Vitamin D and the Risk of Developing Hypertension in the SUN Project: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Assessment
2.3. Outcome Assessment
2.4. Covariates Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-Ric). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Benet’s, A.; Petrovic, M.; Strandberg, T. Hypertension Management in Older and Frail Older Patients. Circ. Res. 2019, 124, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Fairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Raghavan, R.; Zhang, G.; Talegawkar, S.A.; Jacques, P.F. Vitamin D status and prevalence of metabolic syndrome by race and Hispanic origin in US adults: Findings from the 2007–2014 NHANES. Am. J. Clin. Nutr. 2022, 116, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Valer-Martinez, A.; Sayon-Orea, C.; Martinez, J.A.; Basterra-Gortari, F.J.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Vitamin D and risk of developing type 2 diabetes in the SUN project: A prospective cohort study. J. Endocrinol. Invest. 2024. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Vitamin D and Cardiometabolic Outcomes: A Systematic Review Anastassios. Ann. Intern. Med. 2011, 152, 307–314. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Scragg, R.; Sowers, M.F.; Bell, C. Serum 25-hydroxyvitamin D, Ethnicity, and Blood Pressure in the Third National Health and Nutrition Examination Survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of Cardiovascular Risk Factors and the Serum Levels of 25-Hydroxyvitamin D in the United States. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef]
- McMullan, C.J.; Borgi, L.; Curhan, G.C.; Fisher, N.; Forman, J.P. The effect of Vitamin D on renin-angiotensin system activation and blood pressure: A randomized control trial. J. Hypertens. 2016, 35, 822–829. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S.; Buring, J.E. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 2020, 198, 105522. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Apekey, T.A.; Steur, M. Vitamin D and risk of future hypertension: Meta-analysis of 283,537 participants. Eur. J. Epidemiol. 2013, 28, 205–221. [Google Scholar] [CrossRef]
