Adherence to a Healthy Diet and Risk of Multiple Carotid Atherosclerosis Subtypes: Insights from the China MJ Health Check-Up Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Dietary Intakes
2.3. Assessment of Dietary Patterns
3. Covariates
4. Ascertainment of CAS
5. Statistical Analysis
6. Results
6.1. Population Characteristics
6.2. Association between Dietary Patterns and CAS
6.3. Comparison of Dietary Scores and Mediation Analysis
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAS | Carotid atherosclerosis |
| cIMT | Increased carotid intima-media thickness |
| CP | Carotid plaque |
| CVD | Cardiovascular diseases |
| DDS | Dietary diversity score |
| DII | Dietary inflammation index |
| HCDP | High-carbohydrate dietary pattern |
| HFADP | High-fat dietary pattern |
| HFIDP | High-fiber dietary pattern |
| HPDP | High-protein dietary pattern |
| hPDI | Healthful plant-based diet index |
| PURE | Prospective Urban Rural Epidemiology healthy diet |
References
- Chen, W.; Gao, R.; Liu, L.; Zhu, M.; Wang, W.; Wang, Y.; Wu, Z.; Li, H.; Zheng, Z.; Jiang, L.; et al. Outline of the report on cardiovascular diseases in China, 2014. Eur. Heart J. Suppl. 2016, 18, F2–F11. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, X.; Wu, T.; Wang, R.; Wu, X.; Wang, Y.; Feng, Z. Carotid atherosclerosis biomarkers in cardiovascular diseases prevention: A systematic review and bibliometric analysis. Eur. J. Radiol. 2020, 129, 109133. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A. Asymptomatic carotid stenosis and stroke risk. Lancet Neurol. 2021, 20, 698–699. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Deng, Y.; Ma, Y.; Man, S.; Yang, X.; Yu, C.; Lv, J.; Wang, B.; Li, L. National and Provincial-Level Prevalence and Risk Factors of Carotid Atherosclerosis in Chinese Adults. JAMA Netw. Open 2024, 7, e2351225. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Karam, G.; Agarwal, A.; Sadeghirad, B.; Jalink, M.; Hitchcock, C.L.; Ge, L.; Kiflen, R.; Ahmed, W.; Zea, A.M.; Milenkovic, J.; et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: Systematic review and network meta-analysis. BMJ 2023, 380, e072003. [Google Scholar] [CrossRef]
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.A.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560–2579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Han, H.; Hu, Y.; Zhu, L.; Rimm, E.B.; Hu, F.B.; Sun, Q. Associations between plant-based dietary patterns and risks of type 2 diabetes, cardiovascular disease, cancer, and mortality—A systematic review and meta-analysis. Nutr. J. 2023, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Saz-Lara, A.; Battino, M.; Del Saz Lara, A.; Cavero-Redondo, I.; Davalos, A.; Lopez de Las Hazas, M.C.; Visioli, F.; Luceron-Lucas-Torres, M.; Giampieri, F. Differences in carotid to femoral pulse wave velocity and carotid intima media thickness between vegetarian and omnivorous diets in healthy subjects: A systematic review and meta-analysis. Food Funct. 2024, 15, 1135–1143. [Google Scholar] [CrossRef]
- Li, J.; Lee, D.H.; Hu, J.; Tabung, F.K.; Li, Y.; Bhupathiraju, S.N.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary Inflammatory Potential and Risk of Cardiovascular Disease Among Men and Women in the U.S. J. Am. Coll. Cardiol. 2020, 76, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Fanelli Kuczmarski, M.; Brewer, B.C.; Rawal, R.; Pohlig, R.T.; Zonderman, A.B.; Evans, M.K. Aspects of Dietary Diversity Differ in Their Association with Atherosclerotic Cardiovascular Risk in a Racially Diverse US Adult Population. Nutrients 2019, 11, 1034. [Google Scholar] [CrossRef] [PubMed]
- Cacau, L.T.; Levy, J.; Alves, M.A.; Santos, I.S.; Fonseca, M.J.M.; Lotufo, P.A.; Bensenor, I.M.; Marchioni, D.M. Association between dietary patterns and carotid intima-media thickness: Cross-sectional analysis of ELSA-Brasil study. Eur. J. Nutr. 2023, 62, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Li, Y.; Liu, M.; Gan, G.; Zhou, Y.; Luo, X.; Zhang, C.; Xie, J.; Duan, Y.; et al. The Impact of Dietary Diversity, Lifestyle, and Blood Lipids on Carotid Atherosclerosis: A Cross-Sectional Study. Nutrients 2022, 14, 815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Gu, Y.; Zhang, S.; Wang, Y.; Zhang, T.; et al. Relationship Between Dietary Patterns and Carotid Atherosclerosis Among People Aged 50 Years or Older: A Population-Based Study in China. Front. Nutr. 2021, 8, 723726. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wen, Q.; Lyu, J.; Sun, D.; Ma, Y.; Man, S.; Yin, J.; Jin, C.; Tong, M.; Wang, B.; et al. Dietary pattern derived by reduced-rank regression and cardiovascular disease: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Chu, C.H.; Wu, M.H.; Hsu, G.C.; Yang, T.; Chou, W.Y.; Huang, H.P.; Lee, M.S.; Yu, C.P.; Yu, J.C.; et al. Dietary intake of vitamin B(6) and risk of breast cancer in Taiwanese women. J. Epidemiol. 2011, 21, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.F.; Hsu, C.C.; Chiu, T.H.; Lee, C.Y.; Liu, T.T.; Tsao, C.K.; Chuang, S.C.; Hsiung, C.A. Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: A matched cohort study. Br. J. Nutr. 2015, 114, 1313–1320. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.R.; Li, Z.H.; Zhang, Y.J.; Lv, Y.B.; Wang, Z.H.; Shen, D.; Chen, P.L.; Zhong, W.F.; Huang, Q.M.; et al. Association of dietary diversity changes and mortality among older people: A prospective cohort study. Clin. Nutr. 2021, 40, 2620–2629. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, R.; Li, F.; Chen, L.; Wu, K.; Huang, J.; Liu, H.; Huang, Z.; Xu, L.; Yuan, Z.; et al. Association between dietary diversity and cognitive impairment among the oldest-old: Findings from a nationwide cohort study. Clin. Nutr. 2021, 40, 1452–1462. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhong, W.F.; Li, Z.H.; Chen, P.L.; Zhang, Y.J.; Ren, J.J.; Liu, D.; Shen, Q.Q.; Yang, P.; Song, W.Q.; et al. Dietary diversity and frailty among older Chinese people: Evidence from the Chinese Longitudinal Healthy Longevity Study. Am. J. Clin. Nutr. 2023, 117, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, J.; Xuan, J.; Zhu, A.; Ji, J.S.; Liu, X.; Cao, Y.; Zong, G.; Zeng, Y.; Wang, X.; et al. Plant-based dietary patterns in relation to mortality among older adults in China. Nat. Aging 2022, 2, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.; Li, S.; Tu, H.; Jia, J.; Zhao, W.; Xu, A.; Xu, W.; Tsai, M.K.; Chu, D.T.; et al. Association between plant-based dietary pattern and biological aging trajectory in a large prospective cohort. BMC Med. 2023, 21, 310. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition, 2nd ed.; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 5th ed.; Prentice Hall: Upper Saddler River, NJ, USA, 2002. [Google Scholar]
- Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Tang, X.; Zhang, W.; Qian, Y.; Huang, Y.; et al. Adherence to Healthy Lifestyle and Cardiovascular Diseases in the Chinese Population. J. Am. Coll. Cardiol. 2017, 69, 1116–1125. [Google Scholar] [CrossRef]
- Chen, Z.; Peto, R.; Zhou, M.; Iona, A.; Smith, M.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Lancaster, G.; et al. Contrasting male and female trends in tobacco-attributed mortality in China: Evidence from successive nationwide prospective cohort studies. Lancet 2015, 386, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hypponen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef]
- Chen, C.; Lu, F.C.; Department of Disease Control Ministry of Health, P.R.C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17, 1–36. [Google Scholar] [PubMed]
- World Health Organization. Waist circumference and waist–hip ratio. In Proceedings of the WHO Expert Consultation, Geneva, Switzerland, 8–11 December 2008. [Google Scholar]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef]
