Abstract
Metabolic and bariatric surgery (MBS) effectively treats obesity and related comorbidities, though individual responses vary. This systematic review examines how genetic variants influence MBS outcomes in morbidly obese patients. A comprehensive search in PubMed, Embase, Medline, and the Cochrane Library identified 1572 studies, with 52 meeting the inclusion criteria. Two reviewers independently filtered and selected studies, including relevant cross-references. Research focused on polymorphisms in genes such as UCP2, UCP3, 5-HT2C, MC4R, FKBP5, FTO, CAT haplotypes, LYPAL-1, PTEN, FABP-2, CNR1, LEP656, LEP223, GLP-1R, APOA-1, APOE, ADIPOQ, IL-6, PGC1a, TM6SF2, MBOAT7, PNPLA3, TCF7L2, ESR1, GHSR, GHRL, CD40L, DIO2, ACSL5, CG, TAS2R38, CD36, OBPIIa, NPY, BDNF, CLOCK, and CAMKK2. Most studies explored associations with post-surgery weight loss, while some examined metabolic, cardiovascular, taste, and eating behavior effects as well. Understanding the role of genetic factors in weight loss and metabolic outcomes post-MBS can help tailor personalized treatment plans for improved efficacy and long-term success. Further research with larger sample sizes and extended follow-up is needed to clarify the effects of many genetic variants on MBS outcomes in morbidly obese patients.
1. Introduction
Obesity is a significant risk factor for several of the leading causes of mortality worldwide, including cardiovascular disease, stroke, diabetes, and various forms of cancer. According to the latest data of the Institute of Health Metrics and Evaluation (IHME), which are from 2021, obesity is the sixth leading risk factor for death in the world. In 2021, obesity was accountable for 129 million (95% UI 56.0–202) disability-adjusted life years (DALYs) and 3.71 million (1.85–5.66) deaths worldwide [1]. Studies have proven that obesity leads to the development of obesity-related diseases, such as the following: diabetes mellitus type 2 (T2D), metabolic syndrome, hypertension, hyperlipidemia, chronic kidney disease, cardiovascular disease, obstructive sleep apnea, osteoarthritis, malignancies, and metabolic dysfunction-associated steatotic liver disease (MASLD) [2,3,4,5]. Metabolic syndrome, a cluster of metabolic disorders including central obesity, high blood pressure, elevated fasting glucose levels, atherogenic dyslipidemia, and insulin resistance, further exacerbates the health risks associated with obesity. Individuals with metabolic syndrome are at heightened risk for cardiovascular disease, stroke, and T2D, underscoring the urgent need for effective preventive measures and management strategies [6].
Weight loss interventions such as diet, exercise, and metabolic and bariatric surgery (MBS) reduce all-cause mortality in obese adults [7]. MBS is linked to a decreased risk of premature death and the development of obesity-related diseases at the population level, and is considered the most effective treatment option for obesity [8,9]. Older MBS procedures like vertical banded gastroplasty (VBG) have been replaced by safer and more effective operations, with sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) now accounting for approximately 90% of all procedures performed worldwide. Other less common procedures include adjustable gastric banding (AGB or LAGB), biliopancreatic diversion (BPD) with duodenal switch, and one-anastomosis gastric bypass (OAGB), with most MBS now performed using minimally invasive techniques [10,11]. According to the newest guidelines by the American Society of Metabolic and Bariatric Surgery (ASMBS) and the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO), MBS is strongly recommended for patients with a BMI ≥35 kg/m2 regardless of the presence, absence, or severity of comorbidities; it is recommended in patients with T2D and a BMI ≥30 kg/m2 and should be considered in patients with a BMI 30–34.9 kg/m² who have not achieved significant or sustained weight loss or improvement in comorbidities through non-surgical methods. The new guidelines also recommended modifying the BMI thresholds for the Asian population, since traditional BMI thresholds do not apply equally to all populations. In the Asian population, BMI >25 kg/m² indicates clinical obesity and MBS should be considered for individuals with a BMI >27.5 kg/m² [11]. An Expert Modified Delphi Consensus conducted in 2023 concluded that intra-gastric ballooning (IGB) is suitable for Class I obesity regardless of the presence, absence, or severity of comorbidities. SG and RYGB were recommended as viable treatment options for patients with Class I and II obesity, as well as patients with T2D and BMI of ≥30 kg/m². OAGB was deemed suitable for patients with Class II obesity and T2DM regardless of the presence, absence or severity of comorbidities [12]. MBS induces weight loss and remission of obesity-related diseases through several biological mechanisms, many of which are still not completely understood [13,14]. Although MBS is highly effective for long-term weight loss in morbid obesity, about 20% of patients fail to maintain over 50% excess weight loss. Twin and close-relative studies suggested a genetic component to weight loss, indicating that genetic factors may influence the outcomes of MBS [15].
Modern technology and genome-wide association studies (GWASs) have opened a whole new world of theories that allow us to tap into the genetic background of obesity and obesity-related diseases. The genetics of obesity has two distinct forms—syndromic and non-syndromic. The non-syndromic form consists of monogenic obesity (POMC, NPY, LEP, LEPR, MC3R, MC4R, FTO, PC1, GHSR, etc.) and polygenic obesity (UCP1, UCP2, UCP3, ADRB1, ADRB2, ADRB3, SLC6A14, etc.) [16]. Researches in the past decade have been focusing on genes as well as single nucleotide polymorphisms (SNPs) and their association with weight loss, eating behavior, biochemical markers, and cardiovascular and metabolic parameters after MBS [17]. The aim of this systematic review is to examine how genetic variants influence MBS outcomes in morbidly obese patients.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for this study. A systematic review protocol was constructed to describe the rationale and hypothesis and to define the methodology, and it was registered under the registration number INPLASY202470020.
2.1. Selection Criteria
The inclusion criteria for performing the search and selection were the following: studies conducted on adult patients aged 18 years or older diagnosed with morbid obesity and undergoing MBS, studies with a defined genetic variant/polymorphism of interest, and availability of follow-up data after MBS. We excluded studies on non-human subjects (animal studies), observational studies without a pre-surgery and post-surgery comparison (cross-sectional studies), reviews, letters to the editor and preprints, studies that are not available in the English language, and studies that are not freely available.
2.2. Search Strategy
An extensive literature search was performed in PubMed, Embase, Medline, and Cochrane library to identify eligible studies. The search was repeated 4 times and the date of the last search is 19.06.2024. Additional searching of gray literature or hand searching was not performed.
The used search terms were the following: (exp obesity/OR exp morbid obesity/OR exp diabetic obesity/) AND (exp bariatric surgery/) AND ((genetic variant.mp. OR exp genetic variability/) OR (exp gene/) OR (exp single nucleotide polymorphism/ OR exp genetic polymorphism/ OR polymorphism.mp)).
All the search results were summed up and duplicates were removed. For the first selection, two reviewers screened potentially relevant articles based on the title and abstract and according to predefined eligibility criteria. For the second selection, full articles were read to determine further inclusion. Cross-references of relevant studies and reviews were screened.
2.3. Data Extraction
Data from the included studies were extracted independently by two reviewers using a standardized data extraction form. The form included data on the study characteristics (author, year, country), sample size, patient demographics, details of the bariatric surgery performed, genetic variants or polymorphisms examined, follow-up duration, main outcomes measured (e.g., weight loss, metabolic parameters), and the association between the outcomes and the genetic variant/polymorphism. Discrepancies between reviewers were resolved through discussion or consultation with a third reviewer.
2.4. Data Synthesis
For this type of etiology/risk review, a narrative synthesis approach was used to analyze the data. The findings were summarized by integrating the results across studies by design, population, demographic data and outcomes without statistical pooling. Key findings were grouped according to the genetic variant/polymorphism and the type of bariatric surgery. Patterns and differences in outcomes were highlighted, and the potential influence of factors such as patient demographics and follow-up duration were discussed. The quality and risk of bias of the included studies were also assessed and considered in the interpretation of the results.
