Sex-Moderated Divergence between Adult Child and Parental Dietary Behavior Patterns in Relation to Body Mass Condition—Evaluating the Mediating Role of Physical Activity: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Sample Size
2.4. Participants
2.5. Data Collection
2.6. Questionnaire Measurements
2.6.1. Dietary Characteristics
2.6.2. Physical Activity
2.7. Anthropometric and Body Composition Measurements
2.8. Handling and Imputation of Missing Data
2.9. Statistics
3. Results
3.1. Sample Characteristics
3.2. Patterns of Dietary Behaviors in Family Affinity Comparisons
3.3. Prevalence of Overweight among Parents and Adult Children
3.4. Conformity of Overweight in Families—Parent–Child Pairwise Matching
3.5. Specific Dietary Behaviors in Relation to Being Overweight
3.6. Intrinsic and Extrinsic Predictors of Body Weight of Adult Children’s BMI
3.7. The Role of Physical Activity and Inactivity in Relation to Dietary Behaviors and Weight Conditions in Adult Children
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128_9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Youth and Health Risks. 2011. Available online: http://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_R28-en.pdf (accessed on 18 May 2024).
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Davison, B.; Saeedi, P.; Black, K.; Harrex, H.; Haszard, J.; Meredith-Jones, K.; Quigg, R.; Skeaff, S.; Stoner, L.; Wong, J.E.; et al. The Association between Parent Diet Quality and Child Dietary Patterns in Nine- to Eleven-Year-Old Children from Dunedin, New Zealand. Nutrients 2017, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Craigie, A.M.; Lake, A.A.; Kelly, S.A.; Adamson, A.J.; Mathers, J.C. Tracking of obesity-relatedbehaviours from childhood to adulthood: A systematic review. Maturitas 2011, 70, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, J.E.; Mikkilä, V.; Raitakari, O.T. Role of childhood food patterns on adult cardiovascular disease risk. Curr. Atheroscler. Rep. 2014, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- Patrick, H.; Nicklas, T.A. A review of family and social determinants of children’s eating patterns and diet quality. J. Am. Coll. Nutr. 2005, 24, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Cooke, L. Genetic and environmental determinants of children’s food preferences. Br. J. Nutr. 2008, 99, 15–21. [Google Scholar] [CrossRef]
- Pearson, N.; Biddle, S.J.H.; Gorely, T. Family correlates of fruit and vegetable consumption in children and adolescents: A systematic review. Public. Health Nutr. 2009, 12, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.N.; Rollo, M.E.; Watson, J.; Burrows, T.L.; Collins, C.E. Relationships between dietary intakes of children and their parents: A cross-sectional, secondary analysis of families participating in the Family Diet Quality Study. J. Hum. Nutr. Diet. 2015, 28, 443–451. [Google Scholar] [CrossRef]
- Wolnicka, K.; Taraszewska, A.M.; Jaczewska-Schuetz, J.; Jarosx, M. Factors within the family environment such as parents’ dietary habits and fruit and vegetable availability have the greatest influence on fruit and vegetable consumption by Polish children. Public Health Nutr. 2015, 18, 2705–2711. [Google Scholar] [CrossRef]
- Tenjin, K.; Sekine, M.; Yamada, M.; Tatsuse, T. Relationship between Parental Lifestyle and Dietary Habits of Children: A Cross-Sectional Study. J. Epidemiol. 2020, 30, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, N.; Page, P.; Penney, T.L.; Nicholson, S.; Kirk, S.F.; Almiron-Roig, E. Eating at food outlets and leisure places and “on the go” is associated with less-healthy food choices than eating at home and in school in children: Cross-sectional data from the UK National Diet and Nutrition Survey Rolling Program (2008–2014). Am. J. Clin. Nutr. 2018, 107, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Monge-Rojas, R.; Vargas-Quesada, R.; Gómez, G. Role of Residence Area on Diet Diversity and Micronutrient Intake Adequacy in Urban and Rural Costa Rican Adolescents. Nutrients 2022, 14, 5093. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhao, A.; Lan, H.; Mao, S.; Li, P.; Jiang, H.; Wang, P.; Szeto, I.M.Y.; Zhang, Y. Dietary Diversity, Micronutrient Adequacy and Bone Status during Pregnancy: A Study in Urban China from 2019 to 2020. Nutrients 2022, 14, 4690. [Google Scholar] [CrossRef] [PubMed]
