Methylsulfinyl Hexyl Isothiocyanate (6-MSITC) from Wasabi Is a Promising Candidate for the Treatment of Cancer, Alzheimer’s Disease, and Obesity
Abstract
1. Introduction
Search Strategy and Selection Criteria
2. The Chemical and Biological Structure of 6-MSITC
3. Cellular Location and Cytological and Biological Significance of 6-MSITC
4. LADME (Liberation, Absorption, Distribution, Metabolism, Excretion/Elimination) of 6-MSITC
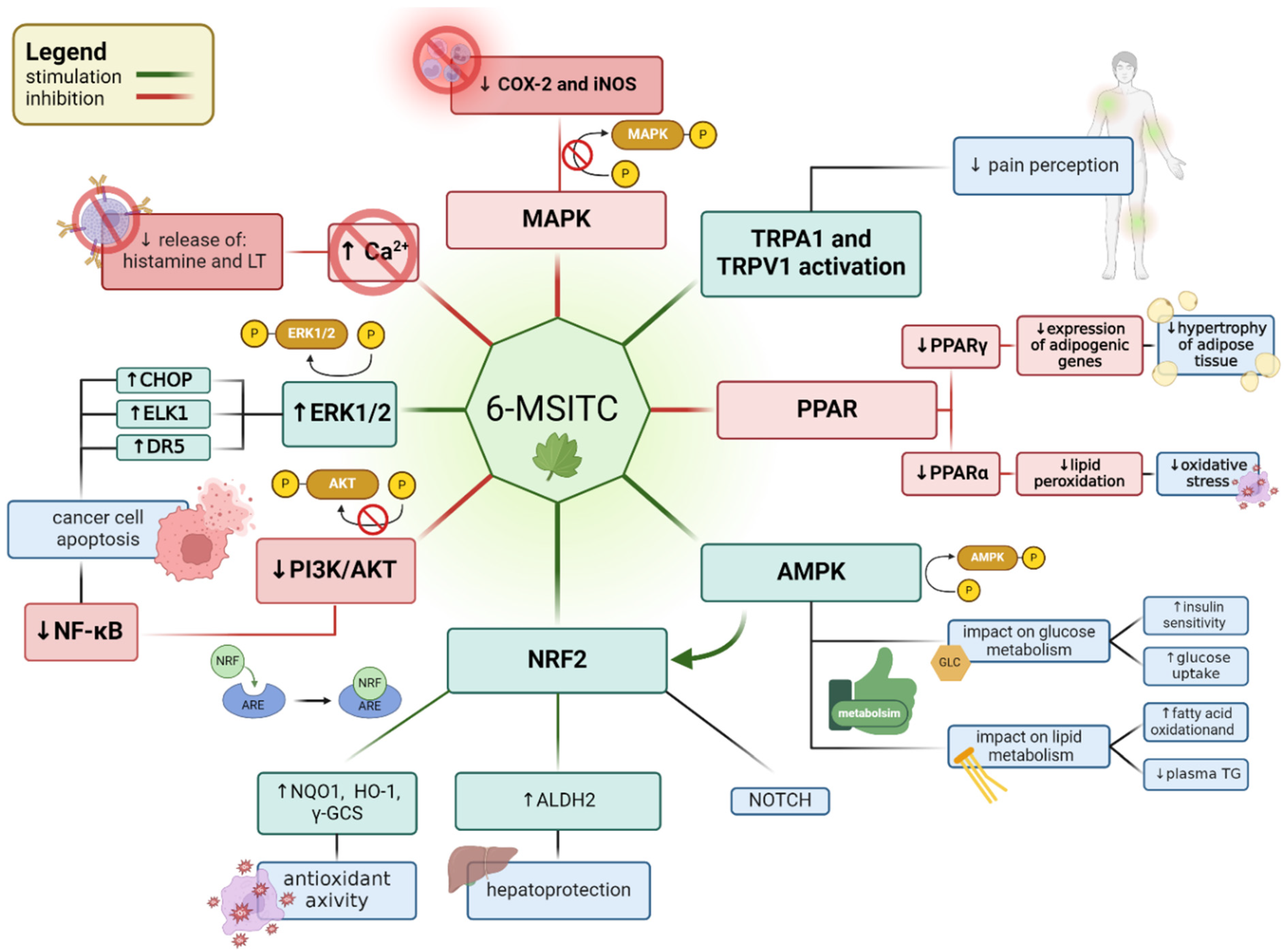
5. Involvement of Wasabi Extracts/6-MSITC in Cellular Signaling Pathways
5.1. PPAR Signaling Pathway
5.2. AMP-Activated Protein Kinase (AMPK) Signaling Pathway
5.3. The Nrf2/Keap1-ARE Pathway
5.4. ERK1/2-ELK1/CHOP/DR5 Pathway
5.5. The MAPK Pathways
5.6. PI3K/AKT/mTOR Pathway
5.7. Inflammatory and Anti-Inflammatory Pathways
6. Other Activities of 6-MSITC
7. 6-MSITC and Cancer
7.1. Cancer Cell Lines
7.2. Colorectal and Stomach Cancer Studies
7.3. Breast Cancer Studies
7.4. Metastasis Studies
8. Neuroprotective Effects of 6-MSITC
8.1. 6-MSITC in Alzheimer’s Disease
8.2. 6-MSITC in Parkinson’s Disease
8.3. 6-MSITC in Chronic Fatigue Syndrome
8.4. 6-MSITC and Cognitive Functions
9. 6-MSITC and the Endothelium
10. 6-MSITC in Diet-Induced Metabolic Syndrome
11. Discussion
12. Summary
13. Concussions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PS | performance status |
| ME | myalgic encephalomyelitis |
| CFS | chronic fatigue syndrome |
| TMT-A | Trail Making test-A |
| STAT3 | signal transducer and activator of transcription 3 |
| NF-κB | nuclear factor |
| ITC | isothiocyanate |
| AMPKα | AMP-activated protein kinase α |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| FOXO1 | Forkhead box protein O1 |
| PPARα | peroxisome proliferator–activated receptor α |
| ALDH2 | aldehyde dehydrogenase |
| ARE | antioxidant response element |
| EC | Endothelial cells |
| mTRPA1 | mouse transient receptor potential ankyrin 1 |
| hTRPA1 | human transient receptor potential ankyrin 1 |
| mTRPV1 | mouse transient receptor potential vanilloid 1 |
| LPS | lipopolysaccharide |
| iNOS | inducible nitric oxide synthase |
| Jak2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinase |
| CCL11 | C-C Motif Chemokine Ligand 11 |
| CCL25 | C-C Motif Chemokine Ligand 25 |
| IL3 | Interleukin 3 |
| IL1ra12 | Interleukin 1 Receptor Antagonist 12 |
| IL8ra | Interleukin 8 Receptor Alpha |
| TNFRSF23 | Tumor Necrosis Factor Receptor Superfamily Member 23 |
| TNFRSF4 | Tumor Necrosis Factor Receptor Superfamily Member 4 |
| TNF-a | tumor necrosis factor-alpha |
References
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Shiffman, J.; Shawar, Y.R.; Åkesson, K.; Ali, N.; Woolf, A.D. Global health policy in the 21st century: Challenges and opportunities to arrest the global disability burden from musculoskeletal health conditions. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101549. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Ogawa, T.; Wang, L.; Katsube, T.; Yamasaki, Y.; Sun, X.; Shiwaku, K. Anti-obesity effects of hot water extract from wasabi (Wasabia japonica Matsum.) leaves in mice fed high-fat diets. Nutr. Res. Pract. 2013, 7, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J. Glucosinolates and their breakdown products in food and food plants. Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Shinoda, S.; Yamori, T.; Sawaki, S.; Nagata, I.; Ryoyama, K.; Fuke, Y. Selective sensitivity to wasabi-derived 6-(methylsulfinyl)hexyl isothiocyanate of human breast cancer and melanoma cell lines studied in vitro. Cancer Detect. Prev. 2005, 29, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Morimitsu, Y.; Hayashi, K.; Nakagawa, Y.; Horio, F.; Uchida, K.; Osawa, T. Antiplatelet and anticancer isothiocyanates in Japanese domestic horseradish, wasabi. Biofactors 2000, 13, 271–276. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Protective Effects of 6-(Methylsulfinyl)hexyl Isothiocyanate on Aβ1-42-Induced Cognitive Deficit, Oxidative Stress, Inflammation, and Apoptosis in Mice. Int. J. Mol. Sci. 2018, 19, 2083. [Google Scholar] [CrossRef]
- Zhang, Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 2012, 33, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Hou, D.X.; Morinaga, O.; Shoyama, Y. Molecular Mechanisms Underlying Anti-Inflammatory Actions of 6-(Methylsulfinyl)hexyl Isothiocyanate Derived from Wasabi (Wasabia japonica). Adv. Pharmacol. Sci. 2012, 2012, 614046. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, C.; Lewis, D.F. Cytochromes P450 in the bioactivation of chemicals. Curr. Top. Med. Chem. 2004, 4, 1767–1788. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, C.; Konsue, N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab. Rev. 2015, 47, 356–373. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Hirose, Y.; Yamada, Y.; Imaida, K.; Tatematsu, K.; Mori, Y.; Mori, H. Chemoprevention of 1,2-dimethylhydrazine-induced colonic preneoplastic lesions in Fischer rats by 6-methylsulfinylhexyl isothiocyanate, a wasabi derivative. Oncol. Lett. 2010, 1, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Hirose, Y.; Yamada, Y.; Imaida, K.; Tatematsu, K.; Mori, Y.; Mori, H. Inhibitory effects of 6-methylsulfinylhexyl isothiocyanate on the development of colonic aberrant crypt foci induced by azoxymethane in rats. Exp. Toxicol. Pathol. 2010, 62, 259–264. [Google Scholar]