- Cleeman, J.I. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: Summary and discussion of the American Society of Echocardiography consensus statement. Prev. Cardiol. 2009, 12, 34–38. [Google Scholar] [CrossRef]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Desvarieux, M.; Ebrahim, S.; Fatar, M.; Hernandez, R.H.; Kownator, S.; et al. Mannheim Intima-Media Thickness Consensus. Cerebrovasc. Dis. 2004, 18, 346–349. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Hayes-Larson, E.; Kezios, K.L.; Mooney, S.J.; Lovasi, G. Who is in this study, anyway? Guidelines for a useful Table 1. J. Clin. Epidemiol. 2019, 114, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.D.; McCann, M.; Katikireddi, S.V.; Thomson, H.; Green, M.J.; Smith, D.J.; Lewsey, J.D. Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): A novel and systematic method for building directed acyclic graphs. Int. J. Epidemiol. 2020, 49, 322–329. [Google Scholar] [CrossRef]
- Spiegelman, D.; Hertzmark, E.; Wand, H.C. Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control 2007, 18, 571–579. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Valeri, L.; VanderWeele, T.J. SAS macro for causal mediation analysis with survival data. Epidemiology 2015, 26, e23–e24. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Shoaibinobarian, N.; Danehchin, L.; Mozafarinia, M.; Hekmatdoost, A.; Eghtesad, S.; Masoudi, S.; Mohammadi, Z.; Mard, A.; Paridar, Y.; Abolnezhadian, F.; et al. The Association between DASH Diet Adherence and Cardiovascular Risk Factors. Int. J. Prev. Med. 2023, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Chalermsri, C.; Ziaei, S.; Ekstrom, E.C.; Muangpaisan, W.; Aekplakorn, W.; Satheannopakao, W.; Rahman, S.M. Dietary diversity associated with risk of cardiovascular diseases among community-dwelling older people: A national health examination survey from Thailand. Front. Nutr. 2022, 9, 1002066. [Google Scholar] [CrossRef] [PubMed]
- Mirrafiei, A.; Jayedi, A.; Shab-Bidar, S. Total and different dietary fiber subtypes and the risk of all-cause, cardiovascular, and cancer mortality: A dose-response meta-analysis of prospective cohort studies. Food Funct. 2023, 14, 10667–10680. [Google Scholar] [CrossRef]
- Lazarova, S.V.; Sutherland, J.M.; Jessri, M. Adherence to emerging plant-based dietary patterns and its association with cardiovascular disease risk in a nationally representative sample of Canadian adults. Am. J. Clin. Nutr. 2022, 116, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Karvonen-Gutierrez, C.A.; Jackson, E.A.; Elliott, M.R.; Appelhans, B.M.; Barinas-Mitchell, E.; Bielak, L.F.; Huang, M.H.; Baylin, A. Western Dietary Pattern Derived by Multiple Statistical Methods Is Prospectively Associated with Subclinical Carotid Atherosclerosis in Midlife Women. J. Nutr. 2020, 150, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Rexrode, K.M. Quality of Plant-Based Diet and Risk of Total, Ischemic, and Hemorrhagic Stroke. Neurology 2021, 96, e1940–e1953. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Biggs, M.L.; Djousse, L.; Durda, P.J.; Kizer, J.R.; Psaty, B.; Reiner, A.P.; Tracy, R.P.; Siscovick, D.; Mukamal, K.J. Intake and Sources of Dietary Fiber, Inflammation, and Cardiovascular Disease in Older US Adults. JAMA Netw. Open 2022, 5, e225012. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Nguyen, L.H.; Song, M.; Wang, D.D.; Franzosa, E.A.; Cao, Y.; Joshi, A.; Drew, D.A.; Mehta, R.; Ivey, K.L.; et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef]
- Abeyrathne, E.; Nam, K.; Huang, X.; Ahn, D.U. Plant- and Animal-Based Antioxidants’ Structure, Efficacy, Mechanisms, and Applications: A Review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides and their health promoting effects: A potential role in atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hebert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients 2018, 10, 200. [Google Scholar] [CrossRef]
- Marx, W.; Veronese, N.; Kelly, J.T.; Smith, L.; Hockey, M.; Collins, S.; Trakman, G.L.; Hoare, E.; Teasdale, S.B.; Wade, A.; et al. The Dietary Inflammatory Index and Human Health: An Umbrella Review of Meta-Analyses of Observational Studies. Adv. Nutr. 2021, 12, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, J.; Lai, R.; Wang, X.; Chen, X.; Tian, W.; Liu, Q.; Li, J.; Ju, J.; Xu, H. Association between dietary inflammatory index and atherosclerosis cardiovascular disease in U.S. adults. Front. Nutr. 2022, 9, 1044329. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Lewis, J.R.; Blekkenhorst, L.C.; Shivappa, N.; Woodman, R.J.; Bondonno, C.P.; Ward, N.C.; Hebert, J.R.; Thompson, P.L.; Prince, R.L.; et al. Dietary inflammatory index in relation to sub-clinical atherosclerosis and atherosclerotic vascular disease mortality in older women. Br. J. Nutr. 2017, 117, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Tylavsky, F.A.; Vitolins, M.Z.; Ockene, J.K.; Hebert, J.R. Longitudinal changes in the dietary inflammatory index: An assessment of the inflammatory potential of diet over time in postmenopausal women. Eur. J. Clin. Nutr. 2016, 70, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Kim, H.; Rebholz, C.M. Higher Ultra-Processed Food Consumption Is Associated with Increased Risk of Incident Coronary Artery Disease in the Atherosclerosis Risk in Communities Study. J. Nutr. 2021, 151, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Montero-Salazar, H.; Donat-Vargas, C.; Moreno-Franco, B.; Sandoval-Insausti, H.; Civeira, F.; Laclaustra, M.; Guallar-Castillon, P. High consumption of ultra-processed food may double the risk of subclinical coronary atherosclerosis: The Aragon Workers’ Health Study (AWHS). BMC Med. 2020, 18, 235. [Google Scholar] [CrossRef]
- Menezes, C.A.; Magalhaes, L.B.; da Silva, J.T.; da Silva Lago, R.M.R.; Gomes, A.N.; Ladeia, A.M.T.; Vianna, N.A.; Oliveira, R.R. Ultra-Processed Food Consumption Is Related to Higher Trans Fatty Acids, Sugar Intake, and Micronutrient-Impaired Status in Schoolchildren of Bahia, Brazil. Nutrients 2023, 15, 381. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Schulze, M.B.; Meigs, J.B.; Manson, J.E.; Rifai, N.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr. 2005, 135, 562–566. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. Diet and inflammation: A link to metabolic and cardiovascular diseases. Eur. Heart J. 2006, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nappo, F.; Esposito, K.; Cioffi, M.; Giugliano, G.; Molinari, A.M.; Paolisso, G.; Marfella, R.; Giugliano, D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002, 39, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Nappo, F.; Giugliano, F.; Di Palo, C.; Ciotola, M.; Barbieri, M.; Paolisso, G.; Giugliano, D. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2003, 78, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Nappo, F.; Giugliano, F.; Giugliano, G.; Marfella, R.; Giugliano, D. Effect of dietary antioxidants on postprandial endothelial dysfunction induced by a high-fat meal in healthy subjects. Am. J. Clin. Nutr. 2003, 77, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.A.; Khaire, A.A. Leptin as a predictive marker for metabolic syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Santaniemi, M.; Kesaniemi, Y.A.; Ukkola, O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur. J. Endocrinol. 2006, 155, 745–750. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Ding, S.; Gan, G.; Li, L.; Li, Y.; Chen, Z.; Duan, Y.; Xie, J.; Cheng, A.S.K. Relationship between lifestyle and metabolic factors and carotid atherosclerosis: A survey of 47,063 fatty and non-fatty liver patients in China. Front. Cardiovasc. Med. 2022, 9, 935185. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/health-topics/healthy-diet#tab=tab_2 (accessed on 15 December 2023).
