Polyphenols: Secondary Metabolites with a Biological Impression
Abstract
1. Introduction
2. Polyphenols’ Categories
2.1. Phenolic Acids
2.2. Flavonoids
2.3. Stilbenes
2.4. Lignans
3. Methods for Extraction and Determination of Polyphenols
3.1. Types of Most Common Extraction Methods
- -
- Ultrasound-Assisted Extraction (UAE)
- -
- Microwave-Assisted Extraction (MAE)
- -
- Ultrasound–Microwave-Assisted Extraction
- -
- Supercritical Fluid Extraction (SFE)
- -
- Other extraction methods
3.2. Types of Most Common Quantification Methods for Polyphenols
- -
- Spectrophotometric Assays
- -
- Gas Chromatography (GC)
- -
- High-Performance Liquid Chromatography (HPLC)
- -
- Other quantification methods
4. Bioavailability of Polyphenols
Bioavailability of Encapsulated Polyphenols or Polyphenols Covered with Liposomes or Nanoparticles and Their Effect of Functionalities
5. Health Benefits of Polyphenols
5.1. Antioxidant Activity
5.2. Anti-Inflammatory Activity
5.3. Antimicrobial Activity
5.4. Antidiabetic Activity
5.5. Skin and Hair Health
5.6. Neuroprotective Effect
5.7. Anti-Tumor and Anticancer Activity
5.8. Other Effects
| Health Benefits | Polyphenols From | Type of Polyphenols | Outcome | References |
|---|---|---|---|---|
| Antioxidant activity | Rhododendron tomentosum | Rosmarinic acid Caffeic acid Chlorogenic acid Rutin Quercetin |
| [185] |
| Rye Bread | * |
| [166] | |
| Red cabbage | * |
| [171] | |
| Herbal tea | Gallic acid Catechin Caffeic acid Ferulic acid Epicatechin Gallate Quercetin Kaempferol |
| [164] | |
| Fabacea | * |
| [188] | |
| Rosa roxburghii | Gallic acid Ellagic acid Gallocatechin Epigallocatechin Catechin Epicatechin |
| [178] | |
| * | 3,4-dihydroxyphenylacetic acid Homovanillic acid Vanillic acid Caffeic acid Gallic acid Phloroglucinol Pelargonidi Ellagic acid |
| [153] | |
| De-oiled rice bran | Vanillin Ferulic acid Sinapic acid Chlorogenic acid |
| [181] | |
| Corn bran | 4-hydroxybenzaldehyde p-coumaric Sinapic acid Ferulic acid | |||
| Amaranthus lividus | * |
| [183] | |
| Banana | 3-Hydroxyphenylpropionic acid Ferulic acid Caffeic acid Anthocyanins Cyanidin 2′-Hydroxyformononetin Quercetin Neoeriocitrin Scopoletin 2′-Hydroxyformononetin |
| [187] | |
| Zhourat | Gallic acid |
| [186] | |
| Leptospermum scoparium | * |
| [190] | |
| Sambucus ebulus | Chlorogenic acid Caffeic acid glucoside 3-p-coumaroylquinic acid 3-p-Feruloylquinic acid Catechin Epicatechin Procyanidin Kaempferol Quercetin Piceid |
| [191] | |
| Rubus spp. | Gallic acid Neochlorogenic acid Procyanidin Catechin Vanillic acid Caffeic acid Epicatechin p-coumaric acid Quercetin Ferulic acid Kaempferol |
| [119] | |
| Thymus serpyllum L. | Rosmarinic acid Luteolin Salvianolic acid |
| [157] | |
| Euphorbia antisyphilitica | * |
| [168] | |
| Herbal tea and green tea | * |
| [172] | |
| Eugenia uniflora leaves Eucalyptus microcorys leaves Myrciaria cauliflora seeds | Ellagic acid Kaempferol Quercetin Myricetin 2,3-Di-O-galloyl-glucose 2,3,6-Tri-O-galloyl-glucose 1,2,3,4,6-Penta-O-galloyl-glucose 4,6-O-HHDP-glucose Gemin Oenothein Isocoriariin Tellimagrandin Pedunculagin Tellimagrandin Eugeniflorin Camptothin Oenothein |
| [152] | |
| Satureja hortensis L. | Rutin Rosmarinic acid |
| [169] | |
| Chamerion angustifolium | Oenothein Quercetin Myricetin Luteolin Kaempferol Gallic acid Chlorogenic acid p-coumaric acid Ellagic acid Benzoic acid etc. |
| [167] | |
| Sargassum wightii | Gallic acid Quercetin Ferulic acid Vanillin |
| [154] | |
| Ulva rigida | ||||
| Gracilaria edulis | ||||
| Pistacia lentiscus L. | Feruloylquinic acid p-coumaroylquinic acid 5-O-caffeoylquinic acid Monogalloyl glucose Gallic acid 5-O-galloylquinic acid Chlorogenic acid Digalloylquinic acid Procyanidin Epicatechin Catechin Epigallocatechin gallate Trigalloylquinic acid p-coumaric acid Myricetin Quercetin Kaempferol Luteolin Apigenin |
| [165] | |
| Amaranthus dubius | 2-O- Caffeoylglucaric acid Ferulic acid 4-Hydroxycinnamic acid Kaempferol Caffeoylquinic acid Myricetin Quercetin |
| [179] | |
| Amaranthus spinosus | Dihydromyricetin Ferulic acid 4-Hydroxycinnamic acid Feruloylquinic acid Kaempferol Caffeoylquinic acid Myricetin Quercetin | |||
| Amaranthus tricolor | 2-O-Caffeoylglucaric acid Ferulic acid 4-Hydroxycinnamic acid Kaempferol Caffeoylquinic acid Myricetin Quercetin | |||
| Amaranthus viridis | Ferulic acid 4-Hydroxycinnamic acid Myricetin Quercetin Quercetin | |||
| Carrot | Gallic acid Protocatechuic acid Vanillic acid 4-hydroxybenzaldehyde |
| [158] | |
| Echinacea Purpurea | Caftaric Chicoric acids Catechins |
| [159] | |
| Malus domestica borkh | Chlorogenic acid p-coumaric acid Quercetin -3-O-galactoside -3-O-arabinoside Phloretin-2′-O-glucoside Catechin Epicatechin Procyanidin |
| [184] | |
| Eucalypts leaf | * |
| [182] | |
| Nigella sativa L. | Gallic acid Hydroquinone Apigenin Naringenin Quercetin Kaempferol Rutin |
| [180] | |
| Ipomoea batatas | Cyanidin Peonidin Pelargonidin |
| [163] | |
| Vitis vinifera L. | Flavan-3-ol Proanthocyanidin Anthocyanins |
| [193] | |
| Coffee silverskin | Caffeoylquinic Feruloylquinic acids |
| [129] | |
| Coffee | * |
| [189] | |
| Polyscias fruticosa roots | * |
| [170] | |
| Chroogomphus rutilus | Protocatechuic acid |
| [160] | |
| Anti-inflammatory activity | Tetraclinis articulata | * |
| [215] |
| Pleurotus ostreatus | Cathechin Sinapic acid Resveratrol etc. |
| [216] | |
| Green tea and red wine | * |
| [217] | |
| Punica granatum L. | Luteolin Rosmarinic acid Quercetin Eriodictyol etc. |
| [371] | |
| Thymus vulgaris | Rosmarinic acid Luteolin etc. | |||
| Rosmarinus officinalis L. | Chlorogenic acid Caffeic acid etc. | |||
| Echinacea purpurea L. | Ellagic acid Gallagic acid etc. | |||
| Maclura tricuspidate Pyrus Montana Naka | Gallic acid Protocatechuic acid Chlorogenic acid p-hydroxybenzoic acid Vanillic acid Caffeic acid Rutin ρ-coumaric acid Ferulic acid Rosmarinic acid Salicylic acid Quercetin Cinnamic acid Taxifolin |
| [214] | |
| Olive Oil | Oleacein Oleocanthal |
| [197] | |
| Finger millet | Protocatechuic acid Catechin Chlorogenic acid Naringin |
| [218] | |
| Kodo millet | Catechin Naringin p-coumaric acid Taxifolin Ferulic acid Sinapic acid Methyl vanillate | |||
| Rhamnus prinoides L’Herit |
Caffeic acid
Protocatechuic acid Kaempferol Gallocatechin Proanthocyanidin Luteolin Quercetin Apigenin Rutin etc. |
| [200] | |
| Petroselinum crispum Apium graveolens Coriandrum sativum | * |
| [206] | |
| Huangjiu | Protocatechuic acid Catechin Chlorogenic acid Vanillic acid Caffeic acid Syringic acid p-coumaric acid Ferulic acid Sinapic acid Rutin Quercetin |
| [210] | |
| Arabidopsis thaliana | Caffeic acid Quercetin Kaempferol Synapic acid Luteolin |
| [196] | |
| Ilex latifolia | Quinic acid Caffeoylquinic acid Shikimic acid Rutin Hyperoside etc. |
| [211] | |
| Cynara scolymus L. | Hydroxytyrosol Verbascoside Apigetrin Oleuropein Quercetin Pinoresinol Apigenin |
| [205] | |
| Acalypha hispida | Gallic acid Quercetin Ellagic acid p-coumaric acid etc. |
| [195] | |
| Lonicera caerulea L. | Chlorogenic acid Caffeic acid Catechin Epicatechin Cyanidin etc. |
| [202] | |
| Prunus domestica L. | Chlorogenic acid p-coumaric acid Rutin etc. |
| [203] | |
| Gaultheria procumbens L. | Protocatechuic acid Caffeoylquinic acid p-hydroxybenzoic acid Vanillic acid Catechin Epicatechin p-coumaric acid Procyanidin Quercetin Kaempferol etc. |
| [201] | |
| Baccaurea ramiflora Lour | Rosmarinic acid |
| [194] | |
| Libidibia ferrea Parapiptadenia rigida Psidium guajava | Catechin Gallic acid |
| [212] | |
| Phaseolus vulgaris bean | Sinapic acid Ferulic acid Naringenin Catechin Quercetin etc. |
| [199] | |
| Verbascum phlomoides | Gallic acid Rosmarinic acid Caffeic acid Ferulic acid Quercetin etc. |
| [198] | |
| Rubus coreanus Miquel | * |
| [209] | |
| Antimicrobial activity | Guizotia abyssinica L. leaf and flower extracts | Tannins Glycosides Flavanoids Phenols |
| [225] |
| Retama monosperma | Flavonoids Tannins Quinones Anthocyanins |
| [3] | |
| Filipendula ulmaria | Quercetin Rutin |
| [223] | |
| Salvia officinalis | Quercetin Apigenin Naringenin Rutin | |||
| Rosmarinus officinalis | Luteolin Eriodictyol | |||
| Sideritis scardica | Quercetin Rutin Epicatechin | |||
| Geranium purpureum | Quercetin Rutin Catechin Epicatechin Hydroxytyrosol | |||
| Banana peels | * |
| [240] | |
| Artemisia aucheri | * |
| [143] | |
| Grape pomace | Anthocyanins Phenolic acid Flavonoids Stilbenes |
| [243] | |
| Alcea rosea | Gallic acid Salicylic acid Pyrogallol Cinnamic acid Catechin Naringin Ferulic acid |
| [142] | |
| Achillea millefolium | Salicylic Succinic acids Folic acid Caffeic acid Kaempferol Luteolin Apigenin and other phenolic and flavonoid compounds |
| [140] | |
| Rheum ribes | Gallic acid Salicylic acid Caffeic acid Cinnamic acid Catechin Ellagic acid Ferulic acid |
| [141] | |
| Lycium chinense Mill. | Quercetin Kaempferol Catechin Flavan-3-ols Coumaric acid Chlorogenic acid Procyanidin |
| [224] | |
| Propolis | p-coumaric acid Ferulic acid Chrysin |
| [219] | |
| * | Combined polyphenols |
| [234] | |
| Punica granatum L. | Ursolic acid Corosolic acid Arjunolic acid |
| [221] | |
| Hibiscus sabdariffa L. | Kaempferol Cyanidin Quercetin |
| [244] | |
| Spirulina | * |
| [367] | |
| Zhourat | Gallic acid etc. |
| [186] | |
| Lantana camara L. | Tetramethylhexadec-2-en-1-ol Linolenic acid 2,6-Dimethoxyphenol 9,12-Octadecadienoic acid |
| [173] | |
| Picea abies L. Larix decidua Mill Pinus sylvestris L. Pseudotsuga menziesii Juniperus communis L. | Gallic acid p-coumaric acid 2,5-dihydroxybenzoic acid 4-hydroxybenzoic acid Chlorogenic acid Caffeic acid Syringic acid Vanillic acid Sinapic acid Ferulic acid Salicylic acid Cinnamic acid Vitexin Apigenin Kaempferol Luteolin Quercetin Naringenin Rutin |
| [230] | |
| Natural polyphenols | TF3 TF2b TF1 TF2a Hesperidin EGCG Myricetin Quercetagetin |
| [235] | |
| * | Apigenin Catechin Luteolin Morin Myricetin Naringin Quercetin Rutin |
| [370] | |
| Olive oil | * |
| [227] | |
| Prunus dulcis | Epicatechin Catechin |
| [236] | |
| Vitis vinifera L. | Gallic acid Coumaric acid Vanillic acid Chlorogenic acid Cyanidin Catechin Caffeic acid Peonidin 3-O-glucoside Epicatechin Luteolin Resveratrol Ferulic acid |
| [233] | |
| Moringa oleifera | Coumaric acid Myricetin Quercetin Kaempferol Resveratrol Naringenin Biochanin A Naringin Catechin |
| [232] | |
| Olive oil | * |
| [231] | |
| Achillea pachycephala Achillea millefolium Achillea nobilis Achillea filipendulina Achillea santolina Achillea aucheri | Chlorogenic acid Caffeic acid Quercetin Luteolin Rutin Ferulic acid |
| [220] | |
| * | Stilbenes Cinnamic Benzoic Flavonoids Coumarins Naphtoquinones |
| [242] | |
| Antidiabetic activity | Solanum anguivi | * |
| [263] |
| Syzygium zeylanicum L. | Gallic acid Catechin Epicatechin Caffeine Quercetin Apigenin Ethyl gallate Rutin Ellagic acid Chlorogenic acid Quercitrin |
| [266] | |
| Cucumis dipsaceus | Rutin Gallic acid |
| [278] | |
| Phaseolus vulgaris L. | * |
| [268] | |
| Gracilaria bursa-pastoris | Gallic acid Catechin 4-hydroxy benzoïc acid Chlorogenic acid Caffeic acid Syringic acid Vanilline p-coumaric acid Sinapic acid Quercetin 7,3′,4′-flavon-3-ol Naringin Rutin Salicylic acid Quercetin Cinnamic acid Luteolin Apigenin Kaempferol Flavone Flavanone |
| [255] | |
| Carica papaya | * |
| [269] | |
| Curcuma longa | Curcumin |
| [273] | |
| Cocos nucifera | Gallic acid Ferulic acid 4-Hydroxycinnamic acid p-coumaric acid Quercetin |
| [274] | |
| Borassus flabellifer | Gallic acid Ferulic acid 4-Hydroxycinnamic acid Quercetin Myricetin-3-O-glucoside | |||
| Vinegar extract | 4-Hydroxybenzoic acid Ferulic acid Salicylic acid Vanillic acid Protocatechuic acid Catechin Ellagic acid Gallic acid Gallocatechin 3-O-gallate Rutin etc. |
| [272] | |
| Vigna radiata L. | Gallic acid Vitexin |
| [368] | |
| Quercus suber Quercus ilex Quercus coccifera Quercus canariensis | Chlorogenic acid |
| [260] | |
| Red wine | Gallic acid Caftaric acid Coutaric acid Malvidin 3-O-glucoside Petunidin 3-O-glucoside |
| [298] | |
| Aerva lanata L. Juss | Gallic acid Protocatechuic acid Caffeic acid Syringic acid 4-hydroxybenzoic acid Vanillic acid Gentisic acid Sinapic acid p-coumaric acid Ferulic acid Rosmarinic acid Isoferulic acid Salicylic acid |
| [270] | |
| Linum usitatissimum | * |
| [267] | |
| Vigna unguiculata | Gentisic acid Coumaric acid Ferulic acid Quercetin |
| [275] | |
| Lonicera caerulea L. | Cyanidin Quercetin Chlorogenic acid Flavan-3-ol Catechin Epicatechin |
| [276] | |
| Propolis | Protocatechuic acid Catechin Caffeic acid Syringic Acid Epicatechin p-coumaric acid Ferulic acid Luteolin |
| [271] | |
| Rosmarinus officinalis L. | * |
| [137] | |
| Lagerstroemia speciosa | Caffeic acid Ellagitannins Flavonoids Quercetin |
| [253] | |
| Peanut shell | Luteolin Pyrogallol Catechol Phloroglucinol Quercetin |
| [16] | |
| Skin and hair effects | Caralluma europaea |
Luteolin
Gallic acid Hesperetin Quercetin Myricetin Ferulic acid Salicylic acid Naringenin |
| [285] |
| Vitis vinifera seed | * |
| [138] | |
| Rhus coriaria | Anthocyanins Flavonoids Phenols Hydrolyzable tannins Gallic acid Quercetin |
| [290] | |
| Penthorum chinense Prush | * |
| [294] | |
| * |
Naringenin
Curcumin |
| [288] | |
| Neuroprotective activity | Propolis | * |
| [318] |
| * | Mix of polyphenols |
| [299] | |
| Phyllanthus emblica L. | Gallic acid Epicatechin Ethly gallate Chebulagic acid Ellagic acid Quercetin |
| [300] | |
| * | Curcumin |
| [321] | |
| Olive | * |
| [319] | |
| * | Resveratrol |
| [305] | |
| * | Curcumin and fatty acid |
| [325] | |
| * | Curcumin |
| [322] | |
| Grape leaves | * |
| [307] | |
| Tea | * |
| [317] | |
| * | Curcumin and fatty acids |
| [372] | |
| Anti-tumor/Anticancer | Cuminum cyminum | * |
| [29] |
| Cerasus humilis | * |
| [346] | |
| Caralluma europaea | Kaempferol Luteolin Trans-ferulic acid Syringic acid |
| [285] | |
| * | Isoeugenol |
| [332] | |
| Camellia sinensis | Epigallocatechin-3-gallate |
| [350] | |
| * | Quercetin Fisetin |
| [330] | |
| Viscum album | Epicatechin Quercetin |
| [373] | |
| Apple | Cyanidin-3-O-arabinoside |
| [334] | |
| Hippophae rhamnoides | Sinapinic acid Ferulic acid Coumaric acid 7-Hydroxycoumarine Kaempferol 5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxo-4H-chromen-3-yl-6-O-(6-deoxy-α-L-mannopyranosyl) hexopyranoside |
| [358] | |
| Artemisia argyi leaf | Neochlorogenic acid Chlorogenic acid Cryptochlorogenic acid Isochlorogenic acid |
| [335] | |
| Ziziphus jujuba | * |
| [340] | |
| Coriandrum sativum L. | Flavonoids Catechins Rutin |
| [336] | |
| Empetrum nigrum | * |
| [329] | |
| Pinus koraiensis bark | Penta-hydroxy flavone |
| [349] | |
| Sabal yapa leaves | Tricin Luteolin Apigenin |
| [352] | |
| Sugarcane | * |
| [331] | |
| Varthemia candicans Peganum harmala Suaeda vermiculata Conyza dioscoridis | * |
| [328] | |
| Euphorbia lathyris | Esculetin Euphorbetin Gaultherin Kaempferol |
| [359] | |
| Ipomoea batatas | Caffeic acid |
| [343] | |
| Vaccinium spp. | Pelargonidin-3-O-galactoside Delphinidin-3-glucoside Chlorogenic acid isomers Epicatechin gallate Malvidin-3-O-glucose Kaempferol-3-rhamnoside Hexose ferulic acid esters Myricetin-3-O-hexose |
| [361] | |
| Thalassia testudinum | * |
| [341] | |