3. Results
A total of 1572 studies were identified from which 260 were duplicates. After the first selection, 109 studies were selected, and after the second selection, only 52 were determined eligible to be included in qualitative synthesis (See Figure 1). Results were organized in a table according to the gene and polymorphism in order to compare them more efficiently (See Table 1).
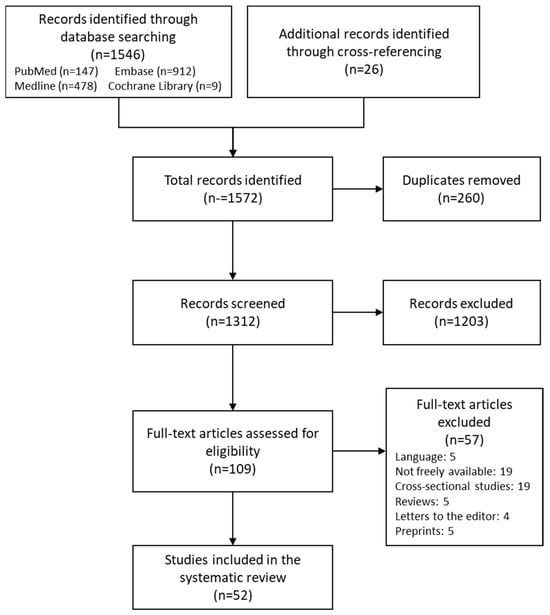
Figure 1.
Flow diagram.

Table 1.
Summary of studies included in the systematic review.
3.1. Uncoupling Protein 2 Gene (UCP2) and Uncoupling Protein 3 Gene (UCP3)
Eight studies about UCP2 were found. For the rs659366 polymorphism, two studies with 164 patients that underwent LAGB and 150 that underwent RYGB showed that the A allele was associated with increased weight loss after 6 and 12 months, respectively [18,19]. Another study performed on 351 patients that underwent RYGB showed no association with % of excess weight loss (%EWL) after 12 months [20]. On the other hand, a study performed on 186 patients that underwent RYGB showed that the A/A genotype is associated with higher BMI, more excess weight, and lower %EWL 18 months after MBS. Patients with the Ins allele had a smaller decrease in BMI 12 months after MBS compared to those with the Del/Del genotype [21].
Regarding the rs660339 polymorphism, three studies concluded that the T allele was associated with weight loss after 6–24 months if the bariatric procedure was LAGB or RYGB, while there were no associations with BMI changes after laparoscopic mini-gastric bypass (LMGB) [19,22,23]. On the other hand, other studies performed on Brazilian patients showed no association with weight loss 12 months and 3 years after RYGB [20,24]. Nicoletti et al. concluded that genetic variants in the UCP2 gene were associated with dietary consumption after RYGB. It was shown that 1 year after MBS patients with rare variants in rs660339 and rs659366 had higher energy and carbohydrate intake. Patient with rare variants for Ala55Val had a higher protein consumption, as well [25].
Two studies found no association between UCP3 rs1800849 polymorphism and weight loss after MBS (RYGB and BPD) [20,26].
3.2. 5-Hydroxytryptamine Receptor 2C Gene (5-HT2C)
5HT2C gene rs3813929 polymorphism (more specifically, the TT genotype) predicted greater body weight loss after RYGB surgery. Post-surgery responsiveness may be related to the control of satiety, involving the interaction between a hormone (serotonin) and its receptor (5-HT2C) [20].
3.3. Melanocortin 4 Receptor Gene (MC4R)
Eight studies about MC4R were found. Women carriers of rs17782313 presented a higher pre-surgical BMI and did not attain a minimum BMI as low as women in the TT group. Furthermore, carriers of the rs17782313 polymorphism exhibited a significantly lower likelihood of achieving a non-obesity BMI compared to non-carriers [27]. On the other hand, in a study on 169 patients undergoing RYGB (78 women and 23 men), no significant association was found between the MC4R polymorphism and post-surgical weight and BMI [28].
Two studies showed that heterozygous MC4R mutations (NM_005912.2 c. 812G>T (p. Cys271Phe, NM_005912.2 c. 919C>T (p. Gln307stop), NM_005912.2 c. 706C>T (p. Arg236Cys), functional mutations, rs2229616, rs52820871, rs17782313, rs34114122) did not affect weight loss and body fat mass (FM) after 12 months of RYGB [29,30]. Another study confirmed that MC4R defects (rs52820871, NM_005912.2 c.594C>T (p. Ile198Ile)) are not associated with a higher complication rate following LAGB [31].
Regarding rs52820871, a study determined that I251L carriers have lower levels of baseline glucose and insulin levels leading to reduced risk of T2D. They are predisposed to better clinical outcome and better weight loss during diet and RYGB [32].
Carriers of MC4R gene variants, in contrast to matched severely obese patients without gene abnormalities and binge eating, exhibited an aggressive form of binge eating disorder associated with the metabolic syndrome and gastrointestinal pathology, with poorer response to massive sustained weight loss from food restriction after LAGB [33]. A study on 300 patients showed that rs79783591 mutation increases obesity risk, but one functional MC4R copy allows short-term weight loss from dietary restriction, phentermine, and RYGB [34].
3.4. FK506-Binding Protein 5 Gene (FKBP5)
Two studies on FKBP5 rs1360780 showed that the T allele is associated with weight loss. A study on 42 Brazilian obese patients showed that carriers of the T allele showed significantly lower weight loss compared to C/C genotype patients 12 to 14 months after MBS. T-allele carriers also experienced an earlier plateau in weight loss post-surgery [35]. Another study in 151 obese patients showed an association between the FKBP5 rs1360780 variant and sex, age, and type of surgery. Male T-allele carriers had higher BMI 24 months after MBS compared to non-carriers, particularly among those who underwent SG. Age also impacted weight loss, with older T-allele carriers exhibiting less favorable outcomes [36].
3.5. Fat Mass and Obesity-Associated Protein Gene (FTO)
Eight studies about FTO were found. One study indicated that the minor allele (G) of the FTO rs16945088 was associated with 3 kg less weight loss compared with common allele homozygotes (A/A), particularly in banding surgery patients, but not in gastric bypass subjects [37].
Studies examining the rs9939609 variant of the FTO gene in the context of MBS provided mixed results regarding its influence on weight loss. Weight loss progressed differently in obese carriers of the FTO gene variant rs9939609 after RYGB. The rs9939609 variant (AA or AT genotypes) did not affect weight loss until two years post-surgery, after which weight loss diminished and weight regain increased [39]. Conversely, other research concluded that FTO genotypes did not significantly influence weight loss two years post-RYGB [42]. This might be due to the pre-surgery vitamin D levels, which were proven to have influence on subsequent weight loss, especially in carriers with two copies of the A allele [41]. Additionally, one study reported higher weight loss at three months in TT carriers of rs9939609, with no significant differences at nine and twelve months post-BPD, but with a significant improvement in biochemical parameters and cardiovascular comorbidities [38]. Another study showed that there was no association between the FTO gene and weight loss 6 months after LSG [40]. However, in patients undergoing RSG, those without the A allele experienced better anthropometric and insulin level improvements, reducing diabetes risk after 12 months [43]. These findings suggest that the impact of the FTO rs9939609 variant on weight loss post-MBS varies and may depend on surgery type and other factors like vitamin D levels.
The rs9939609 and rs1421085 variants were linked to higher post-surgery weight, while rs9930506 and rs1421085 were associated with lower total body weight loss (TBWL) after MBS. Additionally, patients carrying these three polymorphisms exhibited elevated insulin levels and higher HOMA-IR, indicating increased insulin resistance [28].
