Deer Skin Collagen Peptides Bound to Calcium: In Vitro Gastrointestinal Simulation of Digestion, Cellular Uptake and Analysis of Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Ca-DSCP
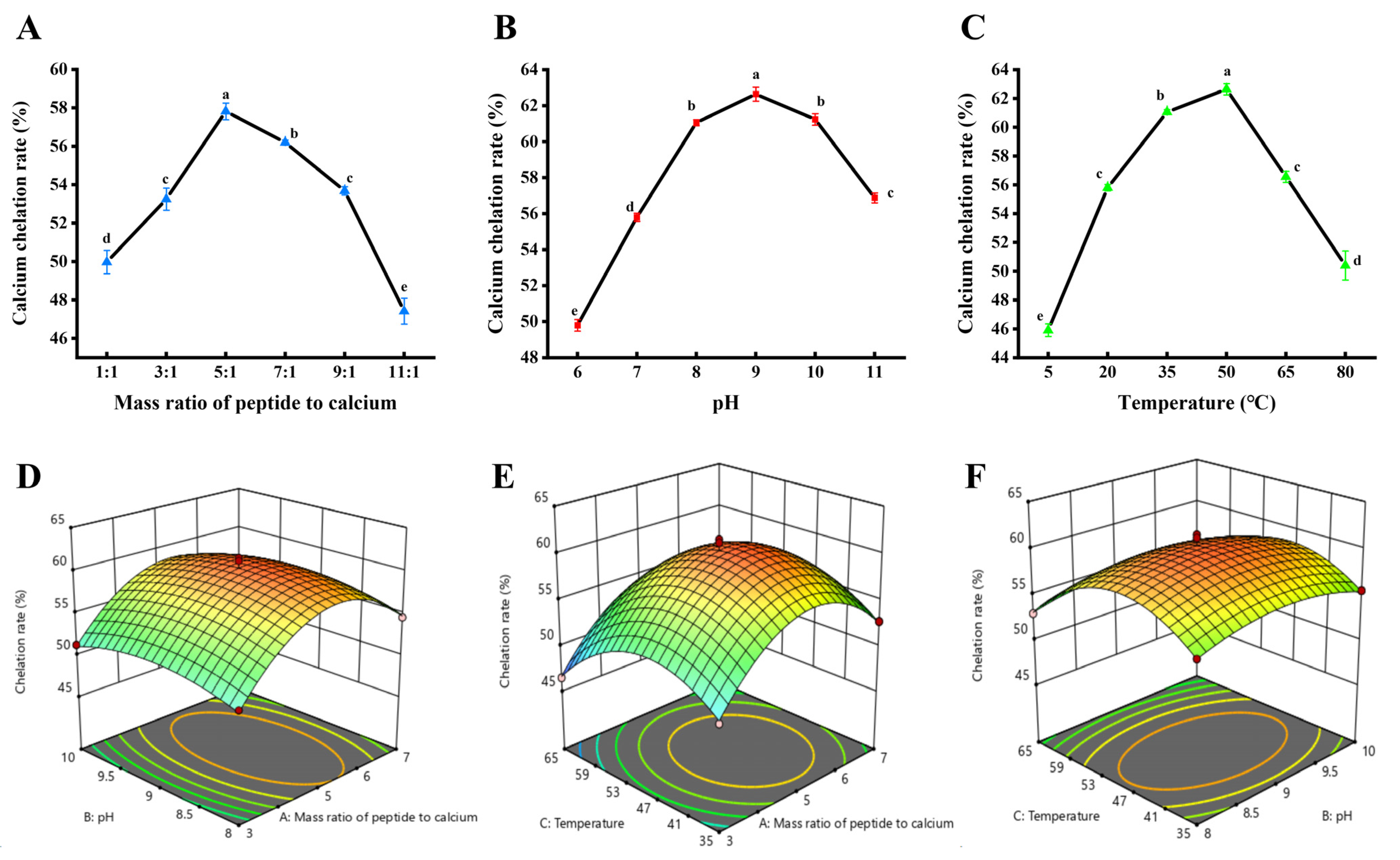
2.2.1. Single-Factor Experiments
2.2.2. Response Surface Experiments
2.2.3. Determination of Calcium Chelation Rate
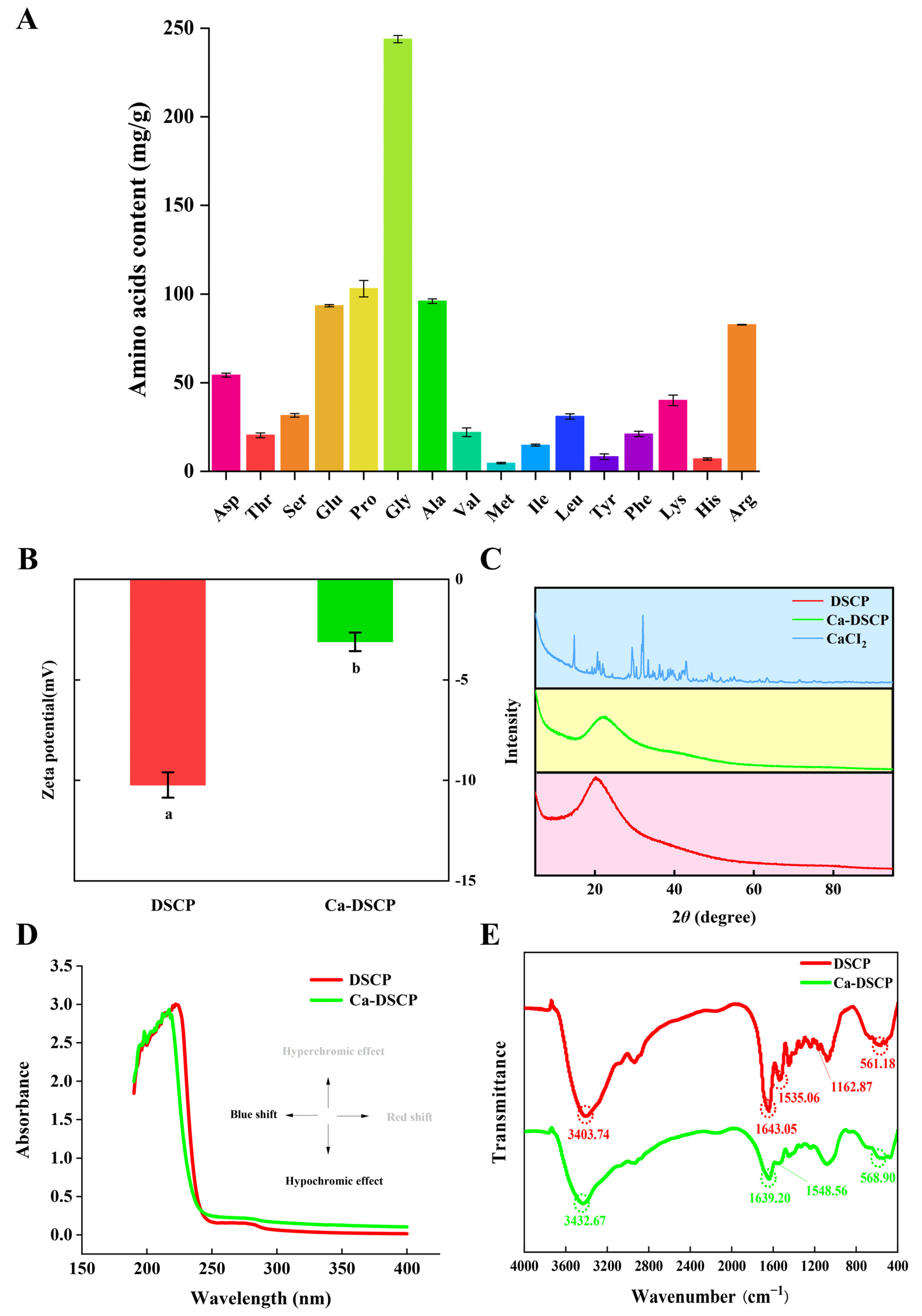
2.3. Amino Acid Analysis
2.4. Zeta Potential and XRD of DSCP and Ca-DSCP
2.5. UV–vis and FTIR of DSCP and Ca-DSCP
2.6. Stability of Ca-DSCP
2.6.1. Acid–Base Stability and Thermal Stability
2.6.2. In Vitro Gastrointestinal Digestion
2.7. Cellular Experiments
2.7.1. Caco-2 Cell Culture
2.7.2. Cytotoxicity Test of Caco-2 Cells
2.7.3. Caco-2 Cells Monolayer Calcium Transport Studies
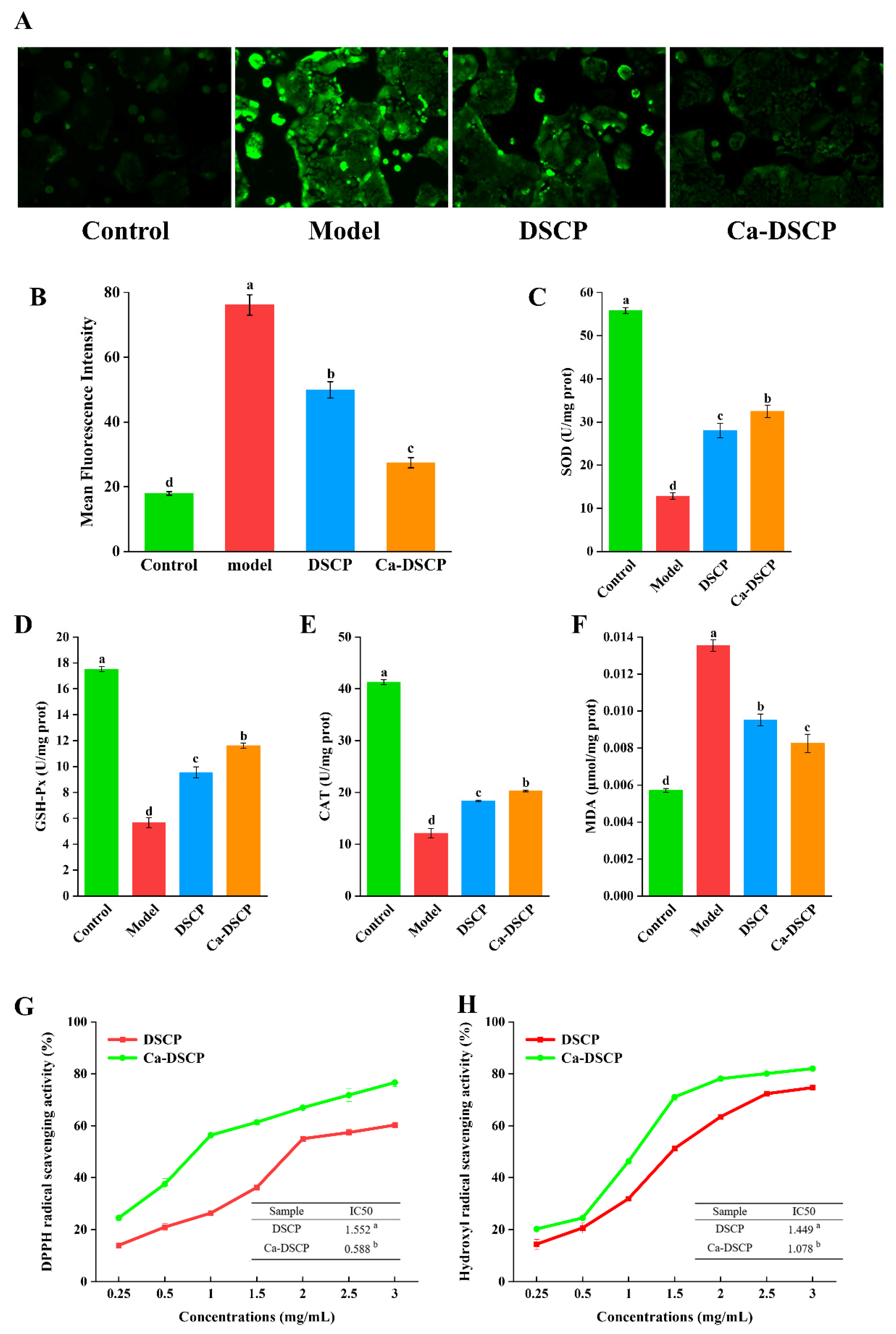
2.7.4. Measurement of Oxidative Stress Indicators
2.8. Antioxidant Capacity of DSCP and Ca-DSCP
2.8.1. DPPH Radical Scavenging Ability Assay
2.8.2. Hydroxyl Radical Scavenging Ability Assay
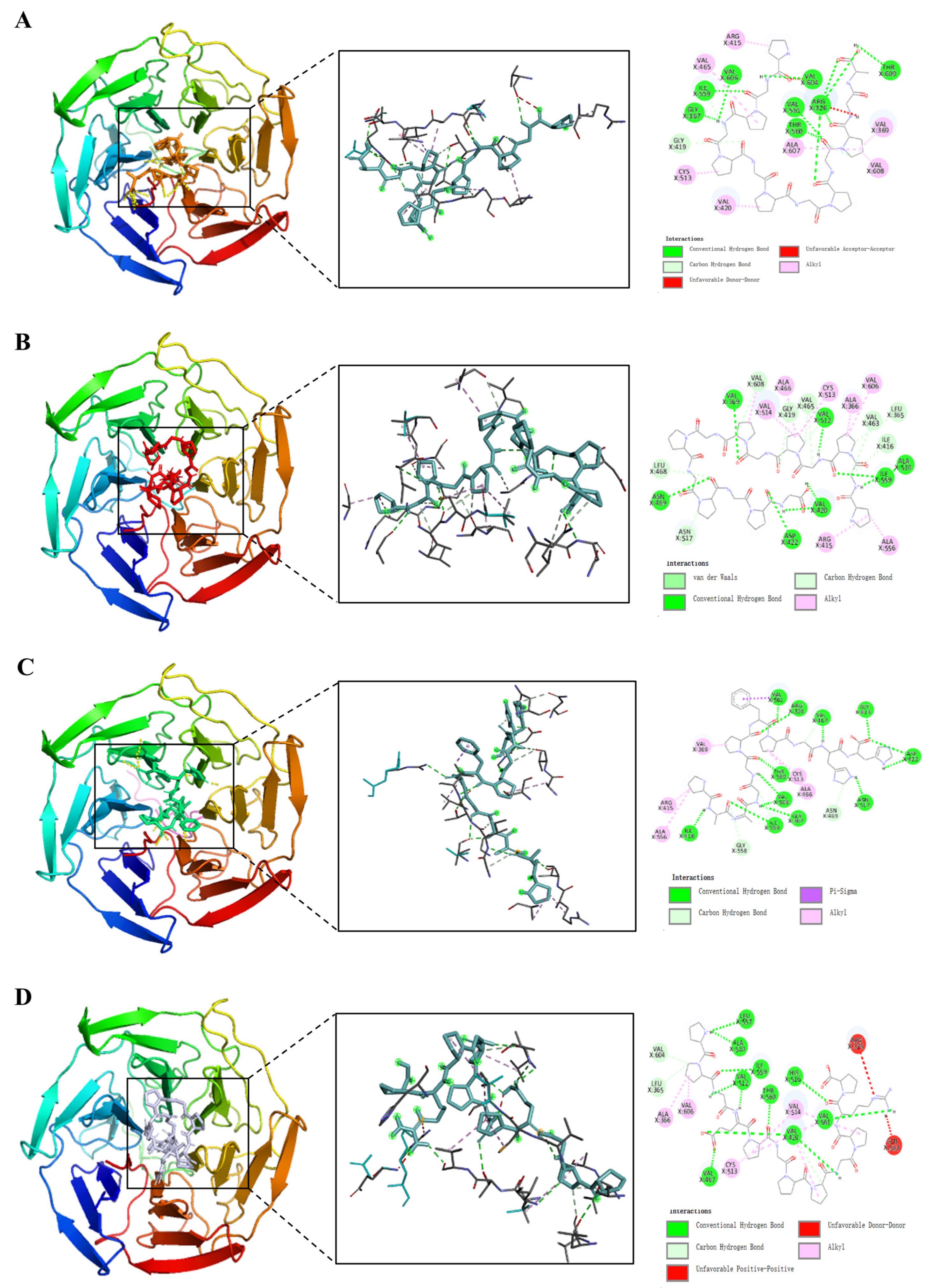
2.9. Bioinformatics and Molecular Docking
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Process Conditions on the Preparation of Ca-DSCP
3.2. Response Surface Analysis for the Preparation of Ca-DSCP
3.3. Amino Acid Composition
3.4. Zeta Potential and XRD Analysis
3.5. Structural Characterization of UV–vis and FTIR Analysis
3.6. Acid–Base Stability and Thermal Stability Analysis
3.7. In Vitro Gastrointestinal Digestion
3.8. Caco-2 Cytotoxicity Test
3.9. Caco-2 Cells Monolayer Calcium Transport Studies
3.10. Analysis of Oxidative Stress Indicators
3.11. DPPH Radical Scavenging Capacity of DSCP and Ca-DSCP
3.12. Hydroxyl Radical Scavenging Capacity of DSCP and Ca-DSCP
3.13. Bioinformatics and Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, C.M.; Peacock, M. Calcium. Adv. Nutr. 2019, 10, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Robinson, L.J.; Huang, C.L.H.; Sun, L.; Friedman, P.A.; Schlesinger, P.H.; Zaidi, M. Calcium and bone disease. Biofactors 2011, 37, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferran, H.A.; Bhasin, S.; Manson, J.E. Preventing Fractures and Falls A Limited Role for Calcium and Vitamin D Supplements? JAMA J. Am. Med. Assoc. 2018, 319, 1552–1553. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.J.; Fan, Y.; Hao, L.; Wang, J.; Xia, C.S.; Wang, J.F.; Hou, H. A comprehensive review of calcium and ferrous ions chelating peptides: Preparation, structure and transport pathways. Crit. Rev. Food Sci. 2023, 63, 4418–4430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, B.W.; Song, Z.H.; Deng, G.Y.; Shi, Y.; Li, T.Y.; Huang, Y.Q. Antioxidant activity analysis of collagen peptide-magnesium chelate. Polym. Test. 2023, 117, 107822. