Juvenile/Peripubertal Exposure to Omega-3 and Environmental Enrichment Differentially Affects CORT Secretion and Adulthood Stress Coping, Sociability, and CA3 Glucocorticoid Receptor Expression in Male and Female Rats
Abstract
1. Introduction
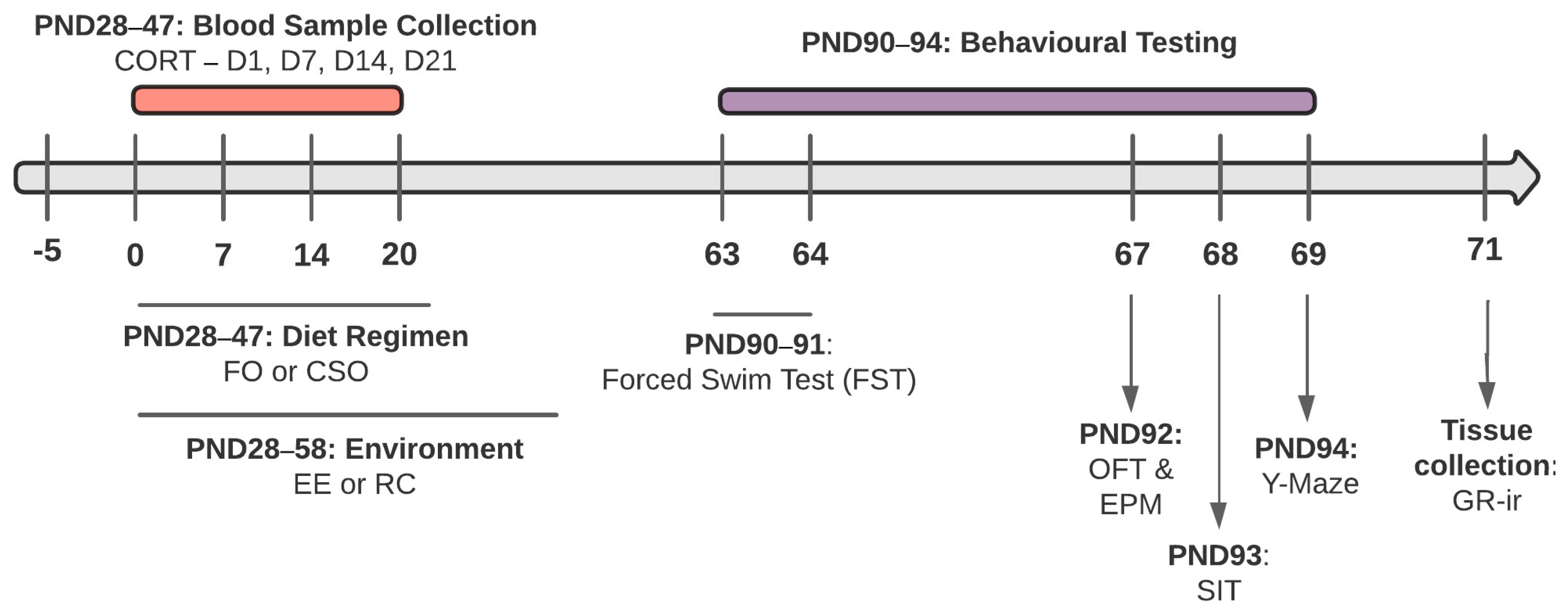
2. Materials and Methods
2.1. Animals
2.2. Dietary Supplementation and Environmental Conditions
2.3. Blood Sampling
2.4. Estrus Cycle Assessment
2.5. Adulthood Behavioural Testing
2.5.1. Repeated Forced Swim Test (FST) Exposure
2.5.2. Open Field Test (OFT)
2.5.3. Elevated Plus Maze (EPM)
2.5.4. Social Interaction Test (SIT)
2.5.5. Y-Maze Passive Avoidance Test (Y-Maze)
2.6. Post-Mortem Brain Tissue Collection
2.7. Blood Corticosterone Assessment Using Enzyme-Linked Immunosorbent Assay
2.8. Immunohistochemical Detection of Glucocorticoid Receptors (GR-ir)
2.9. Quantification of Immunoreactivity
2.10. Statistical Analyses
3. Results
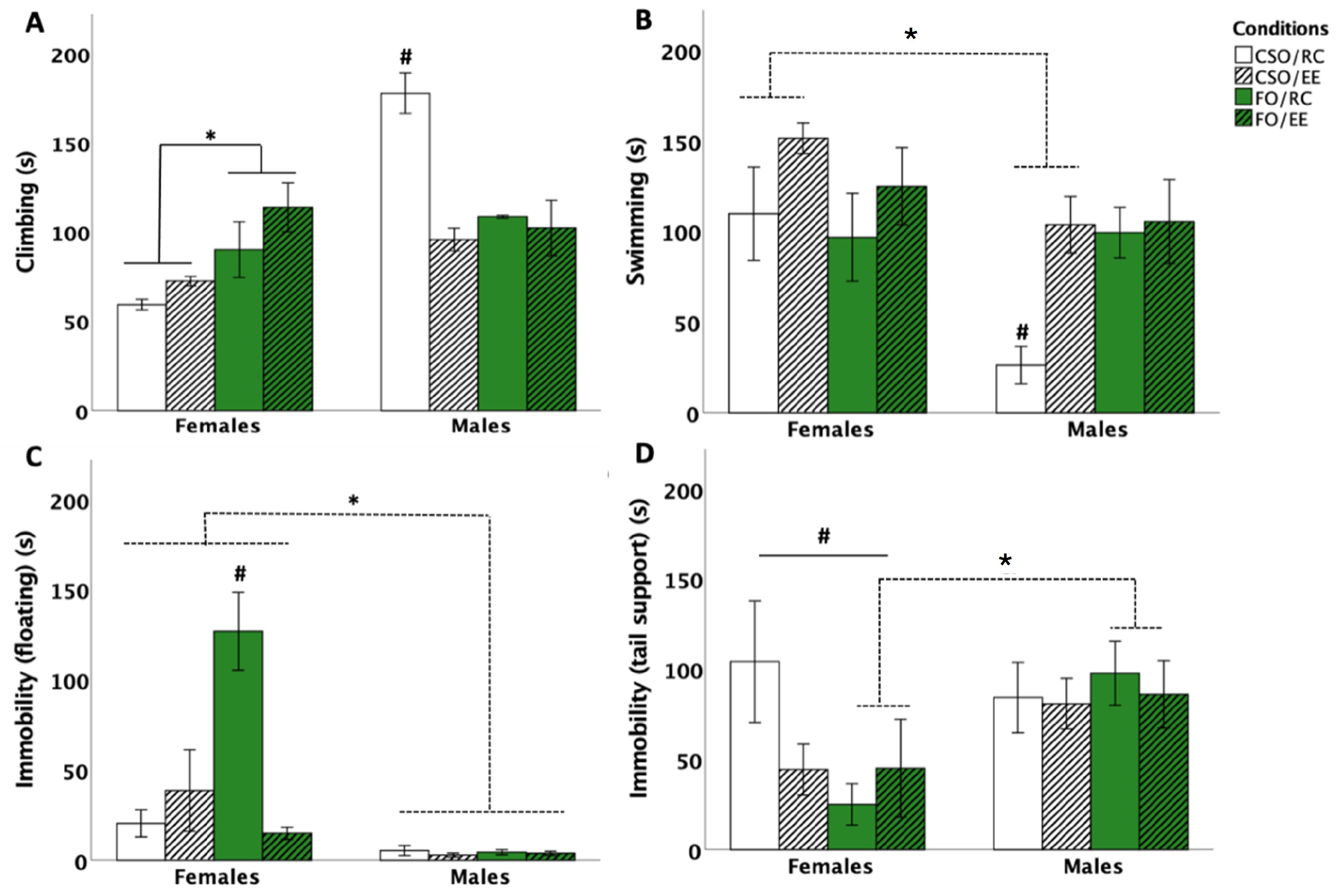
3.1. Behavioural Responses in the FST
3.1.1. Time Spent Climbing
3.1.2. Time Spent Swimming
3.1.3. Time Spent Immobile: Floating and Tail Support
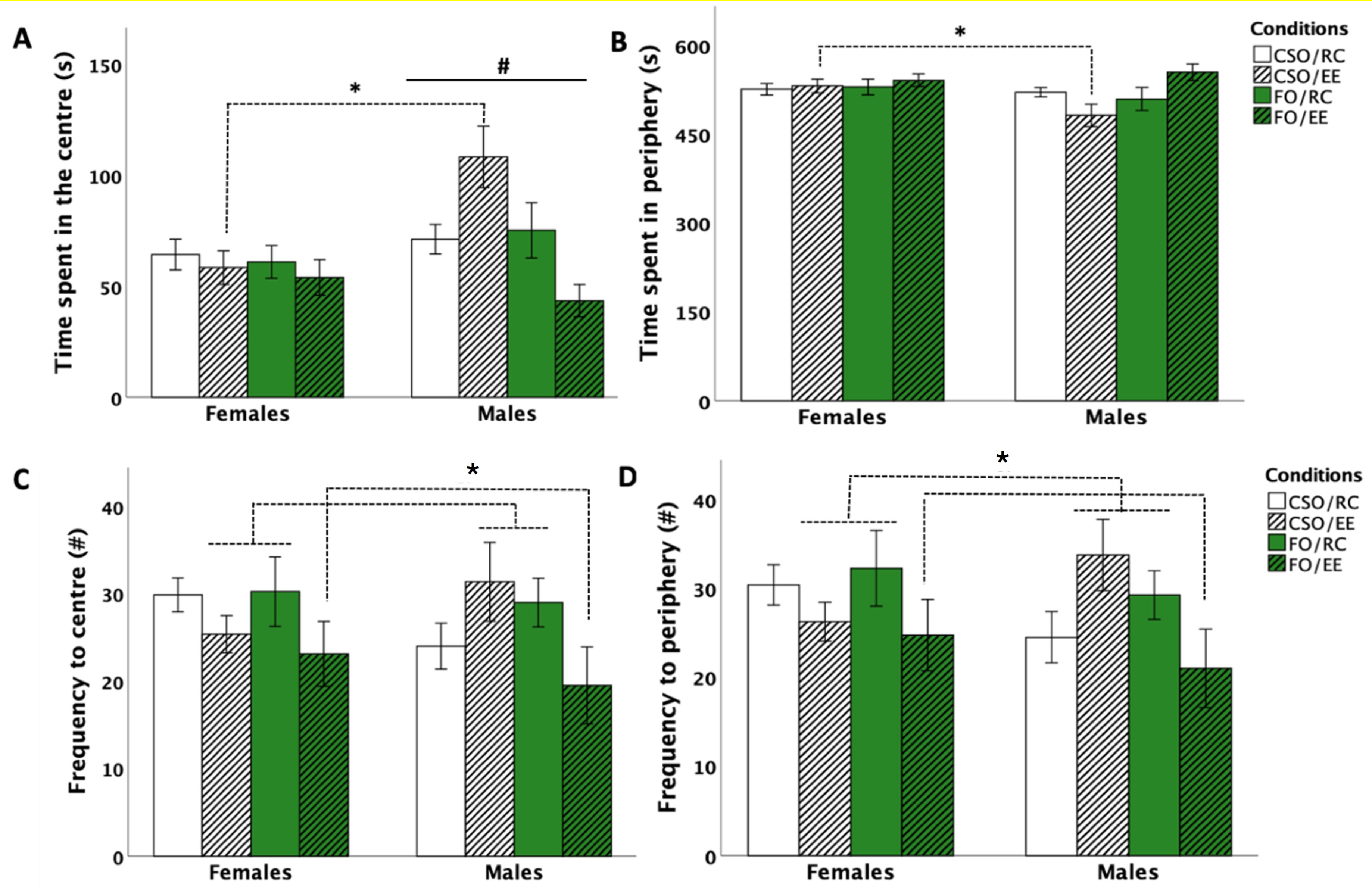
3.2. Open Field Test (OFT)
3.2.1. Time in the Centre Zone
3.2.2. Time in the Peripheral Zone
3.2.3. Frequency of Entries in the Centre and Peripheral Zones
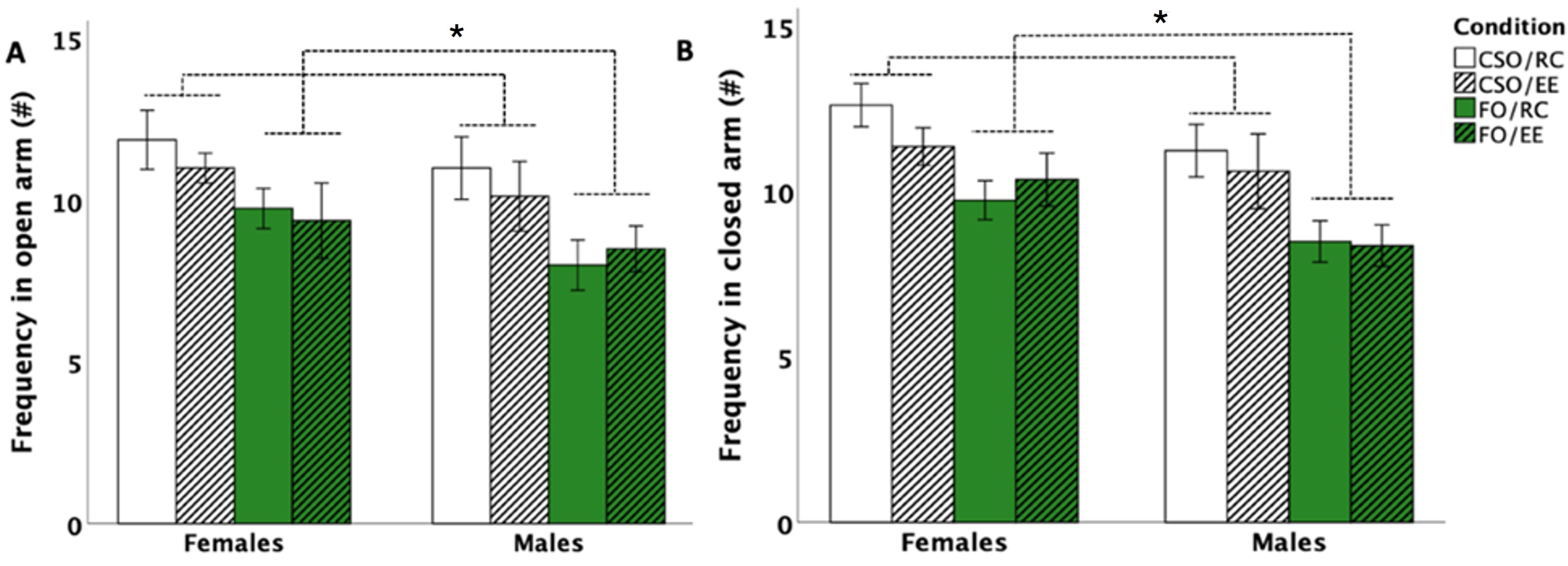
3.3. Elevated Plus Maze (EPM)
3.4. Social Interaction Test (SIT)
3.4.1. Social Interaction
3.4.2. Social Preference
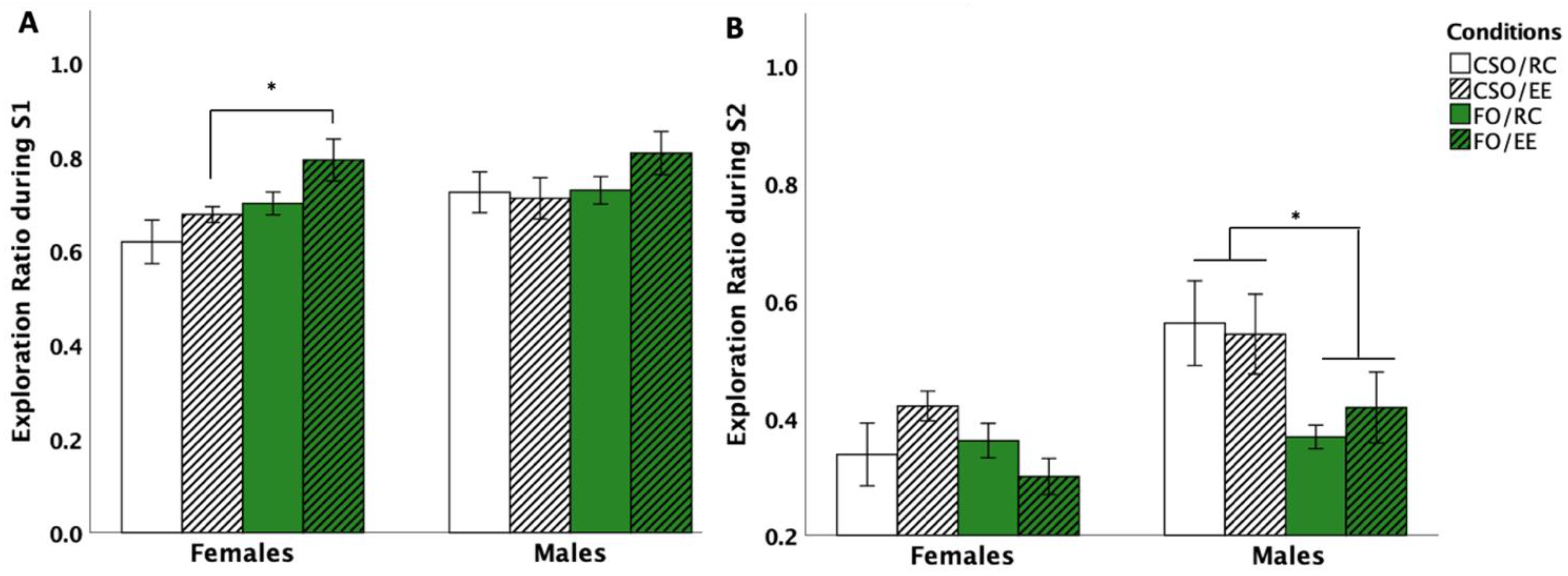
3.5. Y-Maze Avoidance Test
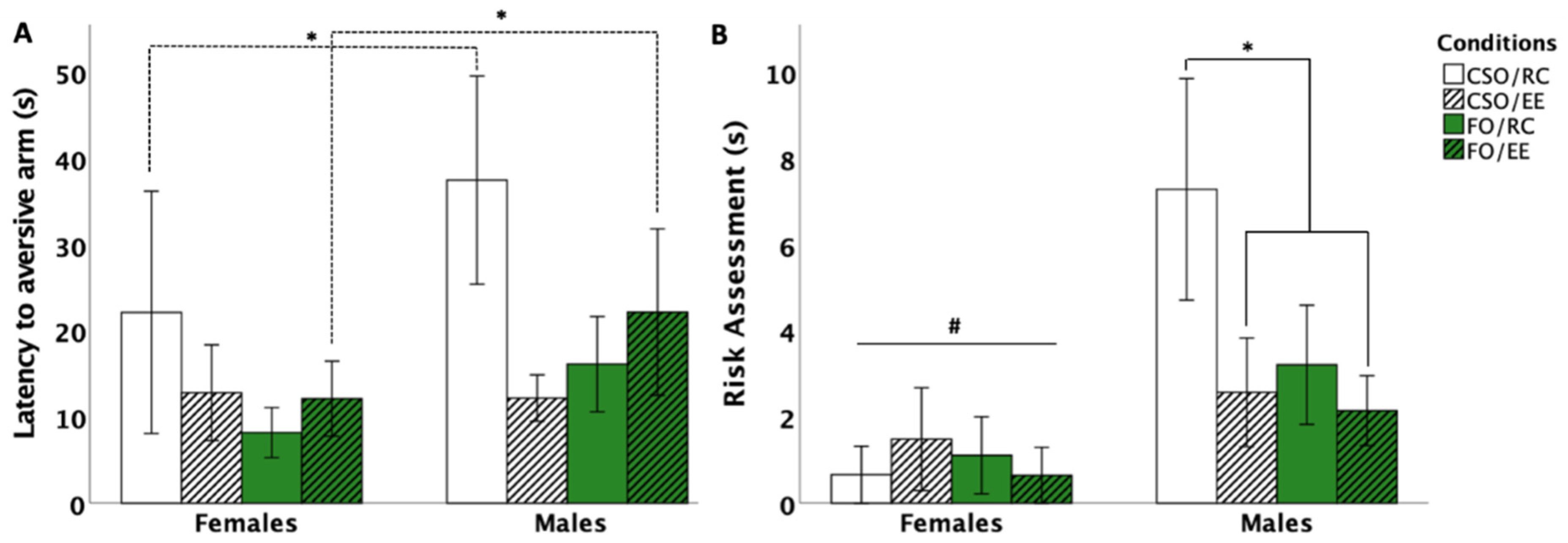
3.5.1. Latency to Aversive Arm Entry
3.5.2. Risk Assessment Behaviour
3.6. Corticosterone Level
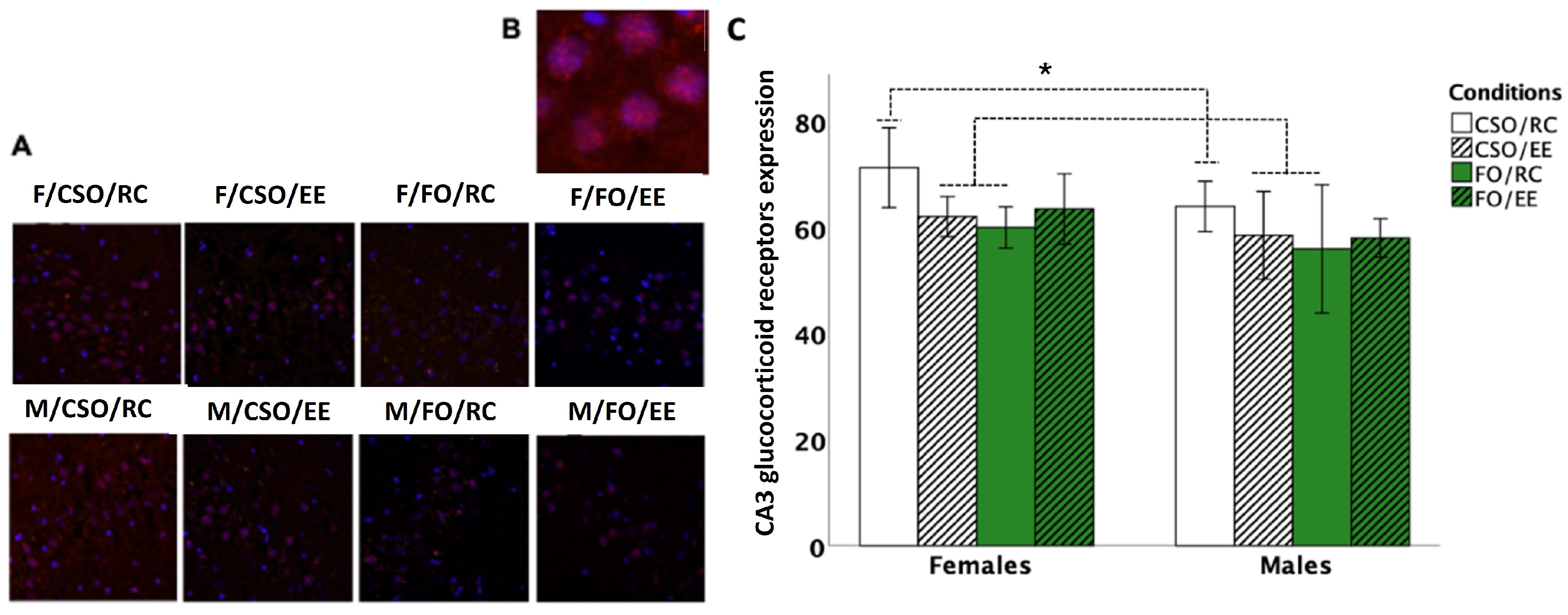
3.7. Glucocorticoid Receptor Immunoreactivity
4. Discussion
4.1. Sexually Dimorphic Influence of FO Supplementation and Housing on Coping Response
4.2. Juvenile FO Supplementation in Non-Deficient Rats Has a Marginal Impact on Adulthood Anxiety Levels
4.3. FO and EE Fostered Sociability in Females While CSO Promoted Social Recognition in Male Rats
4.4. Juvenile Dietary Supplementation Influenced Adulthood Fear Responses in a Sex-Dependent Manner
4.5. Diet and Housing Exerted Distinctive Effects on Adulthood GR-ir Expression at the Hippocampal CA3 Layer
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Pinilla, F. Brain Foods: The Effects of Nutrients on Brain Function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. The Biochemistry of N-3 Polyunsaturated Fatty Acids. J. Biol. Chem. 2002, 277, 8755–8758. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Cansev, M.; Sakamoto, T.; Ulus, I.H. Use of Phosphatide Precursors to Promote Synaptogenesis. Annu. Rev. Nutr. 2009, 29, 59–87. [Google Scholar] [CrossRef]
- Kitajka, K.; Puskás, L.G.; Zvara, Á.; Hackler, L.; Barceló-Coblijn, G.; Yeo, Y.K.; Farkas, T. The Role of N-3 Polyunsaturated Fatty Acids in Brain: Modulation of Rat Brain Gene Expression by Dietary n-3 Fatty Acids. Proc. Natl. Acad. Sci. USA 2002, 99, 2619–2624. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty Acids, Eicosanoids and PPAR Gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- De Lorgeril, M. Mediterranean Diet and Cardiovascular Disease: Historical Perspective and Latest Evidence. Curr. Atheroscler. Rep. 2013, 15, 370. [Google Scholar] [CrossRef]