3.6. Catalase (CAT) Haplotype
Variants in the antioxidant enzyme catalase are linked to hypertension, dyslipidemia, and diabetes. The CAT1 haplotype, comprising homozygous carriers of CATH1 [−844 G, −89 A, −20 T], contrasts with the CAT2 haplotype, which includes heterozygous carriers (CATH1/CATH2) and homozygous CATH2 [−844 A, −89 T, −20 C]. CAT haplotypes may contribute to the variability in metabolic and cardiovascular improvements observed during post-MBS follow-up. Specifically, the CAT1 haplotype appeared to display a more favorable effect on metabolic and cardiovascular parameters following MBS [44].
3.7. Lysophospholipase-like 1 Gene (LYPLAL1)
A study of 251 obese patients who underwent RYGB surgery found that those with the homozygous T allele of SNP rs4846567 near the LYPLAL1 gene had a 7% higher excess BMI loss (EBMIL) compared to other genotypes. These TT-allele carriers also exhibited better eating behavior outcomes, including lower Hunger and Disinhibition scores and higher Cognitive Restraint scores. Additionally, patients with the lowest Hunger scores had a 32% greater EBMIL. These findings suggest that LYPLAL1 genotyping and eating behavior assessments can help predict RYGB surgery success [45].
3.8. Phosphatase and Tensin Homolog Gene (PTEN)
A patient with a PTEN mutation achieved a total body weight loss of 39.4% at 1 year, 48.8% at 2 years, and 44.9% at 3 years post-surgery, comparable to a control group of patients with normal genetic test results. The successful weight loss following Sleeve Gastrectomy suggests it could be an effective treatment for obesity in patients with PTEN mutations. Further research on a bigger sample with long-term follow-up is needed [46].
3.9. Fatty-Acid-Binding Protein 2 Gene (FABP-2)
Two studies found that the FABP2 rs1799883 genotypes did not affect weight loss or clinical outcomes after MBS. Both wild-type and mutant genotype groups showed similar improvements in BMI, weight, FM, and metabolic parameters, with no significant differences in %EWL at 12 months [42,47].
3.10. Cannabinoid Receptor Type 1 Gene (CNR1)
A study of 66 patients found that the G1359A polymorphism in the CB1 receptor did not significantly affect outcomes after biliopancreatic diversion surgery. Both wild-type (G1359G) and mutant (G1359A) groups experienced similar decreases in BMI, weight, waist circumference, blood pressure, glucose, cholesterol, and triacylglycerol levels. The percentage of initial weight loss at one year was also comparable between the groups (33.1% vs. 33.6%) [48].
3.11. Leptin Gene (LEP) and Leptin Receptor Gene (LEPR)
One study found that patients with the AA genotype of rs1137101 experienced significantly higher excess weight loss (%EWL) at 12 months (76.5%) and 24 months compared to those with the GG genotype (52.0%). rs8179183 and rs1805094 genotypes showed no significant association [42]. Conversely, another study revealed that patients with the rs1805094 variant of the LEPR gene had a higher initial %EWL at one year (38.9% vs. 29.9%) and greater total weight loss (50.7 kg vs. 37.2 kg) compared to the wild-type group, despite having a higher baseline weight and BMI [49]. Thus, specific genotypes of rs1137101 and rs1805094 are associated with better weight loss outcomes post-surgery.
3.12. Glucagon-like Peptide-1 Receptor Gene (GLP-1R)
Patients carrying the rs6923761 GG genotype showed lower BMI, weight, and waist circumference compared to A-allele carriers at 12 and 18 months after MBS. The GG genotype was associated with higher initial weight loss percentages at both 12 months (45.6% vs. 39.8%) and 18 months (49.6% vs. 41.3%) compared to A-allele carriers. Biochemical and cardiovascular improvements were similar across both genotype groups [50].
3.13. Apolipoprotein A1 Gene (APOA-I), Apolipoprotein E Gene (APOE), and Adiponectin Gene (ADIPOQ)
In a study involving rs670 polymorphism of the APOA1 gene, both GG and GA/AA groups showed improvements in percent excess weight loss, anthropometric measurements, and biochemical parameters. A-allele carriers (GA/AA) experienced greater reductions in fasting insulin levels at 1, 2, and 3 years post-surgery compared to GG carriers. Similarly, A-allele carriers showed more significant improvements in HOMA-IR levels and increases in HDL-cholesterol levels over the same period [51].
A study that explored associations between APOE polymorphisms and endocrine functions in 62 patients showed that adiponectin levels were significantly lower in the APOE E2 group compared to the E4 group. Adiponectin showed a positive association, while cortisol showed a negative association, across the ordered APOE groups (E2-E3-E4) [52].
In a study exploring the ADIPOQ (rs266729), both genotype groups demonstrated improvements in weight loss and various metabolic markers after MBS. Non-G-allele carriers showed greater reductions in fasting insulin, insulin resistance, total cholesterol, LDL cholesterol, and triglycerides compared to G-allele carriers. Adiponectin levels increased significantly more in non-G-allele carriers over one, two, and three years, particularly in those with the CC genotype [53]. Another study exploring ADIPOQ (rs3774261) revealed decreases in total cholesterol, LDL cholesterol, and triglyceride levels across all genotype groups after MBS. Significant improvements in glucose, insulin, and insulin resistance were observed earlier in subjects with the AA genotype compared to AG and GG genotypes. Adiponectin levels increased significantly in AA-genotype subjects throughout the three-year follow-up, and in AG-genotype subjects at three years. Conversely, GG-genotype subjects did not show significant improvements in these parameters, indicating a delayed response among G-allele carriers of rs3774261 [54]. In a study of 100 patients undergoing RYGB, no association was found with the adiponectin (rs2241766) variant and weight loss after MBS [55].
3.14. Interleukin 6 Gene (IL-6)
Two studies on grade II-III obesity patients that underwent LAGB showed that rs1800795 polymorphism of IL-6 provides the opportunity to predict therapeutic response. One study found that rs1800795 is linked to body composition and fluid distribution in obese individuals both before and six months after LAGB surgery. C-allele carriers exhibited continued cardiovascular risk and higher FM compared to the reference population. They also showed less weight and FM loss after LAGB and experienced a more pronounced negative impact on bone density, suggesting reduced effectiveness of LAGB in obesity treatment for these genotype carriers [56]. The other study highlighted baseline differences in extracellular water and intra-cellular water between rs1800795 carriers (C+) and non-carriers (C−). LAGB surgery significantly reduced weight and BMI in both groups. Post-surgery, C− carriers showed decreases in weight, BMI, and ECW, with increases in body cell mass (BCM) and phase angle (PA). In contrast, C+ carriers experienced reductions in weight, BMI, ICW, and PA, with increases in ECW and resistance (R). C+ carriers also showed greater reductions in BMI and phase angle compared to C− carriers, suggesting potential utility of IL-6 genotyping for predicting body composition changes following LAGB [57].
3.15. Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha Gene PGC1a
In a study of 55 patients undergoing Roux-en-Y gastric bypass, those with the rs8192678 polymorphism showed greater improvements in waist/hip ratio, fasting blood glucose, C-reactive protein, blood leukocyte count, serum interleukin-6, and carotid artery intima–media thickness compared to Gly/Gly patients. This suggests that the rs8192678 polymorphism may predict better metabolic and inflammatory outcomes, potentially lowering atherosclerotic risk post-surgery [58].