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, H.S.; Wang, S.J.; Wang, R.X.; Wang, Z.Z. Casein phosphopeptide-calcium chelate: Preparation, calcium holding capacity and simulated digestion. Food Chem. 2023, 401, 134218. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.J.; Zhang, P.L.; Sun, N.; Lin, S.Y. Elucidating the Calcium-Binding Site, Absorption Activities, and Thermal Stability of Egg White Peptide-Calcium Chelate. Foods 2021, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Li, Y.; Xiong, T.; Feng, Y.; Ma, H.; Akpabli-Tsigbe, N.D.K. Calcium-chelating improved zein peptide stability, cellular uptake, and bioactivity by influencing the structural characterization. Food Res. Int. 2022, 162, 112033. [Google Scholar] [CrossRef] [PubMed]
- Moravčíková, N.; Kasarda, R.; Židek, R.; McEwan, J.C.; Brauning, R.; Landete-Castillejos, T.; Chonco, L.; Ciberej, J.; Pokorádi, J. Traces of Human-Mediated Selection in the Gene Pool of Red Deer Populations. Animals 2023, 13, 2525. [Google Scholar] [CrossRef]
- Rehbein, S.; Lindner, T.; Visser, M.; Lutz, W.; Reindl, H. Distribution, prevalence, and intensity of Sarcocystis infections in sika deer (Cervus nippon) of free-ranging populations in Germany and Austria. Parasitol. Res. 2022, 121, 2079–2086. [Google Scholar] [CrossRef]
- Shirazi, S.; Broomandkhoshbacht, N.; Oppenheimer, J.; Metcalfe, J.Z.; Found, R.; Ives, J.W.; Shapiro, B. Ancient DNA-based sex determination of bison hide moccasins indicates Promontory cave occupants selected female hides for footwear. J. Archaeol. Sci. 2022, 137, 105533. [Google Scholar] [CrossRef]
- Blanco, M.; Vazquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ramella, M.; Pateiro, M.; Barba, F.J.; Franco, D.; Campagnol, P.C.B.; Munekata, P.E.S.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Microencapsulation of healthier oils to enhance the physicochemical and nutritional properties of deer pate. LWT-Food Sci. Technol. 2020, 125, 109223. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Purification and characterization of antioxidative peptides derived from chicken skin gelatin hydrolysate. Food Hydrocolloid 2018, 85, 311–320. [Google Scholar] [CrossRef]
- Saborirad, S.; Baghaei, H.; Hashemi-Moghaddam, H. Optimizing the ultrasonic extraction of polyphenols from mango peel and investigating the characteristics, antioxidant activity and storage stability of extract nanocapsules in maltodextrin/whey protein isolate. Ultrason. Sonochemistry 2024, 103, 106778. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yue, Y.; Pei, Q.; Zhang, S.; Ji, C.; Chen, Y.; Dai, Y.; Dong, L.; Zhu, B.; Lin, X. Excellent iron-chelating capacity of Yesso scallop (Patinopecten yessoensis) skirt hydrolysate fermented by Bacillus subtilis M17-b7. Food Biosci. 