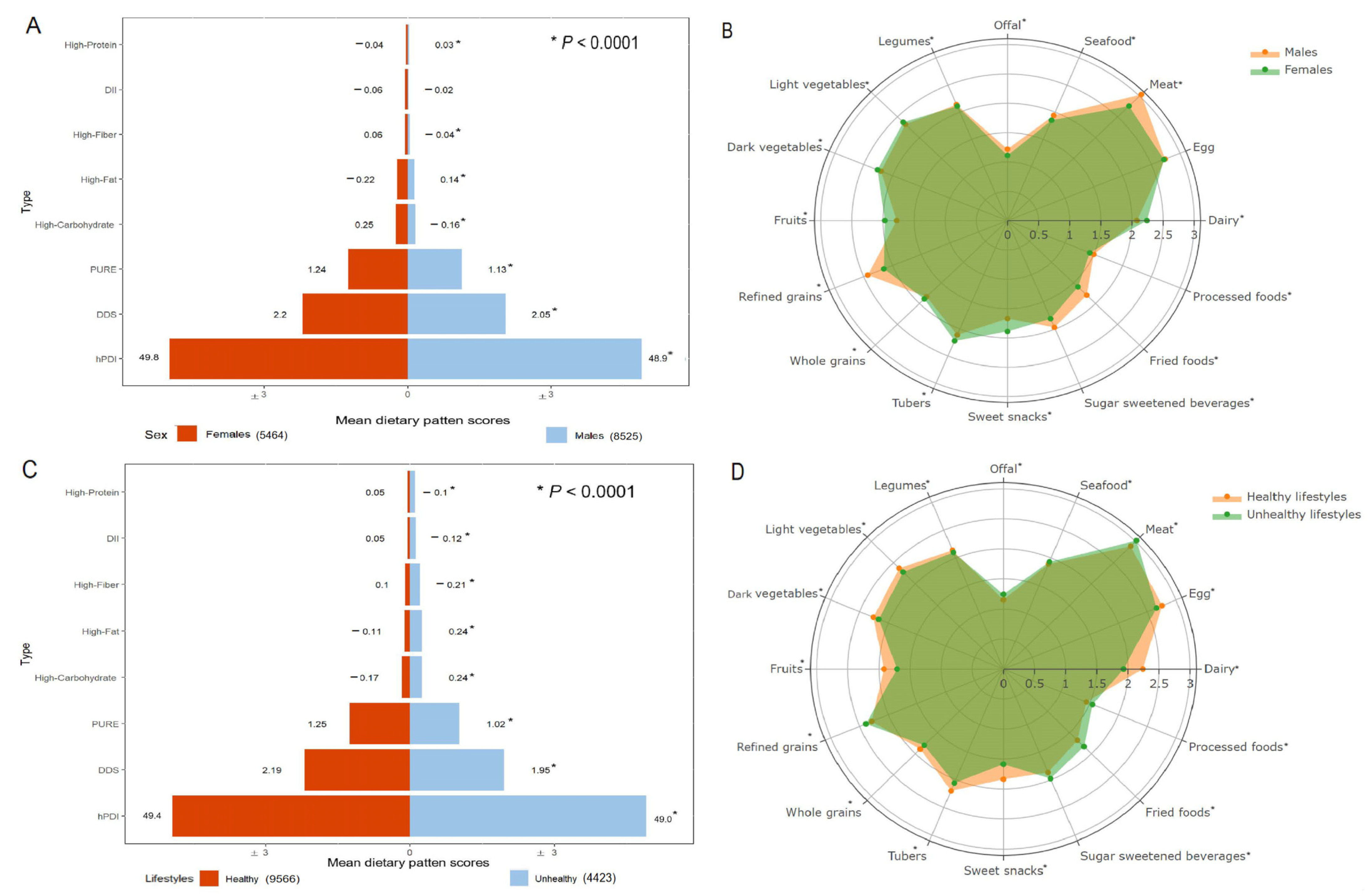
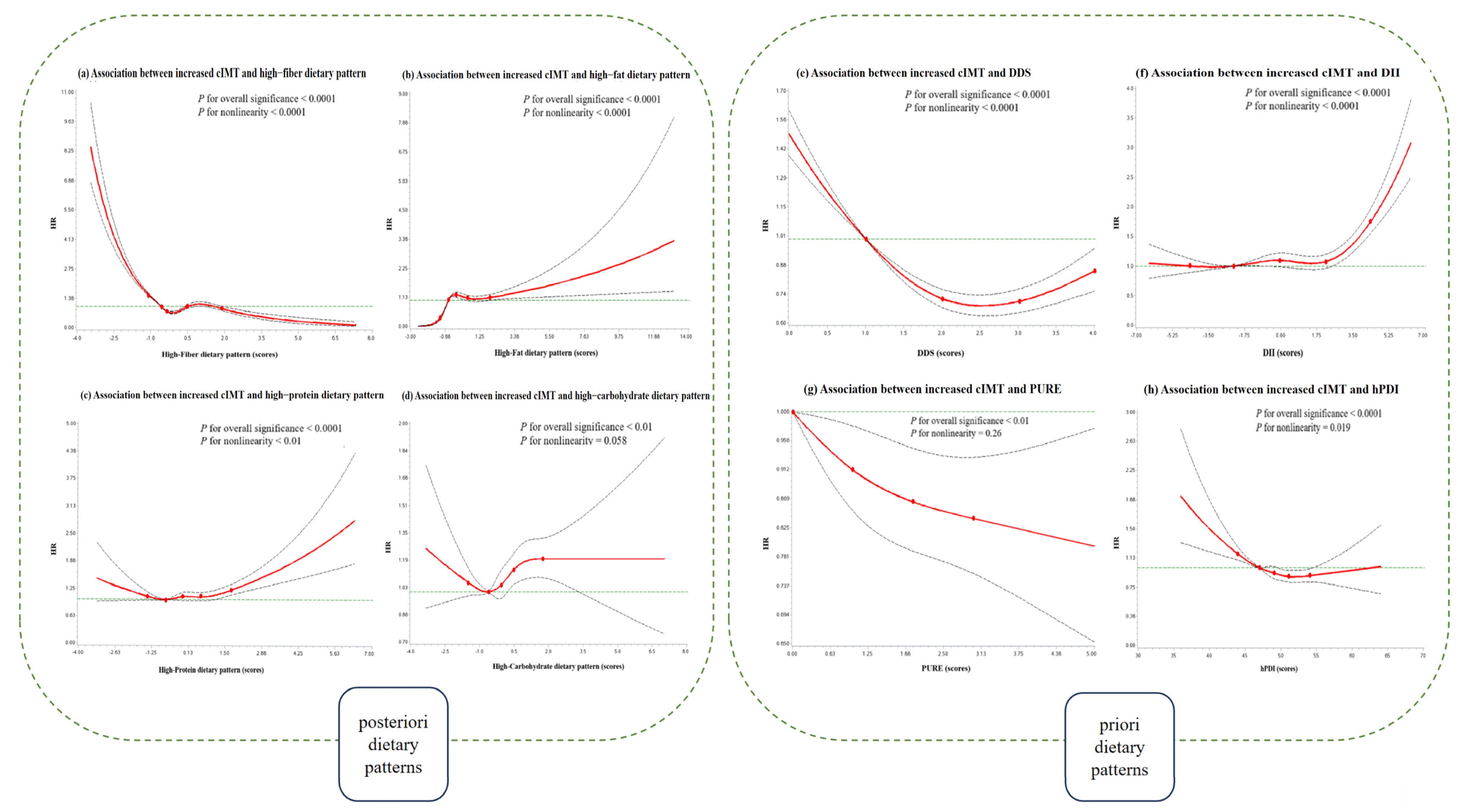
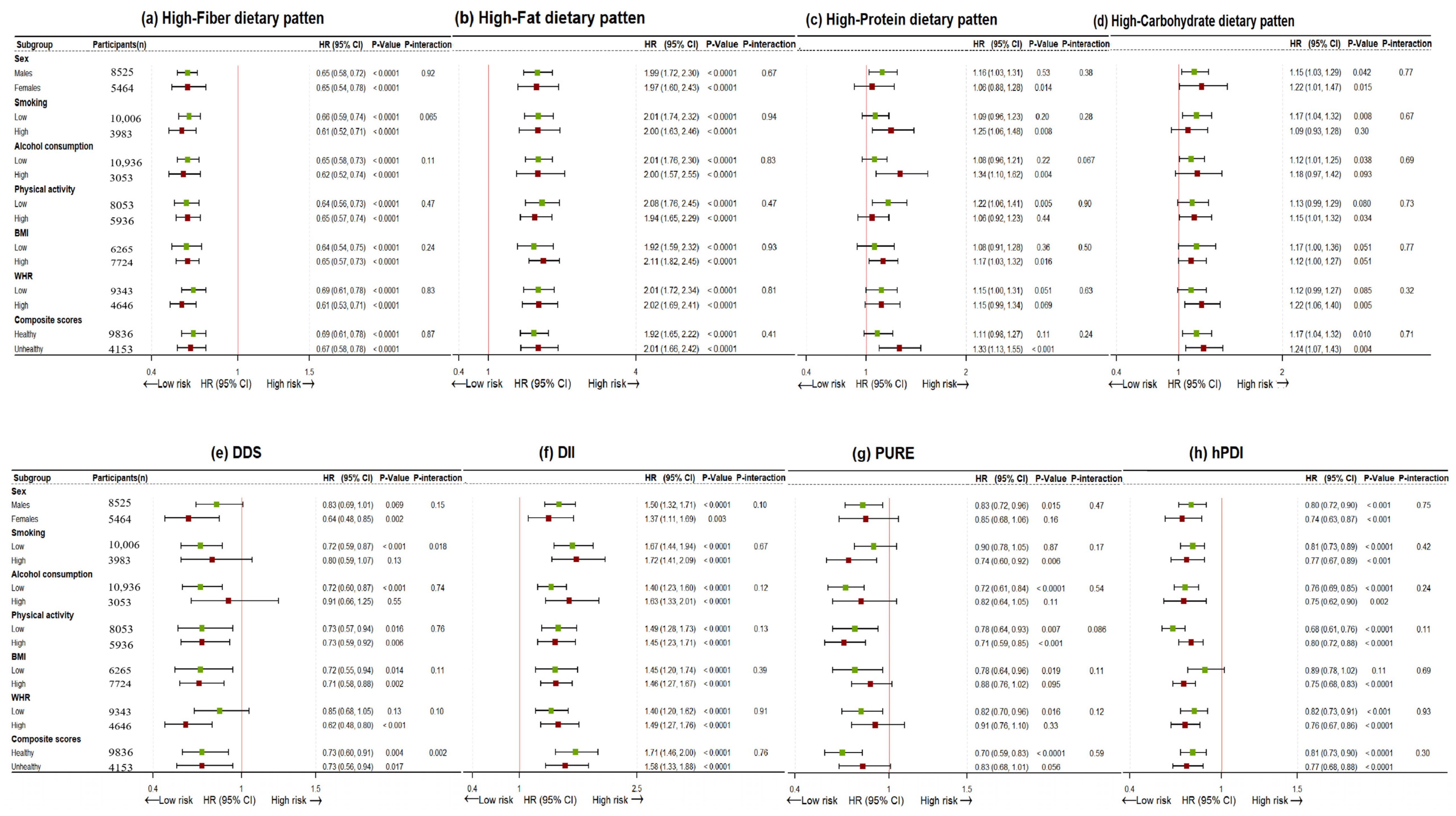
| Characteristics | Total | DDS | DII | PURE | hPDI | High-Fiber | High-Fat | High-Protein | High-Carbohydrate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | ||
| No. of subjects | 13,989 | 2925 | 737 | 3560 | 3462 | 4431 | 1704 | 3945 | 3363 | 3497 | 3498 | 3497 | 3497 | 3498 | 3497 | 3498 | 3497 |
| Median diet score | 1.00 | 4.00 | −3.45 | 3.48 | 0.00 | 3.00 | 46.0 | 52.0 | −0.76 | 1.27 | −0.97 | 1.10 | −1.06 | 1.10 | −1.03 | 0.99 | |
| Age (y) | 41.7 (41.5, 41.8) | 41.8 (41.5, 42.1) | 42.2 (41.6, 42.8) | 41.9 (41.7, 42.2) | 41.4 (41.1, 41.7) | 41.3 (41.1, 41.6) | 41.5 (41.1, 42.0) | 41.0 (40.8, 41.3) | 42.5 (42.3, 42.8) | 41.9 (41.6, 42.2) | 41.7 (41.5, 42.0) | 42.9 (42.6, 43.2) | 39.8 (39.5, 40.0) | 42.0 (41.8, 42.3) | 41.4 (41.1, 41.7) | 40.6 (40.3, 40.9) | 42.9 (42.6, 43.2) |
| Sex (Male, %) | 8525 (60.9) | 2,113 (72.2) | 4197 (57.9) | 2132 (59.9) | 2145 (62.0) | 2856 (64.5) | 974 (57.2) | 2689 (68.2) | 1721 (51.2) | 2241 (64.1) | 2043 (58.4) | 1686 (48.2) | 2520 (72.1) | 2060 (58.9) | 2176 (62.2) | 2675 (76.5) | 1661 (47.5) |
| Ethnic group (Han, %) | 5657 (40.4) | 1254 (42.9) | 304 (41.2) | 1411 (39.6) | 1455 (42.0) | 1949 (44.0) | 638 (37.4) | 1567 (39.7) | 1430 (42.5) | 1449 (41.4) | 1378 (39.4) | 1366 (39.1) | 1504 (43.0) | 1601 (45.8) | 1309 (37.4) | 1434 (41.0) | 1418 (40.5) |
| Educational attainment (>12 y, %) | 12,895 (92.2) | 2591 (88.6) | 675 (91.6) | 3291 (92.4) | 3133 (90.5) | 4030 (91.0) | 1592 (93.4) | 3711 (94.1) | 2998 (89.2) | 3207 (91.7) | 3223 (92.1) | 3217 (92.0) | 3210 (91.8) | 3097 (88.5) | 3320 (94.9) | 3183 (91.0) | 3226 (92.2) |
| Currently married (%) | 12,683 (90.7) | 2647 (90.5) | 668 (90.6) | 3238 (91.0) | 3125 (90.3) | 4040 (91.2) | 1508 (88.5) | 3552 (90.0) | 3044 (90.5) | 3111 (88.9) | 3167 (90.5) | 3229 (92.3) | 3081 (88.1) | 3208 (91.7) | 3154 (90.2) | 3182 (91.0) | 3172 (90.7) |
| Annual income (≥150,000 yuan, %) | 6642 (47.5) | 1209 (41.3) | 408 (55.4) | 1880 (52.8) | 1382 (39.9) | 1896 (42.8) | 930 (54.6) | 2048 (51.9) | 1412 (42.0) | 1481 (42.3) | 1807 (51.7) | 1597 (45.7) | 1735 (49.6) | 1354 (38.7) | 1896 (54.2) | 1681 (48.1) | 1615 (46.2) |
| Hypertension (%) | 1963 (14.0) | 500 (17.1) | 103 (14.0) | 489 (13.7) | 507 (14.6) | 659 (14.9) | 227 (13.3) | 540 (13.7) | 492 (14.6) | 563 (16.1) | 509 (14.6) | 469 (13.4) | 435 (12.4) | 542 (15.5) | 447 (12.8) | 483 (13.8) | 502 (14.4) |
| Diabetes (%) | 665 (4.75) | 231 (7.90) | 53 (7.19) | 180 (5.06) | 176 (5.08) | 223 (5.00) | 80 (4.69) | 194 (4.92) | 154 (4.58) | 199 (5.69) | 191 (5.46) | 175 (5.00) | 154 (4.40) | 173 (4.95) | 169 (4.83) | 192 (5.49) | 159 (4.55) |
| Dyslipidemia (%) | 6896 (49.3) | 1557 (53.2) | 364 (49.4) | 1711 (48.1) | 1763 (50.9) | 2230 (50.3) | 787 (46.2) | 2046 (51.9) | 1540 (45.8) | 1854 (53.0) | 1675 (47.9) | 1679 (48.0) | 1736 (49.6) | 1751 (50.1) | 1717 (49.1) | 1734 (49.6) | 1738 (49.7) |