3.16. Transmembrane 6 Superfamily 2 Human Gene (TM6SF2), Membrane-Bound O-Acyltransferase Domain-Containing Protein 7 Gene (MBOAT7) and Patatin-like Phospholipase Domain-Containing 3 Gene (PNPLA3)
In a study of 84 obese patients undergoing MBS, those with PNPLA3 rs738409 showed higher hepatic triglyceride content, MRI-detected steatosis, and lower serum glucose levels pre-surgery. After one year, these patients experienced greater weight loss and liver fat reduction compared to those with the wild-type allele. The PNPLA3 mutation and initial steatosis grade were key predictors of MASLD improvement, while TM6SF2 (rs58542926) and MBOAT7 (rs641738) variants were not significant. Thus, PNPLA3 rs738409 may predict better hepatic steatosis outcomes after MBS [59].
3.17. Transcription Factor 7-like 2 Gene (TCF7L2)
In a study of 99 morbidly obese patients undergoing RYGB surgery, the TCF7L2 rs7903146 variation influenced the decrease in fasting blood glucose levels over one year. Both groups experienced similar BMI reductions, but T-risk-allele carriers had a smaller decrease in FBG compared to non-carriers. At one year, FBG was higher in T-risk-allele carriers (6.42 vs. 5.36 mmol/L, p = 0.022). The TCF7L2 rs7903146 variation affected FBG reduction independently of weight loss [60].
3.18. Estrogen Receptor 1 Gene (ESR1)
In a study of 508 patients undergoing MBS (LAGB or LMGB), it was shown that the ESR1 rs712221 polymorphism influenced serum uric acid reduction after MBS. The LMGB group showed a greater reduction in serum uric acid levels compared to the LAGB group (−2.3 vs. −1.2 mg/dL, p = 0.002). Additionally, patients with the rs712221 genotype experienced better glycemic control and the greatest reduction in serum uric acid levels post-surgery. This suggests that the type of surgery and the rs712221 polymorphism synergistically affect serum uric acid reduction [61].
3.19. Ghrelin Receptor Gene (GHSR), Preproghrelin Gene (GHRL), and CD40 Ligand Gene (CD40L)
A study of 100 obese patients undergoing MBS found that those carrying the T allele of the GHRL rs696217 variant experienced a significantly greater reduction in BMI 52 weeks after RYGB compared to those with the GG genotype. On the other hand, the C allele of the CD40L rs1126535 variant was associated with a lower BMI reduction at week 52. The GHSR rs490683 and GHSR rs27647 variants did not show any difference between carriers and non-carriers of the mutant allele [55]. Another study including GHSR rs572169 and GHRL rs26802 showed no association with weight loss after RYGB [20].
3.20. Iodothyronine Deiodinase 2 Gene (DIO2)
A study on 182 obese patients undergoing MBS showed that weight loss and comorbidity improvement were similar across all genetic variations of the DIO2 rs225014 variation. The specific rs225014 variation might be linked to earlier onset of severe obesity, but it does not affect surgical outcomes [62].
3.21. Acyl-CoA Synthetase Long Chain Family Member 5 Gene (ACSL5)
In a study of 48 patients undergoing RSG, rs2419621 T-allele carriers experienced greater improvements in EWL%, weight loss, waist circumference, and triglyceride levels 12 months after MBS. Hypertriglyceridemia was eliminated in T-allele carriers by 12 months [63].
3.22. GC Vitamin-D-Binding Protein Gene (GC)
In a study of 76 participants, the rs2282679 GC gene variant influenced 25-hydroxyvitamin D levels and insulin resistance after MBS. G-allele carriers had higher basal insulin levels and HOMA-IR and lower 25-hydroxyvitamin D levels compared to non-carriers. These differences persisted throughout the 12-month follow-up. At 12 months, non-G-allele carriers had 25-hydroxyvitamin D levels above 30 ng/dL, while G-allele carriers had levels below 30 ng/dL. This suggests the G allele is associated with lower 25-hydroxyvitamin D levels and greater insulin resistance, impacting the improvement of these parameters after weight loss surgery [64].
3.23. Taste 2 Receptor Member 38 Gene (TAS2R38), CD36 Gene (CD36), and Odorant-Binding Protein 2A Gene (OBPIIa)
In a study of 51 obese patients undergoing MBS, taste perception changes were observed. PAV haplotype carriers were more likely to be super-tasters after surgery, while AVI haplotype carriers tended to be non-tasters before surgery. However, these changes were not linked to the TAS2R38 gene. All genotypes showed improved fatty acid taste perception post-surgery, especially those with the non-taster variant (AA). The OBPIIa rs2590498 (A/G) locus was associated with changes in overall taste sensitivity, particularly for sweet and sour tastes. GG carriers had a significantly increased total taste score after surgery, unlike AA or AG carriers [65].
3.24. Neuropeptide Y Gene (NPY)
In a study of 147 morbidly obese patients without T2D undergoing MBS, improvements in fasting glucose, insulin, HOMA-IR, and lipid profiles were observed across all genotypes. However, A-allele carriers of the rs16147 NPY gene showed significantly greater reductions in glucose, fasting insulin, and HOMA-IR 1 year after MBS. These results suggest that the A-allele variant is associated with a faster metabolic response after MBS [66].
3.25. Brain-Derived Neurotrophic Factor Gene (BDNF)
In a study of 158 severely obese patients undergoing MBS in Spain, researchers focused on the BDNF rs6265 variant and its impact on weight loss over 24 months. They found that patients with the Met allele tended to achieve better weight loss outcomes (%EWL) after surgery, although this was not statistically significant (p = 0.056). Patients with the Met allele and no T2D achieved greater %EWL and %EBMIL compared to those with T2D or the Val/Val genotype. These findings suggest that BDNF genotype, combined with T2D status, influences weight loss success following MBS [67].
3.26. Clock Circadian Regulator Gene (CLOCK)
In a study involving 375 patients with morbid obesity and 230 controls, the rs1801260 variant showed a protective effect against morbid obesity, while the TT genotype of rs3749474 was strongly associated with morbid obesity. Both SNPs were independently linked to long-term weight loss and weight regain after surgery, regardless of pre-surgery patient characteristics [68].
3.27. Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CAMKK2)
The study assessed how the CAMKK2 NM_001270486.1, c.1614dup (p.Gly539Argfs*3) mutation affects lipid levels before and 12 months after MBS. Both mutation carriers and non-carriers showed increased HDL cholesterol levels, with non-carriers having a more significant percentage change. Non-carriers also experienced greater reductions in triglyceride levels compared to mutation carriers. These findings highlight that, while both groups benefited from surgery, non-carriers demonstrated more pronounced improvements in lipid metabolism [69].
4. Discussion
This systematic review considered fifty-two studies. Our review indicates that there are multiple genetic polymorphisms that have individual effects on weight loss and improvement of obesity-related diseases after MBS. Most of the studies focused on finding associations between the genetic polymorphisms and weight-loss after MBS, but some also explored association with metabolic and cardiovascular comorbidities, as well as eating behavior and taste. The most frequently researched genetic polymorphisms were in the UCP2, MC4R, and FTO genes, each included in eight studies. These genes are prioritized in research due to their well-known roles in energy balance, appetite regulation, and metabolic processes. Based on our narrative synthesis, the UCP2 rs659366 and rs660339 polymorphisms, the FKBP5 rs1360780 polymorphism, and various FTO and MC4R polymorphisms have the biggest potential to be used as predictive markers for the outcome of MBS. Several other polymorphisms, including those in CAT haplotypes, LYPLAL-1, APOA1, APOE, ADIPOQ, PGC-1, MBOAT7, PNPLA3, and TCF7L2, show potential for predicting metabolic improvement outcomes following MBS. However, further research is needed to thoroughly validate and understand their predictive capabilities.