- Mori, Y.; Koide, A.; Fuwa, K.; Kobayashi, Y. N-benzylimidazole for preparation of S9 fraction with multi-induction of metabolizing enzymes in short-term genotoxicity assays. Mutagenesis 2001, 16, 479–486. [Google Scholar] [CrossRef][Green Version]
- Mori, Y.; Tatematsu, K.; Koide, A.; Sugie, S.; Tanaka, T.; Mori, H. Modification by curcumin of mutagenic activation of carcinogenic N-nitrosamines by extrahepatic cytochromes P-450 2B1 and 2E1 in rats. Cancer Sci. 2006, 97, 896–904. [Google Scholar] [CrossRef]
- Mori, Y.; Takahashi, H.; Yamazaki, H.; Toyoshi, K.; Makino, T.; Yokose, Y.; Konishi, Y. Distribution, metabolism and excretion of N-nitrosobis(2-hydroxypropyl)amine in Wistar rats. Carcinogenesis 1984, 5, 1443–1447. [Google Scholar] [CrossRef]
- Morimitsu, Y.; Nakagawa, Y.; Hayashi, K.; Fujii, H.; Kumagai, T.; Nakamura, Y.; Osawa, T.; Horio, F.; Itoh, K.; Iida, K.; et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J. Biol. Chem. 2002, 277, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Fujii, M.; Hou, D.-X. Inhibition of Lipopolysaccharide-Induced Cyclooxygenase-2 Transcription by 6-(Methylsulfinyl) Hexyl Isothiocyanate, a Chemopreventive Compound from Wasabia japonica (Miq.) Matsumura, in Mouse Macrophages. Biochem. Pharmacol. 2005, 70, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Fujii, M.; Hou, D.X. Effects of 6-(methylsulfinyl)hexyl isothiocyanate on cyclooxygenase-2 expression induced by lipopolysaccharide, interferon-gamma and 12-O-tetradecanoylphorbol-13-acetate. Oncol. Rep. 2007, 17, 233–238. [Google Scholar] [PubMed]
- Chen, J.H.; Uto, T.; Tanigawa, S.; Kumamoto, T.; Fujii, M.; Hou, D.X. Expression profiling of genes targeted by bilberry (Vaccinium myrtillus) in macrophages through DNA microarray. Nutr. Cancer. 2008, 60, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Uto, T.; Tanigawa, S.; Yamada-Kato, T.; Fujii, M.; Hou, D.X. Microarray-based determination of anti-inflammatory genes targeted by 6-(methylsulfinyl)hexyl isothiocyanate in macrophages. Exp. Ther. Med. 2010, 1, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Korenori, Y.; Tanigawa, S.; Yamada-Kato, T.; Nagai, M.; He, X.; He, J. Dynamics of Nrf2 and Keap1 in ARE-mediated NQO1 expression by wasabi 6-(methylsulfinyl)hexyl isothiocyanate. J. Agric. Food Chem. 2011, 59, 11975–11982. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Miura, Y.; Nagai, M.; Tominaga, M. Isothiocyanates from Wasabia japonica activate transient receptor potential ankyrin 1 channel. Chem. Senses 2012, 37, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Akita, N.; Nagai, M.; Hayashi, T.; Suzuki, K. 6-Methylsulfinylhexyl isothiocyanate modulates endothelial cell function and suppresses leukocyte adhesion. J. Nat. Med. 2014, 68, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Morroni, F.; Sita, G.; Tarozzi, A.; Cantelli-Forti, G.; Hrelia, P. Neuroprotection by 6-(methylsulfinyl) hexyl isothiocyanate in a 6-hydroxydopamine mouse model of Parkinson’s disease. Brain Res. 2014, 1589, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Fuke, Y.; Hishinuma, M.; Namikawa, M.; Oishi, Y.; Matsuzaki, T. Wasabi-derived 6-(methylsulfinyl)hexyl isothiocyanate induces apoptosis in human breast cancer by possible involvement of the NF-κB pathways. Nutr. Cancer 2014, 66, 879–887. [Google Scholar] [CrossRef]
- Trio, P.Z.; Fujisaki, S.; Tanigawa, S.; Hisanaga, A.; Sakao, K.; Hou, D.X. DNA Microarray Highlights Nrf2-Mediated Neuron Protection Targeted by Wasabi-Derived Isothiocyanates in IMR-32 Cells. Gene Regul. Syst. Biol. 2016, 10, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Trio, P.Z.; Kawahara, A.; Tanigawa, S.; Sakao, K.; Hou, D.X. DNA Microarray Profiling Highlights Nrf2-Mediated Chemoprevention Targeted by Wasabi-Derived Isothiocyanates in HepG2 Cells. Nutr. Cancer 2017, 69, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.X. Wasabi 6-(methylsulfinyl)hexyl isothiocyanate induces apoptosis in human colorectal cancer cells through p53-independent mitochondrial dysfunction pathway. BioFactors 2018, 44, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective Effect of Caffeic Acid Phenethyl Ester in A Mouse Model of Alzheimer’s Disease Involves Nrf2/HO-1 Pathway. Aging Dis. 2018, 9, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.X. Involvement of ERK1/2-mediated ELK1/CHOP/DR5 pathway in 6-(methylsulfinyl)hexyl isothiocyanate-induced apoptosis of colorectal cancer cells. Biosci. Biotechnol. Biochem. 2019, 83, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Muraki, K.; Fuse, Y.; Kobayashi, M. Evaluation of Antioxidant Activity of Spice-Derived Phytochemicals Using Zebrafish. Int. J. Mol. Sci. 2020, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Yuan, S.; Inoue, M.; Yoshioka, Y.; Yamashita, Y.; Ashida, H. 6-(Methylsulfinyl)hexyl isothiocyanate protects acetaldehyde-caused cytotoxicity through the induction of aldehyde dehydrogenase in hepatocytes. Arch. Biochem. Biophys. 2020, 686, 108329. [Google Scholar] [CrossRef] [PubMed]