| MetS (%) | 3850 (27.5) | 988 (33.8) | 201 (27.3) | 907 (25.5) | 1008 (29.1) | 1361 (30.7) | 422 (24.8) | 1087 (27.6) | 893 (26.6) | 1045 (29.9) | 901 (25.8) | 891 (25.5) | 1008 (28.8) | 1093 (31.2) | 866 (24.8) | 971 (27.8) | 936 (26.8) |
| Healthy lifestyle characteristics b | |||||||||||||||||
| Noncurrent smoker (%) | 10,006 (71.5) | 1702 (58.2) | 565 (76.7) | 2686 (75.5) | 2274 (65.7) | 2925 (66.0) | 1320 (77.5) | 2661 (67.5) | 2.601 (77.3) | 2328 (66.6) | 2589 (74.0) | 2621 (74.9) | 2377 (68.0) | 2372 (67.8) | 2628 (75.1) | 2436 (69.6) | 2625 (75.0) |
| Limited alcohol consumption (%) | 10,936 (78.2) | 2004 (68.5) | 593 (80.5) | 2756 (77.4) | 2720 (78.6) | 3345 (75.7) | 1361 (79.9) | 2914 (73.9) | 2787 (82.9) | 2688 (76.8) | 2753 (78.7) | 2849 (81.5) | 2562 (73.2) | 2676 (76.5) | 2795 (79.9) | 2654 (75.9) | 2834 (81.0) |
| Regular PA (%) | 8053 (57.6) | 933 (31.9) | 396 (53.7) | 1788 (50.2) | 1173 (33.9) | 1603 (36.2) | 877 (51.5) | 1655 (42.0) | 1466 (43.6) | 1342 (38.4) | 1720 (49.2) | 1651 (47.2) | 1311 (37.5) | 1275 (36.5) | 1713 (49.0) | 1264 (36.1) | 1756 (50.2) |
| Moderate BMI (%) | 6264 (44.8) | 1169 (40.0) | 337 (45.7) | 1611 (45.3) | 1545 (44.6) | 1908 (43.1) | 776 (45.5) | 1707 (43.3) | 1565 (46.5) | 1558 (44.5) | 1592 (45.5) | 1666 (47.6) | 1459 (41.7) | 1501 (42.9) | 1619 (46.3) | 1552 (44.4) | 1568 (44.8) |
| Moderate WHR (%) | 9343 (66.8) | 1724 (58.9) | 503 (68.3) | 2484 (69.8) | 2197 (63.5) | 2793 (63.0) | 1209 (71.0) | 2581 (65.4) | 2295 (68.2) | 2235 (63.9) | 2417 (69.1) | 2363 (67.6) | 2312 (66.1) | 2204 (63.0) | 2439 (69.7) | 2277 (65.1) | 2394 (68.4) |
| Family history of CVD (%) | 3569 (25.5) | 768 (26.3) | 175 (23.7) | 905 (25.4) | 874 (25.2) | 1074 (24.2) | 474 (27.8) | 1063 (27.0) | 788 (23.4) | 928 (26.5) | 885 (25.3) | 851 (24.3) | 974 (27.8) | 775 (22.2) | 965 (27.6) | 916 (26.2) | 874 (25.0) |
| Total energy intake, kcal/d | 1927.3 (1919.6, 1935.0) | 1688.6 (1675.1, 1702.2) | 2443.9 (2405.1, 2483.2) | 2407.1 (2393.1, 2421.2) | 1547.7 (1538.6, 1556.9) | 1642.8 (1633.3, 1652.5) | 2472.0 (2448.8, 2495.5) | 2057.3 (2042.2, 2072.6) | 1872.4 (1857.4, 1887.4) | 1731.7 (1719.5, 1744.0) | 2287.5 (2271.4, 2303.8) | 1708.8 (1696.5, 1721.1) | 2269.1 (2252.8, 2285.5) | 1677.8 (1665.8, 1689.9) | 2238 (2222.0, 2254.2) | 1915.2 (1900.6, 1929.9) | 2162.8 (2146.3, 2179.4) |
| Sugar-sweetened beverages, g/d | 45.6 (44.8, 46.4) | 44.4 (42.7, 46.2) | 48.7 (45.1, 52.7) | 46.9 (45.2, 48.5) | 42.6 (41.1, 44.2) | 43.2 (41.8, 44.6) | 49.2 (46.8, 51.8) | 53.6 (51.8, 55.4) | 38.5 (37.1, 39.9) | 51.2 (49.4, 53.1) | 44.7 (43.1, 46.3) | 23.8 (23.0, 24.5) | 91.7 (88.9, 94.7) | 43.9 (42.4, 45.5) | 46.2 (44.6, 47.9) | 38.6 (37.2, 40.0) | 53.6 (51.8, 55.6) |
| Vegetable intake, g/d | 337.1 (335.4, 338.7) | 285.4 (282.5, 288.3) | 393.6 (385.7, 401.7) | 442.7 (439.3, 446.2) | 273.4 (271.3, 275.6) | 283.7 (281.7, 285.8) | 454.8 (449.5, 460.2) | 307.3 (304.6, 310.0) | 386.1 (382.4, 389.8) | 266.9 (265.3, 268.5) | 494.4 (491.4, 497.3) | 353.4 (350.0, 356.9) | 334.5 (331.3, 337.8) | 334.3 (331.1, 337.6) | 343.9 (340.6, 347.3) | 353.1 (349.7, 356.6) | 339.6 (336.3, 342.9) |