- Rosenberger, T.A.; Villacreses, N.E.; Hovda, J.T.; Bosetti, F.; Weerasinghe, G.; Wine, R.N.; Harry, G.J.; Rapoport, S.I. Rat Brain Arachidonic Acid Metabolism Is Increased by a 6-day Intracerebral Ventricular Infusion of Bacterial Lipopolysaccharide. J. Neurochem. 2004, 88, 1168–1178. [Google Scholar] [CrossRef]
- Yoshida, K.; Shinohara, H.; Suryono; Haneji, T.; Nagata, T. Arachidonic Acid Inhibits Osteoblast Differentiation through Cytosolic Phospholipase A2-dependent Pathway. Oral Dis. 2007, 13, 32–39. [Google Scholar] [CrossRef]
- Gow, R.V.; Hibbeln, J.R. Omega-3 Fatty Acid and Nutrient Deficits in Adverse Neurodevelopment and Childhood Behaviors. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 555–590. [Google Scholar] [CrossRef]
- Green, M.R.; Barnes, B.; McCormick, C.M. Social Instability Stress in Adolescence Increases Anxiety and Reduces Social Interactions in Adulthood in Male Long–Evans Rats. Dev. Psychobiol. 2013, 55, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, B.; Ryan, T.; Hibbeln, J.R.; Murray, I.T.; Glynn, S.; Ramsden, C.E.; SanGiovanni, J.P.; Davis, J.M. Efficacy of Omega-3 Highly Unsaturated Fatty Acids in the Treatment of Depression. Br. J. Psychiatry 2016, 209, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Nemets, H.; Nemets, B.; Apter, A.; Bracha, Z.; Belmaker, R.H. Omega-3 Treatment of Childhood Depression: A Controlled, Double-Blind Pilot Study. Am. J. Psychiatry 2006, 163, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Ginty, A.T.; Conklin, S.M. Short-Term Supplementation of Acute Long-Chain Omega-3 Polyunsaturated Fatty Acids May Alter Depression Status and Decrease Symptomology among Young Adults with Depression: A Preliminary Randomized and Placebo Controlled Trial. Psychiatry Res. 2015, 229, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of Long-Chain Polyunsaturated Fatty Acids (PUFAs) for the Development and Behaviour of Children. Eur. J. Pediatr. 2010, 169, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Lassek, W.D.; Gaulin, S.J.C. Sex Differences in the Relationship of Dietary Fatty Acids to Cognitive Measures in American Children. Front. Evol. Neurosci. 2011, 3, 5. [Google Scholar] [CrossRef]
- Harland, B.; Dalrymple-Alford, J. Enriched Environment Procedures for Rodents: Creating a Standardized Protocol for Diverse Enrichment to Improve Consistency across Research Studies. BIO-Protocol 2020, 10, e3637. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.M.; Cao, L. A Protocol for Housing Mice in an Enriched Environment. J. Vis. Exp. 2015, 100, e52874. [Google Scholar] [CrossRef]
- Kempermann, G. Environmental Enrichment, New Neurons and the Neurobiology of Individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef]