| Eugenia involucrata | Gallic acid Catechin p-coumaric acid Rutin Myricetin Quercetin |
| [339] | |
| Agrimonia pilosa | Agrimoniin |
| [344] | |
| Peanut skin | Proanthocyanidin-B2 |
| [354] | |
| Extra-virgin olive oil | Oleacein |
| [365] | |
| Cinnamomum cassia | * |
| [366] | |
| Vaccinium macrocarpon | Cyanidin Peonidin |
| [353] | |
| Camellia sinensis | Epigallocatechin |
| [337] | |
| Green tea | Epigallocatechin |
| [342] | |
| Foxtail millet Bran | Vanillic acid Glucosyringic acid Ferulic acid 4-hydroxybenzoic acid Vanillic acid Syringic acid p-coumaric acid Vitexin Ferulic acid Isoferulic acid Biferulic acid 4,4′-dihydroxy-3,5′-dimethoxy,3′-bicinnamic acid |
| [362] | |
| * | Tannic acid |
| [356] | |
| * | Resveratrol Pterostilbene |
| [357] | |
| Olive oil | Oleacein |
| [360] | |
| Caesalpinia spinosa | * |
| [327] | |
| Other effects | Green tea Dehydrated red delicious apple Dark chocolate | * |
| [369] |
| Fucus vesiculosus | * |
| [204] |
6. Polyphenols in Nutritional Aspect
6.1. Maternal and Infant Health
6.2. From Childhood to Elderly
6.3. Athlete Health
7. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.J. Definition of a Plant-Based Diet and Overview of This Special Issue. J. Geriatr. Cardiol. 2017, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Benkhouili, F.Z.; Moutawalli, A.; Ouchari, L.; El Fahime, E.; Benzeid, H.; Doukkali, A.; Zahidi, A. Evaluation of the Content of Polyphenols, Flavonoids and Tannins, the Antioxidant Capacity, and the Antimicrobial Activity of Different Organic and Aqueous Fractions of Stems of Retama Monosperma. Plant Sci. Today 2024, 11. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Arikal, A.O.; Eksi, A. Effect of Cold Storage of Various Pomegranate Cultivars Fruit Juices on Health Promoting Compounds and Their Activities. J. Food Nutr. Res. 2015, 3, 593–598. [Google Scholar] [CrossRef]
- Stefani, M.; Rigacci, S. Beneficial Properties of Natural Phenols: Highlight on Protection against Pathological Conditions Associated with Amyloid Aggregation. BioFactors 2014, 40, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.; Zhang, M.; Han, F.; Liao, W.; Duan, X. Natural Polyphenols for Drug Delivery and Tissue Engineering Construction: A Review. Eur. J. Med. Chem. 2024, 266, 116141. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Karav, S.; Arikal, A.O.; Ekşi, A. Apple Peel Is a Promising Source of Natural Bioactive Compounds That Promote Human Health. J. Food Nutr. Res. 2015, 3, 624–628. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. Classification of Phenolic Compounds in Plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. [Google Scholar]
- Charis, M. Galanakis. In Food Bioactives and Health; Galanakis, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-57468-0. [Google Scholar]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ye, H.; Liu, J.; Wu, L.; Lin, D.; Yu, Y.; Gao, F. Assessment of Anti-Diabetic Activity of Peanut Shell Polyphenol Extracts. J. Zhejiang Univ. B 2018, 19, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An Overview on the Role of Dietary Phenolics for the Treatment of Cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Verma, C. Science and Engineering of Polyphenols: Fundamentals and Industrial Scale Applications; Wiley Online Library: Hoboken, NJ, USA, 2024; ISBN 978-1-394-20390-1. [Google Scholar]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as Antioxidants in Plants Under Abiotic Stresses. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 159–179. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Bondonno, N.P.; Dalgaard, F.; Murray, K.; Gardener, S.L.; Martins, R.N.; Rainey-Smith, S.R.; Cassidy, A.; Lewis, J.R.; Croft, K.D.; et al. Flavonoid Intake and Incident Dementia in the Danish Diet, Cancer, and Health Cohort. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12175. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Dodd, G.; Spencer, J. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and Roles of Stilbenes in Plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [PubMed]
- El Tannir, H.; Houhou, D.; Debs, E.; Koubaa, M.; Jammoul, A.; Azakir, B.; Khalil, M.I.; El Darra, N.; Louka, N. Optimization of Aqueous Extraction of Polyphenols from Cuminum Cyminum Seeds Using Response Surface Methodology and Assessment of Biological Activity. BioTech 2024, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.; Chinnici, F.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Carbú, M.; Guerrero, R.F.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Cantos-Villar, E. Sulfur Free Red Wines through the Use of Grapevine Shoots: Impact on the Wine Quality. Food Chem. 2018, 243, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Valls-Fonayet, J.; Richard, T.; Cantos-Villar, E. A Rapid Quantification of Stilbene Content in Wine by Ultra-High Pressure Liquid Chromatography—Mass Spectrometry. Food Control. 2020, 108, 106821. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research Progress on Classification, Sources and Functions of Dietary Polyphenols for Prevention and Treatment of Chronic Diseases. J. Futur. Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Critical Reviews in Clinical Laboratory Sciences. Crit. Rev. Clin. Lab. Sci. 2010, 47, 1–4. [CrossRef] [PubMed][Green Version]
- Adlercreutz, H. Lignans and Human Health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Chen, J.; Thompson, L.U. Lignans and Tamoxifen, Alone or in Combination, Reduce Human Breast Cancer Cell Adhesion, Invasion and Migration in Vitro. Breast Cancer Res. Treat. 2003, 80, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rickard, S.E.; Yuan, Y.V.; Chen, J.; Thompson, L.U. Dose Effects of Flaxseed and Its Lignan on N-Methyl-N-Nitrosourea-Induced Mammary Tumorigenesis in Rats. Nutr. Cancer 1999, 35, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Dabrosin, C.; Chen, J.; Wang, L.; Thompson, L.U. Flaxseed Inhibits Metastasis and Decreases Extracellular Vascular Endothelial Growth Factor in Human Breast Cancer Xenografts. Cancer Lett. 2002, 185, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bylund, A.; Saarinen, N.; Zhang, J.; Bergh, A.; Widmark, A.; Johansson, A.; Lundin, E.; Adlercreutz, H.; Hallmans, G.; Stattin, P.; et al. Anticancer Effects of a Plant Lignan 7-Hydroxymatairesinol on a Prostate Cancer Model In Vivo. Exp. Biol. Med. 2005, 230, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Teodor, E.D.; Moroeanu, V.; Radu, G.L. Lignans from Medicinal Plants and Their Anticancer Effect. Mini-Reviews Med. Chem. 2020, 20, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Qin, F.; Wang, C.-G.; Kim, D.; Li, J.-J.; Chen, X.-L.; Wang, H.-S.; Lee, S.K. Novel Lignans from Zanthoxylum Nitidum and Antiproliferation Activity of Sesaminone in Osimertinib-Resistant Non-Small Cell Lung Cancer Cells. Bioorganic Chem. 2023, 134, 106445. [Google Scholar] [CrossRef] [PubMed]
- Bayar, İ.; Çağlar Yavuz, S.; Akkoç, S. In Vitro and In Silico Studies on Lignan SecoisolariciresinolL Diglycoside. Ank. Univ. Eczaci. Fak. Derg. 2023, 48, 127–137. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef] [PubMed]
- Tago, R.; Yamauchi, S.; Maruyama, M.; AKIYAMA, K.; SUGAHARA, T.; KISHIDA, T.; KOBA, Y. Structure-Antibacterial Activity Relationship for 9-O,9′-O-Demethyl (+)-Virgatusin. Biosci. Biotechnol. Biochem. 2008, 72, 1032–1037. [Google Scholar] [CrossRef]
- Chen, D.-F.; Zhang, S.-X.; Chen, K.; Zhou, B.-N.; Wang, P.; Cosentino, L.M.; Lee, K.-H. Two New Lignans, Interiotherins A and B, as Anti-HIV Principles from Kadsura Interior. J. Nat. Prod. 1996, 59, 1066–1068. [Google Scholar] [CrossRef]
- Soto-Hernández, M.; García-Mateos, R.; Palma-Tenango, M. (Eds.) Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; ISBN 978-1-78984-033-9. [Google Scholar]
- Pham, T.N.; Lam, T.D.; Nguyen, M.T.; Le, X.T.; Vo, D.-V.N.; Toan, T.Q.; Vo, T.S. Effect of Various Factors on Extraction Efficiency of Total Anthocyanins from Butterfly Pea (Clitoria ternatea L. Flowers) in Southern Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544, 012013. [Google Scholar] [CrossRef]
- Chiriac, E.; Chiţescu, C.; Geană, E.-I.; Gird, C.; Socoteanu, R.; Boscencu, R. Advanced Analytical Approaches for the Analysis of Polyphenols in Plants Matrices—A Review. Separations 2021, 8, 65. [Google Scholar] [CrossRef]
- Soufi, O.; Medouni-Haroune, L.; Bachirbey, M.; Medouni-Adrar, S.; Idir, F.; Heddad, T.; Ouldsaadi, L.; Romero, C.; Madani, K.; Makhlouf-Boulekbache, L. Statistical Optimization of Ultrasound-Assisted Extraction of Polyphenols from Olive Pomace. Sustain. Chem. Pharm. 2023, 36, 101260. [Google Scholar] [CrossRef]
- Vinatoru, M. An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles from Herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, H.; Huang, J.; Chen, Q.; Li, X.; Chen, X.; Liang, J.; Wang, L. Ultrasound-Assisted Extraction of Polyphenols from Pine Needles (Pinus Elliottii): Comprehensive Insights from RSM Optimization, Antioxidant Activity, UHPLC-Q-Exactive Orbitrap MS/MS Analysis and Kinetic Model. Ultrason. Sonochem. 2024, 102, 106742. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Gómez, D.S.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Zamora-Gasga, V.M.; Ragazzo-Sánchez, J.A. Ultrasound Assisted Extraction of Polyphenols from Randia Monantha: Optimization, Characterization and Antifungal Activity. Ind. Crop. Prod. 2024, 209, 117932. [Google Scholar] [CrossRef]
- Riaz, T.; Hayat, Z.; Saleem, K.; Akram, K.; Rehman, H.U.; Rehman, S.U.; Azam, M. Optimization of an Ultrasound-assisted Extraction Method to Obtain Gallic Acid-rich Extracts from Mango Seed Kernels. Food Sci. Nutr. 2024, 12, 4038–4048. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds from Chestnut Processing Waste Using Response Surface Methodology. J. Clean. Prod. 2023, 395, 136452. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Kingwascharapong, P.; Mazumder, M.R.; Rawdkuen, S. Optimization of Polyphenolic Compounds from Gossampinus Malabarica Flowers by Microwave-Assisted Extraction Technology. Futur. Foods 2023, 8, 100271. [Google Scholar] [CrossRef]
- Murugesan, S.; Maran, P.; Venkatesan, M.; Alexander, R.A. Microwave Assisted Extraction of Polyphenols from Pithecellobium Dulce Benth Fruit Peels and Evaluation of Its Anticancer and Antioxidant Activity. Waste Biomass Valorization 2024, 15, 841–855. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Feng, C.-H.; Domínguez-Fernández, N.-M.; Álvarez-Mateos, P. Microwave-Assisted Extraction of Polyphenols from Bitter Orange Industrial Waste and Identification of the Main Compounds. Life 2023, 13, 1864. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.S.; Kumar, P. Simulation and Experimentation on Parameters Influencing Microwave-Assisted Extraction of Bioactive Compounds from Punica Granatum Waste and Its Preliminary Analysis. Food Chem. Adv. 2023, 3, 100344. [Google Scholar] [CrossRef]
- Marđokić, A.; Maldonado, A.E.; Klosz, K.; Molnár, M.A.; Vatai, G.; Bánvölgyi, S. Optimization of Conditions for Microwave-Assisted Extraction of Polyphenols from Olive Pomace of Žutica Variety: Waste Valorization Approach. Antioxidants 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Duke, K.; Syeunda, C.; Brantsen, J.F.; Nindawat, S.; Awika, J.M. Polyphenol Recovery from Sorghum Bran Waste by Microwave Assisted Extraction: Structural Transformations as Affected by Grain Phenolic Profile. Food Chem. 2024, 444, 138645. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.A.; D’Arrigo, M.; Martínez, J.A.; Martínez, J.A. Combinatory and Hyphenated Sample Preparation for the Determination of Bioactive Compounds in Foods. TrAC Trends Anal. Chem. 2010, 29, 553–561. [Google Scholar] [CrossRef]
- Ma, N.B.; Ton, N.M.N.; Le, N.L. Co-Optimization of Polysaccharides and Polyphenols Extraction from Mangosteen Peels Using Ultrasound-Microwave Assisted Extraction (UMAE) and Enzyme-Ultrasound Assisted Extraction (EUAE) and Their Characterization. J. Food Meas. Charact. 2024. [Google Scholar] [CrossRef]
- Bouchez, A.; Vauchel, P.; Périno, S.; Dimitrov, K. Multi-Criteria Optimization Including Environmental Impacts of a Microwave-Assisted Extraction of Polyphenols and Comparison with an Ultrasound-Assisted Extraction Process. Foods 2023, 12, 1750. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Amaresh, Y.S.; Kavyasri, M.; Ashwathanarayana, D.S.; Raghavendra, B.T.; Hiregoudar, S. Chemical Characterization of Supercritical Fluid Extract (SFE) of Ailanthus Excelsa and Estimation of Total Phenols and Flavonoids in SFE. Int. J. Plant Soil Sci. 2024, 36, 81–88. [Google Scholar] [CrossRef]
- Amador-Luna, V.M.; Herrero, M.; Domínguez-Rodríguez, G.; Ibáñez, E.; Montero, L. Enhancing the Bioactivity of Dunaliella Salina Extracts through Ultra-High Pressure Supercritical Fluid Extraction (UHP-SFE). Innov. Food Sci. Emerg. Technol. 2024, 95, 103697. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; López-Yerena, A.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Predicting the Effects of In-Vitro Digestion in the Bioactivity and Bioaccessibility of Antioxidant Compounds Extracted from Chestnut Shells by Supercritical Fluid Extraction—A Metabolomic Approach. Food Chem. 2024, 435, 137581. [Google Scholar] [CrossRef] [PubMed]
- Almehayawi, M.S.; Almuhayawi, M.S.; El-Fadl, S.R.A.; Nagshabandi, M.K.; Tarabulsi, M.K.; Selim, S.; Alruwaili, Y.S.; Mostafa, E.M.; Al Jaouni, S.K.; Abdelghany, T.M. Evaluating the Anti-Yeast, Anti-Diabetic, Wound Healing Activities of Moringa Oleifera Extracted at Different Conditions of Pressure via Supercritical Fluid Extraction. BioResources 2024, 19, 5961–5977. [Google Scholar] [CrossRef]
- Kronholm, J.; Hartonen, K.; Riekkola, M.-L. Analytical Extractions with Water at Elevated Temperatures and Pressures. TrAC Trends Anal. Chem. 2007, 26, 396–412. [Google Scholar] [CrossRef]
- Žagar, T.; Frlan, R.; Kočevar Glavač, N. Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties. Antibiotics 2024, 13, 334. [Google Scholar] [CrossRef]
- HERRERO, M.; CIFUENTES, A.; IBANEZ, E. Sub- and Supercritical Fluid Extraction of Functional Ingredients from Different Natural Sources: Plants, Food-by-Products, Algae and MicroalgaeA Review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Anoraga, S.B.; Shamsudin, R.; Hamzah, M.H.; Sharif, S.; Saputro, A.D.; Basri, M.S.M. Optimization of Subcritical Water Extraction for Pectin Extraction from Cocoa Pod Husks Using the Response Surface Methodology. Food Chem. 2024, 459, 140355. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Vardakas, A.; Vassilev, K.; Nenov, N.; Passon, M.; Shikov, V.; Schieber, A.; Mihalev, K. Combining Enzymatic and Subcritical Water Treatments for Green Extraction of Polyphenolic Co-Pigments from Saffron Tepals. Waste Biomass Valorization 2024, 15, 207–217. [Google Scholar] [CrossRef]
- Karimi Sani, I.; Mehrnoosh, F.; Rasul, N.H.; Hassani, B.; Mohammadi, H.; Gholizadeh, H.; Sattari, N.; Kaveh, M.; Khodaei, S.M.; Alizadeh Sani, M.; et al. Pulsed Electric Field-Assisted Extraction of Natural Colorants; Principles and Applications. Food Biosci. 2024, 61, 104746. [Google Scholar] [CrossRef]
- Hegde, S.; Sivamani, Y.; Muthuraman, A.; Elayaperumal, S. Pulsed Electric Field Extraction. In Bioactive Extraction and Application in Food and Nutraceutical Industries; Springer Protocol; Humana: New York, NY, USA, 2024; pp. 223–253. [Google Scholar]