- Vispute, S.; Mandlik, R.; Sanwalka, N.; Gondhalekar, K.; Khadilkar, A. Dietary Diversity and Food Variety Scores and Their Association with Nutrition and Health Status of Indian Children and Adolescents: A Multicenter Study. Nutrition 2023, 111, 112039. [Google Scholar] [CrossRef] [PubMed]
- Aurino, E. Do boys eat better than girls in India? Longitudinal evidence on dietary diversity and food consumption disparities among children and adolescents. Econ. Hum. Biol. 2017, 25, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Foote, J.A.; Murphy, S.P.; Wilkens, L.R.; Basiotis, P.P.; Carlson, A. Dietary Variety Increases the Probability of Nutrient Adequacy among Adults. J. Nutr. 2004, 134, 1779–1785. [Google Scholar] [CrossRef]
- Suling, M.; Hebestreit, A.; Peplies, J.; Bammann, K.; Nappo, A.; Eiben, G.; Fernández Alvira, J.M.; Verbestel, V.; Kovács, É.; Pitsiladis, Y.P.; et al. Design and Results of the Pretest of the IDEFICS Study. Int. J. Obes. 2011, 35, S30–S44. [Google Scholar] [CrossRef]
- Semmler, C.; Ashcroft, J.; van Jaarsveld, C.H.; Carnell, S.; Wardle, J. Development of overweight in children in relation to parental weight and socio-economic status. Obesity 2009, 17, 814–820. [Google Scholar] [CrossRef]
- Keane, E.; Layte, R.; Harrington, J.; Kearney, P.M.; Perry, I.J. Measured parental weight status and familial socio-economic status correlates with childhood overweight and obesity at age 9. PLoS ONE 2012, 7, e43503. [Google Scholar] [CrossRef]
- Freeman, E.; Fletcher, R.; Collins, C.E.; Morgan, P.J.; Burrows, T.; Callister, R. Preventing and treating childhood obesity: Time to target fathers. Int. J. Obes. 2012, 36, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Naughton, P.; McCarthy, S.N.; McCarthy, M.B. The creation of a healthy eating motivation score and its association with food choice and physical activity in a cross sectional sample of Irish adults. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Smit, E.; Mahoney, S. Physical activity and dietary behavior in US adults and their combined influence on health. Mayo Clin. Proc. 2014, 89, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.A.; Rehman, L.; Kirk, S.F. Understanding gender norms, nutrition, and physical activity in adolescent girls: A scoping review. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Harton, A.; Myszkowska-Ryciak, J.; Laskowski, W.; Gajewska, D. Prevalence of overweight and obesity among adolescents in Poland. J. Health Inequalities 2019, 5, 180–187. [Google Scholar] [CrossRef]
- Domaradzki, J. Congruence between Physical Activity Patterns and Dietary Patterns Inferred from Analysis of Sex Differences in Lifestyle Behaviors of Late Adolescents from Poland: Cophylogenetic Approach. Nutrients 2023, 15, 608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Domaradzki, J. The Discriminant Power of Specific Physical Activity and Dietary Behaviors to Distinguish between Lean, Normal and Excessive Fat Groups in Late Adolescents. Nutrients 2023, 15, 1230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wadolowska, L.; Kowalkowska, J.; Lonnie, M.; Czarnocinska, J.; Jezewska-Zychowicz, M.; Babicz-Zielinska, E. Associations between physical activity patterns and dietary patterns in a representative sample of Polish girls aged 13–21 years: A cross-sectional study (GEBaHealth Project). BMC Public Health 2016, 16, 698. [Google Scholar] [CrossRef]
- Harriss, D.J.; Atkinson, G. Ethical Standards in Sport and Exercise Science Research: 2016 Update. Int. J. Sports Med. 2015, 36, 1121–1124. [Google Scholar] [CrossRef]
- Dalmaijer, E.S.; Nord, C.L.; Astle, D.E. Statistical power for cluster analysis. BMC Bioinform. 2022, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Questionnaire of Eating Behaviors (QEB). Available online: http://www.uwm.edu.pl/edu/lidiawadolowska/pdf/qeb.pdf (accessed on 15 March 2023).