- Uruno, A.; Matsumaru, D.; Ryoke, R.; Saito, R.; Kadoguchi, S.; Saigusa, D.; Saito, T.; Saido, T.C.; Kawashima, R.; Yamamoto, M. Nrf2 Suppresses Oxidative Stress and Inflammation in App Knock-In Alzheimer’s Disease Model Mice. Mol. Cell. Biol. 2020, 40, e00467-19. [Google Scholar] [CrossRef]
- Tanabe, Y.; Akazawa, N.; Nishimaki, M.; Shimizu, K.; Fujii, N.; Takahashi, H. Effects of 6-(Methylsulfinyl)hexyl Isothiocyanate Ingestion on Muscle Damage after Eccentric Exercise in Healthy Males: A Pilot Placebo-Controlled Double-Blind Crossover Study. J. Diet. Suppl. 2022, 19, 656–671. [Google Scholar] [CrossRef]
- Oka, T.; Yamada, Y.; Lkhagvasuren, B.; Nakao, M.; Nakajima, R.; Kanou, M.; Hiramatsu, R.; Nabeshima, Y. Clinical Effects of Wasabi Extract Containing 6-MSITC on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Open-Label Trial. Biopsychosoc. Med. 2022, 16, 26. [Google Scholar] [CrossRef]
- Shimoyama, M.; Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Hosaka, K. 6-(Methylsulfinyl) Hexyl Isothiocyanate Inhibits IL-6 and CXCL10 Production in TNF-α-Stimulated Human Oral Epithelial Cells. Curr. Issues Mol. Biol. 2022, 44, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, F.S.; Tan, Y.P.; Williams, C.M.; Ward, L.C.; Worrall, S.; Panchal, S.K. Wasabi (Eutrema japonicum) Reduces Obesity and Blood Pressure in Diet-Induced Metabolic Syndrome in Rats. Foods 2022, 11, 3435. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xie, K.; Chen, K.; He, Z.; Sakao, K.; Hou, D.X. Involvement of AMP-activated Protein Kinase α/Nuclear Factor (Erythroid-derived 2) Like 2-initiated Signaling Pathway in Cytoprotective Effects of Wasabi 6-(Methylsulfinyl) Hexyl Isothiocyanate. J. Cancer Prev. 2022, 27, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Kawata, N.Y.S.; Saito, T.; Nouchi, H.; Kawashima, R. Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial. Nutrients 2023, 15, 4608. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Kanou, M.; Tokushima, M.; Iwama, Y.; Yamana, K. Oral Administration of 6-Methylsulfinylhexyl Isothiocyanate Extracted from Wasabi Is Safe and Improves the Fatigue and Sleep of Healthy Volunteers. Biopsychosoc. Med. 2023, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Barish, G.D.; Narkar, V.A.; Evans, R.M. PPARδ: A dagger in the heart of the metabolic syndrome. J. Clin. Investig. 2006, 116, 590–597. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Ogawa, T.; Tabata, H.; Katsube, T.; Ohta, Y.; Yamasaki, Y.; Yamasaki, M.; Shiwaku, K. Suppressive effect of hot water extract of wasabi (Wasabia japonica Matsum.) leaves on the differentiation of 3T3-L1 preadipocytes. Food Chem. 2010, 118, 239–244. [Google Scholar] [CrossRef]
- Oowatari, Y.; Ogawa, T.; Katsube, T.; Iinuma, K.; Yoshitomi, H.; Gao, M. Wasabi leaf extracts attenuate adipocyte hypertrophy through PPARγ and AMPK. Biosci. Biotechnol. Biochem. 2016, 80, 1594–1601. [Google Scholar] [CrossRef]
- Van Raalte, D.H.; Li, M.; Pritchard, P.H.; Wasan, K.M. Peroxisome proliferator-activated receptor (PPAR)-alpha: A pharmacological target with a promising future. Pharm. Res. 2004, 21, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Massiah, M.A.; Bozak, R.E.; Hicks, R.J.; Talalay, P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 2001, 98, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response element. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Bray, S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell. Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.F.; Chaudhary, L.; Fausto, A.; Halstead, L.R.; Ory, D.S.; Avioli, L.V.; Cheng, S.L. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J. Biol. Chem. 2001, 276, 14443–14450. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Abrams, S.L.; Lee, J.T.; Chang, F.; Bertrand, F.E.; Navolanic, P.M.; Terrian, D.M.; Franklin, R.A.; D’Assoro, A.B.; et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzym. Regul. 2006, 46, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83, Erratum in Microbiol. Mol. Biol. Rev. 2012, 76, 496.. [Google Scholar] [CrossRef]
- Wu, P.K.; Becker, A.; Park, J.I. Growth Inhibitory Signaling of the Raf/MEK/ERK Pathway. Int. J. Mol. Sci. 2020, 21, 5436. [Google Scholar] [CrossRef]