Uncoupling proteins 2 and 3 belong to an anion-carrier protein family located in the inner mitochondrial membrane. UCP2 exhibits widespread expression across various body tissues, including white adipose tissue, whereas UCP3 is predominantly expressed in skeletal muscle [70]. UCP proteins regulate energy expenditure by uncoupling oxidative phosphorylation in mitochondria, dissipating energy as heat. Decreased UCP activity can lead to reduced energy expenditure, contributing to obesity. Additionally, UCPs influence insulin sensitivity and lipid metabolism, linking them to obesity-related conditions like type 2 diabetes and metabolic syndrome [16,71]. Regarding UCP2 rs659366 polymorphism, the studies we found reported opposing conclusions [18,19,20,21]. For the rs660339 polymorphism, it is interesting to note that different bariatric procedures yielded different results, with the T allele showing association with reduced BMI after LAGB, no association after LMGB, and mixed results after RYGB [19,22,23]. Regarding the UCP3 polymorphisms, both studies showed no association with weight loss or clinical outcome after MBS [20,26]. Further studies should be performed on bigger mixed population samples and with a longer follow-up duration in order to provide more accurate and high-quality data.
The predominant cause of non-syndromic genetic obesity often stems from mutations in the MC4R [72]. MC4R is coding the melanocortin-4-receptor which is a G protein-coupled receptor [73] The melanocortin pathway regulates appetite and energy balance through receptors in the brain, influencing food intake and energy expenditure. Dysregulation can lead to obesity by increasing appetite and reducing metabolism. This pathway’s role in adipogenesis and insulin sensitivity also links it to obesity-related diseases like T2D and cardiovascular disorders [16,71]. The studies we found had contradictory results about the association of MC4R polymorphisms and weight loss after MBS. The limitations for some of these studies were the rarity of patients with MC4R mutations, since there is a great heterogeneity of the disorder, and a short period of follow-up [29,30,31].
The FTO gene produces a nuclear protein known as alpha-ketoglutarate-dependent dioxygenase FTO [74]. The FTO gene regulates body weight and adiposity by influencing appetite and energy expenditure through its role in hypothalamic function. Variants associated with FTO increase food intake and decrease satiety, predisposing individuals to obesity. FTO gene variants also correlate with higher risks of T2D and cardiovascular diseases [16,71]. Different FTO polymorphisms were associated with weight loss after MBS, especially after long periods of follow-up [37,38,39]. Other studies showed no association, but they were performed on a smaller sample and with shorter follow-up [40,42]. In patients undergoing RSG, those without the A allele experienced better anthropometric and insulin level improvements, reducing diabetes risk after 12 months [43]. Patients with the three polymorphisms rs9939609, rs9930506, and rs1421085 had higher insulin and HOMA-IR [28]. It is important to assess pre-surgery vitamin D levels in future studies, since it has been shown they affect weight loss in certain carriers [41].
Studies investigating FKBP5 determined that the T allele was associated with weight loss [35,36] with worse results in older males who have undergone LSG [36]. Future longitudinal studies with larger sample sizes and longer follow-up period might shed more light on the role of FKBP5 variability and its interaction with variables, such as stress, surgery, and sex.
Some genetic polymorphisms were connected to eating habits, such as 5-HT2C [20] and LYPAL-1 [45], and taste changes after MBS, such as TAS2R38, CD36, and OBPII2 [65]. Ghrelin, produced in the stomach, increases appetite by signaling hunger to the brain. Leptin, released by fat cells, suppresses appetite and regulates energy balance. Imbalances, such as elevated ghrelin or leptin resistance, can contribute to overeating and obesity, highlighting their crucial roles in appetite regulation and metabolic health [75]. It was shown that specific genotypes of rs1137101 and rs1805094 of the leptin gene and rs696217 of GHRL are associated with better weight loss outcomes after MBS [42,49,55]. Others, such as CAT haplotypes [44], GLP-1R [50], APOA-1 [51], ADIPOQ [53,54,55], PGC1a [58], TCF7 L2 [60], ESR1 [61], IL-6 gene [56,57], ACSL5 [63], CG [64], NPY [66], and CAMKK2 [69], influenced metabolic, inflammatory, and cardiovascular parameters after MBS. The GLP-1R gene encodes the GLP-1 receptor, crucial in glucose metabolism and satiety regulation [71]. One study showed that the rs6923761 variant was associated with weight loss after BMS [50]. GLP-1 receptor agonists enhance insulin secretion, delay gastric emptying, and reduce appetite, aiding weight loss in obesity and improving metabolic parameters in related diseases like type 2 diabetes [76]. Larger in-depth longitudinal studies with representative samples and a longer follow-up should be performed to further investigate the role of the polymorphisms and these genes on the outcome of different types of MBS.
Only one study was found that investigated the outcome of MBS in obese patients diagnosed with MASLD. It showed that patients with PNPLA3-associated steatohepatitis show better improvement of hepatic steatosis after MBS as compared to carriers of PNPLA3 wild-type alleles; consequently, PNPLA3 rs738409 is a good prognostic factor for MASLD patients undergoing MBS. On the other hand, TM6SF2 rs58542926 or MBOAT7 rs641738 did not have major effect on hepatic steatosis, even if carriers of the MBOAT7 minor allele presented with increased serum glucose, triglycerides, and cholesterol before surgery [59]. However, it should be taken into consideration that the sample size was small and the follow-up was only 12 months.
While this review provides valuable insights, it is important to acknowledge several limitations. The inclusion criteria restrict the review to English-language studies that are freely available, potentially excluding valuable research in other languages or behind paywalls. While the quality and risk of bias were assessed, the reliance on narrative synthesis without statistical pooling may introduce subjectivity, as it lacks the statistical rigor of a meta-analysis. The included studies varied in sample size, genetic variants studied, methodologies, and types of bariatric surgery procedures, which may introduce heterogeneity and affect the generalizability of the results. Additionally, many studies had short follow-up periods, limiting our understanding of the long-term impacts of genetic factors on bariatric surgery outcomes.
Future research should focus on developing polygenic scores in a large, diverse population, because the aggregated effect of the multiple genetic variants, as well as the heterogeneity of the population, will provide a better prognostic tool for the outcomes of MBS. Polygenic scores should extend beyond predicting weight loss to also include effects on metabolism, cardiovascular conditions, and eating behaviors. Key analyses should involve large-scale genome and exome studies to identify relevant polymorphisms. Prioritizing diverse cohorts with extended follow-up will validate findings and explore additional variants. Expanding genetic analysis and study diversity will help create accurate prognostic tools and tailor interventions to individual genetic profiles.
5. Conclusions
This systematic review examined 52 studies focusing on genetic polymorphisms in UCP2, MC4R, and FTO genes, which were the most frequently researched. Findings indicated varied associations between these genes and weight loss outcomes following MBS. The UCP2 rs659366 and rs660339 variants showed inconsistent results across different MBS types. MC4R polymorphisms also yielded mixed results, limited by small sample sizes and short follow-up periods. The FTO gene’s impact varied with surgery type and duration, with some polymorphisms linked to weight loss differences, insulin levels, and diabetes risk. Additional genetic polymorphisms were connected to eating habits, metabolic changes, and cardiovascular parameters. Despite these insights, study limitations like sample size, methodology variability, and short follow-up periods highlight the need for larger, diverse cohorts with extended follow-ups to better understand genetic influences on MBS outcomes.
