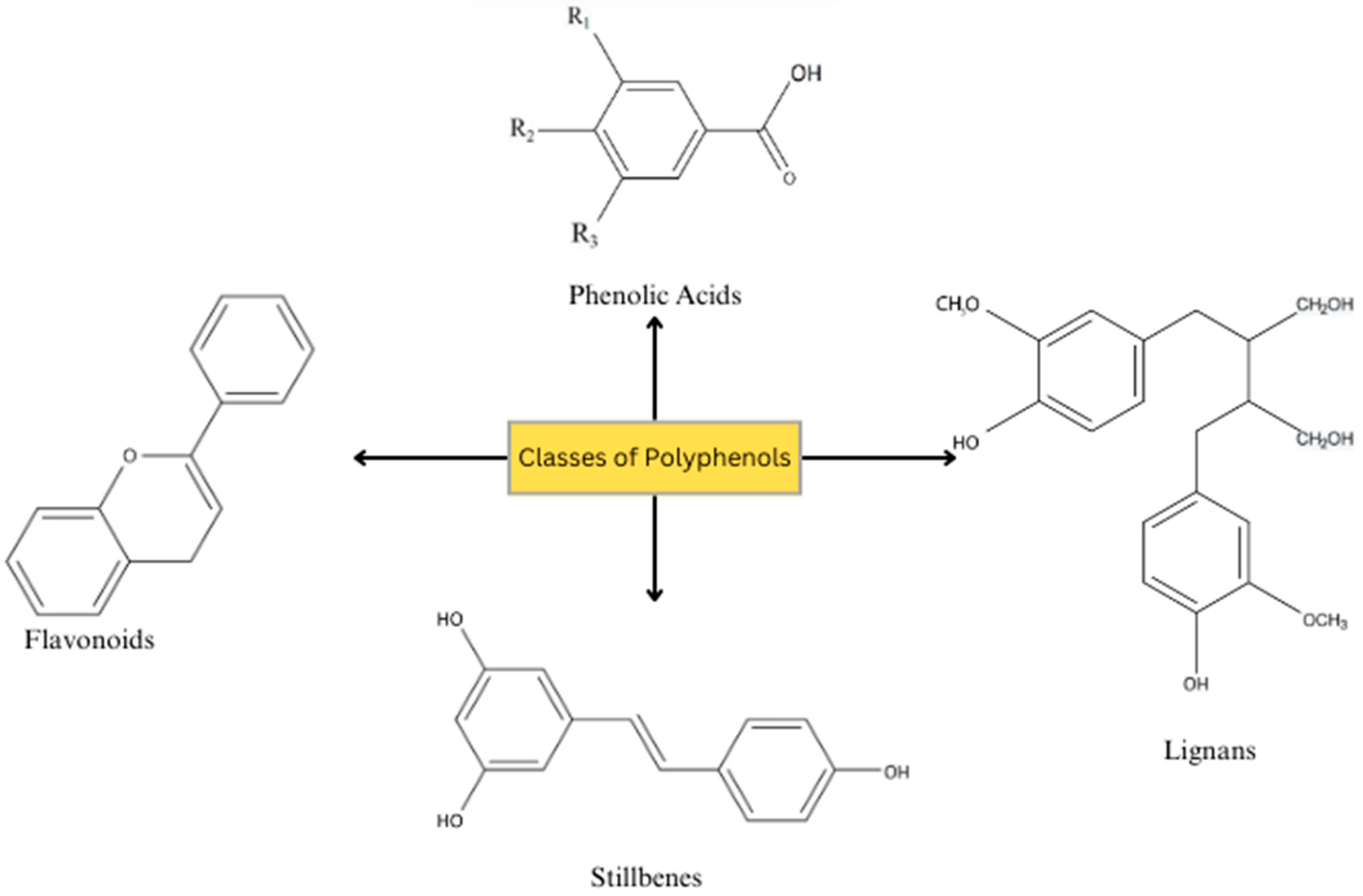
3.1. Chemical Characteristics
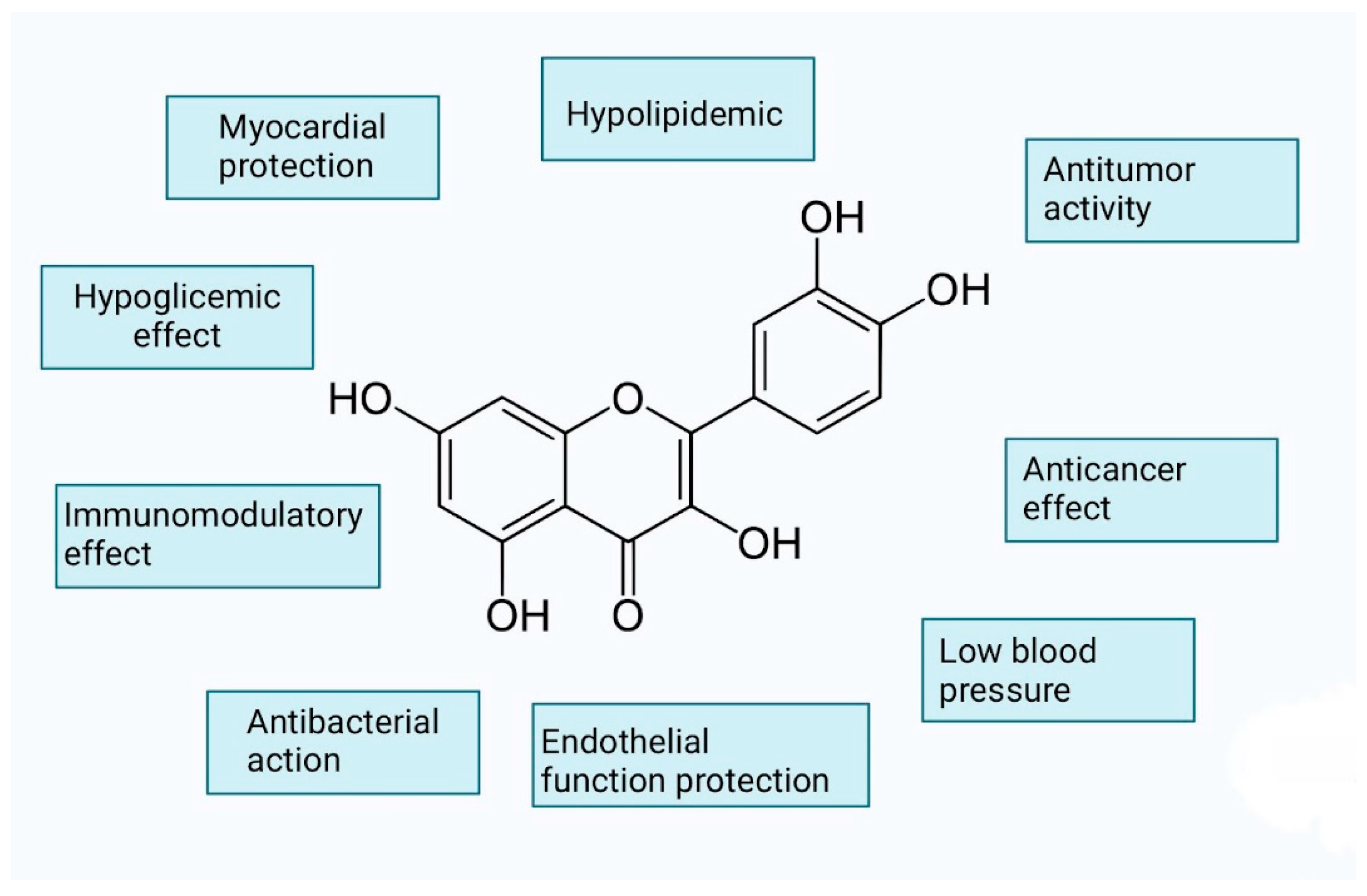
3.2. Flavonoids
3.2.1. Anthoxanthins (Flavanone and Flavanol)
3.2.2. Flavanones
3.2.3. Chalcones
3.3. Stilbenes
3.3.1. Resveratrol’s Impact on Oxidative Stress, Inflammation, and NO Synthesis
3.3.2. Resveratrol and Lipid Oxidation
3.3.3. Resveratrol on Atherosclerosis
3.3.4. Resveratrol and Protective Effect for Heart Injury
3.3.5. Resveratrol and Limitations
| Polyphenol | Source | Cardiovascular Benefits |
|---|---|---|
| Resveratrol | Grapes, red wine, berries | Improves endothelial function, reduces blood pressure, anti-inflammatory effects, decreases LDL oxidation, and inhibits platelet aggregation [76]. |
| Epicatechin | Dark chocolate, green tea | Enhances endothelial function, improves blood flow, lowers blood pressure, reduces oxidative stress, and improves cholesterol profiles [77]. |
| Quercetin | Apples, onions, berries | Anti-inflammatory effects, reduces blood pressure, improves endothelial function, and decreases LDL oxidation [78]. |
| Catechins | Green tea, cocoa, apples | Antioxidant effects, improves endothelial function, reduces blood pressure, lowers cholesterol levels, and enhances nitric oxide availability [79]. |
| Anthocyanins | Berries, red grapes, red cabbage | Reduces oxidative stress, anti-inflammatory effects, improves endothelial function, lowers blood pressure, and enhances nitric oxide production [48]. |
| Flavonols | Tea, onions, kale | Reduces blood pressure and has anti-inflammatory effects [80]. |
4. Carotenoids
4.1. Chemical Characteristics
4.2. Antioxidant Capabilities
4.3. Carotenoids in the Human Body
4.4. Carotenoids and Cardiovascular Diseases
4.5. β-Carotene
4.6. Lycopene
5. Novel Experimental Antioxidant Therapies
5.1. miRNA
5.2. Nanoparticles
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative Stress in Cell Death and Cardiovascular Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9030563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Pagan, L.U.; Gomes, M.J.; Gatto, M.; Mota, G.A.F.; Okoshi, K.; Okoshi, M.P. The Role of Oxidative Stress in the Aging Heart. Antioxidants 2022, 11, 336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velena, A.; Zarkovic, N.; Gall Troselj, K.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1,4-Dihydropyridine Derivatives: Dihydronicotinamide Analogues-Model Compounds Targeting Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 1892412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, G.N.; Gupta, G.; Sharma, P. A Comprehensive Review of Free Radicals, Antioxidants, and Their Relationship with Human Ailments. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709, Erratum in Nat. Rev. Drug Discov. 2021, 20, 652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rabanal-Ruiz, Y.; Llanos-González, E.; Alcain, F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants 2021, 10, 755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crane, F.L.; Hatefi, Y.; Lester, R.L.; Widmer, C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 1957, 25, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S. Coenzyme Q and vitamin E interactions. Methods Enzymol. 2004, 378, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Horn, P.S.; Tang, P.H.; Morrison, J.A.; Miles, L.; DeGrauw, T.; Pesce, A.J. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin. Chim. Acta 2004, 347, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; de la Cruz-Ares, S.; Torres-Peña, J.D.; Alcalá-Diaz, J.F.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 and Cardiovascular Diseases. Antioxidants 2021, 10, 906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergamini, C.; Cicoira, M.; Rossi, A.; Vassanelli, C. Oxidative stress and hyperuricaemia: Pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur. J. Heart Fail. 2009, 11, 444–452. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, S.J.; Hwang, H.Y.; Park, E.J.; Nam, J.H.; Kim, J.; Park, S.I. TGF-beta1 induces cardiac hypertrophic responses via PKC-dependent ATF-2 activation. J. Mol. Cell Cardiol. 2005, 39, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 2004, 1660, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Vieco, E.; Martínez-García, I.; Sequí-Domínguez, I.; Rodríguez-Gutiérrez, E.; Moreno-Herráiz, N.; Pascual-Morena, C. Effect of coenzyme Q10 on cardiac function and survival in heart failure: An overview of systematic reviews and meta-analyses. Food Funct. 