- Poojary, M.M.; Lund, M.N.; Barba, F.J. Pulsed Electric Field (PEF) as an Efficient Technology for Food Additives and Nutraceuticals Development. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–99. [Google Scholar]
- Teh, S.; Niven, B.E.; Bekhit, A.E.A.; Carne, A.; Birch, E.J. Microwave and Pulsed Electric Field Assisted Extractions of Polyphenols from Defatted Canola Seed Cake. Int. J. Food Sci. Technol. 2015, 50, 1109–1115. [Google Scholar] [CrossRef]
- Jara-Quijada, E.; Pérez-Won, M.; Tabilo-Munizaga, G.; Lemus-Mondaca, R.; González-Cavieres, L.; Palma-Acevedo, A.; Herrera-Lavados, C. Liposomes Loaded with Green Tea Polyphenols—Optimization, Characterization, and Release Kinetics Under Conventional Heating and Pulsed Electric Fields. Food Bioprocess Technol. 2024, 17, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Kotsou, K.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Optimizing Extract Preparation from Laurel (Laurus nobilis L.) Leaves Using a Pulsed Electric Field. ChemEngineering 2024, 8, 26. [Google Scholar] [CrossRef]
- Mpakos, D.; Chatzimitakos, T.; Athanasiadis, V.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Optimization of Pulsed Electric Field-Based Extraction of Bioactive Compounds from Cannabis Sativa Leaves. Analytica 2024, 5, 90–106. [Google Scholar] [CrossRef]
- Ye, L.; Luo, W.; Nie, Y.; Chen, M.; Wu, Q.; Yan, P.; Sun, H.; Pei, Y.; Guo, C.; Lin, Y. Phyllanthuse Emblica Polyphenols: Optimization of High-Voltage Pulsed Electric Field Assisted Extraction, an Antioxidant and Anti-Inflammatory Effects in Vitro. J. Dermatol. Sci. Cosmet. Technol. 2024, 100038. [Google Scholar] [CrossRef]
- De Aguiar Saldanha Pinheiro, A.C.; Martí-Quijal, F.J.; Barba, F.J.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Castagnini, J.M.; Tappi, S.; Rocculi, P. Pulsed Electric Fields (PEF) and Accelerated Solvent Extraction (ASE) for Valorization of Red (Aristeus antennatus) and Camarote (Melicertus kerathurus) Shrimp Side Streams: Antioxidant and HPLC Evaluation of the Carotenoid Astaxanthin Recovery. Antioxidants 2023, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Dhowlaghar, N.; Dhanani, T.; Pillai, S.S.; Patil, B.S. Accelerated Solvent Extraction of Red Onion Peel Extract and Its Antimicrobial, Antibiofilm, and Quorum-Sensing Inhibition Activities against Listeria Monocytogenes and Chromobacterium Violaceum. Food Biosci. 2023, 53, 102649. [Google Scholar] [CrossRef]
- Wójciak, M.; Mazurek, B.; Wójciak, W.; Kostrzewa, D.; Żuk, M.; Chmiel, M.; Kubrak, T.; Sowa, I. Optimizing the Extraction of the Polyphenolic Fraction from Defatted Strawberry Seeds for Tiliroside Isolation Using Accelerated Solvent Extraction Combined with a Box–Behnken Design. Molecules 2024, 29, 3051. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Hordiei, K.; Gontova, T.; Trumbeckaite, S.; Yaremenko, M.; Raudone, L. Phenolic Composition and Antioxidant Activity of Tanacetum Parthenium Cultivated in Different Regions of Ukraine: Insights into the Flavonoids and Hydroxycinnamic Acids Profile. Plants 2023, 12, 2940. [Google Scholar] [CrossRef] [PubMed]
- Zulfisa, Z.; Fika, R.; Agusfina, M.; Yonrizon, Y.; Muhsanah, A. Determination of Total Phenolic Content of Ethanol Extract of Broken Bone Twigs (Euphorbia tirucalli Linn.) by Folin-Ciocalteu Method Spectrophotometrically. J. EduHealth 2023, 14, 1326–1331. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, Separation, and Detection Methods for Phenolic Acids and Flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.J.D.; Ferreira, M.R.A.; Randau, K.P.; de Souza, T.P.; Soares, L.A.L. Total Flavonoids Content in the Raw Material and Aqueous Extractives from Bauhinia Monandra Kurz (Caesalpiniaceae). Sci. World J. 2012, 2012, 923462. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent Advances in Anthocyanin Analysis and Characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Khezerlou, A.; Firoozy, S.; Ehsani, A.; Punia Bangar, S. Chitosan-based Film Incorporated with Anthocyanins of Red Poppy (Papaver rhoeas L.) as a Colorimetric Sensor for the Detection of Shrimp Freshness. Int. J. Food Sci. Technol. 2023, 58, 3050–3057. [Google Scholar] [CrossRef]
- Česlová, L.; Kalendová, P.; Dubnová, L.; Pernica, M.; Fischer, J. The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.). Molecules 2023, 28, 6690. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, G.; Zhao, X.; Jiao, L.; Wen, X.; Liu, Y.; Xia, X.; Zhao, C.; Dong, D. Rapid Measurement of Anthocyanin Content in Grape and Grape Juice: Raman Spectroscopy Provides Non-Destructive, Rapid Methods. Comput. Electron. Agric. 2024, 222, 109048. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.-J.E.; Komaitis, M. Analysis of Flavonoids and Phenolic Acids in Greek Aromatic Plants: Investigation of Their Antioxidant Capacity and Antimicrobial Activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Arruda, T.R.; de Castro Leite Junior, B.R.; Marques, C.S.; Bernardes, P.C.; Magalhães, C.G.; Pinheiro, P.F. Emerging Techniques for Extraction and Characterization of Natural Compounds. In Green Products in Food Safety; Elsevier: Amsterdam, The Netherlands, 2023; pp. 29–79. [Google Scholar]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Kamal S, W.J.; Xavier, J. Effect of Heavy Metals on the Pigmentation and Photosynthetic Capability in Jacobaea maritima (L.) Pelser & Meijden. Plant Sci. Today 2023, 10, 192–197. [Google Scholar] [CrossRef]
- Tran, T.K.; Ha, P.T.T.; Henry, R.J.; Nguyen, D.N.T.; Tuyen, P.T.; Liem, N.T. Polyphenol Contents, Gas Chromatography-Mass Spectrometry (GC–MS) and Antibacterial Activity of Methanol Extract and Fractions of Sonneratia Caseolaris Fruits from Ben Tre Province in Vietnam. J. Microbiol. Biotechnol. 2024, 34, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.; Ruiz-Montoya, M.; Díaz, M.J.; Giráldez, I.; Morales, E. Optimization of Bioactive Compounds by Ultrasound Extraction and Gas Chromatography—Mass Spectrometry in Fast-Growing Leaves. Microchem. J. 2023, 193, 109231. [Google Scholar] [CrossRef]
- Sabir, A.M.; Moloy, M.; Bhasin, P.S. Hplc Method Development and Validation: A Review. Int. Res. J. Pharm. 2016, 4, 39–46. [Google Scholar] [CrossRef]
- Roggero, J.-P.; Archier, P.; Coen, S. Chromatography of Phenolics in Wine. In Wine Nutritional and Therapeutic Benefits; ACS Publications: Washington, DC, USA, 1997; pp. 6–11. [Google Scholar]
- Al-Askar, A.A.; Bashir, S.; Mohamed, A.E.; Sharaf, O.A.; Nabil, R.; Su, Y.; Abdelkhalek, A.; Behiry, S.I. Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium Campestre. Separations 2023, 10, 362. [Google Scholar] [CrossRef]
- Orozco-Flores, L.A.; Salas, E.; Rocha-Gutiérrez, B.; Peralta-Pérez, M.D.R.; González-Sánchez, G.; Ballinas-Casarrubias, L. Determination of Polyphenolic Profile of Apple Pomace (Malus Domestica Golden Delicious Variety) by HPLC–MS. ACS Omega 2024, 9, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Suciu, F.; Stoicescu, I.; Lupu, E.; Musuc, A.; Popescu, A.; Mititelu, M.; Roșca, A.; Dumitrescu, D.-E.; Badea, F.; Caraiane, A.; et al. HPLC Analysis of Polyphenolic Compounds in Lysimachia nummularia L. and Comparative Determination of Antioxidant Capacity. Appl. Sci. 2023, 13, 2159. [Google Scholar] [CrossRef]
- Pereira-Coelho, M.; da Silva Haas, I.C.; Vitali, L.; dos Santos Madureira, L.A. Dispersive Pipette Extraction and HPLC-DAD for the Determination of Polyphenols in Grape Juice. Food Anal. Methods 2024, 17, 269–283. [Google Scholar] [CrossRef]
- Ashraf, G.J.; Das, P.; Sahu, R.; Nandi, G.; Paul, P.; Dua, T.K. Impact of Ultrasound-assisted Extraction of Polyphenols and Caffeine from Green Tea Leaves Using High-performance Thin-layer Chromatography. Biomed. Chromatogr. 2023, 37, e5698. [Google Scholar] [CrossRef]
- Jović, M.D.; Agatonovic-Kustrin, S.; Ristivojević, P.M.; Trifković, J.Đ.; Morton, D.W. Bioassay-Guided Assessment of Antioxidative, Anti-Inflammatory and Antimicrobial Activities of Extracts from Medicinal Plants via High-Performance Thin-Layer Chromatography. Molecules 2023, 28, 7346. [Google Scholar] [CrossRef]
- CARIDI, D.; TRENERRY, V.; ROCHFORT, S.; DUONG, S.; LAUGHER, D.; JONES, R. Profiling and Quantifying Quercetin Glucosides in Onion (Allium cepa L.) Varieties Using Capillary Zone Electrophoresis and High Performance Liquid Chromatography. Food Chem. 2007, 105, 691–699. [Google Scholar] [CrossRef]
- Ashmore, P.L.; Valdez, F.; Harbertson, J.F.; Boulton, R.B.; Collins, T.S. Rapid Determination of Free Sulfur Dioxide in Wine and Cider by Capillary Electrophoresis. J. Chromatogr. A 2023, 1695, 463936. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Q.; Zhang, X.; Chen, G. Advances in the Applications of Capillary Electrophoresis to Tobacco Analysis. Curr. Anal. Chem. 2023, 19, 77–99. [Google Scholar] [CrossRef]
- Barbosa, M.F.; Pivatto, M.; Cardoso, A.A.; da Silveira Petruci, J.F. Analysis of Cassine and Spectaline in the Senna Spectabilis Ethanolic Extracts by Capillary Zone Electrophoresis with Indirect UV Detection. Phytochem. Anal. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bamba, T. Application of Supercritical Fluid Chromatography to the Analysis of Hydrophobic Metabolites. J. Sep. Sci. 2008, 31, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lemasson, E.; Bertin, S.; Hennig, P.; Lesellier, E. An Improved Classification of Stationary Phases for Ultra-High Performance Supercritical Fluid Chromatography. J. Chromatogr. A 2016, 1440, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Ul’yanovskii, N.V.; Onuchina, A.A.; Ovchinnikov, D.V.; Faleva, A.V.; Gorbova, N.S.; Kosyakov, D.S. Analytical and Preparative Separation of Softwood Lignans by Supercritical Fluid Chromatography. Separations 2023, 10, 449. [Google Scholar] [CrossRef]
- Ares, A.M.; Toribio, L.; García-Villalba, R.; Villalgordo, J.M.; Althobaiti, Y.; Tomás-Barberán, F.A.; Bernal, J. Separation of Isomeric Forms of Urolithin Glucuronides Using Supercritical Fluid Chromatography. J. Agric. Food Chem. 2023, 71, 3033–3039. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In Vitro Gastrointestinal Digestion Impact on Stability, Bioaccessibility and Antioxidant Activity of Polyphenols from Wild and Commercial Blackberries (Rubus Spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Ceyhan, T.; Çatalkaya, G.; Rajan, L.; Ullah, H.; Daglia, M.; Capanoglu, E. Encapsulated Phenolic Compounds: Clinical Efficacy of a Novel Delivery Method. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Frazzini, S.; Torresani, M.C.; Roda, G.; Dell’Anno, M.; Ruffo, G.; Rossi, L. Chemical and Functional Characterization of the Main Bioactive Molecules Contained in Hulled Cannabis sativa L. Seeds for Use as Functional Ingredients. J. Agric. Food Res. 2024, 16, 101084. [Google Scholar] [CrossRef]
- Xiao, J.B.; Hogger, P. Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future Perspectives. Curr. Med. Chem. 2014, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Polyphenols: Potential Beneficial Effects of These Phytochemicals in Athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Han, L.; Li, X.; Xue, Z.; Zhou, F. Antioxidant and Antitumor Effects and Immunomodulatory Activities of Crude and Purified Polyphenol Extract from Blueberries. Front. Chem. Sci. Eng. 2016, 10, 108–119. [Google Scholar] [CrossRef]
- van de Langerijt, T.M.; O’Callaghan, Y.C.; Tzima, K.; Lucey, A.; O’Brien, N.M.; O’Mahony, J.A.; Rai, D.K.; Crowley, S.V. The Influence of Milk with Different Compositions on the Bioavailability of Blackberry Polyphenols in Model Sports Nutrition Beverages. Int. J. Dairy Technol. 2023, 76, 828–843. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef]
- Franková, H.; Musilová, J.; Árvay, J.; Šnirc, M.; Jančo, I.; Lidiková, J.; Vollmannová, A. Changes in Antioxidant Properties and Phenolics in Sweet Potatoes (Ipomoea batatas L.) Due to Heat Treatments. Molecules 2022, 27, 1884. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Lévèques, A.; Rein, M.; Teml, A.; Schäfer, C.; Hofmann, U.; Li, H.; Schwab, M.; Eichelbaum, M.; Williamson, G. Intestinal Absorption, Metabolism, and Excretion of (–)-Epicatechin in Healthy Humans Assessed by Using an Intestinal Perfusion Technique. Am. J. Clin. Nutr. 2013, 98, 924–933. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Queiroz Barros, H.D.; Maróstica Junior, M.R. Phenolic Compound Bioavailability Using In Vitro and In Vivo Models. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–126. [Google Scholar]
- Moelants, K.R.N.; Lemmens, L.; Vandebroeck, M.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Relation between Particle Size and Carotenoid Bioaccessibility in Carrot- and Tomato-Derived Suspensions. J. Agric. Food Chem. 2012, 60, 11995–12003. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W.; Porter, C.J.H. Formulation of Lipid-Based Delivery Systems for Oral Administration: Materials, Methods and Strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Ali, A.; Oon, C.C.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdylo, A.; Turkiewicz, I.P.; Szumny, A.; Łyczko, J. Volatile and Polyphenol Composition, Anti-Oxidant, Anti-Diabetic and Anti-Aging Properties, and Drying Kinetics as Affected by Convective and Hybrid Vacuum Microwave Drying of Rosmarinus officinalis L. Ind. Crop. Prod. 2020, 151, 112463. [Google Scholar] [CrossRef]
- Tao, K.; Guo, L.; Hu, X.; Fitzgerald, C.; Rouzard, K.; Healy, J.; Tamura, M.; Stock, J.B.; Stock, M.; Pérez, E.; et al. Encapsulated Activated Grape Seed Extract: A Novel Formulation with Anti-Aging, Skin-Brightening, and Hydration Properties. Cosmetics 2021, 9, 4. [Google Scholar] [CrossRef]
- Altan, A.; Yuce, H.; Karataş, Ő.; Taşkan, M.; Gevrek, F.; Çolak, S.; Akbulut, N. Free and Liposome Form of Gallic Acid Improves Calvarial Bone Wound Healing in Wistar Rats. Asian Pac. J. Trop. Biomed. 2020, 10, 156. [Google Scholar] [CrossRef]
- Nateghi, N.; Karimi, E.; Oskoueian, E. Nanoliposome-Encapsulated and Non-Encapsulated Phenolics From Achillea Millefolium and Their Biological Function in Mice Challenged by Campylobacter Jejuni: A Comparative Study. Front. Mol. Biosci. 2022, 8, 832022. [Google Scholar] [CrossRef]
- Shamansoori, M.T.; Karimi, E.; Oskoueian, E. Rheum Ribes Extract-loaded Nanoliposome as a Novel Phytogenic Antibiotic Alternative in Mice Challenged by Escherichia Coli (O157:H7). Biotechnol. Appl. Biochem. 2022, 69, 2540–2549. [Google Scholar] [CrossRef] [PubMed]
- Hassirian, N.; Karimi, E.; Oskoueian, E. Nanoliposome-Encapsulated Phenolic-Rich Fraction from Alcea Rosea as a Dietary Phytobiotic in Mice Challenged by Escherichia Coli. Ann. Microbiol. 2022, 72, 6. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Karimi, E.; Oskoueian, E. Nano-liposomal Encapsulation of Artemisia Aucheri Phenolics as a Potential Phytobiotic against Campylobacter Jejuni Infection in Mice. Food Sci. Nutr. 2022, 10, 3314–3322. [Google Scholar] [CrossRef]
- Ara, T.; Ono, S.; Hasan, M.; Ozono, M.; Kogure, K. Protective Effects of Liposomes Encapsulating Ferulic Acid against CCl4-Induced Oxidative Liver Damage in Vivo Rat Model. J. Clin. Biochem. Nutr. 2023, 72, 22–37. [Google Scholar] [CrossRef]
- Neog, M.K.; Rasool, M. Targeted Delivery of P-Coumaric Acid Encapsulated Mannosylated Liposomes to the Synovial Macrophages Inhibits Osteoclast Formation and Bone Resorption in the Rheumatoid Arthritis Animal Model. Eur. J. Pharm. Biopharm. 2018, 133, 162–175. [Google Scholar] [CrossRef]