| Fruit intake, g/d | 190.5 (188.8, 192.2) | 88.5 (87.3, 89.7) | 256.3 (249.6, 263.2) | 253.2 (249.2, 257.3) | 130.4 (128.3, 132.5) | 157.2 (154.9, 159.6) | 272.2 (265.6, 278.9) | 173.5 (170.6, 176.4) | 219.8 (215.9, 223.8) | 137.3 (135.1, 139.6) | 230.4 (226.6, 234.3) | 199.8 (196.2, 203.4) | 186.7 (183.4, 190.1) | 163.2 (160.4, 166.1) | 219.7 (215.8, 223.6) | 161.5 (158.7, 164.4) | 227.8 (223.9, 231.9) |
| Whole grain intake, g/d | 31.1 (30.6, 31.5) | 23.1 (22.41, 23.81) | 44.6 (42.0, 47.3) | 57.8 (56.4,59.2) | 16.9 (16.4, 17.3) | 24.6 (24.0, 25.2) | 46.0 (44.3, 47.9) | 25.5 (24.8, 26.1) | 41.4 (40.2, 42.6) | 23.2 (22.6, 23.8) | 42.1 (41.0, 43.3) | 27.7 (26.9, 28.4) | 35.4 (34.5, 36.5) | 29.2 (28.4, 30.1) | 34.4 (33.4, 35.4) | 18.5 (18.0, 18.9) | 65.6 (64.1, 67.2) |
| Multivitamin supplement use (%) | 3400 (24.3) | 530 (18.1) | 251 (34.1) | 1019 (28.6) | 672 (19.4) | 850 (19.2) | 561 (32.9) | 1006 (25.5) | 819 (24.4) | 819 (23.4) | 935 (26.7) | 899 (25.7) | 832 (23.8) | 699 (20.0) | 1033 (29.5) | 711 (20.3) | 1032 (29.5) |
| Food Groups | Factor Loadings | DDS a | DII a | PURE a | hPDI a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-Fiber | High-Fat | High-Protein | High-Carbohydrate | Q1 (Least Healthy) | Q4 (Most Healthy) | Q1 (Most Healthy) | Q4 (Least Healthy) | Q1 (Least Healthy) | Q4 (Most Healthy) | Q1 (Least Healthy) | Q4 (Most Healthy) | |
| Dairy products, g per day | 0.01 | −0.17 | 0.62 | 0.28 | 70.5 | 176.5 | 124.6 | 77.1 | 39.6 | 173.2 | 155.4 | 62.0 |
| Eggs, g per day | 0.07 | −0.04 | 0.67 | 0.009 | 21.1 | 53.7 | 37.5 | 24.5 | 25.7 | 39.2 | 41.4 | 23.4 |
| Meat, g per day | 0.05 | 0.31 | 0.54 | −0.25 | 48.3 | 64.2 | 58.0 | 44.3 | 46.2 | 59.9 | 66.1 | 38.5 |
| Seafood, g per day | 0.22 | 0.21 | 0.45 | −0.04 | 20.1 | 43.8 | 35.0 | 16.3 | 18.3 | 43.0 | 32.0 | 20.6 |
| Offal, g per day | 0.09 | 0.51 | 0.11 | −0.07 | 11.6 | 15.9 | 15.8 | 8.49 | 11.0 | 16.1 | 15.8 | 9.43 |
| Legumes, g per day | 0.33 | 0.21 | 0.26 | 0.08 | 49.7 | 118.4 | 91.0 | 35.7 | 42.3 | 100.8 | 57.5 | 67.0 |
| Light vegetables, g per day | 0.87 | −0.04 | 0.03 | −0.03 | 157.0 | 214.2 | 236.0 | 144.2 | 145.6 | 244.2 | 164.2 | 201.7 |
| Dark vegetables, g per day | 0.87 | −0.04 | 0.03 | −0.03 | 145.9 | 197.2 | 222.5 | 138.0 | 143.1 | 224.0 | 155.6 | 188.8 |
| Fruits, g per day | 0.34 | −0.07 | 0.19 | 0.24 | 95.3 | 268.2 | 273.8 | 151.5 | 178.4 | 302.6 | 197.5 | 237.5 |
| Refined grains, g per day | 0.10 | 0.16 | 0.11 | −0.70 | 238.3 | 216.6 | 230.5 | 225.6 | 231.5 | 231.6 | 262.8 | 202.8 |
| Whole grains, g per day | 0.23 | 0.10 | 0.03 | 0.70 | 34.6 | 64.0 | 76.3 | 21.4 | 35.2 | 62.0 | 36.0 | 55.5 |
| Tubers, g per day b | 0.40 | 0.10 | 0.10 | 0.31 | 38.1 | 59.6 | 62.1 | 33.7 | 39.0 | 60.8 | 39.9 | 52.4 |
| Sweet snacks, g per day e | 0.04 | 0.22 | 0.27 | 0.34 | 37.5 | 60.5 | 57.2 | 37.2 | 36.5 | 62.6 | 57.3 | 42.0 |
| Sugar-sweetened beverages, g per day f | −0.03 | 0.48 | 0.03 | 0.09 | 85.1 | 97.8 | 89.6 | 80.8 | 78.8 | 93.7 | 99.9 | 73.5 |