Author Contributions
M.Z.: conceptualization, formal analysis, investigation, methodology, project administration, validation, visualization, writing—original draft, writing—review and editing. A.Z.: formal analysis, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing. T.R.: methodology, supervision, validation, writing—review and editing. T.P.: methodology, supervision, validation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| %EWL | percentage of excess weight loss |
| 5-HT2C | 5-hydroxytryptamine receptor 2C gene |
| ADIPOQ | adiponectin gene |
| ACSL5 | Acyl-CoA Synthetase Long Chain Family Member 5 gene |
| AGB | adjustable gastric banding |
| APOA-1 | apolipoprotein A1 gene |
| APOE | apolipoprotein E gene |
| BDNF | Brain Derived Neurotrophic Factor gene |
| BMI | body mass index |
| BPD | biliopancreatic diversion |
| DALY | disability-adjusted life year |
| MBS | metabolic and bariatric surgery |
| CAMKK2 | Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 |
| CAT haplotype | catalase haplotype |
| CB1 receptor | cannabinoid receptor type 1 |
| CD36 | CD36 gene |
| CD40L | CD40 ligand gene |
| CLOCK | clock circadian regulator gene |
| CNR1 | cannabinoid receptor type 1 gene |
| DIO2 | Iodothyronine deiodinase 2 gene |
| T2D | diabetes mellitus type 2 |
| ESR1 | estrogen receptor 1 gene |
| FABP-2 | fatty-acid-binding protein 2 gene |
| FKBP5 | FK506-binding protein 5 gene |
| FM | fat mass |
| FTO | fat mass and obesity-associated protein gene |
| GC | GC Vitamin-D-Binding Protein gene |
| GHRL | preproghrelin gene |
| GHSR | ghrelin receptor gene |
| GLP-1R | glucagon-like peptide-1 receptor gene |
| GWAS | genome wide association studies |
| HDL | high density lipoprotein |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| IL-6 | interleukin 6 |
| LAGB | laparoscopic adjustable gastric banding |
| LEP | leptin gene |
| LEPR | leptin receptor gene |
| LGALS3 | galectin-3 gene |
| LMGB | laparoscopic mini gastric bypass |
| LSG | laparoscopic sleeve gastrectomy |
| LYPLAL-1 | lysophospholipase-like 1 gene |
| MBOAT7 | membrane-bound O-acyltransferase domain-containing protein 7 gene |
| MC4R | melanocortin 4 receptor gene |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| NPY | Neuropeptide Y gene |
| OBPIIa | Odorant-Binding Protein 2A gene |
| PGC1a | peroxisome proliferator-activated receptor gamma coactivator 1-alpha gene |
| PNPLA3 | patatin-like phospholipase domain-containing 3 gene |
| PTEN | phosphatase and tensin homolog gene |
| RSG | robotic sleeve gastrectomy |
| RYGB | Roux en Y gastric bypass |
| SG | sleeve gastrectomy |
| TAS2R38 | taste 2 receptor member 38 gene |
| TCF7L2 | transcription factor 7-like 2 gene |
| TM6SF2 | transmembrane 6 superfamily 2 human gene |
| UCP2 | uncoupling Protein 2 gene |
| UCP3 | uncoupling Protein 3 gene |
| VBG | vertical banded gastroplasty |
| VLDL | very low-density lipoprotein |
References
- Institute for Health Metrics and Evaluation. GBD 2021 Cause and Risk Summary. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-high-body-mass-index-level-2-risk (accessed on 28 June 2024).
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017, 42, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Rosato, V.; Masarone, M.; Dallio, M.; Federico, A.; Aglitti, A.; Persico, M. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 3415. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Alvarado, G.A.; Otero Rosales, M.C.; Cala Duran, J.C.; Serrano-Gómez, S.; Carrero Barragan, T.Y.; Domínguez Alvarado, P.N.; Ramírez, M.L.; Serrano-Mesa, K.; Lozada-Martinez, I.D.; Narvaez-Rojas, A.R.; et al. The effect of bariatric surgery on metabolic syndrome: A retrospective cohort study in Colombia. Health Sci. Rep. 2023, 6, e1090. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Avenell, A.; Bolland, M.; Hudson, J.; Stewart, F.; Robertson, C.; Sharma, P.; Fraser, C.; MacLennan, G. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: Systematic review and meta-analysis. BMJ 2017, 359, j4849. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Rodrigues, D.; Gomes, L.; Carrilho, F. Short- and long-term mortality after bariatric surgery: A systematic review and meta-analysis. Diabetes Obes. Metab. 2017, 19, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, T.; Guidozzi, N.; Welbourn, R.; Ahmed, A.R.; Markar, S.R. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003206. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Heneghan, H.M. Bariatric Surgery for Obesity. Med. Clin. N. Am. 2018, 102, 165–182. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2023, 33, 3–14. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Chiappetta, S.; Parmar, C.; Shikora, S.A.; Prager, G.; LaMasters, T.; Ponce, J.; Kow, L.; Nimeri, A.; Kothari, S.N.; et al. Current Recommendations for Procedure Selection in Class I and II Obesity Developed by an Expert Modified Delphi Consensus. Sci. Rep. 2024, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lee, H.; Kaura, S.; Yip, J.; Sun, H.; Guan, L.; Han, W.; Ding, Y. Effect of Bariatric Surgery on Metabolic Diseases and Underlying Mechanisms. Biomolecules 2021, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.A. Mechanisms for the metabolic success of bariatric surgery. J. Neuroendocrinol. 2019, 31, e12708. [Google Scholar] [CrossRef] [PubMed]
- Noel, O.F.; Still, C.D.; Gerhard, G.S. Genetics of Bariatric Surgery Outcomes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, P.; Mahalingam, K. Molecular genetics of human obesity: A comprehensive review. Comptes Rendus Biol. 2017, 340, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Perego, L.; Cardellini, M.; Andreozzi, F.; Ricasoli, C.; Vedani, P.; Guzzi, V.; Marchi, M.; Paganelli, M.; Ferla, G.; et al. Impact of common polymorphisms in candidate genes for insulin resistance and obesity on weight loss of morbidly obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. J. Clin. Endocrinol. Metab. 2005, 90, 5064–5069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicoletti, C.F.; de Oliveira, A.P.R.P.; Brochado, M.J.F.; Pinhel, M.A.S.; de Oliveira, B.A.P.; Marchini, J.S.; Dos Santos, J.E.; Salgado, W.; Cury, N.M.; de Araújo, L.F.; et al. The Ala55Val and -866GA polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition 2017, 33, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Novais, P.F.S.; Weber, T.K.; Lemke, N.; Verlengia, R.; Crisp, A.H.; Rasera-Junior, I.; de Oliveira, M.R.M. Gene polymorphisms as a predictor of body weight loss after Roux-en-Y gastric bypass surgery among obese women. Obes. Res. Clin. Pract. 2016, 10, 724–727. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; Rodrigues, M.; Rossoni, E.A.; Sortica, D.A.; Rheinheimer, J.; Moehlecke, M.; Heredia, M.L.D.C.; Horvath, J.D.C.; Kops, N.L.; Trindade, M.R.M.; et al. -866G/A and Ins/Del polymorphisms in UCP2 gene are associated with reduced short-term weight loss in patients who underwent Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2021, 17, 1263–1270. [Google Scholar] [CrossRef]