- Najafi, A.; Taheri, R.A.; Mehdipour, M.; Martínez-Pastor, F.; Rouhollahi, A.A.; Nourani, M.R. Improvement of Post-Thawed Sperm Quality in Broiler Breeder Roosters by Ellagic Acid-Loaded Liposomes. Poult. Sci. 2019, 98, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yi, Z.; Chen, G.; Ma, X.; Su, W.; Deng, Z.; Ma, L.; Tong, Q.; Ran, Y.; Li, X. Carrier-Enhanced Photodynamic Cancer Therapy of Self-Assembled Green Tea Polyphenol-Based Nanoformulations. ACS Sustain. Chem. Eng. 2020, 8, 16372–16384. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Antitumor Activity of Rosmarinic Acid-Loaded Silk Fibroin Nanoparticles on HeLa and MCF-7 Cells. Polymers. 2021, 13, 3169. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, H.; Lu, Z.; Qin, Q.; Jiao, X.; Meng, G.; Liu, W.; Li, G. PH-Driven Preparation of Pea Protein Isolate-Curcumin Nanoparticles Effectively Enhances Antitumor Activity. Int. J. Biol. Macromol. 2024, 256, 128383. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Chen, G.; Chen, X.; Ma, X.; Cui, X.; Sun, Z.; Su, W.; Li, X. Preparation of Strong Antioxidative, Therapeutic Nanoparticles Based on Amino Acid-Induced Ultrafast Assembly of Tea Polyphenols. ACS Appl. Mater. Interfaces 2020, 12, 33550–33563. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols Profile of Pomegranate Leaves and Their Role in Green Synthesis of Silver Nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Fortes, G.A.C.; Camargo, L.T.F.M.; Camargo, A.J.; Ferri, P.H. Antioxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Predicted by Multivariate Regression Tree. LWT 2021, 137, 110366. [Google Scholar] [CrossRef]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules 2022, 27, 3453. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound Assisted Extraction of Selected Edible Macroalgae: Effect on Antioxidant Activity and Quantitative Assessment of Polyphenols by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Duman, H.; Pekdemir, B.; Rocha, J.M.; Oz, F.; Karav, S. Functional Chocolate: Exploring Advances in Production and Health Benefits. Int. J. Food Sci. Technol. 2024, 59, 5303–5325. [Google Scholar] [CrossRef]
- Ozen, I.T.; Karav, S.; Eksi, A. Variability of Sorbitol/Xylitol Content in Pomegranate (Punica granatum) Juice As Affected by Processing Conditions. Int. J. Food Nutr. Sci. 2014, 3, 4–7. [Google Scholar]
- Jovanović, A.A.; Djordjević, V.B.; Petrović, P.M.; Pljevljakušić, D.S.; Zdunić, G.M.; Šavikin, K.P.; Bugarski, B.M. The Influence of Different Extraction Conditions on Polyphenol Content, Antioxidant and Antimicrobial Activities of Wild Thyme. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100328. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of Bound Polyphenols from Carrot Dietary Fiber and Its in Vivo and in Vitro Antioxidant Activity. Food Chem. 2021, 339, 127879. [Google Scholar] [CrossRef]
- Banica, F.; Bungau, S.; Tit, D.M.; Behl, T.; Otrisal, P.; Nechifor, A.C.; Gitea, D.; Pavel, F.-M.; Nemeth, S. Determination of the Total Polyphenols Content and Antioxidant Activity of Echinacea Purpurea Extracts Using Newly Manufactured Glassy Carbon Electrodes Modified with Carbon Nanotubes. Processes 2020, 8, 833. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, M.; Lü, J.; Li, J.; Zhang, K.; Zhi, H.; Zhang, H.; Sun, J. Antioxidant, Anti-inflammatory and Cytotoxic Activities of Polyphenols Extracted from Chroogomphus Rutilus. Chem. Biodivers. 2020, 17, e1900479. [Google Scholar] [CrossRef]
- PM, S.; Tungare, K.; Sunariwal, S.; Sonawane, S. Extraction and Characterization of Polyphenols from Artocarpus Heterophyllus and Its Effect on Oxidative Stability of Peanut Oil. Int. J. Fruit Sci. 2020, 20, S1134–S1155. [Google Scholar] [CrossRef]
- Zunino, S.J.; Storms, D.H.; Stephensen, C.B. Diets Rich in Polyphenols and Vitamin A Inhibit the Development of Type I Autoimmune Diabetes in Nonobese Diabetic Mice. J. Nutr. 2007, 137, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.R.; Kim, I.; Lee, J. Phenolic Composition and Antioxidant Activity of Purple Sweet Potato (Ipomoea batatas (L.) Lam.): Varietal Comparisons and Physical Distribution. Antioxidants 2021, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Pham, H.N.T.; Negus, C. From Herbal Teabag to Infusion—Impact of Brewing on Polyphenols and Antioxidant Capacity. Beverages 2022, 8, 81. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowicz-Budzisz, A.; Pejcz, E.; Spychaj, R.; Harasym, J. Mixed Psyllium Fiber Improves the Quality, Nutritional Value, Polyphenols and Antioxidant Activity of Rye Bread. Foods 2023, 12, 3534. [Google Scholar] [CrossRef] [PubMed]
- Jariene, E.; Lasinskas, M.; Danilcenko, H.; Vaitkeviciene, N.; Slepetiene, A.; Najman, K.; Hallmann, E. Polyphenols, Antioxidant Activity and Volatile Compounds in Fermented Leaves of Medicinal Plant Rosebay Willowherb (Chamerion angustifolium (L.) Holub). Plants 2020, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernández, I.; Aranda-Ledesma, N.E.; Rojas, R.; Tafolla-Arellano, J.C.; Martínez-Ávila, G.C.G. Antioxidant Activity of Polyphenolic Compounds Obtained from Euphorbia Antisyphilitica By-Products. Heliyon 2021, 7, e06734. [Google Scholar] [CrossRef] [PubMed]
- Bimbiraitė-Survilienė, K.; Stankevičius, M.; Šuštauskaitė, S.; Gęgotek, A.; Maruška, A.; Skrzydlewska, E.; Barsteigienė, Z.; Akuņeca, I.; Ragažinskienė, O.; Lukošius, A. Evaluation of Chemical Composition, Radical Scavenging and Antitumor Activities of Satureja hortensis L. Herb Extracts. Antioxidants 2021, 10, 53. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Nguyen, M.T.; Nguyen, V.T.; Le, V.M.; Trieu, L.H.; Le, X.T.; Khang, T.V.; Giang, N.T.L.; Thach, N.Q.; Hung, T.T. The Effects of Different Extraction Conditions on the Polyphenol, Flavonoids Components and Antioxidant Activity of Polyscias Fruticosa Roots. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022067. [Google Scholar] [CrossRef]
- Tan, S.; Lan, X.; Chen, S.; Zhong, X.; Li, W. Physical Character, Total Polyphenols, Anthocyanin Profile and Antioxidant Activity of Red Cabbage as Affected by Five Processing Methods. Food Res. Int. 2023, 169, 112929. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.S.S.; Mazlan, A.N. Quantification of Polyphenols and Antioxidant Activity in Several Herbal and Green Tea Products in Malaysia. Mater. Today Proc. 2020, 31, A106–A113. [Google Scholar] [CrossRef]
- Mansoori, A.; Singh, N.; Dubey, S.K.; Thakur, T.K.; Alkan, N.; Das, S.N.; Kumar, A. Phytochemical Characterization and Assessment of Crude Extracts from Lantana camara L. for Antioxidant and Antimicrobial Activity. Front. Agron. 2020, 2, 582268. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Futur. Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density Functional Theory Study of the Structure–Antioxidant Activity of Polyphenolic Deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K. A Computational Investigation on the Structure, Global Parameters and Antioxidant Capacity of a Polyphenol, Gallic Acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, C.; Chen, Q.; Xie, X.; Fu, X.; Chen, C.; Huang, Q.; Huang, Z.; Dong, H. Identification of Polyphenols from Rosa Roxburghii Tratt Pomace and Evaluation of in Vitro and in Vivo Antioxidant Activity. Food Chem. 2022, 377, 131922. [Google Scholar] [CrossRef]
- House, N.C.; Puthenparampil, D.; Malayil, D.; Narayanankutty, A. Variation in the Polyphenol Composition, Antioxidant, and Anticancer Activity among Different Amaranthus Species. South Afr. J. Bot. 2020, 135, 408–412. [Google Scholar] [CrossRef]
- Tiji, S.; Benayad, O.; Berrabah, M.; El Mounsi, I.; Mimouni, M. Phytochemical Profile and Antioxidant Activity of Nigella Sativa L Growing in Morocco. Sci. World J. 2021, 2021, 6623609. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Yadav, K.; Chaturvedi, K.; Shivhare, U.S.; Yadav, S.K. Impact of Cold Plasma on Extraction of Polyphenol From De-Oiled Rice and Corn Bran: Improvement in Extraction Efficiency, In Vitro Digestibility, Antioxidant Activity, Cytotoxicity and Anti-Inflammatory Responses. Food Bioprocess Technol. 2022, 15, 1142–1156. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; He, Z.; Chen, Y.; Li, Z.; Meng, T.; Li, Y.; Cao, Y. In Vitro and in Vivo Antioxidant Activity of Eucalyptus Leaf Polyphenols Extract and Its Effect on Chicken Meat Quality and Cecum Microbiota. Food Res. Int. 2020, 136, 109302. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.N.; Sarker, U.; Raihan, M.S.; Al-Huqail, A.A.; Siddiqui, M.H.; Oba, S. Influence of Salinity Stress on Color Parameters, Leaf Pigmentation, Polyphenol and Flavonoid Contents, and Antioxidant Activity of Amaranthus Lividus Leafy Vegetables. Molecules 2022, 27, 1821. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Kukhtenko, H.; Bevz, N.; Konechnyi, Y.; Kukhtenko, O.; Jasicka-Misiak, I. Spectrophotometric and Chromatographic Assessment of Total Polyphenol and Flavonoid Content in Rhododendron Tomentosum Extracts and Their Antioxidant and Antimicrobial Activity. Molecules 2024, 29, 1095. [Google Scholar] [CrossRef] [PubMed]
- Menhem, C.; Mattar, J.; Carrillo, C.; Serhan, M. Determination of Polyphenols, Antioxidant Activity, and Antimicrobial Properties of Zhourat Using Different Extraction Conditions. Appl. Food Res. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; BK, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and Characterization of Phenolic Compounds from Australian Grown Bananas and Their Antioxidant Capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Janarny, G.; Ranaweera, K.K.D.S.; Gunathilake, K.D.P.P. Digestive Recovery of Polyphenols, Antioxidant Activity, and Anti-inflammatory Activity of Selected Edible Flowers from the Family Fabaceae. J. Food Biochem. 2022, 46, e14052. [Google Scholar] [CrossRef] [PubMed]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, Ľ.; Capcarová, M.; Čurlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Čapla, J.; et al. The Effect of Roasting on the Total Polyphenols and Antioxidant Activity of Coffee. J. Environ. Sci. Health Part B 2020, 55, 495–500. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Application of Deep Eutectic Solvents in the Extraction of Polyphenolic Antioxidants from New Zealand Manuka Leaves (Leptospermum scoparium): Optimization and Antioxidant Activity. J. Mol. Liq. 2021, 337, 116385. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High Resolution LC-MS/MS Characterization of Polyphenolic Composition and Evaluation of Antioxidant Activity of Sambucus Ebulus Fruit Tea Traditionally Used in Bulgaria as a Functional Food. Food Chem. 2022, 367, 130759. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant Polyphenols Effectively Protect HaCaT Cells from Ultraviolet C–Triggered Necrosis and Suppress Inflammatory Chemokine Expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef]
- Ghidossi, R.; Yammine, S.; Delsart, C.; Vitrac, X.; Mietton Peuchot, M. Characterisation of Polyphenols and Antioxidant Potential of Red and White Pomace By-Product Extracts Using Subcritical Water Extraction. OENO One 2020, 54, 263–278. [Google Scholar] [CrossRef]
- Usha, T.; Middha, S.; Bhattacharya, M.; Lokesh, P.; Goyal, A. Rosmarinic Acid, a New Polyphenol from Baccaurea Ramiflora Lour. Leaf: A Probable Compound for Its Anti-Inflammatory Activity. Antioxidants 2014, 3, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Siraj, M.A.; Shilpi, J.A.; Hossain, M.G.; Uddin, S.J.; Islam, M.K.; Jahan, I.A.; Hossain, H. Anti-Inflammatory and Antioxidant Activity of Acalypha Hispida Leaf and Analysis of Its Major Bioactive Polyphenols by HPLC. Adv. Pharm. Bull. 2016, 6, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; D’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis Thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Costa, M.; Videira, R.A.; Andrade, P.B.; Paiva-Martins, F. Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines 2022, 10, 2990. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.; Colceru-Mihul, S.; Litescu, S.; Panteli, M.; Rasit, I. Correlation between Polyphenol Content and Anti-Inflammatory Activity of Verbascum Phlomoides (Mullein). Pharm. Biol. 2013, 51, 925–929. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Moro, C.; Manchón, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamón, E.; Rostagno, M.; Mateo-Vivaracho, L. In Vitro Anti-Inflammatory Activity of Phenolic Rich Extracts from White and Red Common Beans. Food Chem. 2014, 161, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Munyao Mutie, F.; Xu, Y.-B.; Saleri, F.D.; Hu, G.-W.; Guo, M.-Q. Antioxidant, Anti-Inflammatory Activities and Polyphenol Profile of Rhamnus Prinoides. Pharmaceuticals 2020, 13, 55. [Google Scholar] [CrossRef]
- Michel, P.; Dobrowolska, A.; Kicel, A.; Owczarek, A.; Bazylko, A.; Granica, S.; Piwowarski, J.; Olszewska, M. Polyphenolic Profile, Antioxidant and Anti-Inflammatory Activity of Eastern Teaberry (Gaultheria procumbens L.) Leaf Extracts. Molecules 2014, 19, 20498–20520. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.; Boehm, M.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A. Anti-Inflammatory Activity of Haskap Cultivars Is Polyphenols-Dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Kumar, A.; Zhang, J.Y.; Johnson, S.A.; Chai, S.C.; Arjmandi, B.H. Evidence for Anti-Inflammatory and Antioxidative Properties of Dried Plum Polyphenols in Macrophage RAW 264.7 Cells. Food Funct. 2015, 6, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Bogolitsyn, K.; Dobrodeeva, L.; Samodova, A.; Parshina, A. In Vitro Immunostimulant Activity of the Polyphenolic Extract from the Arctic Brown Algae Fucus Vesiculosus. Plant Foods Hum. Nutr. 2024, 79, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, M.; Affes, H.; Athmouni, K.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Chemicals Compositions, Antioxidant and Anti-Inflammatory Activity of Cynara Scolymus Leaves Extracts, and Analysis of Major Bioactive Polyphenols by HPLC. Evid.-Based Complement. Altern. Med. 2017, 2017, 4951937. [Google Scholar] [CrossRef] [PubMed]
- Derouich, M.; Bouhlali, E.D.T.; Hmidani, A.; Bammou, M.; Bourkhis, B.; Sellam, K.; Alem, C. Assessment of Total Polyphenols, Flavonoids and Anti-Inflammatory Potential of Three Apiaceae Species Grown in the Southeast of Morocco. Sci. Afr. 2020, 9, e00507. [Google Scholar] [CrossRef]
- Barfoot, K.L.; Istas, G.; Feliciano, R.P.; Lamport, D.J.; Riddell, P.; Rodriguez-Mateos, A.; Williams, C.M. Effects of Daily Consumption of Wild Blueberry on Cognition and Urinary Metabolites in School-Aged Children: A Pilot Study. Eur. J. Nutr. 2021, 60, 4263–4278. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and MiRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.; Kim, S.A.; Park, H.K.; Kim, W. Anti-Inflammatory and Anti-Superbacterial Activity of Polyphenols Isolated from Black Raspberry. Korean J. Physiol. Pharmacol. 2013, 17, 73. [Google Scholar] [CrossRef]