| Fried foods, g per day d | −0.07 | 0.71 | 0.004 | −0.02 | 49.8 | 49.8 | 49.3 | 45.9 | 46.9 | 50.4 | 54.1 | 42.9 |
| Processed foods, g per day c | 0.04 | 0.60 | −0.008 | 0.003 | 7.11 | 8.13 | 8.44 | 6.51 | 7.17 | 8.44 | 10.4 | 5.37 |
| Explained variation in food groups (%) | 15.6 | 10.9 | 8.14 | 7.18 | - | - | - | - | - | - | - | - |
| Alcohol, g per day g | 10.3 | 10.3 | 10.3 | 10.3 | 16.3 | 9.86 | 11.2 | 10.2 | 11.6 | 9.33 | 12.3 | 8.40 |
| Cholesterol, mg per day | 547.9 | 547.9 | 547.9 | 547.9 | 456.2 | 805.8 | 677.7 | 409.1 | 461.2 | 717.1 | 710.3 | 419.7 |
| Carbohydrates, %E | 63.7 | 63.7 | 63.7 | 63.7 | 62.7 | 59.9 | 62.7 | 64.6 | 64.9 | 61.9 | 61.9 | 65.3 |
| Fats, %E | 22.6 | 22.6 | 22.6 | 22.6 | 22.7 | 23.9 | 21.8 | 23.0 | 22.1 | 22.7 | 25.0 | 20.5 |
| Saturated, %E | 7.91 | 7.91 | 7.91 | 7.91 | 7.90 | 8.49 | 7.48 | 8.16 | 7.52 | 7.99 | 9.15 | 6.82 |
| Monounsaturated, %E | 7.95 | 7.95 | 7.95 | 7.95 | 7.98 | 8.25 | 7.60 | 8.13 | 7.86 | 7.87 | 8.85 | 7.13 |
| Polyunsaturated, %E | 5.85 | 5.85 | 5.85 | 5.85 | 5.85 | 6.24 | 5.87 | 5.75 | 5.78 | 5.98 | 5.96 | 5.73 |
| Polyunsaturated-to-saturated fat ratio | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.77 | 0.81 | 0.72 | 0.78 | 0.77 | 0.66 | 0.85 |
| Other, %E h | 0.92 | 0.92 | 0.92 | 0.92 | 0.99 | 0.89 | 0.82 | 1.00 | 0.97 | 0.84 | 1.04 | 0.80 |
| Protein, %E | 16.2 | 16.2 | 16.2 | 16.2 | 16.0 | 17.6 | 16.4 | 15.8 | 15.7 | 16.9 | 16.8 | 15.7 |
| Dietary Patterns | n | Increased cIMT | CP | ||||
|---|---|---|---|---|---|---|---|
| Cases (%) (n = 3732) | Incident Rate (Events per 1000 Person-Years) | HR (95% CI) | Cases (%) (n = 2861) | Incident Rate (Events per 1000 Person-Years) | HR (95% CI) | ||
| High-Fiber (range) a | |||||||
| Q1 (−3.44, −0.58) | 3497 | 1387 (39.7) | 133.6 | Ref | 1068 (30.5) | 97.3 | Ref |
| Q2 (−0.58, −0.32) | 3496 | 670 (19.2) | 63.1 | 0.50 (0.45, 0.54) | 516 (14.8) | 47.0 | 0.51 (0.46, 0.57) |
| Q3 (−0.32, 0.44) | 3498 | 760 (21.7) | 72.2 | 0.54 (0.49, 0.59) | 579 (16.6) | 53.1 | 0.55 (0.49, 0.61) |
| Q4 (0.44, 7.38) | 3498 | 915 (26.2) | 86.1 | 0.65 (0.59, 0.71) | 698 (20.0) | 63.1 | 0.65 (0.59, 0.73) |
| p for trend | <0.0001 | <0.0001 | |||||
| HR (95% CI) per 1 SD | 0.77 (0.74, 0.80) | 0.77 (0.74, 0.81) | |||||
| PAR (95% CI) | 0.17 (0.14, 0.20) | 0.17 (0.13, 0.21) | |||||
| High-Fat (range) a | |||||||
| Q1 (−2.51, −0.69) | 3498 | 525 (15.0) | 48.7 | Ref | 417 (11.9) | 37.8 | Ref |
| Q2 (−0.69, −0.20) | 3497 | 1137 (32.5) | 106.7 | 2.15 (1.94, 2.38) | 868 (24.8) | 77.4 | 2.00 (1.78, 2.25) |
| Q3 (−0.20, 0.48) | 3496 | 1087 (31.1) | 105.7 | 2.16 (1.94, 2.40) | 837 (23.9) | 77.5 | 2.04 (1.80, 2.30) |
| Q4 (0.48, 13.1) | 3498 | 983 (28.1) | 94.2 | 1.96 (1.75, 2.20) | 739 (21.1) | 68.1 | 1.84 (1.61, 2.10) |
| p for trend | <0.0001 | <0.0001 | |||||
| HR (95% CI) per 1 SD | 1.13 (1.10, 1.17) | 1.10 (1.06, 1.15) | |||||
| PAR (95% CI) | 0.48 (0.42, 0.53) | 0.44 (0.38, 0.50) | |||||
| High-Protein (range) a | |||||||
| Q1 (−3.29, −0.69) | 3498 | 911 (26.0) | 85.1 | Ref | 691 (19.8) | 62.1 | Ref |