- Bechara, R.G.; Kelly, Á.M. Exercise Improves Object Recognition Memory and Induces BDNF Expression and Cell Proliferation in Cognitively Enriched Rats. Behav. Brain Res. 2013, 245, 96–100. [Google Scholar] [CrossRef]
- Bednarczyk, M.R.; Hacker, L.C.; Fortin-Nunez, S.; Aumont, A.; Bergeron, R.; Fernandes, K.J.L. Distinct Stages of Adult Hippocampal Neurogenesis Are Regulated by Running and the Running Environment. Hippocampus 2011, 21, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.K.; Eadie, B.D.; Ernst, C.; Christie, B.R. Environmental Enrichment and Voluntary Exercise Massively Increase Neurogenesis in the Adult Hippocampus via Dissociable Pathways. Hippocampus 2006, 16, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Neural Consequences of Enviromental Enrichment. Nat. Rev. Neurosci. 2000, 1, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Veena, J.; Srikumar, B.N.; Mahati, K.; Bhagya, V.; Raju, T.R.; Shankaranarayana Rao, B.S. Enriched Environment Restores Hippocampal Cell Proliferation and Ameliorates Cognitive Deficits in Chronically Stressed Rats. J. Neurosci. Res. 2009, 87, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Belz, E.E.; Kennell, J.S.; Czambel, R.K.; Rubin, R.T.; Rhodes, M.E. Environmental Enrichment Lowers Stress-Responsive Hormones in Singly Housed Male and Female Rats. Pharmacol. Biochem. Behav. 2003, 76, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-S.; Long, N.; Pigino, G.; Brady, S.T.; Lazarov, O. Molecular Mechanisms of Environmental Enrichment: Impairments in Akt/GSK3β, Neurotrophin-3 and CREB Signaling. PLoS ONE 2013, 8, e64460. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Hellemans, K.G.C.; Verma, P.; Gorzalka, B.B.; Weinberg, J. Neurobiology of Chronic Mild Stress: Parallels to Major Depression. Neurosci. Biobehav. Rev. 2012, 36, 2085–2117. [Google Scholar] [CrossRef] [PubMed]
- Jeanneteau, F.D.; Lambert, W.M.; Ismaili, N.; Bath, K.G.; Lee, F.S.; Garabedian, M.J.; Chao, M.V. BDNF and Glucocorticoids Regulate Corticotrophin-Releasing Hormone (CRH) Homeostasis in the Hypothalamus. Proc. Natl. Acad. Sci. USA 2012, 109, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Kurahashi, M.; Mori, D.; Iinuma, M.; Tamura, Y.; Mizutani, K.; Shimpo, K.; Sonoda, S.; Azuma, K.; Kubo, K. Hippocampus-Dependent Spatial Memory Impairment Due to Molar Tooth Loss Is Ameliorated by an Enriched Environment. Arch. Oral Biol. 2016, 61, 1–7. [Google Scholar] [CrossRef]
- Akirav, I.; Richter-Levin, G. Biphasic Modulation of Hippocampal Plasticity by Behavioral Stress and Basolateral Amygdala Stimulation in the Rat. J. Neurosci. 1999, 19, 10530–10535. [Google Scholar] [CrossRef]
- Ghashghaei, H.T.; Barbas, H. Pathways for Emotion: Interactions of Prefrontal and Anterior Temporal Pathways in the Amygdala of the Rhesus Monkey. Neuroscience 2002, 115, 1261–1279. [Google Scholar] [CrossRef] [PubMed]