- Golzarand, M.; Shab-Bidar, S.; Koochakpoor, G.; Speakman, J.R.; Djafarian, K. Effect of vitamin D3 supplementation on blood pressure in adults: An updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 663–673. [Google Scholar] [CrossRef]
- He, S.; Hao, X. The effect of vitamin D3 on blood pressure in people with vitamin D deficiency A system review and me-ta-analysis. Medicine 2019, 98, e15284. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Struthers, A.D.; Khan, F.; Jorde, R.; Scragg, R.; Macdonald, H.M.; Alvarez, J.A.; Boxer, R.S.; Dalbeni, A.; Gepner, A.D.; et al. D-PRESSURE Collaboration: Effect of vitamin D supplementation on blood pressure: A systematic review and me-ta-analysis incorporating individual patient data. JAMA Intern. Med. 2015, 175, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cheng, C.; Wang, Y.; Sun, H.; Yu, S.; Xue, Y.; Liu, Y.; Li, W.; Li, X. Effect of Vitamin D on blood pressure and hyper-tension in the general population: An update meta-analysis of cohort studies and randomized controlled trials. Prev. Chronic Dis. 2020, 17, E03. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.O.; de Macedo, L.R.; Silva, M.; Lautner, R.Q. Effect of Vitamin D supplementation on blood pressure in hypertensive individuals with hypovitaminosis D: A systematic review and meta-analysis. J. Hypertens. 2024, 42, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Carlos, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campà, A.; Martínez-González, M.A.; Ruiz-Canela, M. Mediterranean diet and health outcomes in the SUN cohort. Nutrients 2018, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Valer-Martinez, A.; Sayon-Orea, C.; Martínez Hernandez, J.A.; De la Fuente-Arrillaga, C.; Pérez de Rojas, J.; Barcones, F.; Mar-tínez-González, M.A.; Bes-Rastrollo, M. Forecasting levels of serum 25-hydroxyvitamin D based on dietary intake, lifestyle and personal determinants in a sample of Southern Europeans. Br. J. Nutr. 2023, 130, 1814–1822. [Google Scholar] [CrossRef]