2024, 59, 103796. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.X.; Huai, H.P.; Hou, W.Y.; Qi, Y.; Leng, Y.; Liu, X.T.; Wang, X.Y.; Wu, D.; Min, W.H. Purification, Identification, Chelation Mechanism, and Calcium Absorption Activity of a Novel Calcium-Binding Peptide from Peanut (Arachis hypogaea) Protein Hydrolysate. J. Agric. Food Chem. 2023, 71, 11970–11981. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.M.; He, L.C.; Liang, Y.H.; Yue, L.L.; Peng, W.M.; Jin, G.F.; Ma, M.H. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis. Food Chem. 2019, 284, 80–89. [Google Scholar] [CrossRef]
- Song, L.; Chen, Y.; Liu, Z.T.; Zhu, L.X.; Song, L.S.; Zhang, M.R.; Xue, T.R.; Lv, B.F.; Liu, H.P.; Zhang, X.W. Preparation, characterization, and stability assessment of a nano-delivery system loaded with phosvitin phosphopeptide-calcium chelate. Food Biosci. 2023, 56, 103306. [Google Scholar] [CrossRef]
- Cao, Y.; Miao, J.Y.; Liu, G.; Luo, Z.; Xia, Z.M.; Liu, F.; Yao, M.F.; Cao, X.Q.; Sun, S.W.; Lin, Y.Y.; et al. Bioactive Peptides Isolated from Casein Phosphopeptides Enhance Calcium and Magnesium Uptake in Caco-2 Cell Monolayers. J. Agric. Food Chem. 2017, 65, 2307–2314. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ding, L.; Du, Z.Y.; Yu, Z.P.; Liu, J.B. Hydrolysis and Transport of Egg White-Derived Peptides in Caco-2 Cell Monolayers and Everted Rat Sacs. J. Agric. Food Chem. 2019, 67, 4839–4848. [Google Scholar] [CrossRef] [PubMed]
- Kheeree, N.; Kuptawach, K.; Puthong, S.; Sangtanoo, P.; Srimongkol, P.; Boonserm, P.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Discovery of calcium-binding peptides derived from defatted lemon basil seeds with enhanced calcium uptake in human intestinal epithelial cells, Caco-2. Sci. Rep. 2022, 12, 4659. [Google Scholar] [CrossRef]
- Liao, W.W.; Chen, H.; Jin, W.G.; Yang, Z.N.; Cao, Y.; Miao, J.Y. Three Newly Isolated Calcium-Chelating Peptides from Tilapia Bone Collagen Hydrolysate Enhance Calcium Absorption Activity in Intestinal Caco-2 Cells. J. Agric. Food Chem. 2020, 68, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.F.; Liang, Y.J.; Liu, G.; Lin, F.; Wen, X.B. Identification of antioxidant peptides after digestion and absorption of isinglass by serum peptidomics and cellular antioxidant activity analysis. Food Funct. 2023, 14, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Zhur, O.; Yang, Y.; Yin, T.T.; Yan, X.T.; Rao, H.L.; Xun, X.; Dong, X.; Wu, C.L.; He, H.L. Simultaneous preparation of antioxidant peptides and lipids from microalgae by pretreatment with bacterial proteases. Bioresour. Technol. 2022, 348, 126759. [Google Scholar] [CrossRef]