- Petrovich, G.D.; Canteras, N.S.; Swanson, L.W. Combinatorial Amygdalar Inputs to Hippocampal Domains and Hypothalamic Behavior Systems. Brain Res. Rev. 2001, 38, 247–289. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Pruessner, J.; Newhouse, P. Estradiol Levels Modulate Brain Activity and Negative Responses to Psychosocial Stress across the Menstrual Cycle. Psychoneuroendocrinology 2015, 59, 14–24. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, G. Is Depression Normal in Human Beings? A Critique of the Evolutionary Perspective. Int. J. Ment. Health Nurs. 2002, 11, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.M.; Schäfer, M.R.; Klier, C.; Mossaheb, N.; Vijayakumar, N.; Amminger, G.P. Erythrocyte Polyunsaturated Fatty Acid Levels in Young People at Ultra-High Risk for Psychotic Disorder and Healthy Adolescent Controls. Psychiatry Res. 2015, 228, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Kim-Cohen, J.; Maughan, B. Continuities and Discontinuities in Psychopathology between Childhood and Adult Life. J. Child Psychol. Psychiatry 2006, 47, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Chetty, S.; Friedman, A.R.; Taravosh-Lahn, K.; Kirby, E.D.; Mirescu, C.; Guo, F.; Krupik, D.; Nicholas, A.; Geraghty, A.C.; Krishnamurthy, A.; et al. Stress and Glucocorticoids Promote Oligodendrogenesis in the Adult Hippocampus. Mol. Psychiatry 2014, 19, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of Stress throughout the Lifespan on the Brain, Behaviour and Cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Morin, A.; Plamondon, H. Delivery Method Matters: Omega-3 Supplementation by Restricted Feeding Period and Oral Gavage Has a Distinct Impact on Corticosterone Secretion and Anxious Behavior in Adolescent Rats. Nutr. Neurosci. 2020, 25, 169–179. [Google Scholar] [CrossRef]
- Ismail, T.R.; Yap, C.G.; Naidu, R.; Pamidi, N. Enrichment Protocol for Rat Models. Curr. Protoc. 2021, 1, e152. [Google Scholar] [CrossRef]
- Mileva, G.R.; Moyes, C.; Syed, S.; Bielajew, C. Strain Differences and Effects of Environmental Manipulation on Astrocytes (Glial Fibrillary Acidic Protein), Glucocorticoid Receptor, and Microglia (Iba1) Immunoreactivity between Wistar-Kyoto and Wistar Females. Neuropsychobiology 2017, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Milot, M.R.; James, J.S.; Merali, Z.; Plamondon, H. A Refined Blood Collection Method for Quantifying Corticosterone. Lab Anim. 2012, 41, 77–83. [Google Scholar] [CrossRef]
- Goldman, J.M.; Murr, A.S.; Cooper, R.L. The Rodent Estrous Cycle: Characterization of Vaginal Cytology and Its Utility in Toxicological Studies. Birth Defects Res. B. Dev. Reprod. Toxicol. 2007, 80, 84–97. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.C.; Valenzuela, N.; Fai, S.; Bennett, S.A.L. Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. J. Vis. Exp. 2012, 67, e4389. [Google Scholar] [CrossRef]