- Bes-Rastrollo, M.; Pérez-Valdivieso, J.R.; Sánchez-Villegas, A.; Alonso, A.; Martínez-González, M.A. Validation of self-reported weight and body mass index of participants from a cohort of college graduates. Rev. Esp. Obes. 2005, 3, 352–358. [Google Scholar]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and Validation of a Food Frequency Questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- De La Fuente-Arrillaga, C.; Vázquez-Ruiz, Z.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Fernandez-Ballart, J.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Pérez-Bauer, M.; Martínez-González, M.A.; Salas-Salvadó, J.; Martin-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Beunza, J.J.; Delgado-Rodríguez, M.; Martínez-González, M.A. Validation of self reported diagnosis of hypertension in a cohort of university graduates in Spain. BMC Public Health 2005, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Popu-lation. N. Engl. J. Med. 2003, 26, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Lopez, M.; Bes-Rastrollo, M.; Beunza, J.; Fernandez-Montero, A.; Garcia-Lopez, M.; Martinez-Gonzalez, M.A. Validation of metabolic syndrome using medical records in the SUN cohort. BMC Public Health 2011, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Scott, J.B.; Ng, K.; Drake, B.F.; Suarez, E.G.; Hayden, D.L.; Bennett, G.G.; Chandler, P.D.; Hollis, B.W.; Emmons, K.M.; et al. Effect of Vitamin D Supplementation on Blood Pressure in Blacks Clinical Trials. Hypertension 2013, 61, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Giovannucci, E.; Holmes, M.D.; Bischoff-Ferrari, H.A.; Tworoger, S.S.; Willett, W.C.; Curhan, G.C. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 2007, 49, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.; Tikkanen, E.; LifeLines Cohort Study Investigators; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian randomisation study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
- Zhou, A.; Selvanayagam, J.B.; Hyppönen, E. Non-linear Mendelian randomization analyses support a role for vitamin D defi-ciency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef]