- Brennan, A.; Leech, J.T.; Kad, N.M.; Mason, J.M. Selective antagonism of cJun for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.C.; Young, M.R.; Cmarik, J.; Colburn, N.H. Activator protein 1 (AP-1)- and nuclear factor κB (NF-κB)-dependent transcriptional events in carcinogenesis. Free Radic. Biol. Med. 2000, 28, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bishnoi, M.; Khare, P.; Brown, L.; Panchal, S.K. Transient receptor potential (TRP) channels: A metabolic TR(i)P to obesity prevention and therapy. Obes. Rev. 2018, 19, 1269–1292. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Kato, T.; Nagai, M.; Ohnishi, M.; Yoshida, K. Inhibitory effects of wasabi isothiocyanates on chemical mediator release in RBL-2H3 rat basophilic leukemia cells. J. Nutr. Sci. Vitaminol. 2012, 58, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Amador, M.A.; Ribeiro Dos Santos, A.M.; Carvalho, D.C.; Andrade, R.B.; Pereira, E.E.; Fernandes, M.R.; Costa, D.F.; Santos, N.P.; Assumpção, P.P.; et al. Analysis of 12 variants in the development of gastric and colorectal cancers. World J. Gastroenterol. 2017, 23, 8533–8543. [Google Scholar] [CrossRef]
- Tanida, N.; Kawaura, A.; Takahashi, A.; Sawada, K.; Shimoyama, T. Suppressive effect of wasabi (pungent Japanese spice) on gastric carcinogenesis induced by MNNG in rats. Nutr. Cancer 1991, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fuke, Y.; Shinoda, S.; Nagata, I.; Sawaki, S.; Murata, M.; Ryoyama, K.; Koizumi, K.; Saiki, I.; Nomura, T. Preventive effect of oral administration of 6-MITC derived from wasabi against pulmonary metastasis of B16-BL6 mouse melanoma cells. Cancer Detect. Prev. 2006, 30, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Okunishi, I.; Yamada-Kato, T.; Saito, J. The Effects of Wasabi Root-Derived 6-(Methylsulfinyl) Hexyl Isothiocyanate on Neurocognitive Functions in Cognitively Intact Middle-Aged and Older Adults-a Randomized, Double-Blind, Placebo-Controlled Trial. Jpn. Pharmacol. Ther. 2019, 47, 275–286. [Google Scholar]
- Gu, M.-Y.; Kim, J.; Yang, H.O. The Neuroprotective Effects of Justicidin A on Amyloid Beta25-35-Induced Neuronal Cell Death Through Inhibition of Tau Hyperphosphorylation and Induction of Autophagy in SH-SY5Y Cells. Neurochem. Res. 2016, 41, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Carzaniga, R.; Gentleman, S.M.; Van Leuven, F.; Walter, J.; Sastre, M. Glycogen Synthase Kinase 3 Inhibition Promotes Lysosomal Biogenesis and Autophagic Degradation of the Amyloid-β Precursor Protein. Mol. Cell. Biol. 2012, 32, 4410–4418. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.J.; Park, H.R.; Kim, M.E.; Piao, S.; Lee, E.; Jo, D.-G.; Chung, H.Y.; Ha, N.-C.; Mattson, M.P.; Lee, J. Morin Attenuates Tau Hyperphosphorylation by Inhibiting GSK3β. Neurobiol. Dis 2011, 44, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-C. Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) and Mitochondrial Dynamics/Mitophagy in Neurological Diseases. Antioxidants 2020, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.; Ammit, A.J. Targeting P38 MAPK Pathway for the Treatment of Alzheimer’s Disease. Neuropharmacology 2010, 58, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, S.A.L.; Eales, K.L. The Role of P38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease. J. Signal Transduct. 2012, 2012, 649079. [Google Scholar] [CrossRef]
- Sanderson, T.M.; Hogg, E.L.; Collingridge, G.L.; Corrêa, S.A.L. Hippocampal Metabotropic Glutamate Receptor Long-Term Depression in Health and Disease: Focus on Mitogen-Activated Protein Kinase Pathways. J. Neurochem. 2016, 139 (Suppl. S2), 200–214. [Google Scholar] [CrossRef]
- Lauretti, E.; Praticò, D. Glucose Deprivation Increases Tau Phosphorylation via P38 Mitogen-Activated Protein Kinase. Aging Cell 2015, 14, 1067–1074. [Google Scholar] [CrossRef]
- Ito, Y.; Oh-Hashi, K.; Kiuchi, K.; Hirata, Y. P44/42 MAP Kinase and c-Jun N-Terminal Kinase Contribute to the up-Regulation of Caspase-3 in Manganese-Induced Apoptosis in PC12 Cells. Brain Res. 2006, 1099, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.; Heizer, J.W.; Li, Y.; Chapman, K.; Ogden, C.A.; Andreasen, K.; Shapland, E.; Kucera, G.; Mogan, J.; Humann, J.; et al. Transient Receptor Potential Melastatin 2 (TRPM2) Ion Channel Is Required for Innate Immunity against Listeria Monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 11578–11583. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Tarozzi, A.; Rimondini, R.; Hrelia, P. Early Effects of Aβ1-42 Oligomers Injection in Mice: Involvement of PI3K/Akt/GSK3 and MAPK/ERK1/2 Pathways. Behav. Brain Res. 2016, 314, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Wei, J.; Shang, Y.-H.; Huang, H.-C.; Lao, F.-X. Modulation of AβPP and GSK3β by Endoplasmic Reticulum Stress and Involvement in Alzheimer’s Disease. J. Alzheimers Dis 2017, 57, 1157–1170. [Google Scholar] [CrossRef]
- Lucas, J.J.; Hernández, F.; Gómez-Ramos, P.; Morán, M.A.; Hen, R.; Avila, J. Decreased Nuclear Beta-Catenin, Tau Hyperphosphorylation and Neurodegeneration in GSK-3beta Conditional Transgenic Mice. EMBO J. 2001, 20, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, A.; Brewerton, S.; Bell, A.; Sargent, S.; Glover, S.; Hardy, C.; Moore, R.; Calley, J.; Ramachandran, D.; Poidinger, M.; et al. An Unbiased Approach to Identifying Tau Kinases That Phosphorylate Tau at Sites Associated with Alzheimer Disease. J. Biol. Chem. 2013, 288, 23331–23347. [Google Scholar] [CrossRef]