- Lemos, J.C.; Wanat, M.J.; Smith, J.S.; Reyes, B.A.S.; Hollon, N.G.; Van Bockstaele, E.J.; Chavkin, C.; Phillips, P.E.M. Severe Stress Switches CRF Action in the Nucleus Accumbens from Appetitive to Aversive. Nature 2012, 490, 402–406. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A New Animal Model Sensitive to Antidepressant Treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Molendijk, M.L. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast. 2016, 2016, 6503162. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal Models of Anxiety Disorders and Stress. Rev. Bras. Psiquiatr. 2013, 35, S101–S111. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Perez, A.; Barbaro, R.P.; Johns, J.M.; Magnuson, T.R.; Piven, J.; Crawley, J.N. Sociability and Preference for Social Novelty in Five Inbred Strains: An Approach to Assess Autistic-like Behavior in Mice. Genes Brain Behav. 2004, 3, 287–302. [Google Scholar] [CrossRef]
- Kaidanovich-Beilin, O.; Lipina, T.; Vukobradovic, I.; Roder, J.; Woodgett, J.R. Assessment of Social Interaction Behaviors. J. Vis. Exp. 2011, 48, e2473. [Google Scholar] [CrossRef]
- Azogu, I.; Cossette, I.; Mukunzi, J.; Ibeke, O.; Plamondon, H. Sex-Specific Differences in Adult Cognition and Neuroplasticity Following Repeated Combinatory Stress and TrkB Receptor Antagonism in Adolescence. Horm. Behav. 2019, 113, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-08-047513-4. [Google Scholar]
- Morin, A.; Poitras, M.; Plamondon, H. Global Cerebral Ischemia in Adolescent Male Long Evans Rats: Effects of Vanillic Acid Supplementation on Stress Response, Emotionality, and Visuospatial Memory. Behav. Brain Res. 2021, 412, 113403. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.E. Sex Hormones and n-3 Fatty Acid Metabolism. Proc. Nutr. Soc. 2020, 79, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.V. Rodent Models of Depression: Reexamining Validity Without Anthropomorphic Inference. Crit. Rev. Neurobiol. 2003, 15, 143–174. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Green, M.R. From the Stressed Adolescent to the Anxious and Depressed Adult: Investigations in Rodent Models. Neuroscience 2013, 249, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Coping with the Forced Swim Stressor: Current State-of-the-Art. Behav. Brain Res. 2019, 364, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Colom-Lapetina, J.; Begley, S.L.; Johnson, M.E.; Bean, K.J.; Kuwamoto, W.N.; Shansky, R.M. Strain-Dependent Sex Differences in a Long-Term Forced Swim Paradigm. Behav. Neurosci. 2017, 131, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, V.K.; Huang, B.X.; Kim, H.-Y. Effects of Docosahexaenoic Acid on Mouse Brain Synaptic Plasma Membrane Proteome Analyzed by Mass Spectrometry and 16O/18O Labeling. J. Proteome Res. 2011, 10, 5472–5480. [Google Scholar] [CrossRef]
- Campus, P.; Colelli, V.; Orsini, C.; Sarra, D.; Cabib, S. Evidence for the Involvement of Extinction-Associated Inhibitory Learning in the Forced Swimming Test. Behav. Brain Res. 2015, 278, 348–355. [Google Scholar] [CrossRef]