- Theiler-Schwetz, V.; Trummer, C.; Grübler, M.R.; Keppel, M.H.; Zittermann, A.; Tomaschitz, A.; Karras, S.N.; März, W.; Pilz, S.; Gängler, S. Effects of Vitamin D Supplementation on 24-Hour Blood Pressure in Patients with Low 25-Hydroxyvitamin D Levels: A Randomized Controlled Trial. Nutrients 2022, 14, 1360. [Google Scholar] [CrossRef]
- Bressendorff, I.; Brandi, L.; Schou, M.; Nygaard, B.; Frandsen, N.E.; Rasmussen, K.; Ødum, L.; Østergaard, O.V.; Hansen, D. The effect of high dose cholecalciferol on arterial stiffness and peripheral and central blood pressure in healthy humans: A ran-domized controlled trial. PLoS ONE 2016, 11, e0160905. [Google Scholar] [CrossRef]
- Scragg, R.; Slow, S.; Stewart, A.W.; Jennings, L.C.; Chambers, S.T.; Priest, P.C.; Florkowski, C.M.; Camargo, C.A., Jr.; Murdoch, D.R. Long-term high-dose vitamin D3 supplementation and blood pressure in healthy adults a randomized controlled trial. Hypertension 2014, 64, 725–730. [Google Scholar] [CrossRef]
- Wamberg, L.; Kampmann, U.; Stødkilde-Jørgensen, H.; Rejnmark, L.; Pedersen, S.B.; Richelsen, B. Effects of vitamin D sup-plementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—Results from a randomized trial. Eur. J. Intern. Med. 2013, 24, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Gepner, A.D.; Ramamurthy, R.; Krueger, D.C.; Korcarz, C.E.; Binkley, N.; Stein, J.H. A prospective randomized controlled trial of the effects of Vitamin D supplementation on cardiovascular disease risk. PLoS ONE 2012, 7, e36617. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; März, W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system. The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chim. Acta 2010, 411, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.; Birmingham, W.C.; Ocampo, M.; Mohajeri, A. The Role of Vitamin D in Cardiovascular Diseases. Nutrients 2023, 15, 3547. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Liu, Z.Y.; Shi, Y.; Yin, D.W.; Wang, H.; Sha, Y.; Chen, Y.D. Vitamin D and nifedipine in the treatment of Chinese patients with grades I–II essential hypertension: A randomized placebo-controlled trial. Atherosclerosis 2014, 235, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, V.; Mozaianimonfared, A.; Gharakhani, M.; Poorolajal, J. Effect of vitamin D supplementation versus placebo on essential hypertension in patients with vitamin D deficiency: A double-blind randomized clinical trial. J. Clin. Hypertens. 2020, 22, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C. Rationale and plan for vitamin D food fortification: A review and guidance paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Cashman, K.D.; Sheehy, T.; O’Neill, C.M. Is vitamin D deficiency a public health concern for low middle income countries? A systematic literature review. Eur. J. Nutr. 2019, 58, 433–453. [Google Scholar] [CrossRef]
- Rothman, K.J.; Gallacher, J.E.J.; Hatch, E.E. Why representativeness should be avoided. Int. J. Epidemiol. 2013, 42, 1012–1014. [Google Scholar] [CrossRef] [PubMed]

| Quartiles of Predicted Serum 25(OH)D | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| N | 4110 | 4109 | 4109 | 4109 |
| Serum 25(OH)D predicted status (range, ng/mL) | 7.2; 18.7 | 18.8; 19.8 | 19.9; 21.3 | 21.4; 32.7 |
| Age (years) (p25; p75) | 38.8 (31.0; 47.5) | 36.0 (29.0; 45.0) | 32.3 (26.0; 42.0) | 30.0 (24.8; 39.8) |
| Women (%) | 66.1 | 64.1 | 63.9 | 61.0 |
| Smoking status (%) | ||||
| Never | 45.7 | 47.8 | 52.6 | 56.1 |
| Current | 21.9 | 23.3 | 23.1 | 22.0 |
| Former | 32.4 | 28.9 | 24.3 | 21.9 |
| Marital status, married (%) | 58.1 | 52.8 | 42.5 | 35.6 |
| Years of university (years) (p25; p75) | 5.0 (4.0; 5.0) | 5.0 (4.0; 5.0) | 5.0 (4.0; 5.0) | 5.0 (4.0; 5.0) |
| Body Mass Index (kg/m2) (p25; p75) | 25.2 (22.9; 27.8) | 23.1 (21.1; 25.0) | 21.9 (20.2; 23.9) | 21.5 (19.8; 23.5) |
| Weight change (%) 1 | ||||
| No weight change | 21.8 | 26.3 | 30.6 | 30.5 |
| Weight gain | 58.6 | 51.1 | 43.1 | 37.9 |
| Weight loss | 19.6 | 22.6 | 26.3 | 31.6 |
| Physical activity (METs-h/wk) (p25; p75) | 6.9 (1.6; 15.6) | 12.4 (4.5; 21.9) | 19.2 (9.1; 31.2) | 33.4 (18.2; 54.1) |
