Differences in Vitamin A Levels and Their Association with the Atherogenic Index of Plasma and Subclinical Hypothyroidism in Adults: A Cross-Sectional Analysis in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements and Classifications
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Differences in Vitamin A Levels and Their Association with AIP and Thyroid Hormones and Diseases
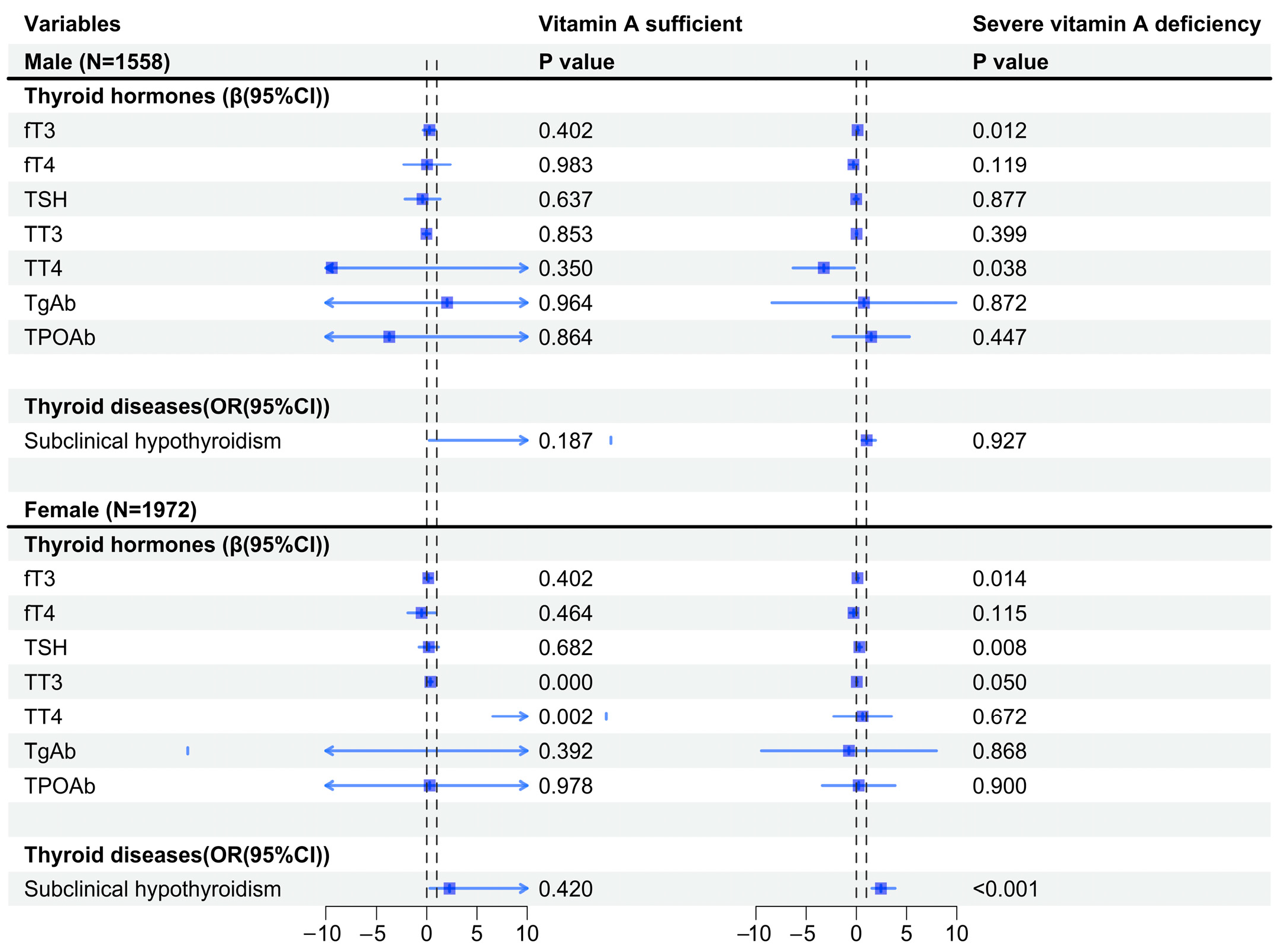
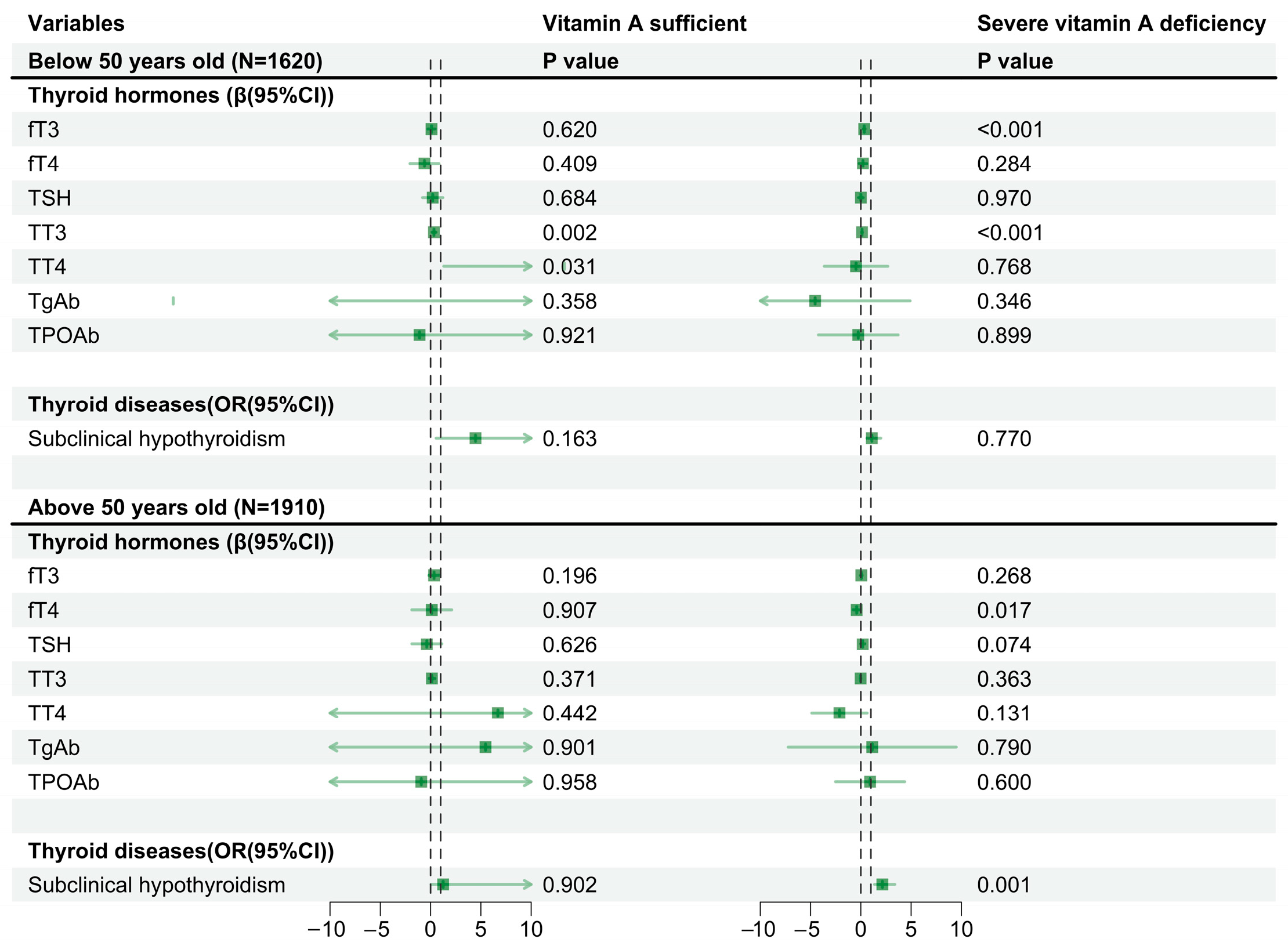
3.3. Differences in Vitamin A Levels and Their Association with AIP and Thyroid Hormones and Diseases, Stratified by Sex and Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Peeters, R.P. Subclinical Hypothyroidism. N. Engl. J. Med. 2017, 376, 2556–2565. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.; da Costa, B.R.; Collet, T.-H.; Feller, M.; Floriani, C.; Bauer, D.C.; Cappola, A.R.; Heckbert, S.R.; Ceresini, G.; Gussekloo, J.; et al. Thyroid Function Within the Normal Range, Subclinical Hypothyroidism, and the Risk of Atrial Fibrillation. Circulation 2017, 136, 2100–2116. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat. Rev. Dis. Prim. 2022, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Razvi, S.; Weaver, J.U.; Vanderpump, M.P.; Pearce, S.H.S. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: Reanalysis of the Whickham Survey cohort. J. Clin. Endocrinol. Metab. 2010, 95, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Fried, L.P.; Arnold, A.M.; Danese, M.D.; Kuller, L.H.; Burke, G.L.; Tracy, R.P.; Ladenson, P.W. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 2006, 295, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.R.; Dabaghi, G.G.; Darouei, B.; Amani-Beni, R. The association of atherogenic index of plasma with cardiovascular outcomes in patients with coronary artery disease: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2024, 23, 119. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, M.; Huang, Q.; Feng, J.; Liu, Z.; Xia, J. The atherogenic index of plasma and carotid atherosclerosis in a community population: A population-based cohort study in China. Cardiovasc. Diabetol. 2023, 22, 125. [Google Scholar] [CrossRef]