- Warden, M.R.; Selimbeyoglu, A.; Mirzabekov, J.J.; Lo, M.; Thompson, K.R.; Kim, S.-Y.; Adhikari, A.; Tye, K.M.; Frank, L.M.; Deisseroth, K. A Prefrontal Cortex–Brainstem Neuronal Projection That Controls Response to Behavioural Challenge. Nature 2012, 492, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.M.; Pase, C.S.; Boufleur, N.; Roversi, K.; Barcelos, R.C.S.; Benvegnú, D.M.; Segat, H.J.; Dias, V.T.; Reckziegel, P.; Trevizol, F.; et al. Exercise Affects Memory Acquisition, Anxiety-like Symptoms and Activity of Membrane-Bound Enzyme in Brain of Rats Fed with Different Dietary Fats: Impairments of Trans Fat. Neuroscience 2011, 195, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 117864691880228. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Nakamoto, Y.; Takahashi, M.; Kitamura, K.; Wakasa, K.; Ishimoto, M. Manipulation of Amino Acid Composition in Soybean Seeds by the Combination of Deregulated Tryptophan Biosynthesis and Storage Protein Deficiency. Plant Cell Rep. 2010, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, B.J.; Sarna, G.S.; Kantamaneni, D.B.; Jackson, A.; Hutson, P.H.; Curzon, G. CSF Tryptophan and Transmitter Amine Turnover May Predict Social Behaviour in the Normal Rat. Brain Res. 1986, 399, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; DiSilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Meal Ingestion, Amino Acids and Brain Neurotransmitters: Effects of Dietary Protein Source on Serotonin and Catecholamine Synthesis Rates. Physiol. Behav. 2009, 98, 156–162. [Google Scholar] [CrossRef]
- Ikemoto, A.; Ohishi, M.; Sato, Y.; Hata, N.; Misawa, Y.; Fujii, Y.; Okuyama, H. Reversibility of N-3 Fatty Acid Deficiency-Induced Alterations of Learning Behavior in the Rat: Level of n-6 Fatty Acids as Another Critical Factor. J. Lipid Res. 2001, 42, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Yimit, D.; Hoxur, P.; Amat, N.; Uchikawa, K.; Yamaguchi, N. Effects of Soybean Peptide on Immune Function, Brain Function, and Neurochemistry in Healthy Volunteers. Nutrition 2012, 28, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H. Stress, Immunity, Cytokines and Depression. Acta Neuropsychiatr. 2002, 14, 251–261. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Thesing, C.S.; Bot, M.; Milaneschi, Y.; Giltay, E.J.; Penninx, B.W.J.H. Omega-3 Polyunsaturated Fatty Acid Levels and Dysregulations in Biological Stress Systems. Psychoneuroendocrinology 2018, 97, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, T.; Hamazaki, K. Fish Oils and Aggression or Hostility. Prog. Lipid Res. 2008, 47, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Augier, S.; Penes, M.C.; Debilly, G.; Miachon, A.S. Polyunsaturated Fatty Acids in the Blood of Spontaneously or Induced Muricidal Male Wistar Rats. Brain Res. Bull. 2003, 60, 161–165. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, S.R.; Christophe, A.B.; Maes, M. In Humans, the Seasonal Variation in Poly-Unsaturated Fatty Acids Is Related to the Seasonal Variation in Violent Suicide and Serotonergic Markers of Violent Suicide. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Re, S.; Zanoletti, M.; Emanuele, E. Aggressive Dogs Are Characterized by Low Omega-3 Polyunsaturated Fatty Acid Status. Vet. Res. Commun. 2008, 32, 225–230. [Google Scholar] [CrossRef]