| TV (hours/day) (p25; p75) * | 1.5 (0.8; 2.0) | 1.4 (0.8; 2.0) | 1.5 (0.8; 2.0) | 1.5 (0.8; 2.0) |
| Siesta (hours/day) (SD) | 0.6 (0.5) | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.5) |
| Sleeping hours (hours/day) (p25; p75) * | 7.3 (7.0; 8.0) | 7.3 (7.0; 8.0) | 7.3 (7.0; 8.0) | 7.3 (7.0; 8.0) |
| Walking time (min/day) (p25; p75) | 15.0 (15.0; 25.0) | 25.0 (15.0; 25.0) | 25.0 (15.0; 45.0) | 45.0 (25.0; 90.0) |
| Summer sun exposure (h/day) (p25; p75) | 0.4 (0.1; 0.9) | 0.8 (0.3; 1.2) | 1.0 (0.5; 1.6) | 1.6 (0.8; 3.0) |
| Skin reaction after sun exposure (%) * | ||||
| Mild reaction | 74.6 | 98.3 | 99.1 | 99.4 |
| Severe reaction | 25.4 | 1.7 | 0.9 | 0.6 |
| Energy intake (kcal/d) (p25; p75) | 2263.2 (1851.0; 2715.1) | 2268.0 (1869.8; 2712.1) | 2346.1 (1941.1; 2786.1) | 2436.0 (2005.4; 2901.7) |
| Carbohydrate intake (% of energy) (p25; p75) | 43.0 (38.4; 47.8) | 43.6 (38.7; 48.1) | 43.8 (39.2; 48.2) | 43.8 (39.2; 48.4) |
| Protein intake (% of energy) (p25; p75) | 18.1 (16.2; 20.4) | 17.9 (16.0; 19.9) | 17.8 (16.0; 20.0) | 18.0 (16.2; 20.1) |
| Fat intake (% of energy) (p25; p75) | 36.8 (32.4; 40.6) | 36.3 (32.6; 40.7) | 36.3 (32.2; 40.5) | 36.2 (32.0; 40.2) |
| Monounsaturated fatty acids intake (% of energy) (p25; p75) | 15.6 (13.7; 18.1) | 15.6 (13.5; 17.9) | 15.5 (13.4; 17.8) | 15.3 (13.3; 17.6) |
| Saturated fatty acids intake (% of energy) (p25; p75) | 12.4 (10.5; 14.5) | 12.5 (10.7; 14.5) | 12.5 (10.6; 14.5) | 12.4 (10.3; 14.4) |
| Polyunsaturated fatty acids intake (% of energy) (p25; p75) | 5.0 (4.1; 6.0) | 5.0 (4.2; 6.0) | 5.0 (4.2; 6.1) | 5.0 (4.1; 6.1) |
| Meat consumption (g/d) (p25; p75) | 169.2 (122.4; 221.7) | 167.4 (121.6; 219.4) | 170.7 (124.3; 221.0) | 171.6 (123.6; 227.2) |
| Trichopoulou’s Mediterranean diet score (p25; p75) | 4.0 (3.0; 5.0) | 4.0 (3.0; 5.0) | 4.0 (3.0; 5.0) | 4.0 (3.0; 6.0) |
| Sodium intake (mg/d) (p25; p75) | 2756 (2064; 3733) | 2799 (2077; 3785) | 2862 (2148; 3861) | 2963 (2191; 3945) |
| Potassium intake (mg/d) (p25; p75) | 4482 (3622; 5468) | 4434 (3616; 5393) | 4584 (3743; 5601) | 4818 (3883; 5937) |