- Inoue, K.; Ritz, B.; Brent, G.A.; Ebrahimi, R.; Rhee, C.M.; Leung, A.M. Association of Subclinical Hypothyroidism and Cardiovascular Disease With Mortality. JAMA Netw. Open 2020, 3, e1920745. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Dehghan, P.; Tajmiri, S. Powdered black cumin seeds strongly improves serum lipids, atherogenic index of plasma and modulates anthropometric features in patients with Hashimoto’s thyroiditis. Lipids Health Dis. 2018, 17, 59. [Google Scholar] [CrossRef]
- Ma, B.; Yang, P.; Gao, J.; Du, L.; Sheng, C.; Usman, T.; Wang, X.; Qu, S. Relationship of Vitamin A and Thyroid Function in Individuals With Obesity and After Laparoscopic Sleeve Gastrectomy. Front. Nutr. 2022, 9, 824193. [Google Scholar] [CrossRef] [PubMed]
- Homma, H.; Watanabe, M.; Inoue, N.; Isono, M.; Hidaka, Y.; Iwatani, Y. Polymorphisms in Vitamin A-Related Genes and Their Functions in Autoimmune Thyroid Disease. Thyroid 2021, 31, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Maurer, M.S. Cardiac Amyloidosis Due to Transthyretin Protein: A Review. JAMA 2024, 331, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Rogstad, T.W.; Sonne, C.; Villanger, G.D.; Ahlstøm; Fuglei, E.; Muir, D.C.; Jørgensen, E.; Jenssen, B.M. Concentrations of vitamin A, E, thyroid and testosterone hormones in blood plasma and tissues from emaciated adult male Arctic foxes (Vulpes lagopus) dietary exposed to persistent organic pollutants (POPs). Environ. Res. 2017, 154, 284–290. [Google Scholar] [CrossRef]
- Sajovic, J.; Meglič, A.; Glavač, D.; Markelj; Hawlina, M.; Fakin, A. The Role of Vitamin A in Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 1014. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Wallscheid, N.; Buettner, F.; Sommerkamp, P.; Klimmeck, D.; Ladel, L.; Thalheimer, F.B.; Pastor-Flores, D.; Roma, L.P.; Renders, S.; Zeisberger, P.; et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 2017, 169, 807–823.e819. [Google Scholar] [CrossRef]
- Wołoszynowska-Fraser, M.U.; Kouchmeshky, A.; McCaffery, P. Vitamin A and Retinoic Acid in Cognition and Cognitive Disease. Annu. Rev. Nutr. 2020, 40, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Hoydal, K.S.; Ciesielski, T.M.; Borrell, A.; Wasik, A.; Letcher, R.J.; Dam, M.; Jenssen, B.M. Relationships between concentrations of selected organohalogen contaminants and thyroid hormones and