2023, 14, 6302–6311. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Q-SYMBIO Study Investigators. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Fladerer, J.P.; Grollitsch, S. Comparison of Coenzyme Q10 (Ubiquinone) and Reduced Coenzyme Q10 (Ubiquinol) as Supplement to Prevent Cardiovascular Disease and Reduce Cardiovascular Mortality. Curr. Cardiol. Rep. 2023, 25, 1759–1767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Campen, L.C.; Visser, F.C.; Visser, C.A. Ejection fraction improvement by beta-blocker treatment in patients with heart failure: An analysis of studies published in the literature. J. Cardiovasc. Pharmacol. 1998, 32 (Suppl. 1), S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Jorat, M.V.; Tabrizi, R.; Kolahdooz, F.; Akbari, M.; Salami, M.; Heydari, S.T.; Asemi, Z. The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2019, 27, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Jorat, M.V.; Tabrizi, R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Mottaghi, R.; Asemi, Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018, 17, 230. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cho, W.J.; Kim, J.K.; Lee, D.C. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: A double-blind randomized controlled study. J. Med. Food 2011, 14, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Tabrizi, R.; Moosazadeh, M.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Chamani, M.; Kolahdooz, F.; Asemi, Z. The Effects of Coenzyme Q10 Supplementation on Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2018, 24, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, Z.; Zhao, D.; Liang, Y.; Dai, S.; Liu, M.; Hou, S.; Dong, X.; Zhaxinima Yang, Y. Effects of Coenzyme Q10 Supplementation on Lipid Profiles in Adults: A Meta-analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2022, 108, 232–249. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Digiesi, V.; Cantini, F.; Oradei, A.; Bisi, G.; Guarino, G.C.; Brocchi, A.; Bellandi, F.; Mancini, M.; Littarru, G.P. Coenzyme Q10 in essential hypertension. Mol. Aspects Med. 1994, 15, s257–s263. [Google Scholar] [CrossRef] [PubMed]
- Flowers, N.; Hartley, L.; Todkill, D.; Stranges, S.; Rees, K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014, 2014, CD010405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohseni, M.; Vafa, M.R.; Hajimiresmail, S.J.; Zarrati, M.; Rahimi-Forushani, A.; Bitarafan, V.; Shidfar, F. Effects of coenzyme q10 supplementation on serum lipoproteins, plasma fibrinogen, and blood pressure in patients with hyperlipidemia and myocardial infarction. Iran. Red. Crescent Med. J. 2014, 16, e16433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liang, Y.; Dai, S.; Hou, S.; Liu, Z.; Liu, M.; Dong, X.; Zhan, Y.; Tian, Z.; Yang, Y. Dose-Response Effect of Coenzyme Q10 Supplementation on Blood Pressure among Patients with Cardiometabolic Disorders: A Grading of Recommendations Assessment, Development, and Evaluation (GRADE)-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 2180–2194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Rodrigo, R.; Gil, D.; Miranda-Merchak, A.; Kalantzidis, G. Antihypertensive role of polyphenols. Adv. Clin. Chem. 2012, 58, 225–254. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paramita, V.; Kusumayanti, H.; Amalia, R.; Leviana, W.; Nisa, Q.A. Application of Flavonoid and Anthocyanin Contents from Rambutan (Nephelium lappaceum) Peel as Natural Dyes on Cotton Fabric. Adv. Sci. Lett. 2018, 24, 9853–9855. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorganic Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Lagunas-Herrera, H.; Tortoriello, J.; Herrera-Ruiz, M.; Martínez-Henández, G.B.; Zamilpa, A.; Santamaría, L.A.; Lorenzana, M.G.; Lombardo-Earl, G.; Jiménez-Ferrer, E. Acute and Chronic Antihypertensive Effect of Fractions, Tiliroside and Scopoletin from Malva parviflora. Biol. Pharm. Bull 2019, 42, 18–25. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, S.; Li, Z.; Chou, S.; Shu, C.; Chen, Y.; Chen, W.; Yang, S.; Yang, Y.; Tian, J.; et al. An updated review on the stability of anthocyanins regarding the interaction with food proteins and polysaccharides. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4378–4401. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.; Geisler, S.; Lisandrelli, R.; Ngoc, H.N.; Ganzera, M.; Schennach, H.; Fuchs, D.; Fuchs, J.E.; Gostner, J.M.; Kurz, K. Pharmacological Targets of Kaempferol Within Inflammatory Pathways—A Hint Towards the Central Role of Tryptophan Metabolism. Antioxidants 2020, 9, 180. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Chen, J.; Yeung, E.Y. Effect of Ginkgo biloba extract on procarcinogen-bioactivating human CYP1 enzymes: Identification of isorhamnetin, kaempferol, and quercetin as potent inhibitors of CYP1B1. Toxicol. Appl. Pharmacol. 2006, 213, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Jin, X.; Zhao, W.; Wu, X.; Zhai, F.; Li, Z. Rutin ameliorates the promotion effect of fine particulate matter on vascular calcification in calcifying vascular cells and ApoE−/− mice. Ecotoxicol. Environ. Saf. 2022, 234, 113410. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Kada, Y.; Kimata, M.; Sakaki, T.; Ikushiro, S. Comparison of metabolism and biological properties among positional isomers of quercetin glucuronide in LPS- and RANKL-challenged RAW264.7 cells. Biosci. Biotechnol. Biochem. 2022, 86, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Mellon, F.; Barron, D.; Sarrazin, G.; Morgan, M.R.; Williamson, G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free Radical Res. 2001, 35, 941–952. [Google Scholar] [CrossRef]
- Bussmann, A.J.C.; Zaninelli, T.H.; Saraiva-Santos, T.; Fattori, V.; Guazelli, C.F.S.; Bertozzi, M.M.; Andrade, K.C.; Ferraz, C.R.; Camilios-Neto, D.; Casella, A.M.B.; et al. The Flavonoid Hesperidin Methyl Chalcone Targets Cytokines and Oxidative Stress to Reduce Diclofenac-Induced Acute Renal Injury: Contribution of the Nrf2 Redox-Sensitive Pathway. Antioxidants 2022, 11, 1261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, H.; Li, J.; Zheng, J.; Zhou, B. Cardamonin attenuates cerebral ischemia/reperfusion injury by activating the HIF-1α/VEGFA pathway. Phytother. Res. 2022, 36, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Luo, Y.; Liu, S.; Li, S.; Li, L.; Ma, Y.; Liu, J. Protective effect of rutin on spinal motor neuron in rats exposed to acrylamide and the underlying mechanism. Neurotoxicology 2023, 95, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Chekalina, N.I. Resveratrol has a positive effect on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiad. Lek. 2017, 70 Pt 2, 286–291. [Google Scholar] [PubMed]
- Chen, H.-E.; Lin, Y.-J.; Lin, I.-C.; Yu, H.-R.; Sheen, J.-M.; Tsai, C.-C.; Huang, L.-T.; Tain, Y.-L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Conesa, M.T.G.; Tomás-Barberán, F.A.; Espín, J.C. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- De Taeye, B.; Smith, L.H.; Vaughan, D.E. Plasminogen activator inhibitor-1: A common denominator in obesity, diabetes and cardiovascular disease. Curr. Opin. Pharmacol. 2005, 5, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Urpi-Sarda, M.; Lamuela-Raventós, R.M.; Martínez-González, M.; Salas-Salvadó, J.; Arós, F.; Fitó, M.; Lapetra, J.; Estruch, R.; Andres-Lacueva, C. High urinary levels of resveratrol metabolites are associated with a reduction in the prevalence of cardiovascular risk factors in high-risk patients. Pharmacol. Res. 2012, 65, 615–620. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of pho tosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef]
- Molfino, A.; Gioia, G.; Rossi Fanelli, F.; Muscaritoli, M. The role for dietary omega-3 fatty acids supplementation in older adults. Nutrients 2014, 6, 4058–4073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, R.R.; Carson, L.; Kwik-Uribe, C.; Evans, E.M.; Erdman, J.W., Jr. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J. Nutr. 2008, 138, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Abudureheman, B.; Yu, X.; Fang, D.; Zhang, H. Enzymatic Oxidation of Tea Catechins and Its Mechanism. Molecules 2022, 27, 942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker, R.S. Carotenoids in human blood and tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76 Pt 3, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Nishino, A.; Yasui, H.; Maoka, T. Reaction of Paprika Carotenoids, Capsanthin and Capsorubin, with Reactive Oxygen Species. J. Agric. Food Chem. 2016, 64, 4786–4792. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W., Jr.; Bierer, T.L.; Gugger, E.T. Absorption and transport of carotenoids. Ann. N. Y Acad. Sci. 1993, 691, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Schwarz, W.; Sundquist, A.R.; Sies, H. cis-trans isomers of lycopene and beta-carotene in human serum and tissues. Arch. Biochem. Biophys. 1992, 294, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The role of carotenoids in human skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Gammone, M.A.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; D’Orazio, N. Prevention of cardiovascular diseases with Carotenoids. Front Biosci. 2017, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L. The oxidation hypothesis of atherosclerosis. Lancet 1994, 344, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Ylä-Herttuala, S.; Yamamoto, R.; Butler, S.; Korpela, H.; Salonen, R.; Nyyssönen, K.; Palinski, W.; Witztum, J.L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 1992, 339, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Lidebjer, C.; Leanderson, P.; Ernerudh, J.; Jonasson, L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.; Voutilainen, S.; Nyyssönen, K.; Salonen, R.; Salonen, J.T. Low plasma lycopene concentration is associated with increased intima-media thickness of the carotid artery wall. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ishii, J.; Kitagawa, F.; Kuno, A.; Kusuhara, Y.; Ochiai, J.; Ichino, N.; Osakabe, K.; Sugimoto, K.; Yamada, H.; et al. Association of serum carotenoid levels with N-terminal pro-brain-type natriuretic peptide: A cross-sectional study in Japan. J. Epidemiol. 2013, 23, 163–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koh, W.P.; Yuan, J.M.; Wang, R.; Lee, Y.P.; Lee, B.L.; Yu, M.C.; Ong, C.N. Plasma carotenoids and risk of acute myocardial infarction in the Singapore Chinese Health Study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 685–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbaraly, T.N.; Fontbonne, A.; Favier, A.; Berr, C. Plasma carotenoids and onset of dysglycemia in an elderly population: Results of the Epidemiology of Vascular Ageing Study. Diabetes Care 2008, 31, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hozawa, A.; Jacobs, D.R. Jr.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.H. Circulating carotenoid concentrations and incident hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) study. J. Hypertens. 2009, 27, 237–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.R.; Zou, Z.Y.; Huang, Y.M.; Xiao, X.; Ma, L.; Lin, X.M. Serum carotenoids in relation to risk factors for development of atherosclerosis. Clin. Biochem. 2012, 45, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goulinet, S.; Chapman, M.J. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Norkus, E.P.; Cristol, L.; Grundy, S.M. beta-Carotene inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta 1991, 1086, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Shaish, A.; Daugherty, A.; O’Sullivan, F.; Schonfeld, G.; Heinecke, J.W. Beta-carotene inhibits atherosclerosis in hypercholesterolemic rabbits. J. Clin. Investig. 1995, 96, 2075–2082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Tomo, P.; Canali, R.; Ciavardelli, D.; Di Silvestre, S.; De Marco, A.; Giardinelli, A.; Pipino, C.; Di Pietro, N.; Virgili, F.; Pandolfi, A. β-Carotene and lycopene affect endothelial response to TNF-α reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012, 56, 217–227. [Google Scholar] [CrossRef] [PubMed]
- D’Odorico, A.; Martines, D.; Kiechl, S.; Egger, G.; Oberhollenzer, F.; Bonvicini, P.; Sturniolo, G.C.; Naccarato, R.; Willeit, J. High plasma levels of alpha- and beta-carotene are associated with a lower risk of atherosclerosis: Results from the Bruneck study. Atherosclerosis 2000, 153, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Laukkanen, J.A.; Mäkikallio, T.H.; Ronkainen, K.; Kurl, S. Low β-carotene concentrations increase the risk of cardiovascular disease mortality among Finnish men with risk factors. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Laukkanen, J.A.; Mäkikallio, T.H.; Ronkainen, K.; Kurl, S. Serum β-carotene and the risk of sudden cardiac death in men: A population-based follow-up study. Atherosclerosis 2013, 226, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Street, D.A.; Comstock, G.W.; Salkeld, R.M.; Schüep, W.; Klag, M.J. Serum antioxidants and myocardial infarction. Are low levels of carotenoids and alpha-tocopherol risk factors for myocardial infarction? Circulation 1994, 90, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Dedoussis, G.V.; Schmidt, H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis 2006, 187, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.Y.; Li, Y.L.; Jiang, C.H.; Liu, Z.Q.; Qu, S.L.; Huang, Y.M. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition 2008, 24, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Sultan Alvi, S.; Ansari, I.A.; Khan, I.; Iqbal, J.; Khan, M.S. Potential role of lycopene in targeting proprotein convertase subtilisin/kexin type-9 to combat hypercholesterolemia. Free Radic. Biol. Med. 2017, 108, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Mills, L.M.; Moir, S.; Masson, L.F. Cardiovascular benefits of lycopene: Fantasy or reality? Proc. Nutr. Soc. 2016, 76, 122–129. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Slivnick, J.; Lampert, B.C. Hypertension and Heart Failure. Heart Fail. Clin. 2019, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Serum lycopene concentrations and carotid atherosclerosis: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2003, 77, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Klipstein-Grobusch, K.; Launer, L.J.; Geleijnse, J.M.; Boeing, H.; Hofman, A.; Witteman, J.C. Serum carotenoids and atherosclerosis. The Rotterdam Study. Atherosclerosis 2000, 148, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Lakka, T.A.; Sivenius, J.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Low serum lycopene concentration is associated with an excess incidence of acute coronary events and stroke: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2001, 85, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2004, 79, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Park, H.W.; Lee, J.H.; Jang, Y.; Lee, J.H. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 2011, 215, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Han, G.M.; Meza, J.L.; Soliman, G.A.; Islam, K.M.; Watanabe-Galloway, S. Higher levels of serum lycopene are associated with reduced mortality in individuals with metabolic syndrome. Nutr. Res. 2016, 36, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Savino, K.; Alunni, G.; Freddio, M.; Senin, U.; Sies, H.; Stahl, W.; Mecocci, P. Plasma lipophilic antioxidants and malondialdehyde in congestive heart failure patients: Relationship to disease severity. Free Radic. Biol. Med. 2002, 32, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Liu, S.; Gaziano, J.M.; Buring, J.E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr. 2003, 133, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2005, 81, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kurata, M.; Suzuki, K.; Hamajima, N.; Hishida, H.; Aoki, K. Cardiovascular disease mortality and serum carotenoid levels: A Japanese population-based follow-up study. J. Epidemiol. 2006, 16, 154–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of Dietary and Supplemental Lycopene on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2020, 11, 1453–1488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed]
- Meili-Butz, S.; Niermann, T.; Fasler-Kan, E.; Barbosa, V.; Butz, N.; John, D.; Brink, M.; Buser, P.T.; Zaugg, C.E. Dimethyl fumarate, a small molecule drug for psoriasis, inhibits Nuclear Factor-kappaB and reduces myocardial infarct size in rats. Eur. J. Pharmacol. 2008, 586, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, Y.; Xiao, Z.; Xu, L.; Wang, P.; Ma, Q. Protective effect of dimethyl fumarate on oxidative damage and signaling in cardiomyocytes. Mol. Med. Rep. 2020, 22, 2783–2790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, M.; Sun, Q.; Zhao, H.; Tao, J.; Yan, D. The Effects of Dimethyl Fumarate on Atherosclerosis in the Apolipoprotein E-Deficient Mouse Model with Streptozotocin-Induced Hyperglycemia Mediated by the Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element (Nrf2/ARE) Signaling Pathway. Med. Sci. Monit. 2019, 25, 7966–7975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, A.; Rizky, L.; Stefanovic, N.; Tate, M.; Ritchie, R.H.; Ward, K.W.; de Haan, J.B. The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activator dh404 protects against diabetes-induced endothelial dysfunction. Cardiovasc. Diabetol. 