- Hendershott, T.R.; Cronin, M.E.; Langella, S.; McGuinness, P.S.; Basu, A.C. Effects of Environmental Enrichment on Anxiety-like Behavior, Sociability, Sensory Gating, and Spatial Learning in Male and Female C57BL/6J Mice. Behav. Brain Res. 2016, 314, 215–225. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Branchi, I.; Cirulli, F.; Chiarotti, F.; Aloe, L.; Alleva, E. Long-Term Effects of the Periadolescent Environment on Exploratory Activity and Aggressive Behaviour in Mice: Social versus Physical Enrichment. Physiol. Behav. 2004, 81, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Aspesi, D.; Choleris, E. Neuroendocrine Underpinning of Social Recognition in Males and Females. J. Neuroendocrinol. 2022, 34, e13070. [Google Scholar] [CrossRef]
- Handa, R.J.; Mani, S.K.; Uht, R.M. Estrogen Receptors and the Regulation of Neural Stress Responses. Neuroendocrinology 2012, 96, 111–118. [Google Scholar] [CrossRef]
- Su, Y.; Tian, Z.; Qi, X.; Luo, D.; Liu, L.; Liu, S.; Zheng, D.; Wei, F.; He, Z.; Guan, Q. Effects of Increasing Intake of Soybean Oil on Synthesis of Testosterone in Leydig Cells. Nutr. Metab. 2021, 18, 53. [Google Scholar] [CrossRef]
- Crane, S.B.; Greenwood, C.E. Dietary Fat Source Influences Neuronal Mitochondrial Monoamine Oxidase Activity and Macronutrient Selection in Rats. Pharmacol. Biochem. Behav. 1987, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Akasaka, D.; Ogasawara, H.; Sato, K.; Miyake, M.; Saito, K.; Takahashi, Y.; Kanaya, T.; Takakura, I.; Hondo, T.; et al. Peripheral Serotonin Enhances Lipid Metabolism by Accelerating Bile Acid Turnover. Endocrinology 2010, 151, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Iwanaga, M.; Harada, E. Possible Regulatory Mechanism of DHA-Induced Anti-Stress Reaction in Rats. Brain Res. 2003, 964, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Mora-Gallegos, A.; Fornaguera, J. The Effects of Environmental Enrichment and Social Isolation and Their Reversion on Anxiety and Fear Conditioning. Behav. Process. 2019, 158, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Barbelivien, A.; Herbeaux, K.; Oberling, P.; Kelche, C.; Galani, R.; Majchrzak, M. Environmental Enrichment Increases Responding to Contextual Cues but Decreases Overall Conditioned Fear in the Rat. Behav. Brain Res. 2006, 169, 231–238. [Google Scholar] [CrossRef] [PubMed]
- De Quervain, D.J.-F.; Aerni, A.; Schelling, G.; Roozendaal, B. Glucocorticoids and the Regulation of Memory in Health and Disease. Front. Neuroendocrinol. 2009, 30, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; Stangl, D.; Jeffries, A.R.; Pariante, C.M.; Thuret, S. The Role of Omega-3 Fatty Acids in Preventing Glucocorticoid-Induced Reduction in Human Hippocampal Neurogenesis and Increase in Apoptosis. Transl. Psychiatry 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Gergerlioglu, H.S.; Oz, M.; Demir, E.A.; Nurullahoglu-Atalik, K.E.; Yerlikaya, F.H. Environmental Enrichment Reverses Cognitive Impairments Provoked by Western Diet in Rats: Role of Corticosteroid Receptors. Life Sci. 2016, 148, 279–285. [Google Scholar] [CrossRef]