vitamins A, E and D in Faroese pilot whales. Environ. Res. 2016, 148, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.R.; Zaki, R.; Hassan, R.; El-Kersh, M.A.; El-Sayed, M.M.; Elmoneam, A.A.A. The impact of vitamin A supplementation on thyroid function and insulin sensitivity: Implication of deiodinases and phosphoenolpyruvate carboxykinase in male Wistar rats. Eur. J. Nutr. 2022, 61, 4091–4105. [Google Scholar] [CrossRef]
- Zheng, C.; Yin, Z.; Zhan, B.; Xu, W.; Ma, Z.F. Pregnant women at risk for iodine deficiency but adequate iodine intake in school-aged children of Zhejiang Province, China. Environ. Geochem. Health 2024, 46, 204. [Google Scholar] [CrossRef]
- Shan, X.Y.; Yan ZO, U.; Huang, L.C.; Jiang, S.; Zhou, W.W.; Qin, Q.L.; Liu, C.Q.; Luo, X.Y.; Lu, J.X.; Mao, D.Q.; et al. Iodine Nutrition, Thyroid-stimulating Hormone, and Related Factors of Postpartum Women from three Different Areas in China: A Cross-sectional Survey. Biomed. Environ. Sci. 2024, 37, 254–265. [Google Scholar] [CrossRef]
- Qin, Z.; Zhou, K.; Li, Y.; Cheng, W.; Wang, Z.; Wang, J.; Gao, F.; Yang, L.; Xu, Y.; Wu, Y.; et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: Results from an observational cohort study in China. Cardiovasc. Diabetol. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Shane, E. Hypercalcemia: A Review. JAMA 2022, 328, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, L.; Zhu, X.; Xu, W.; Zou, Y.; Qi, X.; Fang, J.; Wang, X.; Shi, X.; Sheng, Y.; et al. TT3, a More Practical Indicator for Evaluating the Relationship Between Sarcopenia and Thyroid Hormone in the Euthyroid Elderly Compared with FT3. Clin. Interv. Aging 2023, 18, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Interactions of vitamin A and iodine deficiencies: Effects on the pituitary-thyroid axis. Int. J. Vitam. Nutr. Res. 2007, 77, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Brossaud, J.; Pallet, V.; Corcuff, J.-B. Vitamin A, endocrine tissues and hormones: Interplay and interactions. Endocr. Connect. 2017, 6, R121–R130. [Google Scholar] [CrossRef]