- Wadolowska, L.; Krusińska, B. Procedura Opracowania Danych Żywieniowych z Kwestionariusza QEB [In Polish]. Available online: https://www.uwm.edu.pl/edu/lidiawadolowska (accessed on 15 November 2022).
- IPAQ Research Committee. Guidelines for the Data Processing and Analysis of the International Physical Activity Questionnaire. 2005. Available online: https://sites.google.com/view/ipaq/download (accessed on 15 March 2023).
- PN-EN ISO 9001: 2015; Systemy zarządzania jakością—Wymagania. POLSKI KOMITET NORMALIZACY: Warszawa, Poland, 2015.
- Raymaekers, J.; Rousseeuw, P.J. Transforming variables to central normality. Mach. Learn. 2021, 110, 1–36. [Google Scholar] [CrossRef]
- Allendes Osorio, R.; Tripathi, L.; Mizuguchi, K. CLINE: A web-tool for the comparison of biological dendrogram structures. BMC Bioinf. 2019, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J. Analyse Factorielle de Donnees Mixtes. Rev. Stat. Appliq 2004, 4, 93–111. [Google Scholar]
- Baron, R.M.; Kenny, D.A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psych. 1986, 51, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Cox, M.C. Commentary on “Mediation analysis and categorical variables: The final frontier” by Dawn Iacobucci. J. Cons. Psych. 2012, 22, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Newsom, J.T. Mediation Analysis with Logistic Regression. Available online: https://web.pdx.edu/~newsomj/cdaclass/ho_mediation.pdf (accessed on 15 February 2024).
- Galili, T. Dendextend: An R package for visualizing, adjusting, and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Garnier, S.; Ross, N.; Rudis, R.; Camargo, P.A.; Sciaini, M.; Scherer, C. Viridis(Lite)—Colorblind-Friendly Color Maps for R. 2024. Available online: https://doi.org/10.5281/zenodo.4679423 (accessed on 15 February 2024).
- Morgan, M. BiocManager: Access the Bioconductor Project Package Repository, R Package Version 1.30.19. 2022. Available online: https://CRAN.R-project.org/package=BiocManager (accessed on 15 February 2024).
- Thioulouse, J.; Dray, S.; Dufour, A.; Siberchicot, A.; Jombart, T.; Pavoine, S. Multivariate Analysis of Ecological Data with ade4; Springer: New York, NY, USA, 2018. [Google Scholar]
- Gaylis, J.B.; Levy, S.S.; Kviatkovsky, S.; DeHamer, R.; Hong, M.Y. Relationships between physical activity, food choices, gender and BMI in Southern Californian teenagers. Int. J. Adolesc. Med. Health 2017, 31, 20170067. [Google Scholar] [CrossRef] [PubMed]
- Mazur, J. Health and Health Behaviour of School Children in Poland against the Background of Selected Sociodemographic Conditions; HBSC 2014 Results; Instytut Matki i Dziecka: Warszawa, Poland, 2015. (In Polish) [Google Scholar]
- Górna, S.; Pazdro-Zastawny, K.; Basiak-Rasała, A.; Krajewska, J.; Kolator, M.; Cichy, I.; Rokita, A.; Zatoński, T. Physical activity and sedentary behaviors in Polish children and adolescents. Arch. Pédiatr. 2023, 30, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Harbec, M.J.; Pagani, L.S. Associations between Early Family Meal Environment Quality and Later Well-Being in School-Age Children. J. Dev. Behav. Pediatr. 2018, 39, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Bogl, L.H.; Silventoinen, K.; Hebestreit, A.; Intemann, T.; Williams, G.; Michels, N.; Molnár, D.; Page, A.S.; Pala, V.; Papoutsou, S.; et al. Familial Resemblance in Dietary Intakes of Children, Adolescents, and Parents: Does Dietary Quality Play a Role? Nutrients 2017, 9, 892. [Google Scholar] [CrossRef]