- Saidi, S.; Saoudi, M.; Ben Amar, R. Valorisation of tuna processing waste biomass: Isolation, purification and characterisation of four novel antioxidant peptides from tuna by-product hydrolysate. Environ. Sci. Pollut. Res. 2018, 25, 17383–17392. [Google Scholar] [CrossRef]
- Huang, P.T.; Miao, J.Y.; Li, J.L.; Li, Y.K.; Wang, X.H.; Yu, Y.; Cao, Y. Novel Antioxidant Peptides from Pearl Shell Meat Hydrolysate and Their Antioxidant Activity Mechanism. Molecules 2023, 28, 864. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wu, D.; Fang, L.; Leng, Y.; Wang, X.Y.; Liu, C.L.; Liu, X.T.; Wang, J.; Min, W.H. Anti-inflammatory effect of walnut-derived peptide via the activation of Nrf2/Keap1 pathway against oxidative stress. J. Funct. Foods 2023, 110, 105839. [Google Scholar] [CrossRef]
- Zhai, W.L.; Lin, D.; Mo, R.S.; Zou, X.Z.; Zhang, Y.Q.; Zhang, L.Y.; Ge, Y.H. Process Optimization, Structural Characterization, and Calcium Release Rate Evaluation of Mung Bean Peptides-Calcium Chelate. Foods 2023, 12, 1058. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Z.; Yao, X.; Fu, Y. Mineral-chelating peptides derived from fish collagen: Preparation, bioactivity and bioavailability. LWT 2020, 134, 110209. [Google Scholar] [CrossRef]
- Hu, G.H.; Wang, D.B.; Su, R.N.; Corazzin, M.; Liu, X.M.; Sun, X.Y.; Dou, L.; Liu, C.; Yao, D.; Sun, L.N.; et al. Calcium-binding capacity of peptides obtained from sheep bone and structural characterization and stability of the peptide-calcium chelate. J. Food Meas. Charact. 2022, 16, 4934–4946. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhao, Y.; Zeng, M. Enzymatic preparation and characterization of iron-chelating peptides from anchovy (Engraulis japonicus) muscle protein. Food Res. Int. 2012, 48, 435–441. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Shen, Q.; Qi, L.; Jiang, S.; Guo, Y.; Zhang, C.; Richel, A. Preparation of cattle bone collagen peptides-calcium chelate and its structural characterization and stability. LWT 2021, 144, 111264. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Zhao, H.; Zhao, R.; Xu, Y.; Lyu, X. Preparation of grape seed polypeptide and its calcium chelate with determination of calcium bioaccessibility and structural characterisation. Int. J. Food Sci. Technol. 2021, 56, 166–177. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xu, H.; Li, X.; Hao, X. Preparation of sheep bone collagen peptide–calcium chelate using enzymolysis-fermentation methodology and its structural characterization and stability analysis. RSC Adv. 2020, 10, 11624–11633. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Lu, J.; Gao, F.; Gu, R.-Z.; Lin, F.; Ren, D.-F.; Cai, M.-Y. Preparation, characterization and identification of calcium-chelating Atlantic salmon (Salmo salar L.) ossein oligopeptides. Eur. Food Res. Technol. 2015, 241, 851–860. [Google Scholar] [CrossRef]
