Ramulus Mori (Sangzhi) Alkaloids Alleviate Diabetic Nephropathy through Improving Gut Microbiota Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Cell Culture
2.3. Blood and Urine Biochemical Parameters
2.4. Histopathology
2.5. 16S rRNA Sequencing Analysis
2.6. Untargeted Metabolomics Analysis
2.7. FMT Experiment
2.8. Transmission Electron Microscope (TEM)
2.9. Statistical Analysis
3. Results
3.1. SZ-A Ameliorate Lipid and Glucose Metabolic Disorders in HFD/STZ-Induced Diabetic Mice
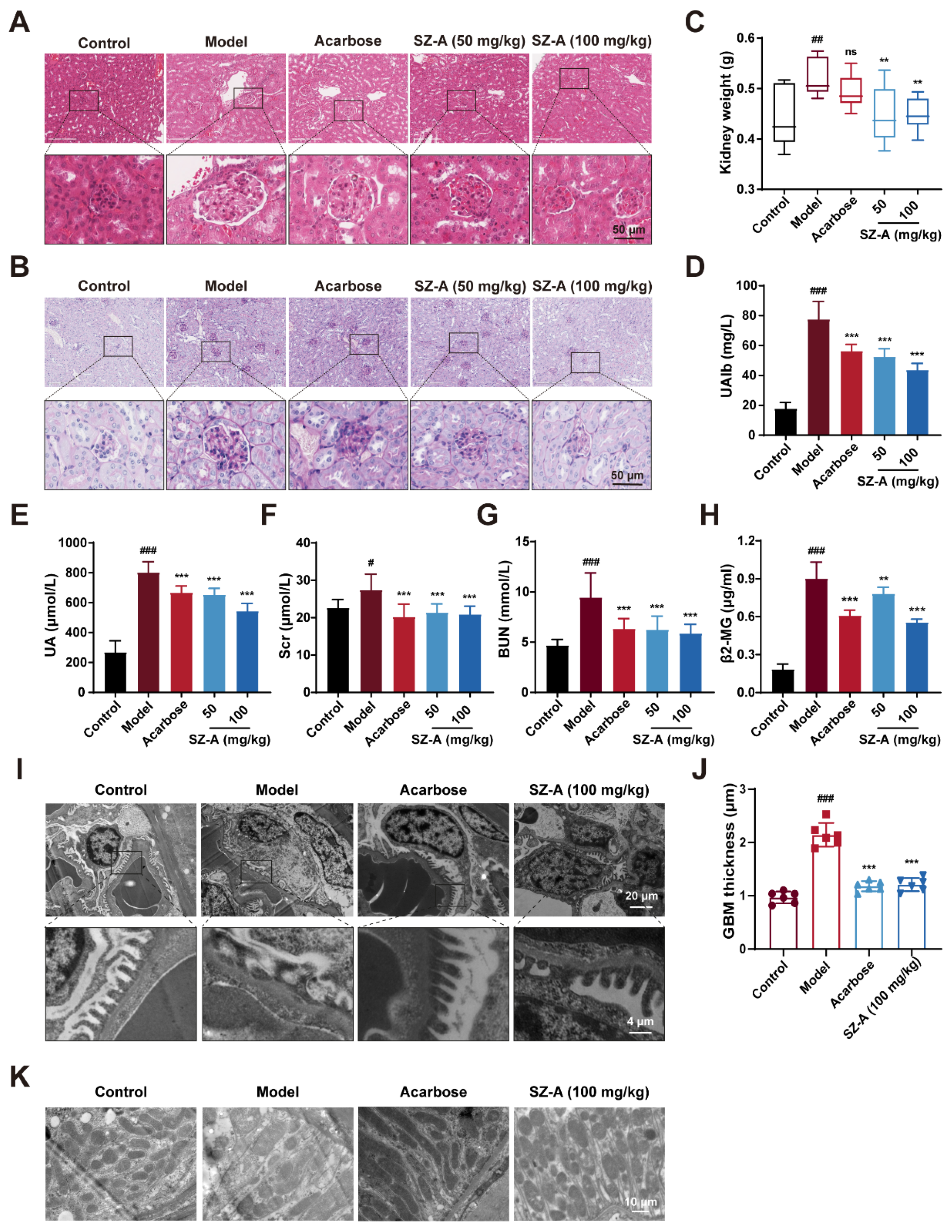
3.2. SZ-A Prevent Kidney Injury in Diabetic Mice
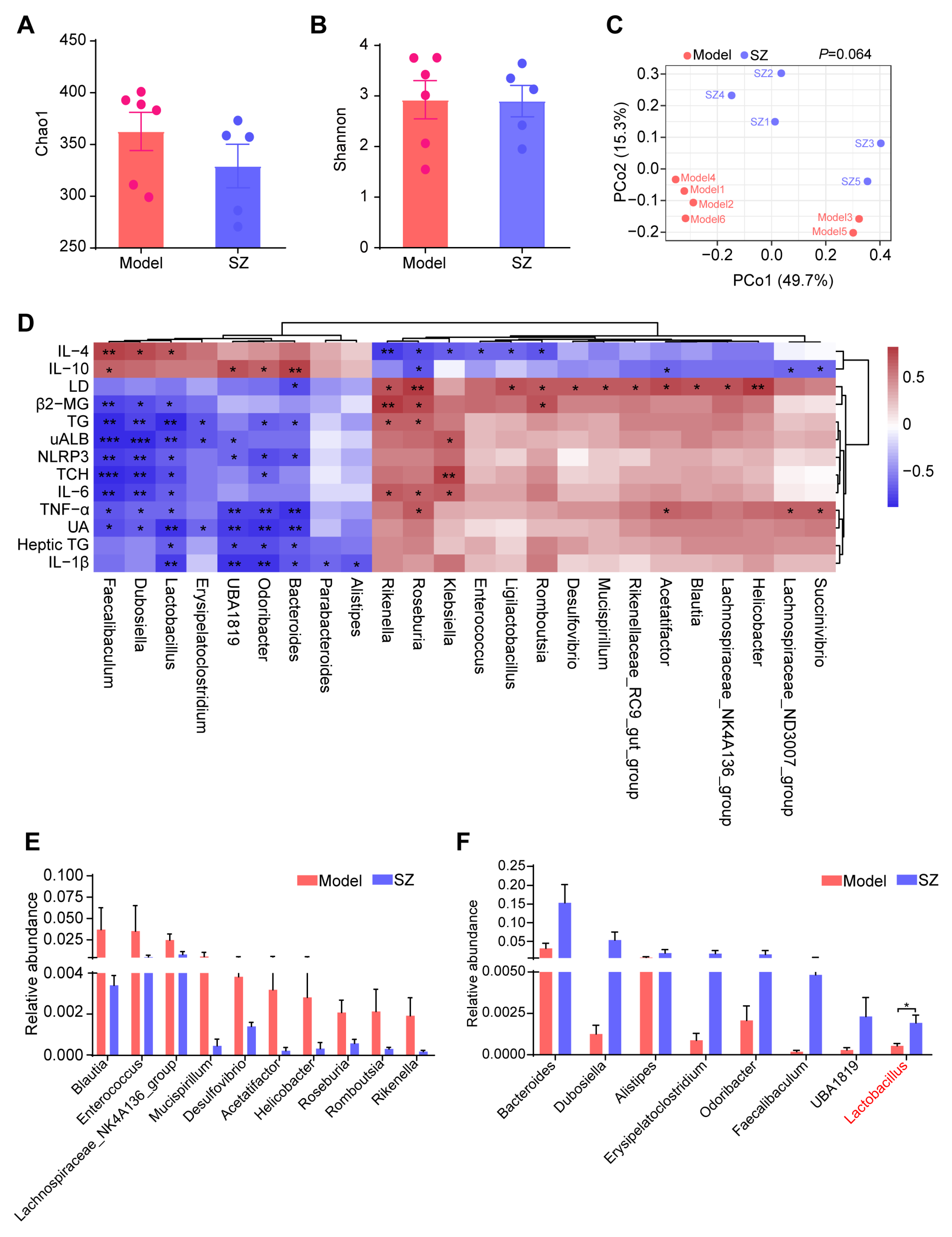
3.3. SZ-A Alters the Composition of Gut Microbiota in STZ and HFD-Induced Mice
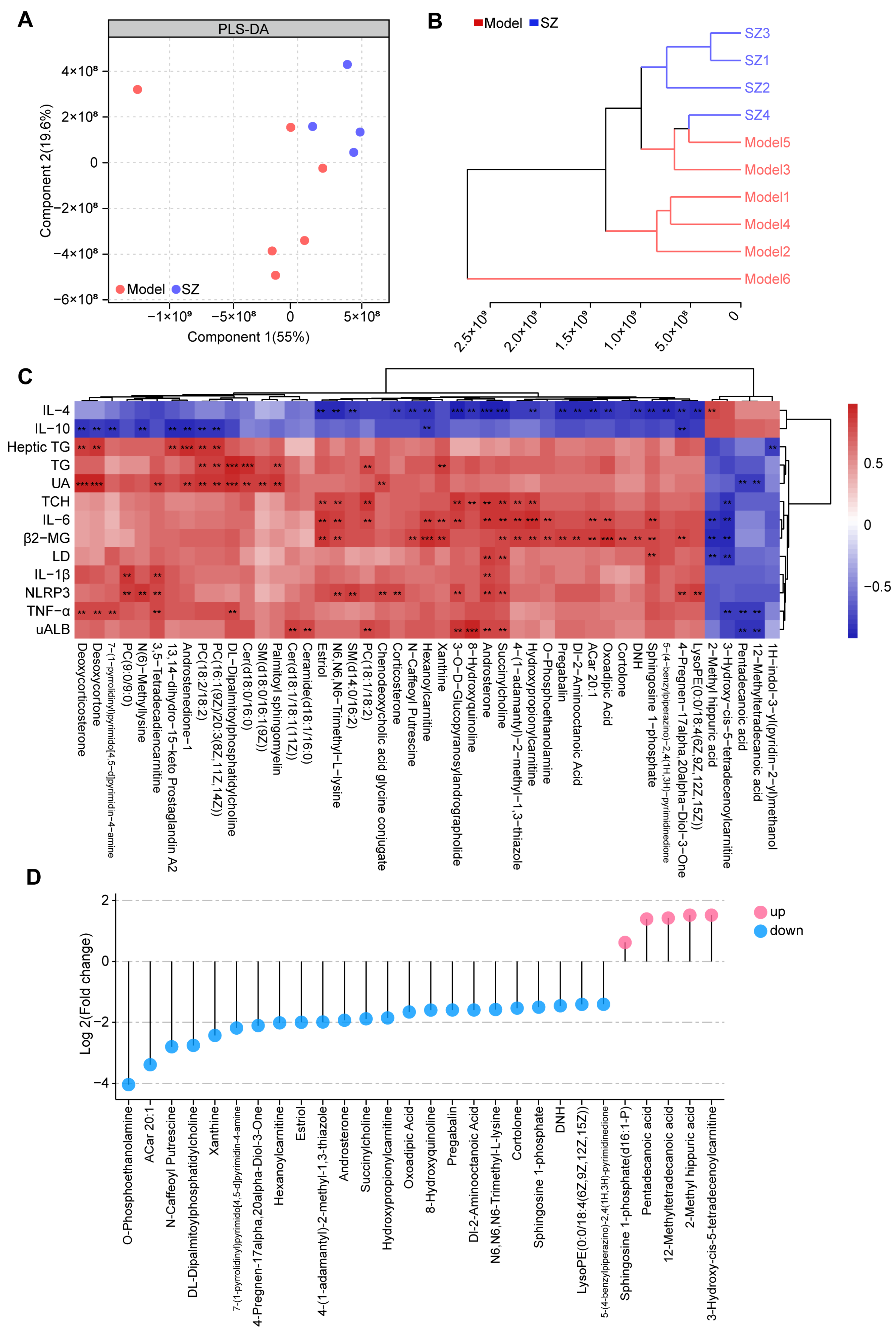
3.4. SZ-A Change the Metabolic Profile in Diabetic Mice
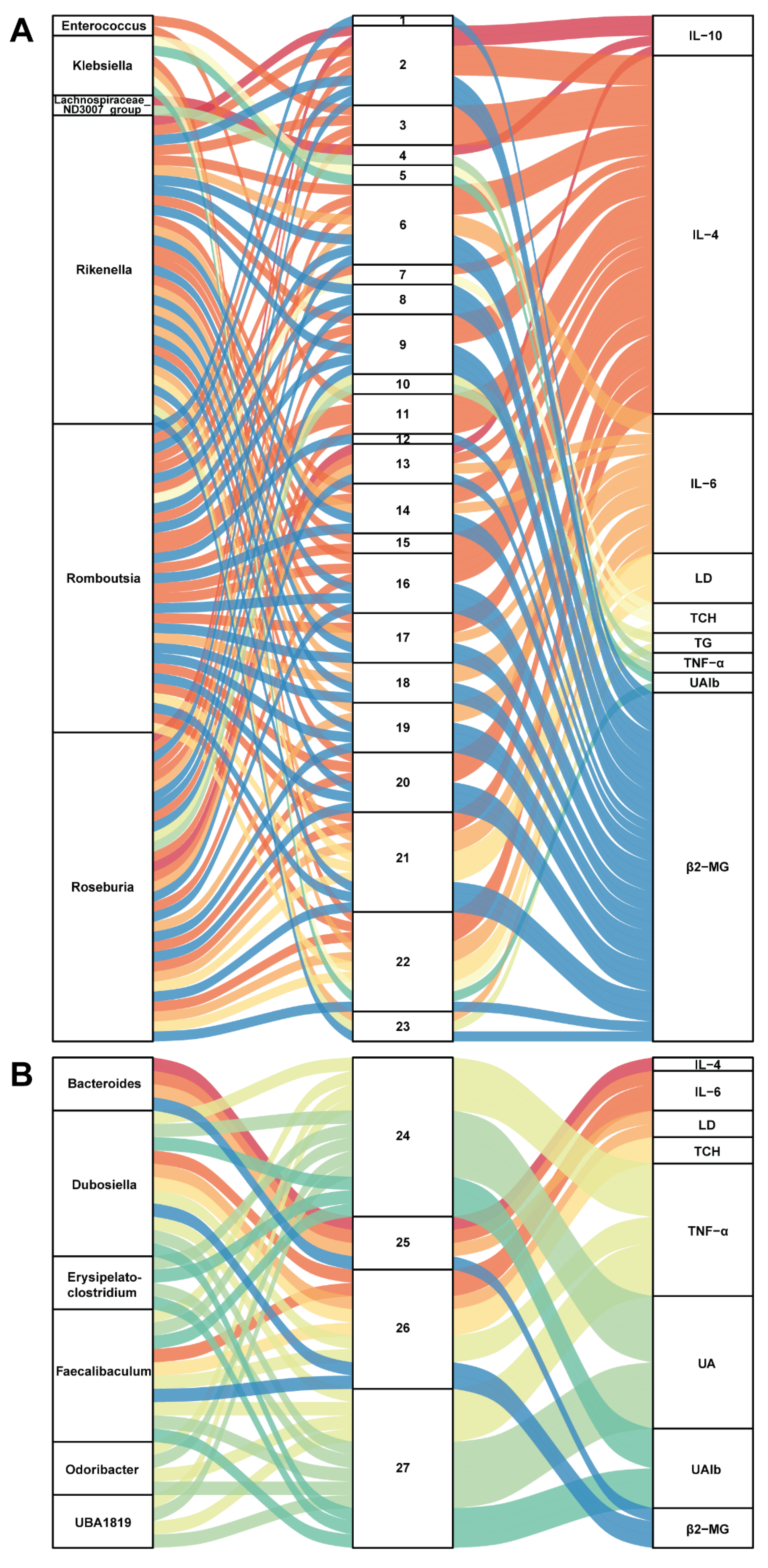
3.5. Correlation between Gut Microbiota and Metabolites in SZ-A-Conditioned Mice
3.6. Gut Microbiota from SZ-A-Treated DN Mice Reverses the Level of Glucose and Lipid in DN Mice
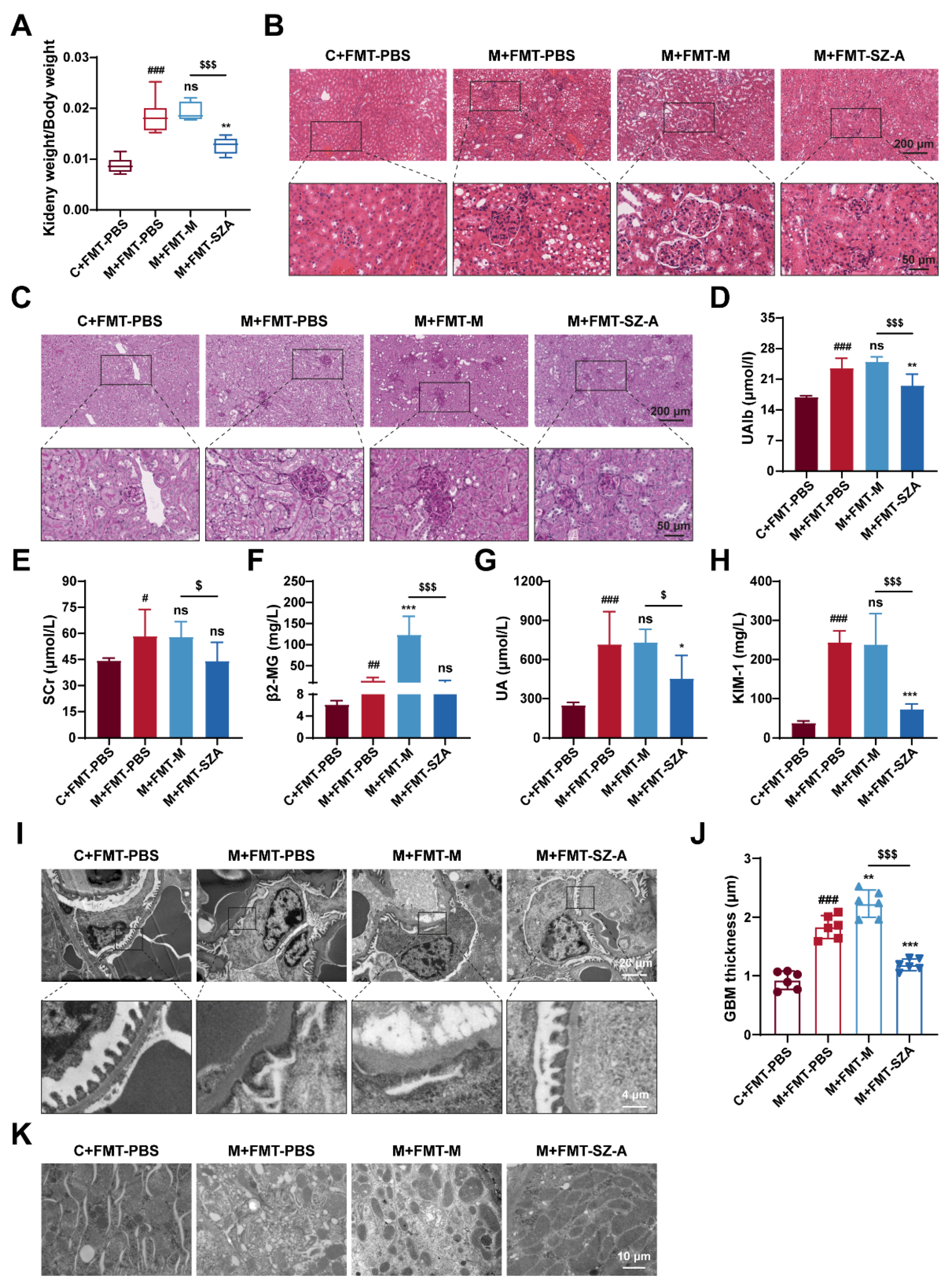
3.7. Gut Microbiota Mediates the Renal Protective Effect of SZ-A
3.8. Pentadecanoic Acid Produced by Gut Microbiota Ameliorates Podocyte Apoptosis In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fineberg, D.; Jandeleit-Dahm, K.A.; Cooper, M.E. Diabetic nephropathy: Diagnosis and treatment. Nat. Rev. Endocrinol. 2013, 9, 713–723. [Google Scholar] [CrossRef]
- Limonte, C.P.; Kretzler, M.; Pennathur, S.; Pop-Busui, R.; de Boer, I.H. Present and future directions in diabetic kidney disease. J. Diabetes Complicat. 2022, 36, 108357. [Google Scholar] [CrossRef]
- Chen, D.Q.; Wu, J.; Li, P. Therapeutic mechanism and clinical application of Chinese herbal medicine against diabetic kidney disease. Front. Pharmacol. 2022, 13, 1055296. [Google Scholar] [CrossRef]