2017, 16, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okafor, O.N.; Farrington, K.; Gorog, D.A. Allopurinol as a therapeutic option in cardiovascular disease. Pharmacol. Ther. 2017, 172, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Hans, N.; Messerli, F.H. Effect of allopurinol on blood pressure: A systematic review and meta-analysis. J. Clin. Hypertens. 2013, 15, 435–442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landmesser, U.; Drexler, H. Allopurinol and endothelial function in heart failure: Future or fantasy? Circulation 2002, 106, 173–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rashid, M.A.; William-Olsson, G. Influence of allopurinol on cardiac complications in open heart operations. Ann. Thorac. Surg. 1991, 52, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Osanai, T.; Kamada, T.; Hanada, H.; Ishizaka, H.; Onodera, H.; Iwasa, A.; Fujita, N.; Kudo, S.; Ohkubo, T.; et al. Effect of allopurinol pretreatment on free radical generation after primary coronary angioplasty for acute myocardial infarction. J. Cardiovasc. Pharmacol. 2003, 41, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, A.; Eftekhari, H.; Casscells, S.W.; Madjid, M. The open-artery hypothesis revisited. Tex. Heart Inst. J. 2006, 33, 345–352. [Google Scholar] [PubMed] [PubMed Central]
- Baldus, S.; Müllerleile, K.; Chumley, P.; Steven, D.; Rudolph, V.; Lund, G.K.; Staude, H.J.; Stork, A.; Köster, R.; Kähler, J.; et al. Inhibition of xanthine oxidase improves myocardial contractility in patients with ischemic cardiomyopathy. Free Radic. Biol. Med. 2006, 41, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altenhöfer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid. Redox Signal 2015, 23, 406–427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Yang, G.; Zhang, X.; Wang, P.; Weng, X.; Yang, Y.; Li, Z.; Fang, M.; Xu, Y.; Sun, A.; et al. Megakaryocytic Leukemia 1 Bridges Epigenetic Activation of NADPH Oxidase in Macrophages to Cardiac Ischemia-Reperfusion Injury. Circulation 2018, 138, 2820–2836. [Google Scholar] [CrossRef] [PubMed]
- Roth Flach, R.J.; Su, C.; Bollinger, E.; Cortes, C.; Robertson, A.W.; Opsahl, A.C.; Coskran, T.M.; Maresca, K.P.; Keliher, E.J.; Yates, P.D.; et al. Myeloperoxidase inhibition in mice alters atherosclerotic lesion composition. PLoS ONE 2019, 14, e0214150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dao, V.T.; Casas, A.I.; Maghzal, G.J.; Seredenina, T.; Kaludercic, N.; Robledinos-Anton, N.; Di Lisa, F.; Stocker, R.; Ghezzi, P.; Jaquet, V.; et al. Pharmacology and Clinical Drug Candidates in Redox Medicine. Antioxid. Redox Signal 2015, 23, 1113–1129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tita, C.; Gilbert, E.M.; Van Bakel, A.B.; Grzybowski, J.; Haas, G.J.; Jarrah, M.; Dunlap, S.H.; Gottlieb, S.S.; Klapholz, M.; Patel, P.C.; et al. A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1321–1332. [Google Scholar] [CrossRef]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Broderick, J.A.; Zamore, P.D. MicroRNA therapeutics. Gene Ther. 2011, 18, 1104–1110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012, 4, 143–159, Erratum in EMBO Mol. Med. 2017, 9, 852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumann, V.; Winkler, J. miRNA-based therapies: Strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med. Chem. 2014, 6, 1967–1984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guan, Y.; Song, X.; Sun, W.; Wang, Y.; Liu, B. Effect of Hypoxia-Induced MicroRNA-210 Expression on Cardiovascular Disease and the Underlying Mechanism. Oxid. Med. Cell Longev. 2019, 2019, 4727283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Jia, Q.; Xinnong, C.; Xie, Y.; Yang, Y.; Zhang, A.; Liu, R.; Zhuo, Y.; Zhang, J. Role of cardiac progenitor cell-derived exosome-mediated microRNA-210 in cardiovascular disease. J. Cell Mol. Med. 2019, 23, 7124–7131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, S.; Huang, M.; Li, Z.; Jia, F.; Ghosh, Z.; Lijkwan, M.A.; Fasanaro, P.; Sun, N.; Wang, X.; Martelli, F.; et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010, 122 (Suppl. 11), S124–S131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karakas, M.; Schulte, C.; Appelbaum, S.; Ojeda, F.; Lackner, K.J.; Münzel, T.; Schnabel, R.B.; Blankenberg, S.; Zeller, T. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur. Heart J. 2017, 38, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Zeller, T. microRNA-based diagnostics and therapy in cardiovascular disease-Summing up the facts. Cardiovasc. Diagn. Ther. 2015, 5, 17–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, B.; Pan, Z.; Lu, Y. The roles of microRNAs in heart diseases: A novel important regulator. Curr. Med. Chem. 2010, 17, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, P.; Di Russo, C.; Rigattieri, S.; Fedele, S.; Todaro, D.; Ferraiuolo, G.; Altamura, G.; Loschiavo, P. MicroRNAs and ischemic heart disease: Towards a better comprehension of pathogenesis, new diagnostic tools and new therapeutic targets. Recent. Pat. Cardiovasc. Drug Discov. 2009, 4, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tan, N.; Yang, J.; Liu, X.; Cao, X.; He, P.; Dong, X.; Qin, S.; Zhang, C. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin. Sci. 2010, 119, 87–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.Q.; Zhang, M.F.; Wen, H.Y.; Hu, C.L.; Liu, R.; Wei, H.Y.; Ai, C.M.; Wang, G.; Liao, X.X.; Li, X. Comparing the diagnostic values of circulating microRNAs and cardiac troponin T in patients with acute myocardial infarction. Clinics 2013, 68, 75–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, L.; Xiong, X.; Liu, Y.; Wang, J. miRNA-1: Functional roles and dysregulation in heart disease. Mol. BioSyst. 2014, 10, 2775–2782. [Google Scholar] [CrossRef]
- Polyakova, E.A.; Zaraiskii, M.I.; Mikhaylov, E.N.; Baranova, E.I.; Galagudza, M.M.; Shlyakhto, E.V. Association of myocardial and serum miRNA expression patterns with the presence and extent of coronary artery disease: A cross-sectional study. Int. J. Cardiol. 2021, 322, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Dudek, K.; Mayr, M. Oxidative stress in atherosclerosis: The role of microRNAs in arterial remodeling. Free Radic. Biol. Med. 2013, 64, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.-L.; Guo, T.; Sun, X.-Y.; Ma, H.; Zhu, M.-L.; Zhao, F.-R.; Xu, P.; Chen, Y.; Wan, G.-R.; et al. Inhibition of Aberrant MicroRNA-133a Expression in Endothelial Cells by Statin Prevents Endothelial Dysfunction by Targeting GTP Cyclohydrolase 1 in Vivo. Circulation 2016, 134, 1752–1765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.Y.; Chiu, J.J. Atherosclerosis and flow: Roles of epigenetic modulation in vascular endothelium. J. Biomed. Sci. 2019, 26, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loyer, X.; Potteaux, S.; Vion, A.C.; Guérin, C.L.; Boulkroun, S.; Rautou, P.E.; Ramkhelawon, B.; Esposito, B.; Dalloz, M.; Paul, J.L.; et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ. Res. 2014, 114, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Q.; Liu, J.; Wang, Y.; Zheng, G.; Lin, L.; Yu, H.; Tang, W.; Huang, Z. Vascular endothelial growth factor A polymorphisms are associated with increased risk of coronary heart disease: A meta-analysis. Oncotarget 2017, 8, 30539–30551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Z.; Huang, W.; Feng, Y.; Cai, W.; Wang, Y.; Wang, X.; Liang, J.; Wani, M.; Chen, J.; Zhu, P.; et al. MicroRNA-377 Regulates Mesenchymal Stem Cell-Induced Angiogenesis in Ischemic Hearts by Targeting VEGF. PLoS ONE 2014, 9, e104666. [Google Scholar] [CrossRef]
- Kim, K.S.; Song, C.G.; Kang, P.M. Targeting Oxidative Stress Using Nanoparticles as a Theranostic Strategy for Cardiovascular Diseases. Antioxid. Redox Signal 2019, 30, 733–746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.; Bae, S.; Hong, D.; Lim, H.; Yoon, J.H.; Hwang, O.; Park, S.; Ke, Q.; Khang, G.; Kang, P.M. H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Sci. Rep. 2013, 3, 2233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, C.; Gwon, S.; Song, C.; Kang, P.M.; Park, S.C.; Jeon, J.; Hwang, D.W.; Lee, D. Fibrin-Targeted and H2O2-Responsive Nanoparticles as a Theranostics for Thrombosed Vessels. ACS Nano 2017, 11, 6194–6203. [Google Scholar] [CrossRef] [PubMed]