- Dejeans, N.; Manié, S.; Hetz, C.; Bard, F.; Hupp, T.; Agostinis, P.; Samali, A.; Chevet, E. Addicted to Secrete—Novel Concepts and Targets in Cancer Therapy. Trends Mol. Med. 2014, 20, 242–250. [Google Scholar] [CrossRef]
- Moussa, N.; Dayoub, N. Exploring the Role of COX-2 in Alzheimer’s Disease: Potential Therapeutic Implications of COX-2 Inhibitors. Saudi Pharm. J. 2023, 31, 101729. [Google Scholar] [CrossRef]
- Jung, H.Y.; Yoo, D.Y.; Nam, S.M.; Kim, J.W.; Kim, W.; Kwon, H.J.; Lee, K.Y.; Choi, J.H.; Kim, D.W.; Yoon, Y.S.; et al. Postnatal Changes in Constitutive Cyclooxygenase-2 Expression in the Mice Hippocampus and Its Function in Synaptic Plasticity. Mol. Med. Rep. 2019, 19, 1996–2004. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Costa, V.; Pomatto, L.C.-D.; Davies, K.J.A. The Proteasome and Oxidative Stress in Alzheimer’s Disease. Antioxid. Redox Signal. 2016, 25, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Hanrott, K.; Gudmunsen, L.; O’Neill, M.J.; Wonnacott, S. 6-Hydroxydopamine-Induced Apoptosis Is Mediated via Extracellular Auto-Oxidation and Caspase 3-Dependent Activation of Protein Kinase Cdelta. J. Biol. Chem. 2006, 281, 5373–5382. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Pathogenesis of Parkinson’s Disease: Dopamine, Vesicles and Alpha-Synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Kume, T.; Muto, C.; Takada-Takatori, Y.; Izumi, Y.; Sugimoto, H.; Akaike, A. Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2 (Nrf2)—Antioxidant-response element (ARE) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J. Pharmacol. Sci. 2011, 115, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef]
- Nouchi, R.; Hu, Q.; Saito, T.; Kawata, N.Y.D.S.; Nouchi, H.; Kawashima, R. Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial. Nutrients 2021, 13, 352. [Google Scholar] [CrossRef]
- Immanuel, J.; Yun, S. Vascular Inflammatory Diseases and Endothelial Phenotypes. Cells 2023, 12, 1640. [Google Scholar] [CrossRef]
- Giesen, P.L.; Nemerson, Y. Tissue factor on the loose. Semin. Thromb. Hemost. 2000, 26, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Schneppenheim, R.; Budde, U. von Willebrand factor: The complex molecular genetics of a multidomain and multifunctional protein. J. Thromb. Haemost. 2011, 9 (Suppl. S1), 209–215. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Chavakis, T. Leukocyte-endothelial interactions in inflammation. J. Cell. Mol. Med. 2009, 13, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Kashima, N.; Seki, T.; Sakurai, H.; Ishii, K.; Ariga, T. Analysis of Volatile Components in Essential Oil of Upland Wasabi and Their Inhibitory Effects on Platelet Aggregation. Biosci. Biotechnol. Biochem. 1994, 58, 2131–2135. [Google Scholar] [CrossRef]
- Mitrovic, B.; Gluvic, Z.M.; Obradovic, M.; Radunovic, M.; Rizzo, M.; Banach, M.; Isenovic, E.R. Non-alcoholic fatty liver disease, metabolic syndrome, and type 2 diabetes mellitus: Where do we stand today? Arch. Med. Sci. 2022, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Lyon, A.R.; Ghadri, J.R.; Templin, C. Takotsubo syndrome: Aetiology, presentation and treatment. Heart 2017, 103, 1461–1469. [Google Scholar] [CrossRef]
- Finkel-Oron, A.; Olchowski, J.; Jotkowitz, A.; Barski, L. Takotsubo cardiomyopathy triggered by wasabi consumption: Can sushi break your heart? BMJ Case Rep. 2019, 12, e230065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| References/ Publication Year | Type and Duration of Experiment | Wasabi/6-MSITC Dose/Medium/Diet | Findings | |
|---|---|---|---|---|
| 1. | [21]/2002 | In vitro/in vivo/5 days rat liver epithelial RL 34 cells | Eagle’a medium 15 μM per 5 days | 6-HITC activated the antioxidant response element (ARE), 6-HITC induced the nuclear localization of the transcription factor Nrf2, which binds to the ARE, and the induction of phase II enzyme genes by 6-HITC was completely blocked in Nrf2 knockout mice. 6-HITC may be a potential activator of the Nrf2/ARE-dependent detoxification pathway. |
| In vivo/7 days Male Wistar Rats | Control diet: 20% casein, 3.5% mineral (93G-MX), 5.0% vitamin (93-VX), 0.2% choline chloride, 5.0% corn oil, 4.0% cellulose powder, 22.1% sucrose, and 44.2% starch | |||
| In vivo/12 days Female ICR mice | Control diet: 20% casein, 3.5% mineral (93G-MX), 5.0% vitamin (93-VX), 0.2% choline chloride, 5.0% corn oil, 4.0% cellulose powder, 22.1% sucrose, and 44.2% starch 15 μM per 5 days | |||
| 2. | [22]/2005 | In vitro Murine macrophage-like RAW264 cells | Dulbecco’s Modified Eagle’s Medium 16 μM 6-MITC. -6-MITC inhibits LPS-induced COX-2 | 6-MITC inhibited LPS-induced COX-2 expression at the signaling level and at the transcription factor/promoter levels. |
| 3. | [22]/2005 | In vitro/39 h Murine macrophage-like RAW264 cells | 37 °C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium containing 10% FBS. | 6-MITC inhibited LPS-induced iNOS expression at the cellular signaling level. LPS induced iNOS expression by activating the Jak2-mediated JNK signaling cascade with the attendant AP-1 activation. 6-MITC blocked LPS-induced iNOS expression through the blockage of the Jak2 signaling cascade, leading to JNK-mediated AP-1 activation. |