- Sunde, H.; Ryder, K.; Bekhit, A.E.-D.A.; Carne, A. Analysis of peptides in a sheep beta lactoglobulin hydrolysate as a model to evaluate the effect of peptide amino acid sequence on bioactivity. Food Chem. 2021, 365, 130346. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, X.; Fang, J.; Wu, H.; Cheng, Y.; Gao, Y.; Chen, X.; Zheng, S.; Liu, S.; Hao, L. Peptide−Calcium Chelate from Antler (Cervus elaphus) Bone Enhances Calcium Absorption in Intestinal Caco-2 Cells and D-gal-Induced Aging Mouse Model. Nutrients 2022, 14, 3738. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, M.; Zhao, H.; Lv, Z.; Liang, L.; Zhang, B. A Novel Insight into Screening for Antioxidant Peptides from Hazelnut Protein: Based on the Properties of Amino Acid Residues. Antioxidants 2022, 11, 127. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- He, L.; Ying, L.; Jingting, X.; Chen, C.; Shuntang, G. Changes in the secondary structures and zeta potential of soybean peptide and its calcium complexes in different solution environments. Food Funct. 2021, 12, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, S.; Yang, Y.; Li, S.; Zhao, Z.; Wu, H. Caseinophosphopeptides Overcome Calcium Phytate Inhibition on Zinc Bioavailability by Retaining Zinc from Coprecipitation as Zinc/Calcium Phytate Nanocomplexes. J. Agric. Food Chem. 2024, 72, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Y.; He, H.; Guo, D.J.; Zhao, M.G.; Hou, T. Two novel calcium delivery systems fabricated by casein phosphopeptides and chitosan oligosaccharides: Preparation, characterization, and bioactive studies. Food Hydrocoll. 2020, 102, 105567. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Q.; Li, M.; Liu, H.; Wang, Q.; Wu, Y.; Niu, L.; Liu, Z. Isolation of a novel calcium-binding peptide from phosvitin hydrolysates and the study of its calcium chelation mechanism. Food Res. Int. 2021, 141, 110169. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, S.; Zhou, G.; Wang, J. Novel Casein-Derived Peptide-Zinc Chelate: Zinc Chelation and Transepithelial Transport Characteristics. J. Agric. Food Chem. 2023, 71, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Bingtong, L.; Yongliang, Z.; Liping, S. Identification and characterization of the peptides with calcium-binding capacity from tilapia (Oreochromis niloticus) skin gelatin enzymatic hydrolysates. J. Food Sci. 2020, 85, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Le, T.D.; Suttikhana, I. Stability and bioactivity of peptides in food matrices based on processing conditions. Food Res. Int. 2023, 168, 112786. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Q.; Zhou, Y.F.; Ma, M.Z.; Zhao, Y.D.; Xiang, X.W.; Shu, C.H.; Zheng, B. Preparation, Structural Characterization, and Stability of Low-Molecular-Weight Collagen Peptides-Calcium Chelate Derived from Tuna Bones. Foods 2023, 12, 3403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.J.; Shi, P.Q.; Li, Y.; Zhuang, Y.L.; You, L.Z.; Liu, L.; Wang, W. A novel ACE-inhibitory hexapeptide from camellia glutelin-2 hydrolysates: Identification, characterization and stability profiles under different food processing conditions. Lwt-Food Sci. Technol. 2021, 147, 111682. [Google Scholar] [CrossRef]
- Feng, L.; Peng, F.; Wang, X.J.; Li, M.; Lei, H.J.; Xu, H.D. Identification and characterization of antioxidative peptides derived from simulated in vitro gastrointestinal digestion of walnut meal proteins. Food Res. Int. 2019, 116, 518–526. [Google Scholar] [CrossRef]
- Cavaliere, C.; Montone, A.M.I.; Aita, S.E.; Capparelli, R.; Cerrato, A.; Cuomo, P.; Laganà, A.; Montone, C.M.; Piovesana, S.; Capriotti, A.L. Production and Characterization of Medium-Sized and Short Antioxidant Peptides from Soy Flour-Simulated Gastrointestinal Hydrolysate. Antioxidants 2021, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.J.; Kim, B.J.; Dallas, D.C. Bioavailability of Peptides Derived from the In Vitro Digestion of Human Milk Assessed by Caco-2 Cell Monolayers. J. Agric. Food Chem. 2022, 70, 7077–7084. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.; Zhuang, J.; Zhang, J.; Shen, F.; Yu, P.; Zhong, H.; Feng, F. Antiaging function of Chinese pond turtle (Chinemys reevesii) peptide through activation of the Nrf2/Keap1 signaling pathway and its structure-activity relationship. Front. Nutr. 2022, 9, 961922. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Tao, H.; Wang, S.; Xing, B.; Wang, Z.; Liu, K.; Shao, Q.; Gao, F. Identification and Functional Validation of Two Novel Antioxidant Peptides in Saffron. Antioxidants 2024, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, K.H.; Huang, P.; Golda-Cepa, M.; Xu, H.; Kotarba, A.; Hakkarainen, M. Scavenging of DPPH by Persistent Free Radicals in Carbonized Particles. Adv. Sustain. Syst. 2023, 7, 2200425. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.X.; Yang, Y.Y.; Zhang, Z.; Yang, K.X.; Sun, M.; Li, C.; Fan, Z.; Du, J.Z. In Situ Enzyme-Induced Self-Assembly of Antimicrobial-Antioxidative Peptides to Promote Wound Healing. Adv. Funct. Mater. 2023, 33, 2214454. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, Y.; Ren, T.Y.; Xiao, P.; Duan, J.A. Novel and efficient techniques in the discovery of antioxidant peptides. Crit. Rev. Food Sci. Nutr. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Miao, J.Y.; Chen, B.B.; Guo, J.B.; Ou, Y.Y.; Liang, X.T.; Yin, Y.Z.; Tong, X.; Cao, Y. Purification, identification, and antioxidative mechanism of three novel selenium-enriched oyster antioxidant peptides. Food Res. Int. 2022, 157, 111359. [Google Scholar] [CrossRef] [PubMed]
- Ortet, P.C.; Muellers, S.N.; Viarengo-Baker, L.A.; Streu, K.; Szymczyna, B.R.; Beeler, A.B.; Allen, K.N.; Whitty, A. Recapitulating the Binding Affinity of Nrf2 for KEAP1 in a Cyclic Heptapeptide, Guided by NMR, X-ray Crystallography, and Machine Learning. J. Am. Chem. Soc. 2021, 143, 3779–3793. [Google Scholar] [CrossRef]
- Abed, D.A.; Goldstein, M.; Albanyan, H.; Jin, H.J.; Hu, L.Q. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents. Acta Pharm. Sin. B 2015, 5, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, K.R.; Beck, P.; Qiu, R.; Bui, V.; Sheng, Z. Precision medicine: Novel PI3K inhibitor as cancer treatment. J. Clin. Oncol. 2022, 40, e15093. [Google Scholar] [CrossRef]



| No. | A-Mass Ratio of Peptide to Calcium | B-pH | C-Temperature | Chelation Rate % |
|---|---|---|---|---|
| 1 | 5:1 | 9 | 50 | 61.52 |
| 2 | 3:1 | 9 | 65 | 46.48 |
| 3 | 7:1 | 9 | 65 | 50.83 |
| 4 | 7:1 | 8 | 50 | 54.58 |
| 5 | 5:1 | 9 | 50 | 59.77 |
| 6 | 7:1 | 10 | 50 | 53.75 |
| 7 | 5:1 | 9 | 50 | 61.19 |
| 8 | 7:1 | 9 | 35 | 52.74 |
| 9 | 3:1 | 10 | 50 | 51.27 |
| 10 | 5:1 | 10 | 65 | 52.27 |
| 11 | 5:1 | 8 | 65 | 52.99 |
| 12 | 3:1 | 8 | 50 | 51.30 |
| 13 | 5:1 | 8 | 35 | 55.30 |
| 14 | 5:1 | 10 | 35 | 55.51 |
| 15 | 5:1 | 9 | 50 | 59.11 |
| 16 | 3:1 | 9 | 35 | 49.57 |
| 17 | 5:1 | 9 | 50 | 60.16 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 305.62 | 9 | 33.96 | 53.59 | <0.0001 | ** |
| A-Mass ratio of peptide to calcium | 21.95 | 1 | 21.95 | 34.63 | 0.0006 | ** |
| B-pH | 0.2244 | 1 | 0.2244 | 0.3542 | 0.5705 | |
| C-Temperature | 13.97 | 1 | 13.97 | 22.04 | 0.0022 | * |
| AB | 0.1681 | 1 | 0.1681 | 0.2653 | 0.6224 | |
| AC | 0.3782 | 1 | 0.3782 | 0.5968 | 0.4651 | |
| BC | 0.2209 | 1 | 0.2209 | 0.3486 | 0.5735 | |
| A2 | 144.90 | 1 | 144.90 | 228.64 | <0.0001 | ** |
| B2 | 12.95 | 1 | 12.95 | 20.43 | 0.0027 | * |
| C2 | 88.18 | 1 | 88.18 | 139.14 | <0.0001 | ** |
| Residual | 4.44 | 7 | 0.6337 | |||
| Lack of fit | 0.4515 | 3 | 0.1505 | 0.1511 | 0.9238 | not significant |
| Pure error | 3.98 | 4 | 0.9962 | |||
| Cor total | 310.06 | 16 |
| Peptide | Mass (Da) | Toxicity | Hydrophobicity (Kcal/mol) | Post-Digestive Fragments | Binding Energy (kcal/mol) | Hydrogen Bonds | Structural Formula | |