- Park, Y.H.; An, M.; Kim, J.K.; Lim, Y.H. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.; Lu, A.; Zhang, Q.; Li, X.; Zhang, N.; Zhang, J.; Wang, S. From metabolomic analysis to quality assessment and biosynthetic insight in traditional Chinese medicine: Mulberry tree as a case study. Phytochem. Anal. 2022, 33, 644–653. [Google Scholar] [CrossRef]
- Jin, D.; Li, L.; Dong, W.; Zhu, X.; Xia, X.; Wang, R.; Ye, J.; Li, R.; Liu, Z.; Xu, X.; et al. Research on Transfer Rate of Heavy Metals and Harmful Elements in Traditional Chinese Medicine Extraction and Refining Processes and Product Health Risk Assessment. Biol. Trace Elem. Res. 2022, 200, 1956–1964. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Ye, H.; Chen, Y.; Yu, J.; Yang, J.; Zhang, X. Randomized, Double-Blinded, Double-Dummy, Active-Controlled, and Multiple-Dose Clinical Study Comparing the Efficacy and Safety of Mulberry Twig (Ramulus Mori, Sangzhi) Alkaloid Tablet and Acarbose in Individuals with Type 2 Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2016, 2016, 7121356. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Cao, H.; Ji, W.; Li, C.; Huan, Y.; Lei, L.; Fu, Y.; Gao, X.; Liu, Y.; et al. Ramulus Mori (Sangzhi) Alkaloids (SZ-A) Ameliorate Glucose Metabolism Accompanied by the Modulation of Gut Microbiota and Ileal Inflammatory Damage in Type 2 Diabetic KKAy Mice. Front. Pharmacol. 2021, 12, 642400. [Google Scholar] [CrossRef]
- Sun, Q.W.; Lian, C.F.; Chen, Y.M.; Ye, J.; Chen, W.; Gao, Y.; Wang, H.L.; Gao, L.L.; Liu, Y.L.; Yang, Y.F. Ramulus Mori (Sangzhi) Alkaloids Ameliorate Obesity-Linked Adipose Tissue Metabolism and Inflammation in Mice. Nutrients 2022, 14, 5050. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: An integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut 2022, 71, 1095–1105. [Google Scholar] [CrossRef]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut 2022, 71, 716–723. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Negm El-Dein, A.; Ezzat, A.; Aly, H.F.; Awad, G.; Farid, M. Lactobacillus-fermented yogurt exerts hypoglycemic, hypocholesterolemic, and anti-inflammatory activities in STZ-induced diabetic Wistar rats. Nutr. Res. 2022, 108, 22–32. [Google Scholar] [CrossRef]
- Hu, Z.B.; Lu, J.; Chen, P.P.; Lu, C.C.; Zhang, J.X.; Li, X.Q.; Yuan, B.Y.; Huang, S.J.; Ruan, X.Z.; Liu, B.C.; et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics 2020, 10, 2803–2816. [Google Scholar] [CrossRef]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K.; et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Liu, D.; Ye, J.; Yan, Y.; Chen, Y.; Wang, H.; Wang, M.; Feng, Y.; Li, R.; Xu, X.; Jiang, Y.; et al. Ramulus mori (Sangzhi) alkaloids regulates gut microbiota disorder and its metabolism profiles in obese mice induced by a high-fat diet. Front. Pharmacol. 2023, 14, 1166635. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef]
- Barri, T.; Dragsted, L.O. UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: Effect of experimental artefacts and anticoagulant. Anal. Chim. Acta 2013, 768, 118–128. [Google Scholar] [CrossRef]
- Luo, L.; Luo, J.; Cai, Y.; Fu, M.; Li, W.; Shi, L.; Liu, J.; Dong, R.; Xu, X.; Tu, L.; et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacol. Res. 2022, 183, 106367. [Google Scholar] [CrossRef]
- Pi, Y.; Wu, Y.; Zhang, X.; Lu, D.; Han, D.; Zhao, J.; Zheng, X.; Zhang, S.; Ye, H.; Lian, S.; et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 2023, 11, 19. [Google Scholar] [CrossRef]