| 4. | [23]/2007 | In vitro/30 h Murine macrophage-like RAW264 cells | Dulbecco’s Modified Eagle’s Medium 15 μM 6-MITC | 6-MITC suppressed COX-2 expression induced by LPS or IFN-y, but not TPA, in murine macrophages. Molecular analysis revealed that LPS, INF-y, and TPA might induce COX-2 expression through different signaling pathways. |
| 5. | [24]/2008 | In vitro/17 h Murine macrophage-like RAW264 cells | 8 μM 6-MSITC, 40 ng/mL LPS Dulbecco’s modified Eagle’s medium | 6-MSITC may target immune and inflammation-related genes, including chemokines, interleukins, and interferons, to exert its anti-inflammatory function. |
| 6. | [25]/2010 | In vitro/21 h Murine macrophage-like RAW264 | Dimethyl sulfoxide final concentration, 0.2% 8 μM 6-MSITC | The genes induced by 6-MSITC are CC chemokines (CCL11 (C-C Motif Chemokine Ligand 11), CCL25 (C-C Motif Chemokine Ligand 25), interleukins IL3 (Interleukin 3) and receptors: IL1ra12 (Interleukin 1 Receptor Antagonist 12), IL8ra (Interleukin 8 Receptor Alpha), TNFRSF23 (Tumor Necrosis Factor Receptor Superfamily Member 23), TNFRSF4 (Tumor Necrosis Factor Receptor Superfamily Member 4) |
| 7. | [16]/2010 | In vivo/12 weeks male F344 rats | Experiment 1 (sixty-six rats divided into seven groups): Groups 1–5: four weekly subcutaneous injections of DMH (40 mg/kg body weight) Groups 2–3: diet containing 200 and 400 ppm of 6-MSITC, respectively, for 5 weeks. Basic diet until the end of the study. Groups 4–5: mixed diet with 200 and 400 ppm 6-MSITC, respectively, from the first week after completion of treatment until the end of the study. Group 6: diet containing 400 ppm 6-MSITC throughout the study. Group 7: control | The dietary administration of 6-MSITC can significantly inhibit the induction of colonic AcF and BcAc by DMH by reducing cell proliferative activity and the protein levels of phase I enzymes. |
| Experiment 2 (nine rats divided into three groups): Group 1: corn oil by gavage—control Groups 2–3: 6-MSITC at a dose of 20 and 40 mg/kg, respectively, in corn oil by gavage | ||||
| 8. | [26]/2011 | In vitro/12 h Human hepatoblastoma HepG2 cells | 0–20 μM Dulbecco’s modified Eagle’s medium | 6-MSITC regulated Nrf2-mediated ARE activation by targeting Nrf2 and Keap1. 6-MSITC reduced the level of Keap1 by modifying Keap1 and enhanced the level of Nrf2 by inhibiting Nrf2 ubiquitination and turnover. Finally, it resulted in a high ratio of Nrf2/Keap1. The surplus Nrf2, compared with Keap1, might bypass Keap1-Cul3 and accumulate in the nucleus to mediate ARE-driven activation. |
| 9. | [27]/2012 | In vitro/in vivo/10 min Male mice C57BL/6 (4–5 weeks; SLC), and TRPA1-deficient mice TRPA1-KO mice, Human embryonic kidney-derived 293 (HEK293) cells | 20 μL 6-MSITC 10 or 30 mM Standard diet-free access Dulbecco’s Modified Eagle’s Medium | The results indicate the following points: (1) 6-MSITC and 6-MTITC activate both mTRPA1 and hTRPA1; (2) 6-MSITC activates mTRPV1; (3) The pharmacological functions of these isothiocyanates may result from TRPA1 activation. |
| 10. | [28]/2014 | In vitro/24 h Primary human umbilical vein endothelial cells (HUVECs) | 0–30 ng/mL Endothelial Cell Growth Medium-2 BulletKit | The antiplatelet and anti-inflammatory effects of 6-MSITC on human umbilical vein endothelial cells (HUVECs) have been demonstrated. 6-MSITC slightly decreased tissue factor expression but did not affect von Willebrand release in activated HUVECs. 6-MSITC modified the generation of activated protein C, which is important for the negative regulation of blood coagulation in normal endothelial cells. 6-MSITC reduced the expression of interleukin-6 and monocytic chemotactic factor protein-1 induced by tumor necrosis factor alpha (TNFa). 6-MSITC significantly attenuated TNFa-induced adhesion of U937 monoblast cells to HUVECs and reduced mRNA expression of cellular vascular adhesion factor-1 and E-selectin in activated endothelial cells. 6-MSITC modifies EC function, inhibits cell adhesion, and 6-MSITC exerts anti-inflammatory effects, suggesting that it may have therapeutic potential as a treatment for vasculitis. |
| 11. | [29]/2014 | In vivo/4 weeks Male mice C57B1/6 (9 weeks old, 25–30g body weight at the beginning of the experiment) Animals were randomly divided into four groups (n 1/4 10–12 per group) | 5 mg/kg 6-MSITC Twice a week Standard diet Free access | Administration of 6-MSITC for a month is able to exert neuroprotective effects in the 6-OHDA model of Parkinson’s disease. Treatment with 6-MSITC resulted in a significant reduction in oxidative stress and apoptotic cell death, leading to the improvement of behavioral disorders, especially motor deficits. |