- Somasuntharam, I.; Boopathy, A.V.; Khan, R.S.; Martinez, M.D.; Brown, M.E.; Murthy, N.; Davis, M.E. Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction. Biomaterials 2013, 34, 7790–7798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seshadri, G.; Sy, J.C.; Brown, M.; Dikalov, S.; Yang, S.C.; Murthy, N.; Davis, M.E. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials 2010, 31, 1372–1379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gray, W.D.; Che, P.; Brown, M.; Ning, X.; Murthy, N.; Davis, M.E. N-acetylglucosamine conjugated to nanoparticles enhances myocyte uptake and improves delivery of a small molecule p38 inhibitor for post-infarct healing. J. Cardiovasc. Transl. Res. 2011, 4, 631–643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soumya, R.S.; Vineetha, V.P.; Raj, P.S.; Raghu, K.G. Beneficial properties of selenium incorporated guar gum nanoparticles against ischemia/reperfusion in cardiomyoblasts (H9c2). Met. Integr. Biometal Sci. 2014, 6, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhao, W.; Dou, Y.; An, H.; Tao, H.; Xu, X.; Jia, Y.; Lu, S.; Zhang, J.; et al. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef] [PubMed]
- Abrescia, P.; Golino, P. Free radicals and antioxidants in cardiovascular diseases. Expert. Rev. Cardiovasc. Ther. 2005, 3, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.T.; Shekhar, H.U. Antioxidant therapy in cardiovascular diseases: Still a matter of debate. Adv. Cytol. Pathol. 2017, 2, 87–88. [Google Scholar] [CrossRef][Green Version]

| Carotenoids | Function | Role in Preventing CVD |
|---|---|---|
| β-Carotene | Inhibits LDL oxidation | Prevents atherosclerosis |
| Decreases TNF-α-induced inflammation in endothelial cells | Reduces risk of CVD by opposing inflammatory oxidative stress | |
| Lycopene | Decreases TNF-α-induced inflammation in endothelial cells | Reduces risk of CVD by opposing inflammatory oxidative stress |
| Inhibits IL-1 secretion | Exerts an anti-atherogenic effect | |
| Increases NO levels | Dilates blood vessels, slowing the progression of atherosclerosis | |
| Regulates PCSK9 and HMGR genes, and increases LDL-R activity | Lowers hypercholesterolemia | |
| Improves the LDL/HDL ratio | Reduces the risk of atherosclerosis and postpones its progression | |
| Reduces accumulation of cholesterol in the aorta | ||
| Inhibits vascular smooth-muscle cell proliferation and foam cell formation |
| Type of miRNA | Role in Onset of CVD |
|---|---|
| miRNA-210 | During hypoxia, miRNA-210 promotes angiogenesis and inhibits cardiomyocyte apoptosis. |
| miRNA-1 | Involved in the differentiation and proliferation of muscle cells. In anemic myocardium, it regulates cardiomyocyte growth and proapoptotic factors. Overexpression increases ROS production. |
| miRNA-133 | Inhibition results in NOS production and may prevent endothelial dysfunction. |
| miRNA-92a | Regulates NOS expression, reduces plaque inflammation, and increases its stability by promoting cell proliferation and angiogenesis. |
| miRNA-206 | Regulates VEGF expression, inhibits viability, and increases apoptosis of endothelial progenitor cells. |
| miRNA-377 | Inhibition of miRNA-377 reduces myocardial fibrosis and improves its function. |
| Type of Nanoparticles | Function of Nanoparticles |
|---|---|
| H2O2-responsive nanoparticles | Anti-inflammatory and anti-apoptotic effects and reductions in further organ damage. |
| Nanoparticles with antioxidant properties | Enables imaging the thrombus and inhibits its formation by scavenging H2O2 and reduces oxidative stress. |
| Nanoparticles carrying SOD | Reduces myocyte apoptosis and improves myocardial function. |
| Nanoparticles carrying N-acetylcysteine | Attenuates myocardial fibrosis. |
| Selenium-based nanoparticles | Reduces ROS production in ischemic cardiomyocytes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases—A Literature Review. Nutrients 2024, 16, 2587. https://doi.org/10.3390/nu16162587
Młynarska E, Hajdys J, Czarnik W, Fularski P, Leszto K, Majchrowicz G, Lisińska W, Rysz J, Franczyk B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases—A Literature Review. Nutrients. 2024; 16(16):2587. https://doi.org/10.3390/nu16162587
Chicago/Turabian StyleMłynarska, Ewelina, Joanna Hajdys, Witold Czarnik, Piotr Fularski, Klaudia Leszto, Gabriela Majchrowicz, Wiktoria Lisińska, Jacek Rysz, and Beata Franczyk. 2024. "The Role of Antioxidants in the Therapy of Cardiovascular Diseases—A Literature Review" Nutrients 16, no. 16: 2587. https://doi.org/10.3390/nu16162587
APA StyleMłynarska, E., Hajdys, J., Czarnik, W., Fularski, P., Leszto, K., Majchrowicz, G., Lisińska, W., Rysz, J., & Franczyk, B. (2024). The Role of Antioxidants in the Therapy of Cardiovascular Diseases—A Literature Review. Nutrients, 16(16), 2587. https://doi.org/10.3390/nu16162587