- Kopp, J.B.; Anders, H.J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Primers 2020, 6, 68. [Google Scholar] [CrossRef]
- Torban, E.; Braun, F.; Wanner, N.; Takano, T.; Goodyer, P.R.; Lennon, R.; Ronco, P.; Cybulsky, A.V.; Huber, T.B. From podocyte biology to novel cures for glomerular disease. Kidney Int. 2019, 96, 850–861. [Google Scholar] [CrossRef]
- Petrazzuolo, A.; Sabiu, G.; Assi, E.; Maestroni, A.; Pastore, I.; Lunati, M.E.; Montefusco, L.; Loretelli, C.; Rossi, G.; Ben Nasr, M.; et al. Broadening horizons in mechanisms, management, and treatment of diabetic kidney disease. Pharmacol. Res. 2023, 190, 106710. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Agarwal, R.; Alpers, C.E.; Bakris, G.L.; Brosius, F.C.; Kolkhof, P.; Uribarri, J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022, 102, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lian, C.F.; Sun, Q.W.; Wang, T.T.; Liu, Y.Y.; Ye, J.; Gao, L.L.; Yang, Y.F.; Liu, S.N.; Shen, Z.F.; et al. Ramulus Mori (Sangzhi) Alkaloids Alleviate High-Fat Diet-Induced Obesity and Nonalcoholic Fatty Liver Disease in Mice. Antioxidants 2022, 11, 905. [Google Scholar] [CrossRef]
- Lei, L.; Huan, Y.; Liu, Q.; Li, C.; Cao, H.; Ji, W.; Gao, X.; Fu, Y.; Li, P.; Zhang, R.; et al. Morus alba L. (Sangzhi) Alkaloids Promote Insulin Secretion, Restore Diabetic β-Cell Function by Preventing Dedifferentiation and Apoptosis. Front. Pharmacol. 2022, 13, 841981. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Xu, C.; Liu, H.; Gao, X.; Li, X.; Lin, W.; Ma, X.; Yang, C.; Hao, M.; Zhao, K.; et al. Effects of mulberry twig alkaloids(Sangzhi alkaloids) and metformin on blood glucose fluctuations in combination with premixed insulin-treated patients with type 2 diabetes. Front. Endocrinol. 2023, 14, 1272112. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, L.; Yan, Q.; Zeng, J.; Ma, X.; Zhao, Y. A natural products solution to diabetic nephropathy therapy. Pharmacol. Ther. 2023, 241, 108314. [Google Scholar] [CrossRef]