| 12. | [30]/2014 | In vitro/in vivo/12 days in vivo: 30 female BALB/c female nude mice with MDA-MB-231 or -453 cells in vitro: breast cancer cell lines (MCF-7, MDA-MB-231, MDA-MB-435S, Hs578T, MDA-MB-453, BT-474, and DU4475 | irradiated CE-7 basal diet—7 days 6.25 mg/kg, 25 mg/kg, and 100 mg/kg of 6-MSITC in sterile deionized water with the use of a stomach sonde needle for 5 days/wk. | The inhibitory effect of 6-MSITC on human breast cancer in a mouse model and the induction of apoptosis in human breast cancer by possible involvement of the NF-κB pathways have been revealed. |
| 13. | [31]/2016 | In vitro/7 h Human neuroblastoma IMR 32 cells (cell no. TKG0207) | 0–20 μM Eagle’s Minimum Essential Medium | Revealed gene expression profiles of Wasabi-derived ITCs in a neuronal cell model, IMR-32. 6-MSITC had the strongest regulation on gene expression among the three ITCs. 6-MSITC could stimulate Nrf2 mediated gene expressions through the stabilization of Nrf2 protein at post transcription. 6-MSITC exerted the neuroprotective effect by activating the Nrf2-mediated oxidative stress response pathway. |
| 14. | [32]/2017 | In vitro/9 h Human hepatoblastoma Hep2G cells (cell no. TKG0205) | 10 mM Dulbecco’s Modified Eagle’s Medium | 6-MSITC was found to be the most potent inducer of the Nrf2-dependent pathway, suggesting an important role of sulfinyl sulfur and carbon chain length in a liver cancer cell model. Furthermore, glutamate metabolism has also been shown to be regulated by 6-MSITC. 6-MSITC exerts a chemopreventive role against cancer through its primary antioxidant activity, activation of Nrf2, and subsequent induction of antioxidant proteins and metabolizing enzymes. |
| 15. | [33]/2018 | In vitro/48 h Human colorectal cancer cell lines HCT116 p53+/+ | 0 or 20 μM Dulbecco’s Modified Eagle’s Medium | 6-MSITC inhibited cell proliferation and induces apoptosis in both HCT116 p53+/+ and HCT116 p53−/− cells through a p53-independent mitochondrial dysfunction pathway. These results suggest that 6-MSITC may be a potential compound for the chemoprevention of colorectal cancer, even in the presence of a p53 mutation. |
| 16. | [34]/2018 | In vivo/29 days Male mice C57B1/6 (9 weeks old, 25–30g body weight at the beginning of the experiment) | 5mg/kg every day Standard diet Free access | 6-MSITC counteracted Aβ1-42 neurotoxicity in mice. These results highlight the interesting neuroprotective activity of 6-MSITC, which reduced apoptosis and neuroinflammation, restored physiological oxidative status, and positively influenced the Nrf2 pathway, resulting in significant behavioral improvement in our Alzheimer’s disease model. These data are promising, but further experimental studies are necessary to confirm the mechanism of action of 6-MSITC, assess its short- and long-term effects, and test its effectiveness in combination with other therapies. |
| 17. | [35]/2019 | In vitro/48 h two types of human colorectal cancer cells (HCT116 p53+/+ and p53−/−) | 20 μM 6-MSITC Dulbecco’s Modified Eagle’s Medium | 6-MSITC was shown to induce cell cycle arrest and apoptosis in both HCT116 p53+/+ and HCT116 p53−/− cells, achieving the same effect. Molecular data showed that activation recruits ERK1/2 and not p53. It has been suggested that 6-MSITC-induced apoptotic cell death via the ELK1/CHOP/DR5 pathway via ERK1/2 is involved in molecular mechanisms. |
| 18. | [36]/2020 | In vivo/12 h + 48 h zebrafish larvae wild-type (AB strain) Nrf2-mutant (nfe2l2afh318) | 6-MSITC (2.5, 5, 10 μM) E3+medium | The activities of 6-(methylsulfinyl)hexyl isothiocyanate were involved in the reduction of arsenite toxicity. The antioxidant activities were all Nrf2-dependent. |
| 19. | [37]/2020 | In vivo/7 days Human hepatocellular carcinoma HepG2 cells 20 male, 8-week-old in vitro C57BL/6J mice randomly assigned to four groups of five each (control and three doses of 6-MSITC administration groups) | 0–40 mg/kg (10, 20–40 mg/kg) Dulbecco’s Modified Eagle’s Medium Normal diet | 6-MSITC increased liver ALDH2 enzyme activity and protein expression by activating the Nrf2/ARE pathway and alleviated acetaldehyde-induced cytotoxicity. Therefore, 6-MSITC may protect hepatocytes against acetaldehyde-induced cytotoxicity. This study represents a potentially effective strategy for preventing the abnormal reaction induced by the ingestion of wasabi extract in the setting of alcohol consumption |
| 20. | [38]/2020 | In vivo/10 months Mice WT, AppNLGF, AppNLGF Keap1FA/FA, AppNLGF Keap1FA/- | 0.4 mg/mL dissolved in water for 10 months 15 mg/kg intraperitoneally (to evaluate the expression of the Nrf2 target Nqo1 gene) | Nrf2 induction improved brain antioxidant function and attenuates pathological neuroinflammation in the AppNLGF mouse model. Additionally, this study provides significant evidence supporting the concept that Nrf2 activation inhibits the onset and/or progression of Alzheimer’s disease (AD), indicating that the Keap1-Nrf2 system is a promising target for drug development for neurocognitive disorders, including AD. |