- Sampedro-Piquero, P.; Begega, A.; Arias, J.L. Increase of Glucocorticoid Receptor Expression after Environmental Enrichment: Relations to Spatial Memory, Exploration and Anxiety-Related Behaviors. Physiol. Behav. 2014, 129, 118–129. [Google Scholar] [CrossRef]
- Shilpa, B.; Bhagya, V.; Harish, G.; Srinivas Bharath, M.; Shankaranarayana Rao, B. Environmental Enrichment Ameliorates Chronic Immobilisation Stress-Induced Spatial Learning Deficits and Restores the Expression of BDNF, VEGF, GFAP and Glucocorticoid Receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 76, 88–100. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Li, L.; Wang, H.; Gong, X.; Xu, H.; Wu, L.; Yan, C. Environmental Enrichment Modulates HPA Axis Reprogramming in Adult Male Rats Exposed to Early Adolescent Stress. Neurosci. Res. 2021, 172, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, L.; Braschi, C.; Spolidoro, M.; Begenisic, T.; Sale, A.; Maffei, L. Nurturing Brain Plasticity: Impact of Environmental Enrichment. Cell Death Differ. 2010, 17, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yuan, M.; Guo, Y.-S.; Shen, X.-Y.; Gao, Z.-K.; Bi, X. The Role of Enriched Environment in Neural Development and Repair. Front. Cell. Neurosci. 2022, 16, 890666. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Dinger, N.; Levine, B.S. Stress Produced by Gavage Administration in the Rat. Contemp. Top. Lab. Anim. Sci. 2000, 39, 17–21. [Google Scholar]
- Cait, J.; Cait, A.; Scott, R.W.; Winder, C.B.; Mason, G.J. Conventional Laboratory Housing Increases Morbidity and Mortality in Research Rodents: Results of a Meta-Analysis. BMC Biol. 2022, 20, 15. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raymond, J.; Morin, A.; Bradley-Garcia, M.; Plamondon, H. Juvenile/Peripubertal Exposure to Omega-3 and Environmental Enrichment Differentially Affects CORT Secretion and Adulthood Stress Coping, Sociability, and CA3 Glucocorticoid Receptor Expression in Male and Female Rats. Nutrients 2024, 16, 2350. https://doi.org/10.3390/nu16142350
Raymond J, Morin A, Bradley-Garcia M, Plamondon H. Juvenile/Peripubertal Exposure to Omega-3 and Environmental Enrichment Differentially Affects CORT Secretion and Adulthood Stress Coping, Sociability, and CA3 Glucocorticoid Receptor Expression in Male and Female Rats. Nutrients. 2024; 16(14):2350. https://doi.org/10.3390/nu16142350
Chicago/Turabian StyleRaymond, Julie, Alexandre Morin, Meenakshie Bradley-Garcia, and Hélène Plamondon. 2024. "Juvenile/Peripubertal Exposure to Omega-3 and Environmental Enrichment Differentially Affects CORT Secretion and Adulthood Stress Coping, Sociability, and CA3 Glucocorticoid Receptor Expression in Male and Female Rats" Nutrients 16, no. 14: 2350. https://doi.org/10.3390/nu16142350
APA StyleRaymond, J., Morin, A., Bradley-Garcia, M., & Plamondon, H. (2024). Juvenile/Peripubertal Exposure to Omega-3 and Environmental Enrichment Differentially Affects CORT Secretion and Adulthood Stress Coping, Sociability, and CA3 Glucocorticoid Receptor Expression in Male and Female Rats. Nutrients, 16(14), 2350. https://doi.org/10.3390/nu16142350