- Tang, G.; Li, S.; Zhang, C.; Chen, H.; Wang, N.; Feng, Y. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B. 2021, 11, 2749–2767. [Google Scholar] [CrossRef]
- Patial, V.; Katoch, S.; Chhimwal, J.; Singh, P.P.; Suresh, P.S.; Padwad, Y. Tinospora cordifolia activates PPARγ pathway and mitigates glomerular and tubular cell injury in diabetic kidney disease. Phytomedicine 2021, 91, 153663. [Google Scholar] [CrossRef]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef]
- Ji, J.L.; Shi, H.M.; Li, Z.L.; Jin, R.; Qu, G.T.; Zheng, H.; Wang, E.; Qiao, Y.Y.; Li, X.Y.; Ding, L.; et al. Satellite cell-derived exosome-mediated delivery of microRNA-23a/27a/26a cluster ameliorates the renal tubulointerstitial fibrosis in mouse diabetic nephropathy. Acta Pharmacol. Sin. 2023, 44, 2455–2468. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Ji, W.; Fu, Y.; Cao, H.; Huan, Y.; Lei, L.; Gao, X.; Chen, L.; Feng, C.; et al. New anti-diabetic drug Morus alba L. (Sangzhi) alkaloids (SZ-A) improves diabetic nephropathy through ameliorating inflammation and fibrosis in diabetic rats. Front. Med. 2023, 10, 1164242. [Google Scholar] [CrossRef]
- Krukowski, H.; Valkenburg, S.; Madella, A.M.; Garssen, J.; van Bergenhenegouwen, J.; Overbeek, S.A.; Huys, G.R.B.; Raes, J.; Glorieux, G. Gut microbiome studies in CKD: Opportunities, pitfalls and therapeutic potential. Nat. Rev. Nephrol. 2023, 19, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Ye, F.; Shen, Z.; Xie, M. Alpha-glucosidase inhibition from a Chinese medical herb (Ramulus mori) in normal and diabetic rats and mice. Phytomedicine 2002, 9, 161–166. [Google Scholar] [CrossRef]