| Q2 (−0.69, −0.10) | 3497 | 904 (25.9) | 86.5 | 1.02 (0.93, 1.12) | 701 (20.1) | 64.4 | 1.02 (0.92, 1.13) |
| Q3 (−0.10, 0.57) | 3496 | 884 (25.3) | 83.6 | 1.03 (0.94, 1.14) | 689 (19.7) | 64.0 | 1.06 (0.95, 1.18) |
| Q4 (0.57, 6.40) | 3498 | 1033 (29.5) | 99.1 | 1.13 (1.02, 1.25) | 780 (22.3) | 71.8 | 1.12 (0.99, 1.25) |
| p for trend | 0.040 | 0.087 | |||||
| HR (95% CI) per 1 SD | 1.04 (1.00, 1.08) | 1.03 (1.00, 1.08) | |||||
| PAR (95% CI) | 0.04 (0.01, 0.07) | 0.04 (0.003, 0.07) | |||||
| High-Carbohydrate (range) a | |||||||
| Q1 (−3.32, −0.61) | 3498 | 924 (24.8) | 86.7 | Ref | 696 (19.9) | 62.7 | Ref |
| Q2 (−0.61, −0.05) | 3497 | 895 (25.1) | 85.3 | 1.05 (0.96, 1.15) | 678 (19.4) | 62.1 | 1.05 (0.95, 1.17) |
| Q3 (−0.05, 0.49) | 3496 | 910 (26.4) | 85.2 | 1.05 (0.96, 1.16) | 694 (19.9) | 62.6 | 1.05 (0.94, 1.17) |
| Q4 (0.49, 7.04) | 3498 | 1003 (28.7) | 97.2 | 1.17 (1.07, 1.29) | 793 (22.7) | 73.4 | 1.20 (1.08, 1.34) |
| p for trend | 0.009 | 0.011 | |||||
| HR (95% CI) per 1 SD | 1.03 (1.00, 1.07) | 1.04 (1.00, 1.08) | |||||
| PAR (95% CI) | 0.05 (0.02, 0.08) | 0.05 (0.02, 0.08) | |||||
| DDS (range) b | |||||||
| Q1 [0.00, 1.00] | 2925 | 986 (33.7) | 110.1 | Ref | 754 (25.8) | 80.6 | Ref |
| Q2 [2.00] | 7244 | 1743 (24.1) | 78.9 | 0.75 (0.69, 0.81) | 1333 (18.4) | 58.0 | 0.76 (0.69, 0.83) |
| Q3 [3.00] | 3083 | 807 (26.2) | 89.9 | 0.78 (0.70, 0.86) | 624 (20.2) | 66.9 | 0.81 (0.72, 0.91) |
| Q4 [4.00, 7.00] | 737 | 196 (26.6) | 92.5 | 0.74 (0.63, 0.87) | 150 (20.4) | 67.5 | 0.72 (0.59, 0.87) |
| p for trend | <0.0001 | <0.001 | |||||
| HR (95% CI) per 1 SD | 0.89 (0.85, 0.92) | 0.90 (0.86, 0.93) | |||||
| PAR (95% CI) | 0.07 (0.03, 0.10) | 0.07 (0.03, 0.11) | |||||
| DII (range) b | |||||||
| Q1 (−6.39, −2.26) | 3560 | 930 (26.1) | 86.7 | Ref | 701 (19.7) | 62.9 | Ref |
| Q2 (−2.26, −0.01) | 3477 | 892 (25.7) | 84.3 | 1.13 (1.03, 1.25) | 688 (19.8) | 62.6 | 1.14 (1.02, 1.27) |
| Q3 (−0.01, 2.22) | 3490 | 872 (25.0) | 83.7 | 1.22 (1.09, 1.35) | 695 (19.9) | 64.0 | 1.25 (1.11, 1.41) |
| Q4 (2.22, 6.37) | 3462 | 1038 (30.0) | 99.5 | 1.66 (1.48, 1.87) | 777 (22.4) | 71.3 | 1.59 (1.39, 1.82) |
| p for trend | <0.0001 | <0.0001 | |||||
| HR (95% CI) per 1 SD | 1.24 (1.19, 1.30) | 1.22 (1.16, 1.29) | |||||
| PAR (95% CI) | 0.13 (0.06, 0.19) | 0.14 (0.06, 0.21) | |||||
| PURE (range) b | |||||||
| Q1 [0] | 4431 | 1157 (26.1) | 90.1 | Ref | 881 (19.9) | 65.8 | Ref |
| Q2 [1.0] | 4902 | 1331 (27.2) | 88.3 | 0.86 (0.79, 0.93) | 1026 (20.9) | 65.2 | 0.87 (0.79, 0.95) |
| Q3 [2.0] | 2952 | 784 (26.6) | 87.1 | 0.77 (0.70, 0.85) | 606 (20.5) | 65.1 | 0.80 (0.71, 0.90) |
| Q4 [3.0, 5.0] | 1704 | 460 (27.0) | 88.0 | 0.76 (0.67, 0.87) | 348 (20.4) | 63.5 | 0.75 (0.65, 0.87) |
| p for trend | <0.0001 | <0.0001 | |||||
| HR (95% CI) per 1 SD | 0.90 (0.87, 0.94) | 0.90 (0.86, 0.95) | |||||
| PAR (95% CI) | 0.05 (0.01, 0.08) | 0.05 (0.02, 0.09) | |||||
| hPDI (range) b | |||||||
| Q1 [36.0, 47.0] | 3945 | 1208 (30.6) | 101.2 | Ref | 923 (23.4) | 74.0 | Ref |