- Peng, L.; Ai-lati, A.; Ji, Z.; Chen, S.; Mao, J. Polyphenols Extracted from Huangjiu Have Anti-Inflammatory Activity in Lipopolysaccharide Stimulated RAW264.7 Cells. RSC Adv. 2019, 9, 5295–5301. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Hu, T.; Jiang, J.-G.; Zhao, J.-W.; Zhu, W. Antioxidant and Anti-Inflammatory Effects of Polyphenols Extracted from Ilex Latifolia Thunb. RSC Adv. 2018, 8, 7134–7141. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, A.A.; Soares, L.A.L.; Assunção Ferreira, M.R.; de Souza Neto, M.A.; da Silva, G.R.; de Araújo, R.F.; Guerra, G.C.B.; de Melo, M.C.N. Quantification of Polyphenols and Evaluation of Antimicrobial, Analgesic and Anti-Inflammatory Activities of Aqueous and Acetone–Water Extracts of Libidibia Ferrea, Parapiptadenia Rigida and Psidium Guajava. J. Ethnopharmacol. 2014, 156, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Sarıtaş, S.; Duman, H.; Karav, S. Nutritional and Functional Aspects of Fermented Algae. Int. J. Food Sci. Technol. 2024, 59, 5270–5284. [Google Scholar] [CrossRef]
- Sim, S.-H.; Choi, H.K.; Lee, D.E.; Na, S.C.; Hwang, D.I.; Bin Oh, H.; Lim, Y.T.; Kim, T.-Y.; Kim, D.-W. Changes in Physiologically Active Ingredients and Anti-Inflammatory Properties of Underutilized Wild Vegetables by Complex Fermentation Using Beneficial Microorganisms. Food Sci. Preserv. 2024, 31, 287–297. [Google Scholar] [CrossRef]
- Labhar, A.; Ahidar, N.; El-Mernissi, Y.; Benamari, O.; Ait Alla, K.; Jahjah, S.; Salhi, A.; Ahari, M.; Abdellah, E.; Amhamdi, H. Phytochemical, Antioxidant, and Anti-Inflammatory Properties of Tetraclinis Articulata (Vahl) Masters Extracts from Al Hoceima Province. E3S Web Conf. 2024, 527, 01016. [Google Scholar] [CrossRef]
- Bassi, S.; Benvenuti, M.; Mirata, S.; Di Piazza, S.; Salis, A.; Damonte, G.; Zotti, M.; Scarfì, S. Enhanced Antioxidant and Anti-Inflammatory Activity of the Extracts of Pleurotus Ostreatus Edible Mushroom Grown on Lavandula Angustifolia Residues. Food Biosci. 2024, 60, 104382. [Google Scholar] [CrossRef]
- Gulua, L.; Shakulashvili, N.; Tskokilauri, A.; Kotorashvili, T.; Pazouki, N.; Rodrigues, K.; Aslani, M.; Ghorbani, M.; Mgbedo, N.E. Saba Rayyani Polyphenol Content, Antioxidant and Anti-Inflammatory Activities of Georgian Wine and Green Tea. World J. Biol. Pharm. Health Sci. 2024, 18, 246–250. [Google Scholar] [CrossRef]
- Khare, P.; Maurya, R.; Bhatia, R.; Mangal, P.; Singh, J.; Podili, K.; Bishnoi, M.; Kondepudi, K.K. Polyphenol Rich Extracts of Finger Millet and Kodo Millet Ameliorate High Fat Diet-Induced Metabolic Alterations. Food Funct. 2020, 11, 9833–9847. [Google Scholar] [CrossRef]
- Nichitoi, M.M.; Josceanu, A.M.; Isopescu, R.D.; Isopencu, G.O.; Geana, E.-I.; Ciucure, C.T.; Lavric, V. Polyphenolics Profile Effects upon the Antioxidant and Antimicrobial Activity of Propolis Extracts. Sci. Rep. 2021, 11, 20113. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M.; Miroliaei, M. Variation in Polyphenolic Profiles, Antioxidant and Antimicrobial Activity of Different Achillea Species as Natural Sources of Antiglycative Compounds. Chem. Biodivers. 2018, 15, e1800075. [Google Scholar] [CrossRef]
- Sun, S.; Huang, S.; Shi, Y.; Shao, Y.; Qiu, J.; Sedjoah, R.-C.A.-A.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, Isolation, Characterization and Antimicrobial Activities of Non-Extractable Polyphenols from Pomegranate Peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional Analysis of Scottish Honeys with Antimicrobial Activity against Antibiotic-Resistant Bacteria Reveals Novel Antimicrobial Components. LWT-Food Sci. Technol. 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Papastavropoulou, K.; Oz, E.; Oz, F.; Proestos, C. Polyphenols from Plants: Phytochemical Characterization, Antioxidant Capacity, and Antimicrobial Activity of Some Plants from Different Sites of Greece. Separations 2022, 9, 186. [Google Scholar] [CrossRef]
- Kruczek, A.; Krupa-Małkiewicz, M.; Lachowicz, S.; Oszmiański, J.; Ochmian, I. Health-Promoting Capacities of In Vitro and Cultivated Goji (Lycium chinense Mill.) Fruit and Leaves; Polyphenols, Antimicrobial Activity, Macro- and Microelements and Heavy Metals. Molecules 2020, 25, 5314. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Mansoori, A.; Prajapati, N.; Tripathi, J.; Sharma, K.; Kumar, A.; Narayan Das, S. Phytochemical Screening and Antimicrobial Activities of Guizotia abyssinica L. Leaf and Flower Extracts. J. Nat. Pestic. Res. 2024, 9, 100083. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial Activity and Action Approach of the Olive Oil Polyphenol Extract Against Listeria Monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial Activity of Phenolic Compounds Identified in Wild Mushrooms, SAR Analysis and Docking Studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A.K.; Kumar, M.; Atri, P. Antiviral Activity of Plant Polyphenols. J. Pharm. Res. 2012, 5, 2402–2412. [Google Scholar]
- Dziedzinski, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M. Identification of Polyphenols from Coniferous Shoots as Natural Antioxidants and Antimicrobial Compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef]
- Fei, P.; Ali, M.A.; Gong, S.; Sun, Q.; Bi, X.; Liu, S.; Guo, L. Antimicrobial Activity and Mechanism of Action of Olive Oil Polyphenols Extract against Cronobacter Sakazakii. Food Control. 2018, 94, 289–294. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.-H.; Sasireka, A.; Chandrasekaran, M.; Chung, I.-M. Polyphenol Composition and Antimicrobial Activity of Various Solvent Extracts from Different Plant Parts of Moringa Oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry by-Products. Food Control. 2018, 92, 516–522. [Google Scholar] [CrossRef]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 9, 1721. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sk, M.F.; Sonawane, A.; Kar, P.; Sadhukhan, S. Plant-Derived Natural Polyphenols as Potential Antiviral Drugs against SARS-CoV-2 via RNA-dependent RNA Polymerase (RdRp) Inhibition: An in-Silico Analysis. J. Biomol. Struct. Dyn. 2021, 39, 6249–6264. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.-W.; Bae, J.-Y.; Heo, J.; Kim, D.; Han, S.-Z.; Park, M.-S. Aronia Melanocarpa and Its Components Demonstrate Antiviral Activity against Influenza Viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory Effect of Pomegranate (Punica granatum L.) Polyphenol Extracts on the Bacterial Growth and Survival of Clinical Isolates of Pathogenic Staphylococcus Aureus and Escherichia Coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative Assessment of Total Polyphenols, Antioxidant and Antimicrobial Activity of Different Tea Varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Chaudhry, F.; Ahmad, M.L.; Hayat, Z.; Ranjha, M.M.A.N.; Chaudhry, K.; Elboughdiri, N.; Asmari, M.; Uddin, J. Extraction and Evaluation of the Antimicrobial Activity of Polyphenols from Banana Peels Employing Different Extraction Techniques. Separations 2022, 9, 165. [Google Scholar] [CrossRef]
- Thomson, C.; Garcia, A.L.; Edwards, C.A. Interactions between Dietary Fibre and the Gut Microbiota. Proc. Nutr. Soc. 2021, 80, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.; Noviello, M.; Calabrese, F.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- El Majdoub, Y.O.; Ginestra, G.; Mandalari, G.; Dugo, P.; Mondello, L.; Cacciola, F. The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity. Nutrients 2021, 13, 2360. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Zidorn, C.; Biernasiuk, A.; Komsta, Ł.; Granica, S. Polyphenols from Impatiens (Balsaminaceae) and Their Antioxidant and Antimicrobial Activities. Ind. Crop. Prod. 2016, 86, 262–272. [Google Scholar] [CrossRef]
- Mokhtar, M.; Ginestra, G.; Youcefi, F.; Filocamo, A.; Bisignano, C.; Riazi, A. Antimicrobial Activity of Selected Polyphenols and Capsaicinoids Identified in Pepper (Capsicum annuum L.) and Their Possible Mode of Interaction. Curr. Microbiol. 2017, 74, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Milenkovic, M.; Vidovic, B.; Todorovic, Z.; Sobajic, S. Correlation between Antimicrobial, Antioxidant Activity, and Polyphenols of Alkalized/Nonalkalized Cocoa Powders. J. Food Sci. 2017, 82, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Kaur, A.; Singh, N.; Nim, L.; Shevkani, K.; Kaur, H.; Arora, D.S. In Vitro Antioxidant and Antimicrobial Properties of Jambolan (Syzygium cumini) Fruit Polyphenols. LWT-Food Sci. Technol. 2016, 65, 1025–1030. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, Antioxidant and Antimicrobial Activities of Leaf and Bark Extracts of Solidago canadensis L. Ind. Crop. Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Benbelaïd, F.; Khadir, A.; Bendahou, M.; Zenati, F.; Bellahsene, C.; Muselli, A.; Costa, J. Antimicrobial Activity of Rosmarinus Eriocalyx Essential Oil and Polyphenols: An Endemic Medicinal Plant from Algeria. J. Coast. Life Med. 2016, 4, 39–44. [Google Scholar] [CrossRef]
- Liu, Z.; Zhai, J.; Han, N.; Yin, J. Assessment of Anti-Diabetic Activity of the Aqueous Extract of Leaves of Astilboides Tabularis. J. Ethnopharmacol. 2016, 194, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Díaz-de-Cerio, E.; Verardo, V.; Gómez-Caravaca, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Exploratory Characterization of Phenolic Compounds with Demonstrated Anti-Diabetic Activity in Guava Leaves at Different Oxidation States. Int. J. Mol. Sci. 2016, 17, 699. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ren, X.; He, K.; Chen, X.; Zhang, S.; Roller, M.; Zheng, B.; Zheng, Q.; Ho, C.-T.; Bai, N. The Anti-Diabetic Effect of Eight Lagerstroemia Speciosa Leaf Extracts Based on the Contents of Ellagitannins and Ellagic Acid Derivatives. Food Funct. 2020, 11, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- IM, K.; Issac, A.; NM, J.; Ninan, E.; Maliakel, B.; Kuttan, R. Effects of the Polyphenol Content on the Anti-Diabetic Activity of Cinnamomum Zeylanicum Extracts. Food Funct. 2014, 5, 2208. [Google Scholar] [CrossRef] [PubMed]
- Ouahabi, S.; Loukili, E.H.; Daoudi, N.E.; Chebaibi, M.; Ramdani, M.; Rahhou, I.; Bnouham, M.; Fauconnier, M.-L.; Hammouti, B.; Rhazi, L.; et al. Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Gracilaria Bursa-Pastoris Extracts. Mar. Drugs 2023, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Hartung, N.M.; Fischer, J.; Ostermann, A.I.; Willenberg, I.; Rund, K.M.; Schebb, N.H.; Garscha, U. Impact of Food Polyphenols on Oxylipin Biosynthesis in Human Neutrophils. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic Acid in Emblica Officinalis Exerts Anti-Diabetic Activity through the Action on β-Cells of Pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.S.; Mini, S. Polyphenols Rich Hibiscus Rosa Sinensis Linn. Petals Modulate Diabetic Stress Signalling Pathways in Streptozotocin-Induced Experimental Diabetic Rats. J. Funct. Foods 2016, 20, 31–42. [Google Scholar] [CrossRef]
- Nie, T.; Cooper, G.J.S. Mechanisms Underlying the Antidiabetic Activities of Polyphenolic Compounds: A Review. Front. Pharmacol. 2021, 12, 798329. [Google Scholar] [CrossRef]
- Mezni, F.; Stiti, B.; Fkiri, S.; Ayari, F.; Ben Slimane, L.; Ksouri, R.; Khaldi, A. Phenolic Profile and in Vitro Anti-Diabetic Activity of Acorn from Four African Quercus Species (Q. Suber, Q. Canariensis, Q. Coccifera and Q. Ilex). South Afr. J. Bot. 2022, 146, 771–775. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Ojo, A.B.; Adanlawo, I.G. Antioxidant, Antidiabetic, and Anti-Inflammatory Activities of Flavonoid-Rich Fractions of Solanum Anguivi Lam. Fruit: In Vitro and Ex Vivo Studies. Heliyon 2024, 10, e31895. [Google Scholar] [CrossRef] [PubMed]
- Sabu, M.C.; Smitha, K.; Kuttan, R. Anti-Diabetic Activity of Green Tea Polyphenols and Their Role in Reducing Oxidative Stress in Experimental Diabetes. J. Ethnopharmacol. 2002, 83, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, S.; Liu, Y.; Huang, M.-T.; Ho, C.-T. Anti-Diabetic Activity of Chemically Profiled Green Tea and Black Tea Extracts in a Type 2 Diabetes Mice Model via Different Mechanisms. J. Funct. Foods 2013, 5, 1784–1793. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Bui, T.-B.-H.; Pham, V.-H.; Tran, M.-D.; Nguyen, Q.-V. Syzygium zeylanicum (L.) DC. Polyphenols Exhibit Anti-Diabetic Activity by Modulation of ACC1, SGLT1, and GLP-1 Genes and Restoration of Gut Microbiota in Overfeeding and High Glucose Exposure-Induced Diabetic Zebrafish. J. Funct. Foods 2024, 112, 105921. [Google Scholar] [CrossRef]
- Draganescu, D.; Andritoiu, C.; Hritcu, D.; Dodi, G.; Popa, M.I. Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology 2021, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Pang, W.; Sun, W.; Lu, B.; Zou, L.; Zhang, D.; Wang, Y. Metallothionein–Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota. Foods 2023, 12, 3139. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Beg, O.U.; Rafie, A.R.; Kanwal, S.; Ovalle-Cisneros, A.; Faison, M.O.; Siddiqui, R.A. Characterization of Green and Yellow Papaya (Carica papaya) for Anti-Diabetic Activity in Liver and Myoblast Cells and Wound-Healing Activity in Fibroblast Cells. Nutrients 2023, 15, 1929. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Gawlik-Dziki, U.; Nowak, R. Antioxidant, Anti-Inflammatory, and Anti-Diabetic Activity of Phenolic Acids Fractions Obtained from Aerva lanata (L.) Juss. Molecules 2021, 26, 3486. [Google Scholar] [CrossRef] [PubMed]
- El Adaouia Taleb, R.; Djebli, N.; Chenini, H.; Sahin, H.; Kolayli, S. In Vivo and in Vitro Anti-diabetic Activity of Ethanolic Propolis Extract. J. Food Biochem. 2020, 44, e13267. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, Z.; Zhao, Y.; Kang, C.; Zhang, X.; Tian, Y.; Yu, J.; Cao, H.; Wang, M. The Anti-Diabetic Activity of Polyphenols-Rich Vinegar Extract in Mice via Regulating Gut Microbiota and Liver Inflammation. Food Chem. 2022, 393, 133443. [Google Scholar] [CrossRef] [PubMed]
- Vaithiyalingam, M.; Sumathi, D.L.; Sabarathinam, S. Isolation and In Silico Study of Curcumin from Curcuma Longa and Its Anti-Diabetic Activity. Appl. Biochem. Biotechnol. 2023, 195, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A.; Job, J.T.; Moothakoottil Kuttithodi, A.; Sasidharan, A.; Benil, P.B.; Ramesh, V.; Farouk Elsadek, M.; Rizwana, H.; Essam El-Din, M.M. Proximate Composition, Antioxidant, Anti-Inflammatory and Anti-Diabetic Properties of the Haustorium from Coconut (Cocos nucifera L.) and Palmyra Palm (Borassus flabellifer L.). J. King Saud Univ.-Sci. 2023, 35, 102404. [Google Scholar] [CrossRef]
- Moloto, M.R.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of Phenolic Compounds, Carotenoids, Amino Acid Composition, In Vitro Antioxidant and Anti-Diabetic Activities in the Leaves of Seven Cowpea (Vigna unguiculata) Cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.B.K.H.; Rupasinghe, H.P.V. Polyphenols Composition and Anti-Diabetic Properties in Vitro of Haskap (Lonicera caerulea L.) Berries in Relation to Cultivar and Harvesting Date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, S.; Huang, Y.; Huang, M.; Zhao, P.; Ma, X.; Wen, Y.; Wang, Q.; Yang, X. Anti-Diabetic Activity of a Polyphenol-Rich Extract from Phellinus Igniarius in KK-Ay Mice with Spontaneous Type 2 Diabetes Mellitus. Food Funct. 2018, 9, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.M.; Jagadeesan, G.; Kannan, G.; Jegan Raj, F.; Annadurai, Y.; Piramanayagam, S.; Thangaraj, P. Exploring the Hypoglycaemic Efficacy of Bio-Accessed Antioxidative Polyphenolics in Thermally Processed Cucumis Dipsaceus Fruits—An in Vitro and in Silico Study. Food Chem. 2024, 435, 137577. [Google Scholar] [CrossRef] [PubMed]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef]