- Yang, S.; Mi, J.; Liu, Z.; Wang, B.; Xia, X.; Wang, R.; Liu, Y.; Li, Y. Pharmacokinetics, Tissue Distribution, and Elimination of Three Active Alkaloids in Rats after Oral Administration of the Effective Fraction of Alkaloids from Ramulus Mori, an Innovative Hypoglycemic Agent. Molecules 2017, 22, 1616. [Google Scholar] [CrossRef]
- Ottosson, F.; Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Almgren, P.; Fernandez, C.; Melander, O.; Orho-Melander, M. Connection Between BMI-Related Plasma Metabolite Profile and Gut Microbiota. J. Clin. Endocrinol. Metab. 2018, 103, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, X.; Gao, Y.; Lv, C.; Gao, Z.; Liu, Y.; Wang, Y.; Li, S.; Wang, Z. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165764. [Google Scholar] [CrossRef]
- Bai, Y.F.; Wang, S.W.; Wang, X.X.; Weng, Y.Y.; Fan, X.Y.; Sheng, H.; Zhu, X.T.; Lou, L.J.; Zhang, F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Romero, M.; Olivares, M.; Jiménez, R.; Duarte, J. The Probiotic Lactobacillus fermentum Prevents Dysbiosis and Vascular Oxidative Stress in Rats with Hypertension Induced by Chronic Nitric Oxide Blockade. Mol. Nutr. Food Res. 2018, 62, e1800298. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Zhu, M.; Yang, B.; Yang, Y.; Jia, X.; Feng, L. Moutan Cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 2022, 206, 849–860. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Gurung, M.; Li, Z.; García-Jaramillo, M.; Greer, R.; Gaulke, C.; Bauchinger, F.; You, H.; Pederson, J.W.; Vasquez-Perez, S.; et al. Transkingdom interactions between Lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes. Nat. Commun. 