- Chaker, L.; Ligthart, S.; Korevaar, T.I.M.; Hofman, A.; Franco, O.H.; Peeters, R.P.; Dehghan, A. Thyroid function and risk of type 2 diabetes: A population-based prospective cohort study. BMC Med. 2016, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, K.B.; Bhatt, P.A.; Shah, J.S. Association between altered thyroid state and insulin resistance. J. Pharmacol. Pharmacother. 2012, 3, 156–160. [Google Scholar] [CrossRef]
- O’kane, S.M.; Mulhern, M.S.; Pourshahidi, L.K.; Strain, J.J.; Yeates, A.J. Micronutrients, iodine status and concentrations of thyroid hormones: A systematic review. Nutr. Rev. 2018, 76, 418–431. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Keshavarz, S.A.; Eshraghian, M.; Ostadrahimi, A.; Saboor-Yaraghi, A.A. The effect of vitamin A supplementation on thyroid function in premenopausal women. J. Am. Coll. Nutr. 2012, 31, 268–274. [Google Scholar] [CrossRef]
- Capriello, S.; Stramazzo, I.; Bagaglini, M.F.; Brusca, N.; Virili, C.; Centanni, M. The relationship between thyroid disorders and vitamin A.: A narrative minireview. Front. Endocrinol. 2022, 13, 968215. [Google Scholar] [CrossRef]
- Rabbani, E.; Golgiri, F.; Janani, L.; Moradi, N.; Fallah, S.; Abiri, B.; Vafa, M. Randomized Study of the Effects of Zinc, Vitamin A, and Magnesium Co-supplementation on Thyroid Function, Oxidative Stress, and hs-CRP in Patients with Hypothyroidism. Biol. Trace Element Res. 2021, 199, 4074–4083. [Google Scholar] [CrossRef]
- Balamurugan, V.; Maradi, R.; Joshi, V.; Shenoy, B.; Goud, M. Dyslipidaemia and inflammatory markers as the risk predictors for cardiovascular disease in newly diagnosed premenopausal hypothyroid women. J. Med. Biochem. 2023, 42, 58–66. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Ghareeb, D.A.; Talat, H.A.; Sarhan, E.M. High fat diet induced insulin resistance and elevated retinol binding protein 4 in female rats; treatment and protection with Berberis vulgaris extract and vitamin A. Pak J. Pharm. Sci. 2013, 26, 1189–1195. [Google Scholar]
- Lyu, Y.; Xiu, Q.; Zuo, H.; Xu, G.; Cui, X.; Sun, Z.; Mi, R.; Wu, L. Effect of vitamin A on the relationship between maternal thyroid hormones in early pregnancy and fetal growth: A prospective cohort study. Front. Nutr. 2022, 9, 980853. [Google Scholar] [CrossRef]
- Elfimova, A.; Tipisova, E.; Bichkaeva, F.; Molodovskaya, I.; Vlasova, O.; Gretskaya, T. Relationship of vitamin A and thyroid function in Arctic residents. Probl. Nutr. 2023, 92, 66–73. [Google Scholar] [CrossRef]