- Bharadvaja, N.; Gautam, S.; Singh, H. Natural Polyphenols: A Promising Bioactive Compounds for Skin Care and Cosmetics. Mol. Biol. Rep. 2023, 50, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Dua, P.; Heiland, M.F.; Kracen, A.C.; Deshields, T.L. Cancer-related Hair Loss: A Selective Review of the Alopecia Research Literature. Psychooncology 2017, 26, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Takeshita, S.; Tomiya, C.; Koikeda, T. Effect of Polyphenols from Water Chestnut Pericarp on Hair-Related Quality of Life in Healthy Middle-Aged, and Older Subjects: A Randomized, Placebo-Controlled, double-blinded, parallel-group, comparison study. Glycative Stress Res. 2024, 11, 21–32. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Chebaibi, M.; Galvão de Azevedo, R.; Conte, R.; Slighoua, M.; Mssillou, I.; Kiokias, S.; de Freitas Gomes, A.; Soares Pontes, G.; Bousta, D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma Europaea Extracts. Molecules 2023, 28, 1780. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, T.; Mathew, L. Free Radical Scavenging Activity from Leaves of Acacia nilotica (L.) Wild. Ex Delile, an Indian Medicinal Tree. Food Chem. Toxicol. 2010, 48, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Hegde, A.R.; Shetty, P.K.; Gollavilli, H.; Managuli, R.S.; Kalthur, G.; Mutalik, S. Sunscreen Creams Containing Naringenin Nanoparticles: Formulation Development and in Vitro and in Vivo Evaluations. Photodermatol. Photoimmunol. Photomed. 2018, 34, 69–81. [Google Scholar] [CrossRef]
- Curcumin Loaded Nano Cubosomal Hydrogel: Preparation, In Vitro Characterization and Antibacterial Activity. Chem. Sci. Trans. 2015, 4, 75–80. [CrossRef]
- Gabr, S.A.; Alghadir, A.H. Evaluation of the Biological Effects of Lyophilized Hydrophilic Extract of Rhus Coriaria on Myeloperoxidase (MPO) Activity, Wound Healing, and Microbial Infections of Skin Wound Tissues. Evid.-Based Complement. Altern. Med. 2019, 2019, 5861537. [Google Scholar] [CrossRef]
- Pinsuwan, S.; Amnuaikit, T.; Ungphaiboon, S.; Itharat, A. Liposome-Containing Hibiscus Sabdariffa Calyx Extract Formulations with Increased Antioxidant Activity, Improved Dermal Penetration and Reduced Dermal Toxicity. J. Med. Assoc. Thai. 2010, 93 (Suppl. S7), S216–S226. [Google Scholar] [PubMed]
- Hanamura, T.; Uchida, E.; Aoki, H. Skin-Lightening Effect of a Polyphenol Extract from Acerola (Malpighia emarginata DC.) Fruit on UV-Induced Pigmentation. Biosci. Biotechnol. Biochem. 2008, 72, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.; Afrin, S.; Reboredo-Rodriguez, P.; Cianciosi, D.; Mezzetti, B.; Quiles, J.; Bompadre, S.; Battino, M.; Giampieri, F. Strawberry-Based Cosmetic Formulations Protect Human Dermal Fibroblasts against UVA-Induced Damage. Nutrients 2017, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Lee, J.; Park, S.H.; Kim, Y.A.; Park, B.J.; Oh, J.; Sung, G.-H.; Aravinthan, A.; Kim, J.-H.; Kang, H.; et al. Antiphotoaging and Antimelanogenic Effects of Penthorum Chinense Pursh Ethanol Extract Due to Antioxidant- and Autophagy-Inducing Properties. Oxidative Med. Cell. Longev. 2019, 2019, 9679731. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsu, C.H. Does Supplementation with Green Tea Extract Improve Acne in Post-Adolescent Women? A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2016, 25, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Affonso, R.C.L.; Voytena, A.P.L.; Fanan, S.; Pitz, H.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Varela, L.A.C.; et al. Phytochemical Composition, Antioxidant Activity, and the Effect of the Aqueous Extract of Coffee (Coffea arabica L.) Bean Residual Press Cake on the Skin Wound Healing. Oxidative Med. Cell. Longev. 2016, 2016, 1923754. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Morton, K.R.; Lee, J.W.; Hartman, R.; Lee, G. Adverse Childhood Experiences and Depressive Symptoms: Protective Effects of Dietary Flavonoids. J. Psychosom. Res. 2020, 131, 109957. [Google Scholar] [CrossRef] [PubMed]
- Sabadashka, M.; Hertsyk, D.; Strugała-Danak, P.; Dudek, A.; Kanyuka, O.; Kucharska, A.Z.; Kaprelyants, L.; Sybirna, N. Anti-Diabetic and Antioxidant Activities of Red Wine Concentrate Enriched with Polyphenol Compounds under Experimental Diabetes in Rats. Antioxidants 2021, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, F.; Salimei, C.; Pastore, D.; Malatesta, G.; Ricordi, C.; Donadel, G.; Bellia, A.; Rovella, V.; Tafani, M.; Garaci, E.; et al. The Protective Effect of a Unique Mix of Polyphenols and Micronutrients against Neurodegeneration Induced by an In Vitro Model of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 3110. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, X.; Ma, C.; Sun, L.; Feng, Y.; He, Z.; Zhang, H. Purification of Polyphenols from Phyllanthus emblica L. Pomace Using Macroporous Resins: Antioxidant Activity and Potential Anti-Alzheimer’s Effects. J. Food Sci. 2022, 87, 1244–1256. [Google Scholar] [CrossRef]
- Hoppe, J.B.; Coradini, K.; Frozza, R.L.; Oliveira, C.M.; Meneghetti, A.B.; Bernardi, A.; Pires, E.S.; Beck, R.C.R.; Salbego, C.G. Free and Nanoencapsulated Curcumin Suppress β-Amyloid-Induced Cognitive Impairments in Rats: Involvement of BDNF and Akt/GSK-3β Signaling Pathway. Neurobiol. Learn. Mem. 2013, 106, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bi, W.; Cheng, A.; Freire, D.; Vempati, P.; Zhao, W.; Gong, B.; Janle, E.M.; Chen, T.-Y.; Ferruzzi, M.G.; et al. Targeting Multiple Pathogenic Mechanisms with Polyphenols for the Treatment of Alzheimer’s Disease-Experimental Approach and Therapeutic Implications. Front. Aging Neurosci. 2014, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, R.; Chia, S.; Pisani, K.; Ruggeri, F.S.; Xu, C.K.; Šneideris, T.; Perni, M.; Sarwat, S.; Joshi, P.; Kumita, J.R.; et al. A Dopamine Metabolite Stabilizes Neurotoxic Amyloid-β Oligomers. Commun. Biol. 2021, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, Z.; Han, X.; Li, S.; Jiang, X.; Chen, S.; Zhang, J.; Lu, H. Neuroprotective Effect of Resveratrol via Activation of Sirt1 Signaling in a Rat Model of Combined Diabetes and Alzheimer’s Disease. Front. Neurosci. 2020, 13, 1400. [Google Scholar] [CrossRef] [PubMed]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic Impact of Grape Leaves Polyphenols on Certain Biochemical and Neurological Markers in AlCl3-Induced Alzheimer’s Disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef]
- Qi, Y.; Shang, L.; Liao, Z.; Su, H.; Jing, H.; Wu, B.; Bi, K.; Jia, Y. Intracerebroventricular Injection of Resveratrol Ameliorated Aβ-Induced Learning and Cognitive Decline in Mice. Metab. Brain Dis. 2019, 34, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Malar, D.S.; Prasanth, M.I.; Brimson, J.M.; Sharika, R.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. Neuroprotective Properties of Green Tea (Camellia sinensis) in Parkinson’s Disease: A Review. Molecules 2020, 25, 3926. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, Q.; Sun, Q.; Chen, C.; Xu, L.; Ding, Y.; Zhou, P. (−)-Epigallocatechin-3-Gallate (EGCG) Inhibits Fibrillation, Disaggregates Amyloid Fibrils of α-Synuclein, and Protects PC12 Cells against α-Synuclein-Induced Toxicity. RSC Adv. 2017, 7, 32508–32517. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG Redirects Amyloidogenic Polypeptides into Unstructured, off-Pathway Oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Quan, Z.; Wong, W.; Guo, J.; Zhang, R.; Yang, Q.; Dai, R.; McGeer, P.L.; Qing, H. Epigallocatechin Gallate (EGCG) Inhibits Alpha-Synuclein Aggregation: A Potential Agent for Parkinson’s Disease. Neurochem. Res. 2016, 41, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, T.; Yue, F.; Li, X.; Wang, P.; Li, Y.; Chan, P.; Yu, S. Tea Polyphenols Alleviate Motor Impairments, Dopaminergic Neuronal Injury, and Cerebral α-Synuclein Aggregation in MPTP-Intoxicated Parkinsonian Monkeys. Neuroscience 2015, 286, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.H.; Mandel, S. Green Tea Polyphenol (–)-epigallocatechin-3-gallate Prevents N -methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Dopaminergic Neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Maor, G.; Youdim, M.B.H. Iron and α-Synuclein in the Substantia Nigra of MPTP-Treated Mice: Effect of Neuroprotective Drugs R-Apomorphine and Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate. J. Mol. Neurosci. 2004, 24, 401–416. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Naz, F.; Jyoti, S. Effect of Curcumin on Lifespan, Activity Pattern, Oxidative Stress, and Apoptosis in the Brains of Transgenic Drosophila Model of Parkinson’s Disease. Biomed Res. Int. 2014, 2014, 606928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Xie, S.P.; Saw, W.T.; Ho, P.G.H.; Wang, H.Y.; Zhou, L.; Zhao, Y.; Tan, E.K. The Therapeutic Implications of Tea Polyphenols Against Dopamine (DA) Neuron Degeneration in Parkinson’s Disease (PD). Cells 2019, 8, 911. [Google Scholar] [CrossRef]
- Mamashli, F.; Meratan, A.A.; Ghasemi, A.; Obeidi, N.; Salmani, B.; Atarod, D.; Pirhaghi, M.; Moosavi-Movahedi, F.; Mohammad-Zaheri, M.; Shahsavani, M.B.; et al. Neuroprotective Effect of Propolis Polyphenol-Based Nanosheets in Cellular and Animal Models of Rotenone-Induced Parkinson’s Disease. ACS Chem. Neurosci. 2023, 14, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, G.; Brunetti, G.; Scuto, M.; Trovato Salinaro, A.; Calabrese, E.J.; Crea, R.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Enhancement by Olive Polyphenols in C. Elegans Wild Type and Parkinson’s Models. Int. J. Mol. Sci. 2020, 21, 3893. [Google Scholar] [CrossRef]
- Chongtham, A.; Agrawal, N. Curcumin Modulates Cell Death and Is Protective in Huntington’s Disease Model. Sci. Rep. 2016, 6, 18736. [Google Scholar] [CrossRef]
- Aditi, K.; Singh, A.; Shakarad, M.N.; Agrawal, N. Management of Altered Metabolic Activity in Drosophila Model of Huntington’s Disease by Curcumin. Exp. Biol. Med. 2022, 247, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Elifani, F.; Amico, E.; Pepe, G.; Capocci, L.; Castaldo, S.; Rosa, P.; Montano, E.; Pollice, A.; Madonna, M.; Filosa, S.; et al. Curcumin Dietary Supplementation Ameliorates Disease Phenotype in an Animal Model of Huntington’s Disease. Hum. Mol. Genet. 2019, 28, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.A.; Zhu, C.; Medvedeva, V.; Lerner, R.P.; Patassini, S.; Franich, N.R.; Maiti, P.; Frautschy, S.A.; Zeitlin, S.; Levine, M.S.; et al. Improvement of Neuropathology and Transcriptional Deficits in CAG 140 Knock-in Mice Supports a Beneficial Effect of Dietary Curcumin in Huntington’s Disease. Mol. Neurodegener. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Tafakhori, A.; Togha, M.; Okhovat, A.A.; Siassi, F.; Eshraghian, M.R.; Sedighiyan, M.; Djalali, M.; Mohammadzadeh Honarvar, N.; Djalali, M. The Synergistic Effects of ω-3 Fatty Acids and Nano-Curcumin Supplementation on Tumor Necrosis Factor (TNF)-α Gene Expression and Serum Level in Migraine Patients. Immunogenetics 2017, 69, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Jafarieh, A.; Sarraf, P.; Sedighiyan, M.; Yousefi, A.; Tafakhori, A.; Abdollahi, H.; Salehinia, F.; Djalali, M. The Neuromodulatory Effects of ω-3 Fatty Acids and Nano-Curcumin on the COX-2/INOS Network in Migraines: A Clinical Trial Study from Gene Expression to Clinical Symptoms. Endocrine, Metab. Immune Disord.-Drug Targets 2019, 19, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Karimi, E.; Sarraf, P.; Tafakhori, A.; Siri, G.; Salehinia, F.; Sedighiyan, M.; Asanjarani, B.; Badeli, M.; Abdollahi, H.; et al. The Omega-3 and Nano-Curcumin Effects on Vascular Cell Adhesion Molecule (VCAM) in Episodic Migraine Patients: A Randomized Clinical Trial. BMC Res. Notes 2021, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Prieto, K.; Cao, Y.; Mohamed, E.; Trillo-Tinoco, J.; Sierra, R.A.; Urueña, C.; Sandoval, T.A.; Fiorentino, S.; Rodriguez, P.C.; Barreto, A. Polyphenol-Rich Extract Induces Apoptosis with Immunogenic Markers in Melanoma Cells through the ER Stress-Associated Kinase PERK. Cell Death Discov. 2019, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Diab, T.A.; Donia, T.; Saad-Allah, K.M. Characterization, Antioxidant, and Cytotoxic Effects of Some Egyptian Wild Plant Extracts. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 13. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Dang, S.; Han, S.; Yun, C.; Wang, W.; Wang, H. Optimized Ultrasound-Assisted Extraction of Total Polyphenols from Empetrum Nigrum and Its Bioactivities. J. Chromatogr. B 2021, 1173, 122699. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Zhao, X.-H. Bioactivity of Two Polyphenols Quercetin and Fisetin against Human Gastric Adenocarcinoma AGS Cells as Affected by Two Coexisting Proteins. Molecules 2022, 27, 2877. [Google Scholar] [CrossRef]
- Prakash, M.D.; Stojanovska, L.; Feehan, J.; Nurgali, K.; Donald, E.L.; Plebanski, M.; Flavel, M.; Kitchen, B.; Apostolopoulos, V. Anti-Cancer Effects of Polyphenol-Rich Sugarcane Extract. PLoS ONE 2021, 16, e0247492. [Google Scholar] [CrossRef] [PubMed]
- Erden Tayhan, S.; Bilgin, S.; Yıldırım, A.; Koç, E. Biological Screening of Polyphenol Derivatives for Anti-Proliferative, Anti-Apoptotic and Anti-Migrative Activities in Human Breast Cancer Cell Lines MCF-7. Chem. Biodivers. 2023, 20, e202200872. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Qu, H.; Wu, Y.; Wang, Z.; Wang, L. Study on Antitumor, Antioxidant and Immunoregulatory Activities of the Purified Polyphenols from Pinecone of Pinus Koraiensis on Tumor-Bearing S180 Mice in Vivo. Int. J. Biol. Macromol. 2017, 94, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, Z.; Zhao, W.; Liu, J. Assessment of the Anti-Tumor Activity of Cyanidin-3-O-Arabinoside from Apple against APN, JAK, and EZH2 Target Proteins. Food Biosci. 2022, 48, 101788. [Google Scholar] [CrossRef]
- Wu, W.; Xiang, F.; He, F. Polyphenols from Artemisia Argyi Leaves: Environmentally Friendly Extraction under High Hydrostatic Pressure and Biological Activities. Ind. Crop. Prod. 2021, 171, 113951. [Google Scholar] [CrossRef]
- Mechchate, H.; Costa de Oliveira, R.; Es-safi, I.; Vasconcelos Mourão, E.M.; Bouhrim, M.; Kyrylchuk, A.; Soares Pontes, G.; Bousta, D.; Grafov, A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals 2021, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green Tea Polyphenol EGCG Attenuates MDSCs-Mediated Immunosuppression through Canonical and Non-Canonical Pathways in a 4T1 Murine Breast Cancer Model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Polyphenols from the Bark of Rhus Verniciflua and Their Biological Evaluation on Antitumor and Anti-Inflammatory Activities. Phytochemistry 2013, 92, 113–121. [Google Scholar] [CrossRef]