| Calcium intake (mg/d) (p25; p75) | 1145 (886; 1444) | 1132 (881; 1417) | 1173 (921; 1482) | 1231 (953; 1552) |
| Magnesium intake (mg/d) (p25; p75) | 393 (321; 479) | 389 (322; 472) | 404 (335; 486) | 423 (347; 512) |
| Following specific diet (%) * | 10.4 | 7.2 | 6.3 | 7.5 |
| Between-meal snacking (%) | 38.1 | 31.9 | 32.8 | 34.6 |
| Alcohol intake (g/d) (p25; p75) | 2.3 (0.6; 8.1) | 2.8 (1.0; 8.6) | 3.2 (1.0; 8.5) | 3.2 (1.0; 8.5) |
| Sugar-sweetened beverage (servings/d) (p25; p75) 2* | 0.1 (0.0; 0.1) | 0.1 (0.0; 0.1) | 0.1 (0.0; 0.4) | 0.1 (0.0; 0.4) |
| Total vitamin D intake (mcg/d) 3 (p25; p75) | 4.8 (3.5; 6.9) | 4.9 (3.6; 7.6) | 5.2 (3.8; 10.5) | 5.6 (3.9; 11.0) |
| Vitamin D supplementation (%) | 6.4 | 7.8 | 9.3 | 10.9 |
| Vitamin D supplementation (mcg/d) 4 (p25; p75) | 2.1 (0.3; 5.0) | 2.1 (0.7; 5.0) | 3.9 (0.7; 5.0) | 5.0 (0.7; 5.0) |
| Total dietary fiber intake (g/d) (p25; p75) | 20.3 (15.3; 26.4) | 20.0 (15.3; 26.1) | 20.8 (16.1; 26.8) | 21.9 (16.7; 28.6) |
| Caffeine intake (mg/d) (p25; p75) | 5.0 (2.0; 6.0) | 5.0 (2.0; 6.0) | 5.0 (1.0; 6.0) | 5.0 (1.0; 6.0) |
| Use of analgesic drugs (%) | 12.5 | 11.2 | 9.2 | 9.3 |
| Family History of Hypertension (%) | 45.1 | 41.9 | 38.0 | 36.4 |
| Prevalent Hypercholesterolemia (%) | 17.9 | 14.9 | 12.3 | 11.6 |
| Prevalent Hypertriglyceridemia (%) | 7.4 | 5.2 | 3.8 | 3.6 |
| Q1 | Q2 | Q3 | Q4 | p Trend | Continuous per + 1 ng/mL d | |
|---|---|---|---|---|---|---|
| Predicted serum 25(OH)D a | 17.75 (16.63; 18.33) | 19.35 (19.05; 19.63) | 20.52 (20.20; 20.87) | 22.48 (21.78; 23.59) | ||
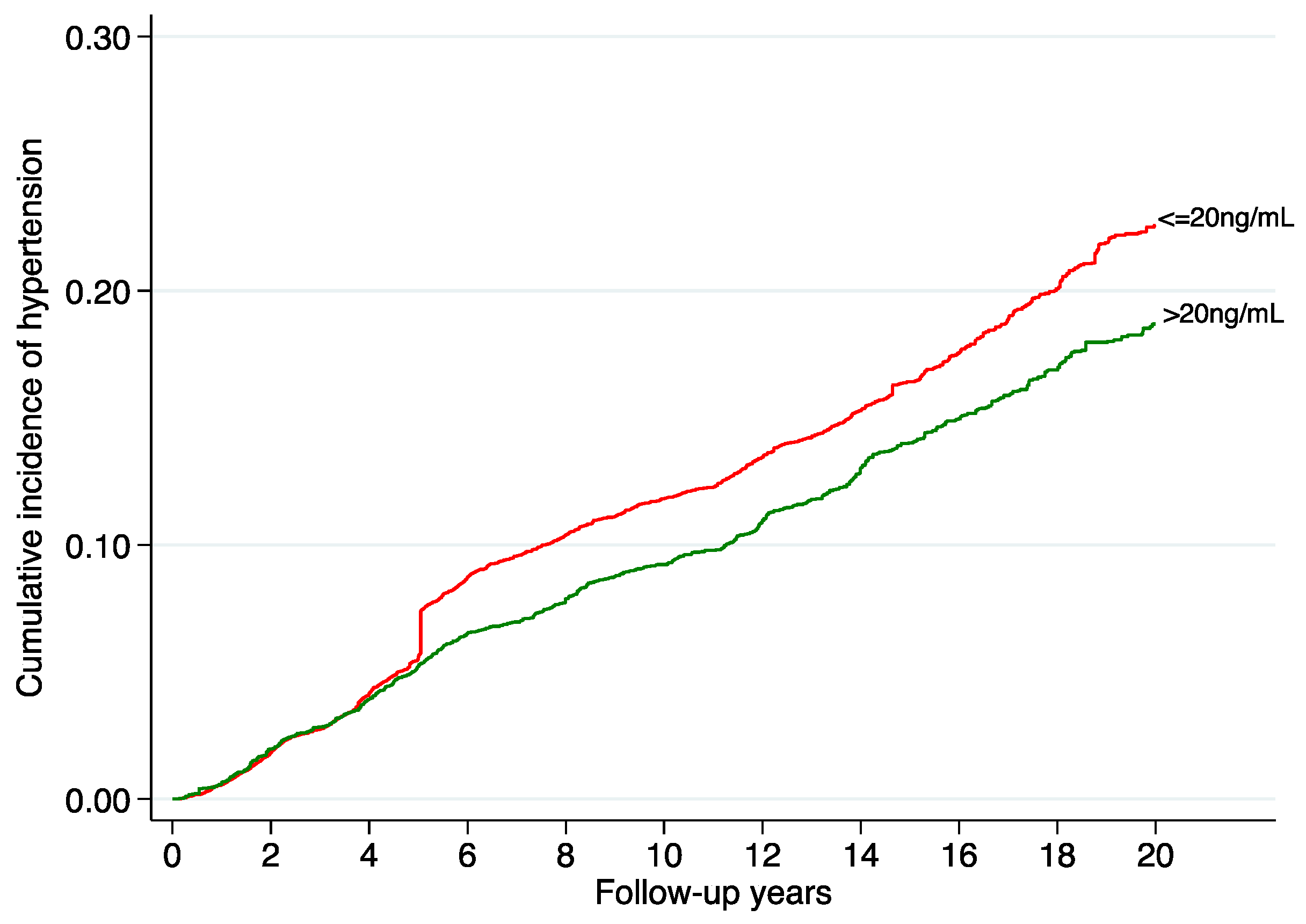
| Cases of Incident HT | 842 | 637 | 447 | 412 | ||
| Person years | 46,144 | 48,251 | 50,020 | 49,075 | ||
| Incident rate 10−3 years−1 | 18.2 | 13.2 | 8.9 | 8.4 | ||
| Age and sex adjusted model | 1.00 (reference) | 0.79 (0.71–0.87) | 0.59 (0.52–0.66) | 0.58 (0.52–0.66) | <0.001 | 0.90 (0.88–0.92) |
| Multiple-adjusted model1 b | 1.00 (reference) | 0.86 (0.77–0.95) | 0.66 (0.58–0.75) | 0.67 (0.58–0.77) | <0.001 | 0.92 (0.90–0.94) |
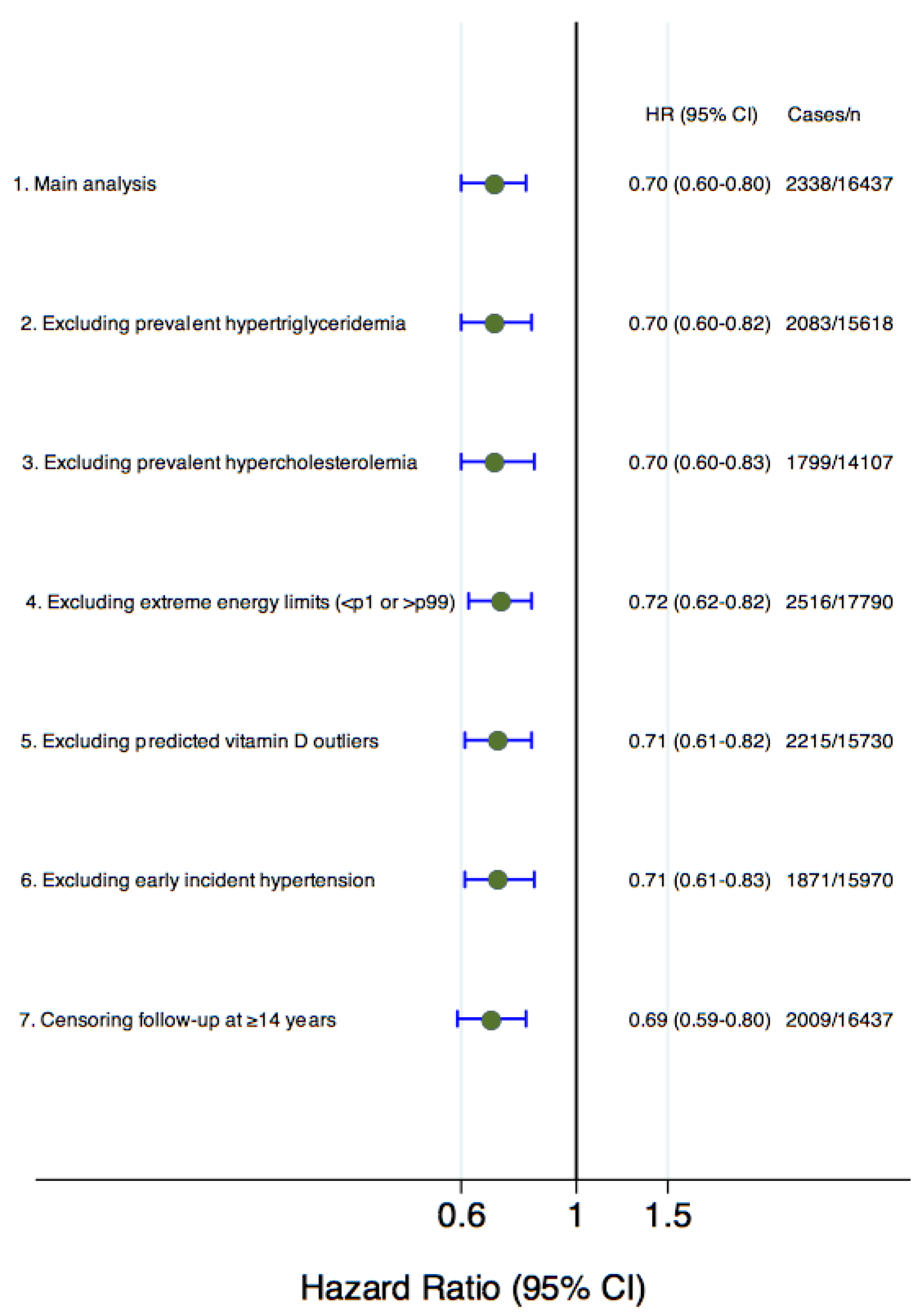
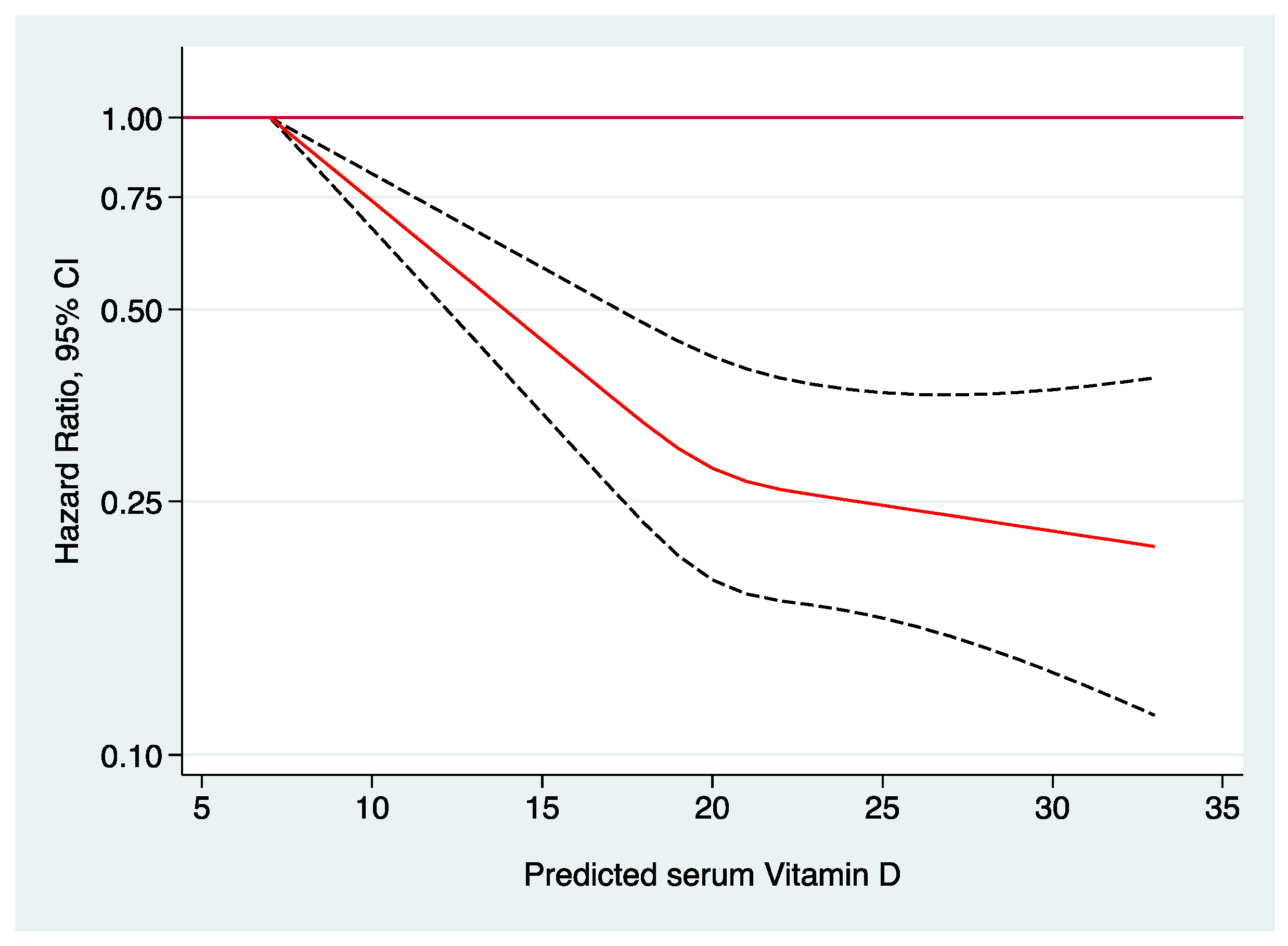