- Sapiejka, E.; Krzyżanowska, P.; Walkowiak, D.; Wenska-Chyży, E.; Szczepanik, M.; Cofta, S.; Pogorzelski, A.; Skorupa, W.; Walkowiak, J. Vitamin A status and its determinants in patients with cystic fibrosis. Acta Sci. Pol. Technol. Aliment. 2017, 16, 345–354. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Lee, C.; Stoeckel, L.E.; Menke, A.; Cowie, C.C. Cognitive function among older adults with diabetes and prediabetes, NHANES 2011–2014. Diabetes Res. Clin. Pr. 2021, 178, 108939. [Google Scholar] [CrossRef]
- AL Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Déjardin, F.; Sparwasser, T.; Bérard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288.e1275. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Hu, Z.; Li, Y.; Gattu, S.; Ruhn, K.A.; Raj, P.; Herz, J.; Hooper, L.V. Serum amyloid A delivers retinol to intestinal myeloid cells to promote adaptive immunity. Science 2021, 373, eabf9232. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Sidhaye, A.R.; Wondisford, F.E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 2014, 10, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Jooste, P.L.; Mabapa, N.S.; Schoeman, S.; Biebinger, R.; Mushaphi, L.F.; Mbhenyane, X. Vitamin A supplementation in iodine-deficient African children decreases thyrotropin stimulation of the thyroid and reduces the goiter rate. Am. J. Clin. Nutr. 2007, 86, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Cakmak, M.; Darcin, T.; Inan, O.; Gurel, O.M.; Bilgic, M.A.; Bavbek, N.; Akcay, A. Subclinical hypothyroidism in combination with vitamin D deficiency increases the risk of impaired left ventricular diastolic function. Endocr. Regul. 2015, 49, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.; Taha, S.; Mahfouz, K.; Ibrahim, M.M.; Aoun, A.H. Vitamin B12 (Cobalamin) Deficiency in Overt and Subclinical Primary Hypothyroidism. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 11795514221086634. [Google Scholar] [CrossRef] [PubMed]
- El-Eshmawy, M.M.; Arafa, M.M.; Elzehery, R.R.; Elhelaly, R.M.; Elrakhawy, M.M.; El-Baiomy, A.A. Relationship between vitamin A deficiency and the thyroid axis in clinically stable patients with liver cirrhosis related to hepatitis C virus. Appl. Physiol. Nutr. Metab. 2016, 41, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Domínguez, G.; Menon, P.; E Black, R. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021, 397, 1388–1399. [Google Scholar] [CrossRef]