- Girardelo, J.R.; Munari, E.L.; Dallorsoleta, J.C.S.; Cechinel, G.; Goetten, A.L.F.; Sales, L.R.; Reginatto, F.H.; Chaves, V.C.; Smaniotto, F.A.; Somacal, S.; et al. Bioactive Compounds, Antioxidant Capacity and Antitumoral Activity of Ethanolic Extracts from Fruits and Seeds of Eugenia involucrata DC. Food Res. Int. 2020, 137, 109615. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, R.; Li, H.; Wang, Y.; Luo, Y.; Zou, J.; Zhao, B.; Chen, S. New Insight into the Joint Significance of Dietary Jujube Polysaccharides and 6-Gingerol in Antioxidant and Antitumor Activities. RSC Adv. 2021, 11, 33219–33234. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; González, K.; Mesta, F.; Couder, B.; Tavarez, Z.; Zavala, R.; Hernandez, I.; Garrido, G.; Rodeiro, I.; Vanden Berghe, W. Polyphenolic Fraction Obtained From Thalassia Testudinum Marine Plant and Thalassiolin B Exert Cytotoxic Effects in Colorectal Cancer Cells and Arrest Tumor Progression in a Xenograft Mouse Model. Front. Pharmacol. 2020, 11, 592985. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer Effects of Epigallocatechin-3-Gallate Nanoemulsion on Lung Cancer Cells through the Activation of AMP-Activated Protein Kinase Signaling Pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Nagane, M.; Aihara, N.; Kamiie, J.; Miyanabe, M.; Hiraki, S.; Luo, X.; Nakanishi, I.; Shoji, Y.; Matsumoto, K.; et al. Lipid-Soluble Polyphenols from Sweet Potato Exert Antitumor Activity and Enhance Chemosensitivity in Breast Cancer. J. Clin. Biochem. Nutr. 2021, 68, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.A.; Sheichenko, O.P.; Fedotcheva, N.I. New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent. Pharmaceutics 2021, 13, 2089. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cadena, A.; Urueña, C.; Prieto, K.; Martinez-Usatorre, A.; Donda, A.; Barreto, A.; Romero, P.; Fiorentino, S. Immune-System-Dependent Anti-Tumor Activity of a Plant-Derived Polyphenol Rich Fraction in a Melanoma Mouse Model. Cell Death Dis. 2016, 7, e2243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, J.; Wang, Y.; Zhao, Z.; Wang, Z.; Li, W. The Anti-Tumor Activity and Critical Active Compounds of Polyphenols from Chinese Dwarf Cherry (Cerasus humilis). J. Berry Res. 2023, 13, 211–225. [Google Scholar] [CrossRef]
- Yi, J.; Wang, Z.; Bai, H.; Yu, X.; Jing, J.; Zuo, L. Optimization of Purification, Identification and Evaluation of the in Vitro Antitumor Activity of Polyphenols from Pinus Koraiensis Pinecones. Molecules 2015, 20, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Wang, Z.; Bai, H.; Li, L.; Zhao, H.; Cheng, C.; Zhang, H.; Li, J. Polyphenols from Pinecones of Pinus Koraiensis Induce Apoptosis in Colon Cancer Cells through the Activation of Caspase in Vitro. RSC Adv. 2016, 6, 5278–5287. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Zhu, Y.; Wang, Z. Pinus Koraiensis Polyphenols: Structural Identification, in Vitro Antioxidant Activity, Immune Function and Inhibition of Cancer Cell Proliferation. Food Funct. 2021, 12, 4176–4198. [Google Scholar] [CrossRef]
- da Luz, J.R.D.; López, J.A.; Ferreira, M.P.; de Sousa, R.M.; e Silva, S.V.; das Graças Almeida, M.; Araujo-Silva, G. In Vitro Antithrombotic, Antitumor and Antiangiogenic Activities of Green Tea Polyphenols and Its Main Constituent Epigallocatechin-3-Gallate. Processes 2022, 11, 76. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Ha, W.; Lin, Y.; Jiang, K.; Yang, J.-L.; Shi, Y.-P. Polyphenols Isolated from Xanthoceras Sorbifolia Husks and Their Anti-Tumor and Radical-Scavenging Activities. Molecules 2016, 21, 1694. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.A.; El Raey, M.A.; Ibrahim, A.Y.; Abdelfattah, M.A.O.; Abdelmageed, A.M.; Sobeh, M. A Sulfated Polyphenols-Rich Extract from Sabal Yapa Exhibits Antitumor Activities in Ehrlich Ascites Carcinoma. Saudi J. Biol. Sci. 2021, 28, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xue, L.; Tata, A.; Song, M.; Neto, C.C.; Xiao, H. Bioactive Components of Polyphenol-Rich and Non-Polyphenol-Rich Cranberry Fruit Extracts and Their Chemopreventive Effects on Colitis-Associated Colon Cancer. J. Agric. Food Chem. 2020, 68, 6845–6853. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shi, A.; Wang, N.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Tao, Y.; Wang, Q.; et al. Polyphenolic Proanthocyanidin-B2 Suppresses Proliferation of Liver Cancer Cells and Hepatocellular Carcinogenesis through Directly Binding and Inhibiting AKT Activity. Redox Biol. 2020, 37, 101701. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Luna, M.; Rucker, N.; Wong, S.; Gomer, C.J. Pro-Apoptotic and Anti-Inflammatory Properties of the Green Tea Constituent Epigallocatechin Gallate Increase Photodynamic Therapy Responsiveness. Lasers Surg. Med. 2011, 43, 644–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bona, N.P.; Pedra, N.S.; Azambuja, J.H.; Soares, M.S.P.; Spohr, L.; Gelsleichter, N.E.; de M. Meine, B.; Sekine, F.G.; Mendonça, L.T.; de Oliveira, F.H.; et al. Tannic Acid Elicits Selective Antitumoral Activity in Vitro and Inhibits Cancer Cell Growth in a Preclinical Model of Glioblastoma Multiforme. Metab. Brain Dis. 2020, 35, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in Vitro and in Vivo Analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, C.; Cui, M.; Guo, H.; Chen, S.; Du, J.; Li, H.; Li, Z. Polyphenols from Hippophae Rhamnoides Suppressed Colon Cancer Growth by Regulating MiRNA-Mediated Cell Cycle Arrest and Apoptosis in Vitro and in Vivo. J. Funct. Foods 2021, 87, 104780. [Google Scholar] [CrossRef]
- Mesas, C.; Martínez, R.; Ortíz, R.; Galisteo, M.; López-Jurado, M.; Cabeza, L.; Perazzoli, G.; Melguizo, C.; Porres, J.M.; Prados, J. Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer. Nutrients 2021, 13, 566. [Google Scholar] [CrossRef]
- Juli, G.; Oliverio, M.; Bellizzi, D.; Gallo Cantafio, M.E.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-Tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the Biological Activity of Anthocyanins and Polyphenols in Blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shan, S.; Zhang, C.; Shi, J.; Li, H.; Li, Z. Inhibitory Effects of Bound Polyphenol from Foxtail Millet Bran on Colitis-Associated Carcinogenesis by the Restoration of Gut Microbiota in a Mice Model. J. Agric. Food Chem. 2020, 68, 3506–3517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, Q.; Yang, C.; Yu, P.; et al. Anti-Cancer Activities of Tea Epigallocatechin-3-Gallate in Breast Cancer Patients under Radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef]
- Yi, J.; Cheng, C.; Li, X.; Zhao, H.; Qu, H.; Wang, Z.; Wang, L. Protective Mechanisms of Purified Polyphenols from Pinecones of Pinus Koraiensis on Spleen Tissues in Tumor-Bearing S180 Mice in Vivo. Food Funct. 2017, 8, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. MiRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gou, N.; Yuan, E.; Ren, J. Bioactivity-Oriented Purification of Polyphenols from Cinnamomum Cassia Presl. with Anti-Proliferation Effects on Colorectal Cancer Cells. Plant Foods Hum. Nutr. 2020, 75, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Alshuniaber, M.A.; Krishnamoorthy, R.; AlQhtani, W.H. Antimicrobial Activity of Polyphenolic Compounds from Spirulina against Food-Borne Bacterial Pathogens. Saudi J. Biol. Sci. 2021, 28, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Chanput, W.P.; Sae-tan, S. Gut Microbiota Modulation, Anti-Diabetic and Anti-Inflammatory Properties of Polyphenol Extract from Mung Bean Seed Coat (Vigna radiata L.). Nutrients 2022, 14, 2275. [Google Scholar] [CrossRef] [PubMed]
- de Jesús Romero-Prado, M.M.; Curiel-Beltrán, J.A.; Miramontes-Espino, M.V.; Cardona-Muñoz, E.G.; Rios-Arellano, A.; Balam-Salazar, L. Dietary Flavonoids Added to Pharmacological Antihypertensive Therapy Are Effective in Improving Blood Pressure. Basic Clin. Pharmacol. Toxicol. 2015, 117, 57–64. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of Flavonoids on Antimicrobial Activity of Microorganisms Present in Dental Plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef]
- Ozturk, T.; Ávila-Gálvez, M.Á.; Mercier, S.; Vallejo, F.; Bred, A.; Fraisse, D.; Morand, C.; Pelvan, E.; Monfoulet, L.-E.; González-Sarrías, A. Impact of Lactic Acid Bacteria Fermentation on (Poly)Phenolic Profile and In Vitro Antioxidant and Anti-Inflammatory Properties of Herbal Infusions. Antioxidants 2024, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Sarraf, P.; Javanbakht, M.H.; Honarvar, N.M.; Hatami, M.; Soveyd, N.; Tafakhori, A.; Sedighiyan, M.; Djalali, M.; Jafarieh, A.; et al. A Novel Combination of ω-3 Fatty Acids and Nano-Curcumin Modulates Interleukin-6 Gene Expression and High Sensitivity C-Reactive Protein Serum Levels in Patients with Migraine: A Randomized Clinical Trial Study. CNS Neurol. Disord.-Drug Targets 2018, 17, 430–438. [Google Scholar] [CrossRef]
- PALFI SALAVAT, M.-C. Polyphenols Content and In Vitro Antitumour Activity of Hydroal Coholic Ectract of Viscum Album In Two Pigmented and Unpigmented Skin Cancer Cell Lines. Farmacia 2022, 70, 807–815. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Zhou, B.; Li, H.; Zeng, J.; Gao, W. Anti-Diabetic Activity in Type 2 Diabetic Mice and α-Glucosidase Inhibitory, Antioxidant and Anti-Inflammatory Potential of Chemically Profiled Pear Peel and Pulp Extracts (Pyrus spp.). J. Funct. Foods 2015, 13, 276–288. [Google Scholar] [CrossRef]
- Kumar, K.; Issac, A.; Ninan, E.; Kuttan, R.; Maliakel, B. Enhanced Anti-Diabetic Activity of Polyphenol-Rich de-Coumarinated Extracts of Cinnamomum Cassia. J. Funct. Foods 2014, 10, 54–64. [Google Scholar] [CrossRef]
- Parigi, S.M.; Eldh, M.; Larssen, P.; Gabrielsson, S.; Villablanca, E.J. Breast Milk and Solid Food Shaping Intestinal Immunity. Front. Immunol. 2015, 6, 415. [Google Scholar] [CrossRef] [PubMed]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of Sweet Cherry Polyphenols on Enhanced Osteoclastogenesis Associated With Childhood Obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Polyphenols and Athletic Performance: A Review on Human Data. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Tanaka, M.; Nakayama, J. Development of the Gut Microbiota in Infancy and Its Impact on Health in Later Life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gallego, C.; Garcia-Mantrana, I.; Salminen, S.; Collado, M.C. The Human Milk Microbiome and Factors Influencing Its Composition and Activity. Semin. Fetal Neonatal Med. 2016, 21, 400–405. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Michels, K.B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 2018, 141, e20172437. [Google Scholar] [CrossRef]
- Stinson, L.F. Establishment of the Early-Life Microbiome: A DOHaD Perspective. J. Dev. Orig. Health Dis. 2020, 11, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lochhead, P.; Ko, Y.; Claggett, B.; Leong, R.W.; Ananthakrishnan, A.N. Systematic Review with Meta-analysis: Breastfeeding and the Risk of Crohn’s Disease and Ulcerative Colitis. Aliment. Pharmacol. Ther. 2017, 46, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; Ferretti, P.; Gorfer, V.; Pedrotti, A.; Tett, A.; et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems 2017, 2, e00164-16. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ferrario, C.; Mangifesta, M.; Viappiani, A.; Ferretti, P.; Gorfer, V.; et al. Exploring Vertical Transmission of Bifidobacteria from Mother to Child. Appl. Environ. Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk Derived Antimicrobial Bioactive Peptides: A Review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Zhu, Y.; Yang, J.; Sun, L.; Chai, X.; Wang, Y. Characteristic Chromatographic Fingerprint Study of Short-Chain Fatty Acids in Human Milk, Infant Formula, Pure Milk and Fermented Milk by Gas Chromatography–Mass Spectrometry. Int. J. Food Sci. Nutr. 2016, 67, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Sánchez, D.; Casanova-Crespo, S.; Rodríguez-Lagunas, M.J.; Castell, M.; Massot-Cladera, M.; Pérez-Cano, F.J. A Maternal Diet Enriched in Fiber and Polyphenols during Pregestation, Gestation, and Lactation Has an Intestinal Trophic Effect in Both the Dam and the Offspring. In Proceedings of the IECN 2023, Online, 1–15 November 2023; MDPI: Basel, Switzerland; p. 2. [Google Scholar]
- Alemu, A.W.; Doepel, L. Fenugreek (Trigonella foenum-graecum L.) as an Alternative Forage for Dairy Cows. Animal 2011, 5, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Akbağ, H.I.; Savaş, T.; Karagül Yüceer, Y. The Effect of Fenugreek Seed (Trigonella foenum-graecum) Supplementation on the Performance and Milk Yield Characteristics of Dairy Goats. Arch. Anim. Breed. 2022, 65, 385–395. [Google Scholar] [CrossRef]
- Al-Shaikh, M.A.; Al-Mufarrej, S.I.; Mogawer, H.H. Effect of Fenugreek Seeds (Trigonella foenumgraecum L) on Lactational Performance of Dairy Goat. J. Appl. Anim. Res. 1999, 16, 177–183. [Google Scholar] [CrossRef]
- Kwan, S.H.; Abdul-Rahman, P.S. Clinical Study on Plant Galactagogue Worldwide in Promoting Women’s Lactation: A Scoping Review. Plant Foods Hum. Nutr. 2021, 76, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.A.; Kumar, P. Fenugreek: A Review on Its Nutraceutical Properties and Utilization in Various Food Products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Sevrin, T.; Alexandre-Gouabau, M.-C.; Castellano, B.; Aguesse, A.; Ouguerram, K.; Ngyuen, P.; Darmaun, D.; Boquien, C.-Y. Impact of Fenugreek on Milk Production in Rodent Models of Lactation Challenge. Nutrients 2019, 11, 2571. [Google Scholar] [CrossRef] [PubMed]
- Sevrin, T.; Boquien, C.-Y.; Gandon, A.; Grit, I.; de Coppet, P.; Darmaun, D.; Alexandre-Gouabau, M.-C. Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion. Genes 2020, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Hussain, T.; Hameed, A.; Shahzad, M.; Mazhar, M.U.; Yang, G. Dietary Moringa Oleifera Alters Periparturient Plasma and Milk Biochemical Indicators and Promotes Productive Performance in Goats. Front. Vet. Sci. 2022, 8, 787719. [Google Scholar] [CrossRef] [PubMed]
- Kekana, T.W.; Marume, U.; Nherera-Chokuda, F.V. Prepartum Supplementation of Moringa Oleifera Leaf Meal: Effects on Health of the Dam, Colostrum Quality, and Acquisition of Immunity in the Calf. J. Dairy Sci. 2022, 105, 5813–5821. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Aguirre, G.; Mendoza-Taco, M.M.; Arcos-Álvarez, D.N.; Piñeiro-Vázquez, A.T.; Moo-Huchin, V.M.; Canul-Solís, J.R.; Castillo-Sánchez, L.; Ramírez-Bautista, M.A.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Effect of Feeding Lactating Ewes with Moringa Oleifera Leaf Extract on Milk Yield, Milk Composition and Preweaning Performance of Ewe/Lamb Pair. Animals 2020, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Jeong, C.H.; Seo, H.G.; Han, S.G. Moringa Extract Attenuates Inflammatory Responses and Increases Gene Expression of Casein in Bovine Mammary Epithelial Cells. Animals 2019, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, G.; Wang, Y.; Zhang, Y. Moringa Oleifera Leaf Flavonoids Protect Bovine Mammary Epithelial Cells from Hydrogen Peroxide-induced Oxidative Stress in Vitro. Reprod. Domest. Anim. 2020, 55, 711–719. [Google Scholar] [CrossRef]
- Mustofa; Yuliani, F.S.; Purwono, S.; Sadewa, A.H.; Damayanti, E.; Heriyanto, D.S. Polyherbal Formula (ASILACT®) Induces Milk Production in Lactating Rats through Upregulation of α-Lactalbumin and Aquaporin Expression. BMC Complement. Med. Ther. 2020, 20, 368. [Google Scholar] [CrossRef]