- Liou, T.-H.; Chen, H.-H.; Wang, W.; Wu, S.-F.; Lee, Y.-C.; Yang, W.-S.; Lee, W.-J. ESR1, FTO, and UCP2 genes interact with bariatric surgery affecting weight loss and glycemic control in severely obese patients. Obes. Surg. 2011, 21, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Lee, W.-J.; Wang, W.; Huang, M.-T.; Lee, Y.-C.; Pan, W.-H. Ala55Val polymorphism on UCP2 gene predicts greater weight loss in morbidly obese patients undergoing gastric banding. Obes. Surg. 2007, 17, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Cury, N.; Malek-Zadeh, C.; Junior, S.; Marchini, J. UCP2 ALA55VAL polymorphism are not associated with weight loss after three years of Roux-en Y gastric bypass: PO519. Ann. Nutr. Metab. 2013, 63, 503. [Google Scholar]
- Nicoletti, C.F.; Kimura, B.M.; de Oliveira, B.A.P.; de Pinhel, M.A.S.; Salgado, W.; Marchini, J.S.; Nonino, C.B. Role of UCP2 polymorphisms on dietary intake of obese patients who underwent bariatric surgery. Clin. Obes. 2016, 6, 354–358. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Pacheco, D.; Aller, R.; González Sagrado, M.; Izaola, O.; Terroba, M.C.; Cuellar, L.; Conde, R.; Martin, T.; Perez Castrillon, J.L. Influence of -55CT polymorphism of UCP3 gene on surgical results of biliopancreatic diversion. Obes. Surg. 2010, 20, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Resende, C.M.M.; Durso, D.F.; Borges, K.B.G.; Pereira, R.M.; Rodrigues, G.K.D.; Rodrigues, K.F.; Silva, J.L.P.; Rodrigues, E.C.; Franco, G.R.; Alvarez-Leite, J.I. The polymorphism rs17782313 near MC4R gene is related with anthropometric changes in women submitted to bariatric surgery over 60 months. Clin. Nutr. 2018, 37, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Perez-Luque, E.; Daza-Hernandez, E.S.; Figueroa-Vega, N.; Cardona-Alvarado, M.I.; Muñoz-Montes, N.; Martinez-Cordero, C. Interaction Effects of FTO and MC4R Polymorphisms on Total Body Weight Loss, Post-Surgery Weight, and Post-Body Mass Index after Bariatric Surgery. Genes 2024, 15, 391. [Google Scholar] [CrossRef] [PubMed]
- Aslan, I.R.; Campos, G.M.; Calton, M.A.; Evans, D.S.; Merriman, R.B.; Vaisse, C. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes. Surg. 2011, 21, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Valette, M.; Poitou, C.; Le Beyec, J.; Bouillot, J.-L.; Clement, K.; Czernichow, S. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS ONE 2012, 7, e48221. [Google Scholar] [CrossRef]
- Peterli, R.; Peters, T.; von Flüe, M.; Hoch, M.; Eberle, A.N. Melanocortin-4 receptor gene and complications after gastric banding. Obes. Surg. 2006, 16, 189–195. [Google Scholar] [CrossRef]
- Mirshahi, U.L.; Still, C.D.; Masker, K.K.; Gerhard, G.S.; Carey, D.J.; Mirshahi, T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J. Clin. Endocrinol. Metab. 2011, 96, E2088–E2096. [Google Scholar] [CrossRef] [PubMed]
- Potoczna, N.; Branson, R.; Kral, J.G.; Piec, G.; Steffen, R.; Ricklin, T.; Hoehe, M.R.; Lentes, K.-U.; Horber, F.F. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J. Gastrointest. Surg. 2004, 8, 971–981; discussion 981–982. [Google Scholar] [CrossRef]
- Salazar-Valencia, I.G.; Villamil-Ramírez, H.; Barajas-Olmos, F.; Guevara-Cruz, M.; Macias-Kauffer, L.R.; García-Ortiz, H.; Hernández-Vergara, O.; Díaz de Sandy-Galán, D.A.; León-Mimila, P.; Centeno-Cruz, F.; et al. Effect of the Melanocortin 4-Receptor Ile269Asn Mutation on Weight Loss Response to Dietary, Phentermine and Bariatric Surgery Interventions. Genes 2022, 13, 2267. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, I.B.; Fries, G.R.; Bücker, J.; Scotton, E.; von Diemen, L.; Kauer-Sant’Anna, M. The FKBP5 polymorphism rs1360780 is associated with lower weight loss after bariatric surgery: 26 months of follow-up. Surg. Obes. Relat. Dis. 2016, 12, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.; Caixàs, A.; Arenas, C.; Rigla, M.; Crivillés, S.; Cardoner, N.; Rosa, A. Role of the FKBP5 polymorphism rs1360780, age, sex, and type of surgery in weight loss after bariatric surgery: A follow-up study. Surg. Obes. Relat. Dis. 2020, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Sarzynski, M.A.; Jacobson, P.; Rankinen, T.; Carlsson, B.; Sjöström, L.; Bouchard, C.; Carlsson, L.M.S. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int. J. Obes. 2011, 35, 676–683. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Aller, R.; Conde, R.; Izaola, O.; Pacheco, D.; Sagrado, M.G.; Primo, D. Effects of RS9939609 gene variant in FTO gene on weight loss and cardiovascular risk factors after biliopancreatic diversion surgery. J. Gastrointest. Surg. 2012, 16, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.K.; Resende, C.M.M.; Durso, D.F.; Rodrigues, L.A.A.; Silva, J.L.P.; Reis, R.C.; Pereira, S.S.; Ferreira, D.C.; Franco, G.R.; Alvarez-Leite, J. A single FTO gene variant rs9939609 is associated with body weight evolution in a multiethnic extremely obese population that underwent bariatric surgery. Nutrition 2015, 31, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Balasar, Ö.; Çakır, T.; Erkal, Ö.; Aslaner, A.; Çekiç, B.; Uyar, M.; Bülbüller, N.; Oruç, M.T. The effect of rs9939609 FTO gene polymorphism on weight loss after laparoscopic sleeve gastrectomy. Surg. Endosc. 2016, 30, 121–125. [Google Scholar] [CrossRef]
- Bandstein, M.; Schultes, B.; Ernst, B.; Thurnheer, M.; Schiöth, H.B.; Benedict, C. The Role of FTO and Vitamin D for the Weight Loss Effect of Roux-en-Y Gastric Bypass Surgery in Obese Patients. Obes. Surg. 2015, 25, 2071–2077. [Google Scholar] [CrossRef]
- Kops, N.L.; Vivan, M.A.; Horvath, J.D.C.; de Castro, M.L.D.; Friedman, R. FABP2, LEPR223, LEP656, and FTO Polymorphisms: Effect on Weight Loss 2 Years After Bariatric Surgery. Obes. Surg. 2018, 28, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Izaola, O.; Primo, D.; López, J.J.; Pacheco, D. A single FTO gene variant rs9939609 is associated with weight change and insulin resistance improvement in response to a robotic sleeve gastrectomy in individuals with severe obesity. Surg. Obes. Relat. Dis. 2023, 19, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Alili, R.; Nivet-Antoine, V.; Saldmann, A.; Golmard, J.-L.; Cottart, C.-H.; Laguillier, C.; Giral, P.; Beaudeux, J.-L.; Bouillot, J.-L.; Poitou, C.; et al. Human catalase gene promoter haplotype and cardiometabolic improvement after bariatric surgery. Gene 2018, 656, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Bandstein, M.; Mwinyi, J.; Ernst, B.; Thurnheer, M.; Schultes, B.; Schiöth, H.B. A genetic variant in proximity to the gene LYPLAL1 is associated with lower hunger feelings and increased weight loss following Roux-en-Y gastric bypass surgery. Scand. J. Gastroenterol. 2016, 51, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Cooiman, M.I.; Kleinendorst, L.; van der Zwaag, B.; Janssen, I.M.C.; Berends, F.J.; van Haelst, M.M. Genetic analysis in the bariatric clinic; impact of a PTEN gene mutation. Mol. Genet. Genom. Med. 2019, 7, e00632. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Sagrado, M.G.; Izaola, O.; Terroba, M.C.; Cuellar, L.; Conde, R.; Martin, T. Influence of Ala54Thr polymorphism of fatty acid-binding protein-2 on clinical results of biliopancreatic diversion. Nutrition 2008, 24, 300–304. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Pacheco, D.; Aller, R.; Gonzalez Sagrado, M.; Conde, R.; Izaola, O.; Cuellar, L.; Terroba, M.C.; Martin, T.; Ventosa, M. G 1359A polymorphism of the cannabinoid receptor gene (CNR1) and clinical results of biliopancreatic diversion. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 197–201. [Google Scholar] [PubMed]