| Multiple-adjusted model2 c | 1.00 (reference) | 0.88 (0.79–0.98) | 0.68 (0.60–0.77) | 0.70 (0.60–0.80) | <0.001 | 0.93 (0.91–0.95) |
| N | Incident HT | Q1 | Q2 | Q3 | Q4 | p for Interaction | |
|---|---|---|---|---|---|---|---|
| Age | 0.060 | ||||||
| Age ≥ 50 years | 2103 | 703 | 1.00 (reference) | 0.87 (0.71–1.06) | 0.85 (0.67–1.06) | 0.79 (0.61–1.05) | |
| Age < 50 years | 14,334 | 1635 | 1.00 (reference) | 0.88 (0.77–0.99) | 0.61 (0.53–0.71) | 0.64 (0.54–0.76) | |
| Sex | 0.280 | ||||||
| Women | 10,483 | 992 | 1.00 (reference) | 0.83 (0.70–0.97) | 0.66 (0.54–0.80) | 0.75 (0.61–0.93) | |
| Men | 5954 | 1346 | 1.00 (reference) | 0.94 (0.81–1.09) | 0.72 (0.61–0.85) | 0.68 (0.56–0.82) | |
| Overweight | 0.692 | ||||||
| Overweight/Obese | 4267 | 1155 | 1.00 (reference) | 1.01 (0.87–1.17) | 0.84 (0.69–1.03) | 0.80 (0.62–1.04) | |
| Normal weight | 12,170 | 1183 | 1.00 (reference) | 0.93 (0.78–1.10) | 0.78 (0.65–0.93) | 0.86 (0.70–1.05) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valer-Martinez, A.; Bes-Rastrollo, M.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Sayon-Orea, C. Vitamin D and the Risk of Developing Hypertension in the SUN Project: A Prospective Cohort Study. Nutrients 2024, 16, 2351. https://doi.org/10.3390/nu16142351
Valer-Martinez A, Bes-Rastrollo M, Martinez JA, Martinez-Gonzalez MA, Sayon-Orea C. Vitamin D and the Risk of Developing Hypertension in the SUN Project: A Prospective Cohort Study. Nutrients. 2024; 16(14):2351. https://doi.org/10.3390/nu16142351
Chicago/Turabian StyleValer-Martinez, Ana, Maira Bes-Rastrollo, Jose Alfredo Martinez, Miguel Angel Martinez-Gonzalez, and Carmen Sayon-Orea. 2024. "Vitamin D and the Risk of Developing Hypertension in the SUN Project: A Prospective Cohort Study" Nutrients 16, no. 14: 2351. https://doi.org/10.3390/nu16142351
APA StyleValer-Martinez, A., Bes-Rastrollo, M., Martinez, J. A., Martinez-Gonzalez, M. A., & Sayon-Orea, C. (2024). Vitamin D and the Risk of Developing Hypertension in the SUN Project: A Prospective Cohort Study. Nutrients, 16(14), 2351. https://doi.org/10.3390/nu16142351