| Variables | Vitamin A-Sufficient | Severe Vitamin A Deficiency | p-Value |
|---|---|---|---|
| Sex (N (%)) | |||
| Male | 1515 (46.06) | 43 (17.84) | <0.001 |
| Female | 1774 (53.94) | 198 (82.16) | |
| Age group (N (%)) | |||
| Below 50 years old | 1445 (43.93) | 175 (72.61) | <0.001 |
| Above 50 years old | 1844 (56.07) | 66 (27.39) | |
| Height (cm, mean ± SD) | 162.96 ± 8.6 | 160.58 ± 6.61 | <0.001 |
| Weight (kg, mean ± SD) | 63.11 ± 11.29 | 56.31 ± 8.75 | <0.001 |
| BMI (kg/m2, mean ± SD) | 23.71 ± 3.55 | 21.85 ± 3.26 | <0.001 |
| AIP | 0.03 ± 0.33 | −0.24 ± 0.24 | <0.001 |
| Thyroid hormones (median, IQR) | |||
| fT3 | 4.82 (4.46, 5.24) | 4.54 (4.25, 5.02) | <0.001 |
| fT4 | 17.2 (15.8, 18.71) | 16.58 (15.49, 17.93) | <0.001 |
| TSH | 2.03 (1.43, 2.93) | 2.17 (1.45, 3.25) | 0.150 |
| TT3 | 1.71 (1.53, 1.9) | 1.64 (1.46, 1.86) | 0.009 |
| TT4 | 109.2 (97.62, 121.3) | 109.41 (98.72, 121) | 0.160 |
| TgAb | 14.95 (12.86, 17.53) | 15.63 (12.95, 19.77) | 0.080 |
| TPOAb | 8.67 (6.63, 11.53) | 8.25 (6.47, 11.09) | 0.172 |
| Subclinical hypothyroidism (N (%)) | |||
| No | 2967 (90.21) | 213 (88.38) | 0.554 |
| Yes | 322 (9.79) | 28 (11.62) | |
| Smoking status (N (%)) | |||
| No | 2514 (76.44) | 218 (90.46) | <0.001 |
| Yes | 775 (23.56) | 23 (9.54) | |
| Vitamin D levels (N (%)) | |||
| Sufficient | 741 (22.56) | 23 (9.54) | <0.001 |
| Deficiency | 2543 (77.44) | 218 (90.46) | |
| Urine iodine levels (N (%)) | |||
| Sufficient | 1895 (67.20) | 141 (70.50) | 0.415 |
| Deficiency | 925 (32.80) | 59 (29.50) |
| Variables | Vitamin A Sufficient | Severe Vitamin A Deficiency | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| β (95%CI) | p-Value | β (95%CI) | p-Value | β (95%CI) | p-Value | β (95%CI) | p-Value | |
| Thyroid hormones | ||||||||
| fT3 | 0.18 (−0.11, 0.47) | 0.217 | 0.18 (−0.13, 0.48) | 0.257 | 0.21 (0.14, 0.27) | 0.000 | 0.12 (0.05, 0.18) | 0.001 |
| fT4 | −0.57 (−1.73, 0.58) | 0.332 | −0.38 (−1.56, 0.81) | 0.536 | −0.18 (−0.43, 0.07) | 0.161 | −0.29 (−0.54, −0.03) | 0.029 |
| TSH | 0.13 (−0.67, 0.93) | 0.753 | 0.06 (−0.80, 0.91) | 0.894 | 0.10 (−0.05, 0.25) | 0.175 | 0.14 (−0.01, 0.30) | 0.074 |