|---|---|---|---|---|---|---|---|---|
| Quantity | Binding Sites | |||||||
| A | 1014.11 | None | 16.14 | PGPGPGPGPGPGA | −12.1 | 13 | VAL561, VAL604, VAL606, ILE559, GLY367, GLY419, THR,560, THR609, ARG326 |  |
| B | 1097.2 | None | 16.93 | PGPGPGPGPGPGPG | −11.9 | 18 | LEU365, LEU468, ASN469, ASN517, VAL369, VAL420, VAL463, VAL465, VAL512, VAL608, GLY419, ILE416, ILE559, ASP442, ALA510 |  |
| C | 1044.14 | None | 15.72 | PAAGGPFPGHH | −11.2 | 17 | ILE416, ILE559, GLY367, GLY423, GLY558, VAL467, VAL561, VAL606, THR560, ASN469, ASN517, ASP422, ARG362 | 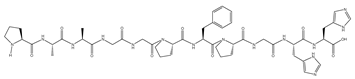 |
| D | 1154.29 | None | 17.77 | PPGEPGPPGPRP | −11.0 | 14 | VAL420, VAL467, VAL512, VAL516, VAL604, LEU365, LEU557, ALA510, ILE559, THR560, HIS516, |  |
| E | 1142.24 | None | 16.91 | PGPGPGPGQAPPGG | −10.9 | 6 | ILE559, VAL420, VAL606, GLY367, ARG326 |  |
| F | 1003.08 | None | 14.26 | PGPSPGPGPSPG | −10.9 | 13 | VAL418, VAL420, VAL463, VAL465, VAL467, ASP422, THR560, ARG326 |  |
| G | 873.96 | None | 14.2 | PGPAGPPGAPG | −10.9 | 8 | VAL369, VAL418, VAL420, VAL463, VAL465, ASP422, ASN469, IEU365 | 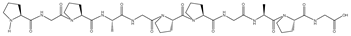 |
| H | 1171.34 | None | 15.11 | MPGPGPGPGPGPGP | −10.8 | 15 | ARG326, GLN563, GLY367, GLY419, GLY564, ASN469, VAL512, ILE559, LEU557, ALA510, VAL606 | 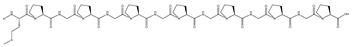 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, R.; Sun, L.; Liu, J.; Gao, F.; Guo, X.; Shi, M.; Guo, P.; Chen, W.; Zong, Y.; Geng, J.; et al. Deer Skin Collagen Peptides Bound to Calcium: In Vitro Gastrointestinal Simulation of Digestion, Cellular Uptake and Analysis of Antioxidant Activity. Nutrients 2024, 16, 2585. https://doi.org/10.3390/nu16162585
Du R, Sun L, Liu J, Gao F, Guo X, Shi M, Guo P, Chen W, Zong Y, Geng J, et al. Deer Skin Collagen Peptides Bound to Calcium: In Vitro Gastrointestinal Simulation of Digestion, Cellular Uptake and Analysis of Antioxidant Activity. Nutrients. 2024; 16(16):2585. https://doi.org/10.3390/nu16162585
Chicago/Turabian StyleDu, Rui, Li Sun, Jinze Liu, Fusheng Gao, Xiangjuan Guo, Meiling Shi, Pengli Guo, Weijia Chen, Ying Zong, Jianan Geng, and et al. 2024. "Deer Skin Collagen Peptides Bound to Calcium: In Vitro Gastrointestinal Simulation of Digestion, Cellular Uptake and Analysis of Antioxidant Activity" Nutrients 16, no. 16: 2585. https://doi.org/10.3390/nu16162585
APA StyleDu, R., Sun, L., Liu, J., Gao, F., Guo, X., Shi, M., Guo, P., Chen, W., Zong, Y., Geng, J., Zhao, Y., & He, Z. (2024). Deer Skin Collagen Peptides Bound to Calcium: In Vitro Gastrointestinal Simulation of Digestion, Cellular Uptake and Analysis of Antioxidant Activity. Nutrients, 16(16), 2585. https://doi.org/10.3390/nu16162585