- de Luis, D.A.; Aller, R.; Sagrado, M.G.; Izaola, O.; Terroba, M.C.; Cuellar, L.; Conde, R.; Martin, T. Influence of lys656asn polymorphism of leptin receptor gene on surgical results of biliopancreatic diversion. J. Gastrointest. Surg. 2010, 14, 899–903. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Pacheco, D.; Aller, R.; Izaola, O. Role of the rs6923761 gene variant in glucagon-like peptide 1 receptor gene on cardiovascular risk factors and weight loss after biliopancreatic diversion surgery. Ann. Nutr. Metab. 2014, 65, 259–263. [Google Scholar] [CrossRef]
- de Luis, D.A.; Pacheco, D.; Izaola, O.; Primo, D.; Aller, R. The association of the rs670 variant of APOA1 gene with insulin resistance and lipid profile in morbid obese patients after a biliopancreatic diversion surgery. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8472–8479. [Google Scholar] [CrossRef]
- Farup, P.G.; Jansen, A.; Hestad, K.; Aaseth, J.O.; Rootwelt, H. APOE Polymorphism and Endocrine Functions in Subjects with Morbid Obesity Undergoing Bariatric Surgery. Genes 2022, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Calvo, S.G.; Pacheco, D.; Ovalle, H.F.; Aller, R. Adiponectin gene variant RS rs266729: Relation to lipid profile changes and circulating adiponectin after bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.; Izaola, O.; Primo, D.; de Luis, D. Role of the variant rs3774261 of adiponectin gene on adiponectin levels and ratio adiponectin/leptin after biliopancreatic diversion in morbid obese subjects. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, E.; Santini, E.; Seghieri, M.; Giannini, L.; Coppedè, F.; Rossi, C.; Dardano, A.; Solini, A. Heterozygosity for the rs696217 SNP in the Preproghrelin Gene Predicts Weight Loss After Bariatric Surgery in Severely Obese Individuals. Obes. Surg. 2017, 27, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Carbonelli, M.G.; Bianchi, A.; Iacopino, L.; Fiorito, R.; Di Daniele, N.; de Lorenzo, A. Body composition changes after laparoscopic adjustable gastric banding: What is the role of -174GC interleukin-6 promoter gene polymorphism in the therapeutic strategy? Int. J. Obes. 2012, 36, 369–378. [Google Scholar] [CrossRef][Green Version]
- Di Renzo, L.; Carbonelli, M.G.; Bianchi, A.; Domino, E.; Migliore, M.R.; Rillo, G.; Iacopino, L.; Di Daniele, N.; de Lorenzo, A. Impact of the -174 G C IL-6 polymorphism on bioelectrical parameters in obese subjects after laparoscopic adjustable gastric banding. J. Obes. 2012, 2012, 208953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geloneze, S.R.; Geloneze, B.; Morari, J.; Matos-Souza, J.R.; Lima, M.M.; Chaim, E.A.; Pareja, J.C.; Velloso, L.A. PGC1α gene Gly482Ser polymorphism predicts improved metabolic, inflammatory and vascular outcomes following bariatric surgery. Int. J. Obes. 2012, 36, 363–368. [Google Scholar] [CrossRef]
- Krawczyk, M.; Jiménez-Agüero, R.; Alustiza, J.M.; Emparanza, J.I.; Perugorria, M.J.; Bujanda, L.; Lammert, F.; Banales, J.M. PNPLA3 p.I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg. Obes. Relat. Dis. 2016, 12, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Rouskas, K.; Cauchi, S.; Raverdy, V.; Yengo, L.; Froguel, P.; Pattou, F. Weight loss independent association of TCF7 L2 gene polymorphism with fasting blood glucose after Roux-en-Y gastric bypass in type 2 diabetic patients. Surg. Obes. Relat. Dis. 2014, 10, 679–683. [Google Scholar] [CrossRef]
- Wang, W.; Liou, T.-H.; Lee, W.-J.; Hsu, C.-T.; Lee, M.-F.; Chen, H.-H. ESR1 gene and insulin resistance remission are associated with serum uric acid decline for severely obese patients undergoing bariatric surgery. Surg. Obes. Relat. Dis. 2014, 10, 14–22. [Google Scholar] [CrossRef]
- Benenati, N.; Bufano, A.; Cantara, S.; Ricci, C.; Marzocchi, C.; Ciuoli, C.; Sannino, I.; Tirone, A.; Voglino, C.; Vuolo, G.; et al. Type 2 deiodinase p.Thr92Ala polymorphism does not affect the severity of obesity and weight loss after bariatric surgery. Sci. Rep. 2022, 12, 10643. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.; Izaola, O.; Primo, D.; Pacheco, D. The gene variant rs2419621 of ACYL-CoA synthetase long-chain 5 gene is associated with weight loss and metabolic changes in response to a robotic sleeve gastrectomy in morbid obese subjects. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7037–7043. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.; Izaola, O.; Primo, D.; López Gómez, J.J.; Pacheco, D. Role of rs2282679 in 25-hydroxyvitamin D levels and insulin resistance after a sleeve gastrectomy. Nutr. Diabetes 2024, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pintus, S.; Mastinu, M.; Fantola, G.; Moroni, R.; Pepino, M.Y.; Barbarossa, I.T. Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes. Nutrients 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.; Izaola, O.; Primo, D.; de Luis, D. Allele a of the rs16147 variant of neuropeptide Y predicts early metabolic improvements after bariatric surgery with biliopancreatic diversion in morbid obese subjects. Clin. Nutr. Open Sci. 2021, 36, 26–34. [Google Scholar] [CrossRef]
- Peña, E.; Caixàs, A.; Arenas, C.; Pareja, R.; León-Mengíbar, J.; Rigla, M.; Powell, T.R.; Cardoner, N.; Rosa, A. Influence of the BDNF Val66Met polymorphism on weight loss after bariatric surgery: A 24-month follow-up. Surg. Obes. Relat. Dis. 2021, 17, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Torrego-Ellacuría, M.; Barabash, A.; Matía-Martín, P.; Sánchez-Pernaute, A.; Torres, A.J.; Calle-Pascual, A.L.; Rubio-Herrera, M.A. Influence of CLOCK Gene Variants on Weight Response after Bariatric Surgery. Nutrients 2022, 14, 3472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Wen, J.; Ju, L.; Ren, Z.; Zhang, T.; Liu, Y. Whole-exome sequencing combined with postoperative data identify c.1614dup (CAMKK2) as a novel candidate monogenic obesity variant. Front. Endocrinol. 2024, 15, 1334342. [Google Scholar] [CrossRef] [PubMed]
- Souza de Oliveira, M.; Sachs Nique, P.; Crispim, D.; Marmontel de Souza, B. The association of uncoupling proteins 1, 2, and 3 with weight loss variability after bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2020, 16, 1858–1868. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Vos, N.; Oussaada, S.M.; Cooiman, M.I.; Kleinendorst, L.; ter Horst, K.W.; Hazebroek, E.J.; Romijn, J.A.; Serlie, M.J.; Mannens, M.M.A.M.; van Haelst, M.M. Bariatric Surgery for Monogenic Non-syndromic and Syndromic Obesity Disorders. Curr. Diabetes Rep. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Mokrosiński, J.; Mendes de Oliveira, E.; Li, C.; Sharp, S.J.; Luan, J.; Brouwers, B.; Ayinampudi, V.; Bowker, N.; Kerrison, N.; et al. Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity. Cell 2019, 177, 597–607.e9. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. FTO Alpha-Ketoglutarate Dependent Dioxygenase [Homo Sapiens (Human)]: Gene ID: 79068. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=79068 (accessed on 26 June 2024).
- Espinoza García, A.S.; Martínez Moreno, A.G.; Reyes Castillo, Z. The role of ghrelin and leptin in feeding behavior: Genetic and molecular evidence. Endocrinol. Diabetes Y Nutr. 2021, 68, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Wilbon, S.S.; Kolonin, M.G. GLP1 Receptor Agonists-Effects beyond Obesity and Diabetes. Cells 2023, 13, 65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).