- Liu, H.; Hua, Y.; Luo, H.; Shen, Z.; Tao, X.; Zhu, X. An Herbal Galactagogue Mixture Increases Milk Production and Aquaporin Protein Expression in the Mammary Glands of Lactating Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 760585. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Aviello, G.; Capasso, F.; Savino, F.; Izzo, A.A.; Lembo, F.; Borrelli, F. Silymarin BIO-C®, an Extract from Silybum Marianum Fruits, Induces Hyperprolactinemia in Intact Female Rats. Phytomedicine 2009, 16, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Soka, S.; Alam, H.; Boenjamin, N.; Agustina, T.W.; Suhartono, M.T. Effect of Sauropus Androgynus Leaf Extracts on the Expression of Prolactin and Oxytocin Genes in Lactating BALB/C Mice. Lifestyle Genom. 2010, 3, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.E.; Khojandi, M.; Illig, K.; Huth, J.; Andreassen, B. The Influence of Prolactin Secretion on Human Lactation. J. Clin. Endocrinol. Metab. 1975, 40, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Faraz, A.; Waheed, A.; Nazir, M.M.; Hameed, A.; Tauqir, N.A.; Mirza, R.H.; Ishaq, H.M.; Bilal, R.M. Impact of Oxytocin Administration on Milk Quality, Reproductive Performance and Residual Effects in Dairy Animals—A Review. Punjab Univ. J. Zool. 2020, 35, 61–67. [Google Scholar] [CrossRef]
- Sani, N.A.; Kawu, M.U.; Bako, I.G. Effects of Launaea Taraxacifolia and Resveratrol on Milk Yield and Serum Prolactin and Oxytocin Levels: A Lactogenic Study. Int. J. Vet. Sci. Med. 2019, 7, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wang, N.; Yao, B.; Zhong, Y.; Lin, Y.; You, T. Quercetin Improves Postpartum Hypogalactia in Milk-deficient Mice via Stimulating Prolactin Production in Pituitary Gland. Phytother. Res. 2018, 32, 1511–1520. [Google Scholar] [CrossRef]
- Cao, L.; Wang, T.; Mi, X.; Ji, P.; Zhao, X.; Zhang, Y. Exploring the Action Mechanism of the Active Ingredient of Quercetin in Ligustrum Lucidum on the Mouse Mastitis Model Based on Network Pharmacology and Molecular Biology Validation. Evid.-Based Complement. Altern. Med. 2022, 2022, 4236222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, S.; Zhao, H.; Li, L.; Li, Y.; Tu, Y.; Jiang, L.; Zhao, G. Lipidomic Profiling Using GC and LC-MS/MS Revealed the Improved Milk Quality and Lipid Composition in Dairy Cows Supplemented with Citrus Peel Extract. Food Res. Int. 2022, 161, 111767. [Google Scholar] [CrossRef]
- Penczynski, K.J.; Krupp, D.; Bring, A.; Bolzenius, K.; Remer, T.; Buyken, A.E. Relative Validation of 24-h Urinary Hippuric Acid Excretion as a Biomarker for Dietary Flavonoid Intake from Fruit and Vegetables in Healthy Adolescents. Eur. J. Nutr. 2017, 56, 757–766. [Google Scholar] [CrossRef]
- Ziauddeen, N.; Rosi, A.; Del Rio, D.; Amoutzopoulos, B.; Nicholson, S.; Page, P.; Scazzina, F.; Brighenti, F.; Ray, S.; Mena, P. Dietary Intake of (Poly)Phenols in Children and Adults: Cross-Sectional Analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014). Eur. J. Nutr. 2019, 58, 3183–3198. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-Rich Foods in the Mediterranean Diet Are Associated with Better Cognitive Function in Elderly Subjects at High Cardiovascular Risk. J. Alzheimers Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Souza, M.A.; de Paiva, P.G.; Pérez-Jiménez, J.; do Carmo Castro Franceschini, S.; Ribeiro, A.Q. Estimated Dietary Intake and Major Food Sources of Polyphenols in Elderly of Viçosa, Brazil: A Population-Based Study. Eur. J. Nutr. 2018, 57, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Hernández-Galiot, A. Intake of Nutrient and Non-Nutrient Dietary Antioxidants. Contribution of Macromolecular Antioxidant Polyphenols in an Elderly Mediterranean Population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Dietary Intake and Food Contributors of Polyphenols in Adults and Elderly Adults of Sao Paulo: A Population-Based Study. Br. J. Nutr. 2016, 115, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, O.-H.; Sung, M.-K. Effects of Phenol-Depleted and Phenol-Rich Diets on Blood Markers of Oxidative Stress, and Urinary Excretion of Quercetin and Kaempferol in Healthy Volunteers. J. Am. Coll. Nutr. 2003, 22, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Williams, C.M. Effects of a Single Dose of a Flavonoid-Rich Blueberry Drink on Memory in 8 to 10 y Old Children. Nutrition 2015, 31, 531–534. [Google Scholar] [CrossRef]
- Darzi, M.; Abbasi, K.; Ghiasvand, R.; Akhavan Tabib, M.; Rouhani, M.H. The Association between Dietary Polyphenol Intake and Attention-Deficit Hyperactivity Disorder: A Case-Control Study. BMC Pediatr. 2022, 22, 700. [Google Scholar] [CrossRef] [PubMed]
- Mengfan, F.; Zhang, C.; Liu, B.; Liu, S.; Shi, D.; Chanf, X. Effect of Polyphenol Compound Formula on Relieving Exercise Fatigue in Mice. Food Mach. 2023, 39, 145–152,161. [Google Scholar] [CrossRef]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.; Medina-Remón, A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Portillo, M.; Moreno, J.; et al. Polyphenol Levels Are Inversely Correlated with Body Weight and Obesity in an Elderly Population after 5 Years of Follow Up (The Randomised PREDIMED Study). Nutrients 2017, 9, 452. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Bernardi, S.; Del Bo’, C.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; Gonzalez-Dominguez, R.; Peron, G.; Zamora-Ros, R.; et al. Effect of a Polyphenol-Rich Dietary Pattern on Intestinal Permeability and Gut and Blood Microbiomics in Older Subjects: Study Protocol of the MaPLE Randomised Controlled Trial. BMC Geriatr. 2020, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J. Genetic Approaches for Sports Performance: How Far Away Are We? Sports Med. 2019, 49, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.; Nguyen, C.; Shetty, M.; Oppezzo, M.; Barrack, M.; Fredericson, M. Popular Dietary Trends’ Impact on Athletic Performance: A Critical Analysis Review. Nutrients 2023, 15, 3511. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Fortinguerra, S.; Caudullo, G.; Buriani, A. Deciphering the Role of Polyphenols in Sports Performance: From Nutritional Genomics to the Gut Microbiota toward Phytonutritional Epigenomics. Nutrients 2020, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Angeles-Valencia, M.; Morales-González, Á.; Madrigal-Santillán, E.O.; Morales-Martínez, M.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Gutiérrez-Salinas, J.; Esquivel-Chirino, C.; Chamorro-Cevallos, G.; et al. Oxidative Stress, Mitochondrial Function and Adaptation to Exercise: New Perspectives in Nutrition. Life 2021, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Slattery, K.; Bentley, D.; Coutts, A.J. The Role of Oxidative, Inflammatory and Neuroendocrinological Systems During Exercise Stress in Athletes: Implications of Antioxidant Supplementation on Physiological Adaptation During Intensified Physical Training. Sports Med. 2015, 45, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Suzuki, K.; Castell, L. A Short Overview of Changes in Inflammatory Cytokines and Oxidative Stress in Response to Physical Activity and Antioxidant Supplementation. Antioxidants 2020, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, K.H. Polyphenol Supplementation: Benefits for Exercise Performance or Oxidative Stress? Sports Med. 2014, 44, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a Polyphenol-Enriched Protein Powder on Exercise-Induced Inflammation and Oxidative Stress in Athletes: A Randomized Trial Using a Metabolomics Approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cerullo, G.; Bianco, A.; Neri, M.; Gennaro, F.; Charrier, D.; Moro, T. Not Only Protein: Dietary Supplements to Optimize the Skeletal Muscle Growth Response to Resistance Training: The Current State of Knowledge. J. Hum. Kinet. 2024, 91, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in Exercise Performance and Prevention of Exercise-Induced Muscle Damage. Oxid. Med. Cell. Longev. 2013, 2013, 825928. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; D Hayes, L.; Milic, M.; Padulo, J. Herbal Medicine for Sports: A Review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Keli, S.O. Dietary Flavonoids, Antioxidant Vitamins, and Incidence of Stroke. Arch. Intern. Med. 1996, 156, 637. [Google Scholar] [CrossRef]
- Otsuka, Y.; Egawa, K.; Kanzaki, N.; Izumo, T.; Rogi, T.; Shibata, H. Quercetin Glycosides Prevent Dexamethasone-Induced Muscle Atrophy in Mice. Biochem. Biophys. Rep. 2019, 18, 100618. [Google Scholar] [CrossRef] [PubMed]
- MacRae, H.S.-H.; Mefferd, K.M. Dietary Antioxidant Supplementation Combined with Quercetin Improves Cycling Time Trial Performance. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Sgrò, P.; Ceci, R.; Lista, M.; Patrizio, F.; Sabatini, S.; Felici, F.; Sacchetti, M.; Bazzucchi, I.; Duranti, G.; Di Luigi, L. Quercetin Modulates IGF-I and IGF-II Levels After Eccentric Exercise-Induced Muscle-Damage: A Placebo-Controlled Study. Front. Endocrinol. 2021, 12, 745959. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Gelabert-Rebato, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Lopez-Rios, L.; Wiebe, J.C.; Martin-Rodriguez, S.; Arteaga-Ortiz, R.; Dorado, C.; et al. Supplementation with a Mango Leaf Extract (Zynamite®) in Combination with Quercetin Attenuates Muscle Damage and Pain and Accelerates Recovery after Strenuous Damaging Exercise. Nutrients 2020, 12, 614. [Google Scholar] [CrossRef]
- de Sousa, B.R.V.; de Lima Tavares Toscano, L.; de Almeida Filho, E.J.B.; Sena, K.F.; Costa, M.S.; de Souza Cunha, R.C.; de Souza Siqueira Quintans, J.; Heimfarth, L.; Marques, A.T.B.; da Silva, D.F.; et al. Purple Grape Juice Improves Performance of Recreational Runners, but the Effect Is Genotype Dependent: A Double Blind, Randomized, Controlled Trial. Genes Nutr. 2022, 17, 9. [Google Scholar] [CrossRef]
- de Lima Tavares Toscano, L.; Silva, A.S.; de França, A.C.L.; de Sousa, B.R.V.; de Almeida Filho, E.J.B.; da Silveira Costa, M.; Marques, A.T.B.; da Silva, D.F.; de Farias Sena, K.; Cerqueira, G.S.; et al. A Single Dose of Purple Grape Juice Improves Physical Performance and Antioxidant Activity in Runners: A Randomized, Crossover, Double-Blind, Placebo Study. Eur. J. Nutr. 2020, 59, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Jones, K.E.; Sidhu, R.S.; Haykowsky, M.; Czubryt, M.P.; Gordon, T.; Dyck, J.R.B. Improvements in Skeletal Muscle Strength and Cardiac Function Induced by Resveratrol during Exercise Training Contribute to Enhanced Exercise Performance in Rats. J. Physiol. 2012, 590, 2783–2799. [Google Scholar] [CrossRef]
- Hart, N.; Sarga, L.; Csende, Z.; Koltai, E.; Koch, L.G.; Britton, S.L.; Davies, K.J.A.; Kouretas, D.; Wessner, B.; Radak, Z. Resveratrol Enhances Exercise Training Responses in Rats Selectively Bred for High Running Performance. Food Chem. Toxicol. 2013, 61, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health Benefits of Resveratrol: Evidence from Clinical Studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of Resveratrol on Glucose Control and Insulin Sensitivity: A Meta-Analysis of 11 Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Maeda, S.; Akazawa, N.; Zempo-Miyaki, A.; Choi, Y.; Ra, S.-G.; Imaizumi, A.; Otsuka, Y.; Nosaka, K. Attenuation of Indirect Markers of Eccentric Exercise-Induced Muscle Damage by Curcumin. Eur. J. Appl. Physiol. 2015, 115, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Chino, K.; Ohnishi, T.; Ozawa, H.; Sagayama, H.; Maeda, S.; Takahashi, H. Effects of Oral Curcumin Ingested before or after Eccentric Exercise on Markers of Muscle Damage and Inflammation. Scand. J. Med. Sci. Sports 2019, 29, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Chino, K.; Sagayama, H.; Lee, H.J.; Ozawa, H.; Maeda, S.; Takahashi, H. Effective Timing of Curcumin Ingestion to Attenuate Eccentric Exercise-Induced Muscle Soreness in Men. J. Nutr. Sci. Vitaminol. 2019, 65, 82–89. [Google Scholar] [CrossRef]
- Wang, I.-L.; Hsiao, C.-Y.; Li, Y.-H.; Meng, F.-B.; Huang, C.-C.; Chen, Y.-M. Nanobubbles Water Curcumin Extract Reduces Injury Risks on Drop Jumps in Women: A Pilot Study. Evid.-Based Complement. Altern. Med. 2019, 2019, 8647587. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.B.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced Inflammatory and Muscle Damage Biomarkers Following Oral Supplementation with Bioavailable Curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.; Hu, J. Effect of Curcumin on the Diversity of Gut Microbiota in Ovariectomized Rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of Resveratrol on Gut Microbiota and Fat Storage in a Mouse Model with High-Fat-Induced Obesity. Food Funct. 2014, 5, 1241. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid.-Based Integr. Med. 2018, 23, 2515690X1879072. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Gut Microbiota in Phytopharmacology: A Comprehensive Overview of Concepts, Reciprocal Interactions, Biotransformations and Mode of Actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Atan, R.M.; Ersoy, G.; Çakıcı, Ç. Effects of Hardaliye, a Fermented Grape Drink, on Oxidative Stress, Lipid Profile, and Blood Pressure in Young Soccer Players: A Randomized Controlled Trial. J. Am. Nutr. Assoc. 2024, 43, 356–364. [Google Scholar] [CrossRef]
- Valder, S.; Staltner, R.; Bizjak, D.A.; Esatbeyoglu, T.; Herdegen, V.; Köpsel, M.; Kostov, T.; Bergheim, I.; Diel, P. Effect of Sugar- and Polyphenol-Rich, Diluted Cloudy Apple Juice on the Intestinal Barrier after Moderate Endurance Exercise and in Ultra-Marathon Runners. Nutrients 2024, 16, 1353. [Google Scholar] [CrossRef]
- Morton, L.; Paton, C.; Braakhuis, A. The Effects of Polyphenol Supplementation on BDNF, Cytokines and Cognition in Trained Male Cyclists Following Acute Ozone Exposure during High-Intensity Cycling. Nutrients 2024, 16, 233. [Google Scholar] [CrossRef]
- Griffin, É.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, Á.M. Aerobic Exercise Improves Hippocampal Function and Increases BDNF in the Serum of Young Adult Males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Bos, I.; De Boever, P.; Int Panis, L.; Meeusen, R. Physical Activity, Air Pollution and the Brain. Sports Med. 2014, 44, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Revi, N.; Rengan, A.K. Impact of Dietary Polyphenols on Neuroinflammation-Associated Disorders. Neurol. Sci. 2021, 42, 3101–3119. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Maldonado, A.; Rentería, I.; García-Suárez, P.C.; Moncada-Jiménez, J.; Freire-Royes, L.F. The Impact of High-Intensity Interval Training on Brain Derived Neurotrophic Factor in Brain: A Mini-Review. Front. Neurosci. 2018, 12, 839. [Google Scholar] [CrossRef] [PubMed]

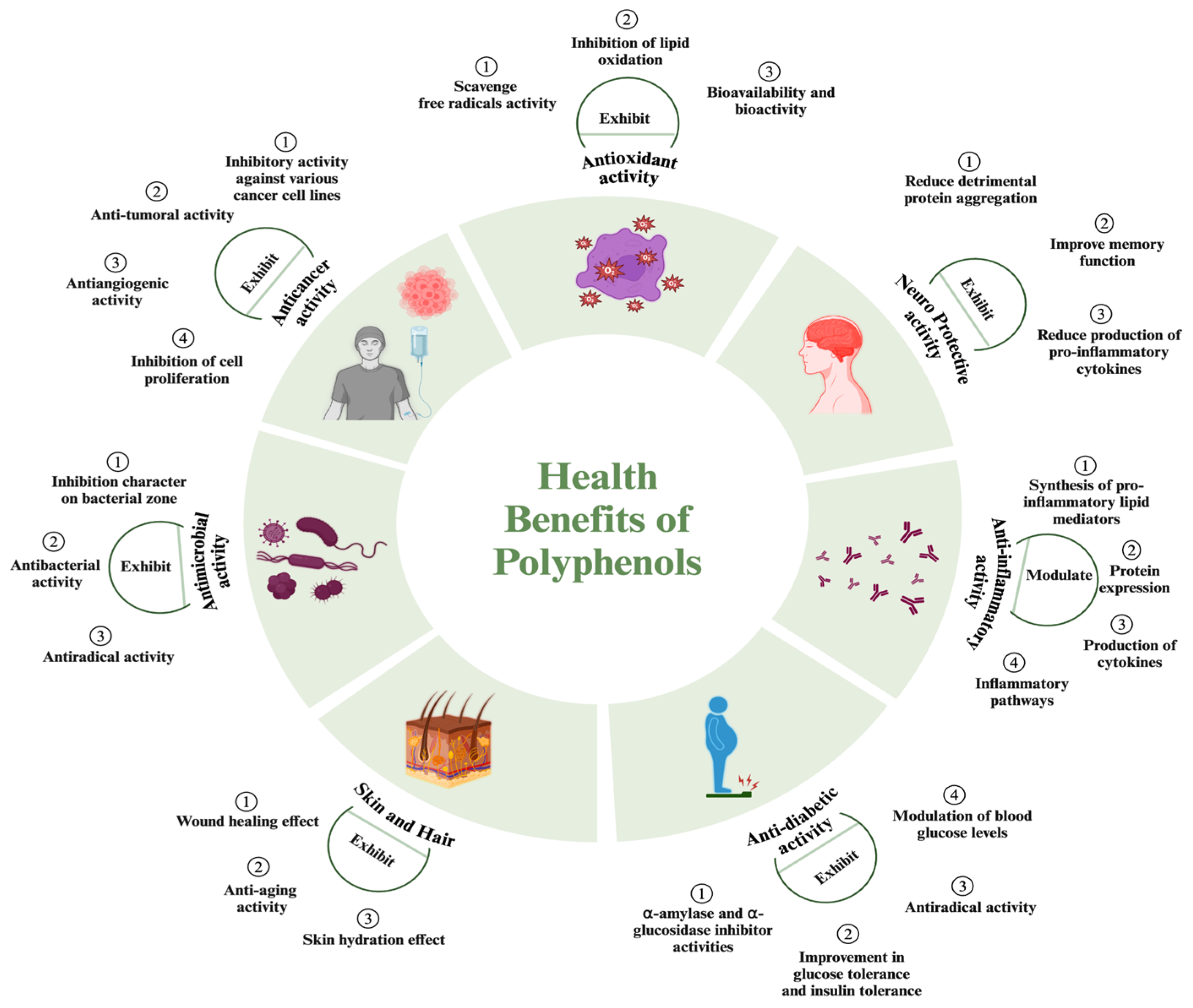
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolat, E.; Sarıtaş, S.; Duman, H.; Eker, F.; Akdaşçi, E.; Karav, S.; Witkowska, A.M. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients 2024, 16, 2550. https://doi.org/10.3390/nu16152550
Bolat E, Sarıtaş S, Duman H, Eker F, Akdaşçi E, Karav S, Witkowska AM. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients. 2024; 16(15):2550. https://doi.org/10.3390/nu16152550
Chicago/Turabian StyleBolat, Ecem, Sümeyye Sarıtaş, Hatice Duman, Furkan Eker, Emir Akdaşçi, Sercan Karav, and Anna Maria Witkowska. 2024. "Polyphenols: Secondary Metabolites with a Biological Impression" Nutrients 16, no. 15: 2550. https://doi.org/10.3390/nu16152550
APA StyleBolat, E., Sarıtaş, S., Duman, H., Eker, F., Akdaşçi, E., Karav, S., & Witkowska, A. M. (2024). Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients, 16(15), 2550. https://doi.org/10.3390/nu16152550