2021, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ouyang, Y.; Chen, X.; Chen, R.; Ruan, Q.; Farag, M.A.; Chen, X.; Zhao, C. Hypoglycaemic and anti-ageing activities of green alga Ulva lactuca polysaccharide via gut microbiota in ageing-associated diabetic mice. Int. J. Biol. Macromol. 2022, 212, 97–110. [Google Scholar] [CrossRef]
- Gu, K.; Wu, A.; Yu, B.; Zhang, T.; Lai, X.; Chen, J.; Yan, H.; Zheng, P.; Luo, Y.; Luo, J.; et al. Iron overload induces colitis by modulating ferroptosis and interfering gut microbiota in mice. Sci. Total Environ. 2023, 905, 167043. [Google Scholar] [CrossRef]
- Igarashi, M.; Morimoto, M.; Suto, A.; Nakatani, A.; Hayakawa, T.; Hara, K.; Kimura, I. Synthetic dietary inulin, Fuji FF, delays development of diet-induced obesity by improving gut microbiota profiles and increasing short-chain fatty acid production. PeerJ 2020, 8, e8893. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, H.; Yin, X.; Zhao, H.; Ma, L.; Yan, M.; Peng, L.; Wang, Q.; Dong, X.; Li, P. Tangshen formula modulates gut Microbiota and reduces gut-derived toxins in diabetic nephropathy rats. Biomed. Pharmacother. 2020, 129, 110325. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Macchietto, M.G.; Liu, X.; Lu, Y.; Ma, Y.; Guo, H.; Saqui-Salces, M.; Bernlohr, D.A.; Chen, C.; Shen, S.; et al. Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity. Int. J. Obes. 2021, 45, 143–154. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Hernandez-Sanabria, E.; Calatayud Arroyo, M.; Van de Wiele, T. Supplementation of a propionate-producing consortium improves markers of insulin resistance in an in vitro model of gut-liver axis. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E742–E749. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, Y.; Su, J.; Zhu, B.; Wang, S.; Liu, K.; Wang, H.; Shi, S.; Zhang, Q.; Qin, L.; et al. Roles of gut microbiota and metabolites in a homogalacturonan-type pectic polysaccharide from Ficus pumila Linn. fruits mediated amelioration of obesity. Carbohydr. Polym. 2020, 248, 116780. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Chong, P.C.T.; Hsieh, C.C.; Lin, Y.T.; Ye, C.H.; Khumsupan, D.; Lu, J.J.; Yu, W.C.; Cheng, K.W.; Yap, K.Y.; et al. Identification of anti-fibrotic and pro-apoptotic bioactive compounds from Ganoderma formosanum and their possible mechanisms in modulating TGF-β1-induced lung fibrosis. J. Ethnopharmacol. 2024, 327, 118008. [Google Scholar] [CrossRef]
- Pereira, P.R.; Carrageta, D.F.; Oliveira, P.F.; Rodrigues, A.; Alves, M.G.; Monteiro, M.P. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med. Res. Rev. 2022, 42, 1518–1544. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Xu, S.; Zhang, B.; Sun, X. Ramulus Mori (Sangzhi) Alkaloids Alleviate Diabetic Nephropathy through Improving Gut Microbiota Disorder. Nutrients 2024, 16, 2346. https://doi.org/10.3390/nu16142346
Liu W, Xu S, Zhang B, Sun X. Ramulus Mori (Sangzhi) Alkaloids Alleviate Diabetic Nephropathy through Improving Gut Microbiota Disorder. Nutrients. 2024; 16(14):2346. https://doi.org/10.3390/nu16142346
Chicago/Turabian StyleLiu, Wenxiu, Saijun Xu, Bin Zhang, and Xiaobo Sun. 2024. "Ramulus Mori (Sangzhi) Alkaloids Alleviate Diabetic Nephropathy through Improving Gut Microbiota Disorder" Nutrients 16, no. 14: 2346. https://doi.org/10.3390/nu16142346
APA StyleLiu, W., Xu, S., Zhang, B., & Sun, X. (2024). Ramulus Mori (Sangzhi) Alkaloids Alleviate Diabetic Nephropathy through Improving Gut Microbiota Disorder. Nutrients, 16(14), 2346. https://doi.org/10.3390/nu16142346