| 21. | [39]/2022 | In vivo, randomized placebo-controlled, double-blind/4 days Human, eight male | 9 mg Standard diet | Consumption of 6-MSITC does not affect the concentration of calpain-1 in the blood and does not cause muscle damage or changes in inflammatory markers. |
| 22. | [40]/2022 | In vivo/12 weeks Human, fifteen patients (three males, twelve females, age 20–58 years) | orally administered wasabi extract (9.6 mg of 6-MSITC/day) | 6-MSITC improved PS, frequency of headache and myalgia, neurocognitive symptoms such as brain fog, difficulty finding appropriate words, photophobia, and psychological vitality in patients with ME/CFS. In accordance with the effects on subjective symptoms, it also improved the PPT and scores on the TMT-A. Currently, treatment for neuro-cognitive dysfunction in ME/CFS patients is lacking. |
| 23. | [41]/2022 | In vitro/76 h TR146 cell line human oral epithelial cell line | 1.5625–25 μM 50 μM—influence the viability of TR146 cells. Ham’s F12 medium | 6-MSITC could suppress IL-6 and CXCL10 production in TNF-α-treated human oral epithelial cells (TR146 cells) by inhibiting the activation of STAT3 and NF-κB pathways. |
| 24. | [42]/2022 | In vivo/16 weeks 48 male Wistar rats (8 to 9 weeks old) weighing 330–340 g | 5% wasabi powder Group 1: corn starch diet (C)—16 weeks normal drinking water Group 2: corn starch diet supplemented with 5% wasabi powder (CW) − 8 weeks (C) + 8 weeks (CW) normal drinking water Group 3: high-carbohydrate, high-fat diet (H)—16 weeks 25% fructose (w/v) in drinking water Group 4: high-carbohydrate, high-fat diet supplemented with 5% wasabi powder (HW) − 8 weeks (H) + 8 weeks (HW) 25% fructose (w/v) in drinking water | Tasmanian wasabi attenuated the changes in acute inflammation in the heart and lipid deposition in the liver and attenuated hypertension and obesity in diet-induced metabolic syndrome in Wistar rats. |
| 25. | [43]/2022 | In vitro/12 h, 24 h/48 h Human hepatocellular carcinoma HepG2 cells | 0–20 M Dulbecco’s Modified Eagle’s Medium | 6-MSITC significantly alleviated CFA-induced formation of thiobarbituric acid-reactive substances and fat accumulation. 6-MSITC enhanced phosphorylation of AMPKα, upregulated the expression of Nrf2, NQO1, heme oxygenase 1, FOXO1, and Siruin1, and downregulated the expression of PPARα. The AMPKα/Nrf2-mediated signaling pathways might be involved in the cytoprotective effects of Wasabi 6-MSITC against metabolic lipid stress. |
| 26. | [44]/2023 | In vivo/12 weeks Human 60 years and over 19 male 53 female | one 6-MSITC capsule that contained 100 mg wasabi extract powder containing 6-MSITC (0.8 mg) | Consumption of 0.8 mg of 6-MSITC for 12 weeks significantly improved memory functioning, including episodic and working memory, compared with the placebo group, but we did not observe significant improvements in other cognitive functions. This study is the first to demonstrate that 6-MSITC benefits memory function in healthy older adults. |
| 27. | [45]/2023 | In vivo/4 weeks Human, 20 healthy volunteers who were experiencing daily fatigue | powder containing 6-MSITC (4.8 mg/day of 6-MSITC) | 6-MSITC did not improve fatigue after a mental task, but fatigue before the mental task, sleep, and mood were improved significantly. No changes were observed in autonomic nerve function, stress, or immune markers. |
| In vivo, double-blind, placebo-controlled method/4 weeks Human overdose safety: 30 healthy volunteers | the extract powder (up to 16 mg/day of 6-MSITC for 4 weeks) | No changes in the parameters or side effects were observed, and the results showed that high doses of the extract powder containing 6-MSITC were safe. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowiak-Wieczorek, J.; Malesza, M.; Malesza, I.; Hadada, T.; Winkler-Galicki, J.; Grzelak, T.; Mądry, E. Methylsulfinyl Hexyl Isothiocyanate (6-MSITC) from Wasabi Is a Promising Candidate for the Treatment of Cancer, Alzheimer’s Disease, and Obesity. Nutrients 2024, 16, 2509. https://doi.org/10.3390/nu16152509
Bartkowiak-Wieczorek J, Malesza M, Malesza I, Hadada T, Winkler-Galicki J, Grzelak T, Mądry E. Methylsulfinyl Hexyl Isothiocyanate (6-MSITC) from Wasabi Is a Promising Candidate for the Treatment of Cancer, Alzheimer’s Disease, and Obesity. Nutrients. 2024; 16(15):2509. https://doi.org/10.3390/nu16152509
Chicago/Turabian StyleBartkowiak-Wieczorek, Joanna, Michał Malesza, Ida Malesza, Tomasz Hadada, Jakub Winkler-Galicki, Teresa Grzelak, and Edyta Mądry. 2024. "Methylsulfinyl Hexyl Isothiocyanate (6-MSITC) from Wasabi Is a Promising Candidate for the Treatment of Cancer, Alzheimer’s Disease, and Obesity" Nutrients 16, no. 15: 2509. https://doi.org/10.3390/nu16152509
APA StyleBartkowiak-Wieczorek, J., Malesza, M., Malesza, I., Hadada, T., Winkler-Galicki, J., Grzelak, T., & Mądry, E. (2024). Methylsulfinyl Hexyl Isothiocyanate (6-MSITC) from Wasabi Is a Promising Candidate for the Treatment of Cancer, Alzheimer’s Disease, and Obesity. Nutrients, 16(15), 2509. https://doi.org/10.3390/nu16152509