| Q2 (47.0, 49.0] | 3408 | 874 (25.7) | 85.5 | 0.88 (0.81, 0.96) | 653 (19.2) | 61.2 | 0.85 (0.76, 0.94) |
| Q3 (49.0, 51.0] | 3273 | 842 (25.7) | 87.3 | 0.87 (0.80, 0.96) | 667 (20.4) | 66.4 | 0.90 (0.82, 0.99) |
| Q4 (51.0, 64.0] | 3363 | 808 (24.0) | 78.1 | 0.78 (0.71, 0.85) | 618 (18.4) | 57.6 | 0.79 (0.72, 0.88) |
| p for trend | <0.0001 | <0.0001 | |||||
| HR (95% CI) per 1 SD | 0.91 (0.88, 0.94) | 0.92 (0.89, 0.96) | |||||
| PAR (95% CI) | 0.06 (0.04, 0.09) | 0.06 (0.03, 0.09) | |||||
| AUC (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| High-Fiber Dietary Pattern c, d, e, f, g, h | High-Fat Dietary Pattern c, d, e, f, g, h | High-Protein Dietary Pattern a, b | High-Carbohydrate Dietary Pattern a, b | DDS a, b | DII a, b | PURE a, b | hPDI a, b | |
| Increased cIMT | 0.56 (0.55, 0.57) | 0.55 (0.54, 0.56) | 0.51 (0.50, 0.52) | 0.52 (0.50, 0.53) | 0.52 (0.51, 0.53) | 0.52 (0.51, 0.53) | 0.51 (0.50, 0.52) | 0.52 (0.51, 0.53) |
| CP | 0.56 (0.55, 0.57) | 0.54 (0.53, 0.55) | 0.51 (0.50, 0.52) | 0.52 (0.50, 0.53) | 0.52 (0.51, 0.53) | 0.52 (0.51, 0.53) | 0.51 (0.50, 0.52) | 0.52 (0.51, 0.52) |
| Dietary Patterns | CAS | No. of MetS | Incident Rate (Events per 1000 Person-Years) | HR (95% CI) NIE | HR (95% CI) NDE a | HR (95% CI) TE | PM (%) b | p-Value |
|---|---|---|---|---|---|---|---|---|
| High-Fiber | Increased cIMT | 3850 | 88.5 | 0.993 (0.988, 0.998) | 0.851 (0.824, 0.879) | 0.845 (0.818, 0.873) | 3.70 | 0.011 |
| High-Fat | Increased cIMT | 3850 | 88.5 | 1.007 (1.002, 1.012) | 1.168 (1.131, 1.207) | 1.176 (1.138, 1.215) | 4.41 | 0.011 |
| High-Fiber | CP | 3850 | 65.2 | 0.993 (0.988, 0.998) | 0.853 (0.823, 0.885) | 0.848 (0.817, 0.880) | 3.88 | 0.011 |
| High-Fat | CP | 3850 | 65.2 | 1.007 (1.002, 1.013) | 1.143 (1.101, 1.013) | 1.151 (1.108, 1.195) | 5.38 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Deng, Y.; Ma, Y.; Man, S.; Yang, X.; Yu, C.; Lv, J.; Liu, H.; Wang, B.; Li, L. Adherence to a Healthy Diet and Risk of Multiple Carotid Atherosclerosis Subtypes: Insights from the China MJ Health Check-Up Cohort. Nutrients 2024, 16, 2338. https://doi.org/10.3390/nu16142338
Fu J, Deng Y, Ma Y, Man S, Yang X, Yu C, Lv J, Liu H, Wang B, Li L. Adherence to a Healthy Diet and Risk of Multiple Carotid Atherosclerosis Subtypes: Insights from the China MJ Health Check-Up Cohort. Nutrients. 2024; 16(14):2338. https://doi.org/10.3390/nu16142338
Chicago/Turabian StyleFu, Jingzhu, Yuhan Deng, Yuan Ma, Sailimai Man, Xiaochen Yang, Canqing Yu, Jun Lv, Hui Liu, Bo Wang, and Liming Li. 2024. "Adherence to a Healthy Diet and Risk of Multiple Carotid Atherosclerosis Subtypes: Insights from the China MJ Health Check-Up Cohort" Nutrients 16, no. 14: 2338. https://doi.org/10.3390/nu16142338
APA StyleFu, J., Deng, Y., Ma, Y., Man, S., Yang, X., Yu, C., Lv, J., Liu, H., Wang, B., & Li, L. (2024). Adherence to a Healthy Diet and Risk of Multiple Carotid Atherosclerosis Subtypes: Insights from the China MJ Health Check-Up Cohort. Nutrients, 16(14), 2338. https://doi.org/10.3390/nu16142338