| TT3 | 0.27 (0.11, 0.43) | 0.001 | 0.26 (0.09, 0.43) | 0.003 | 0.07 (0.03, 0.10) | 0.000 | 0.03 (0.00, 0.07) | 0.048 |
| TT4 | 10.09 (0.52,19.66) | 0.040 | 11.29 (1.24, 21.34) | 0.029 | −1.59 (−3.59, 0.41) | 0.120 | −1.19 (−3.32, 0.94) | 0.274 |
| TgAb | −19.80 (−62.92, 23.32) | 0.369 | −16.80 (−63.45, 29.85) | 0.481 | −1.16 (−7.20, 4.88) | 0.706 | −0.03 (−6.43, 6.37) | 0.993 |
| TPOAb | 0.96 (−16.53, 18.45) | 0.914 | −0.46 (−19.24, 18.33) | 0.962 | −0.34 (−2.85, 2.17) | 0.790 | 0.82 (−1.84, 3.48) | 0.544 |
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Thyroid diseases | ||||||||
| Subclinical hypothyroidism | 3.15 (0.51, 19.32) | 0.216 | 6.07 (0.81, 45.74) | 0.080 | 4.13 (0.58, 29.25) | 0.155 | 1.66 (1.07, 2.58) | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, G.; Chen, M.; Huang, L.; Mo, Z.; Su, D.; Gu, S.; Guo, F.; Wang, Y.; Chen, Z.; Zhang, R.; et al. Differences in Vitamin A Levels and Their Association with the Atherogenic Index of Plasma and Subclinical Hypothyroidism in Adults: A Cross-Sectional Analysis in China. Nutrients 2024, 16, 2613. https://doi.org/10.3390/nu16162613
Mao G, Chen M, Huang L, Mo Z, Su D, Gu S, Guo F, Wang Y, Chen Z, Zhang R, et al. Differences in Vitamin A Levels and Their Association with the Atherogenic Index of Plasma and Subclinical Hypothyroidism in Adults: A Cross-Sectional Analysis in China. Nutrients. 2024; 16(16):2613. https://doi.org/10.3390/nu16162613
Chicago/Turabian StyleMao, Guangming, Manman Chen, Lichun Huang, Zhe Mo, Danting Su, Simeng Gu, Fanjia Guo, Yuanyang Wang, Zhijian Chen, Ronghua Zhang, and et al. 2024. "Differences in Vitamin A Levels and Their Association with the Atherogenic Index of Plasma and Subclinical Hypothyroidism in Adults: A Cross-Sectional Analysis in China" Nutrients 16, no. 16: 2613. https://doi.org/10.3390/nu16162613
APA StyleMao, G., Chen, M., Huang, L., Mo, Z., Su, D., Gu, S., Guo, F., Wang, Y., Chen, Z., Zhang, R., Lou, X., Wang, X., Hu, J., Gu, F., & Dong, B. (2024). Differences in Vitamin A Levels and Their Association with the Atherogenic Index of Plasma and Subclinical Hypothyroidism in Adults: A Cross-Sectional Analysis in China. Nutrients, 16(16), 2613. https://doi.org/10.3390/nu16162613