- Blissett, J.; Meyer, C.; Haycraft, E. Maternal and paternal controlling feeding practices with male and female children. Appetite 2006, 47, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Brann, L.S.; Skinner, J.D. More controlling child-feeding practices are found among parents of boys with an average body mass index compared with parents of boys with a high body mass index. J. Am. Diet. Assoc. 2005, 105, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Frankel, L.A.; Kuno, C.B. The moderating role of parent gender on the relationship between restrictive feeding and a child’s self-regulation in eating: Results from mother-only samples may not apply to both parents. Appetite 2019, 143, 104424. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.W.; van Assema, P.; Sleddens, E.F.; de Vries, N.K.; Kremers, S.P. Associations between general parenting, restrictive snacking rules, and adolescent’s snack intake. The roles of fathers and mothers and interparental congruence. Appetite 2015, 87, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Hendy, H.M.; Williams, K.E.; Camise, T.S.; Eckman, N.; Hedemann, A. The Parent Mealtime Action Scale (PMAS). Development and association with children’s diet and weight. Appetite 2009, 52, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.; Harris, H.A.; Mallan, K.M.; Daniels, L.; Thorpe, K. Measurement invariance of the Feeding Practices and Structure Questionnaire-28 among a community of socioeconomically disadvantaged mothers and fathers. Appetite 2018, 120, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.N.; Dundar, C.; Terzi, Ö. Prevalence of overweight and obesity among school children and parents: A cross-sectional study. Rural. Remote Health 2021, 21, 6773. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.; Kundakçı, G.A.; Dereli, F.; Oztornacı, B.O.; Cetişli, N.E. Obesity prevalence and associated characteristics in primary school students according to age and gender. J. Curr. Pediatr. 2019, 17, 127–140. [Google Scholar] [CrossRef]
- Wang, V.H.; Min, J.; Xue, H.; Du, S.; Xu, F.; Wang, H.; Wang, Y. What factors may contribute to sex differences in childhood obesity prevalence in China? Public Health Nutr. 2018, 21, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 May 2024).
- World Obesity Federation. Global Obesity Observatory, Prevalence of Adult Overweight & Obesity. Available online: https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity (accessed on 20 May 2024).
- Korzycka, M.; Bójko, M.; Radiukiewicz, K.; Dzielska, A.; Oblacińska, A.; Fijałkowska, A. Zdrowie Dzieci w Pandemii COVID-19 [Children’s Health in the COVID-19 Pandemic.]; Instytut Matki i Dziecka: Warszawa, Poland, 2022. [Google Scholar]
- El Ansari, W.; Stock, C.; Mikolajczyk, R.T. Relationships between food consumption and living arrangements among university students in four European countries—A cross-sectional study. Nutr. J. 2012, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; McNaughton, S.A.; Gall, S.L.; Otahal, P.; Dwyer, T.; Venn, A.J. Associations between partnering and parenting transitions and dietary habits in young adults. J. Acad. Nutr. Diet. 2015, 117, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Dohle, S.; Siegrist, M. Time for change? Food choices in the transition to cohabitation and parenthood. Public Health Nutr. 2014, 17, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Myszkowska-Ryciak, J.; Harton, A.; Lange, E.; Laskowski, W.; Wawrzyniak, A.; Hamulka, J.; Gajewska, D. Reduced Screen Time is Associated with Healthy Dietary Behaviors but Not Body Weight Status among Polish Adolescents. Report from the Wise Nutrition—Healthy Generation Project. Nutrients 2020, 12, 1323. [Google Scholar] [CrossRef]
- Tsujiguchi, H.; Hori, D.; Kambayashi, Y.; Hamagishi, T.; Asakura, H.; Mitoma, J.; Kitaoka, M.; Anyenda, E.O.; Nguyen, T.T.T.; Yamada, Y.; et al. Relationship between screen time and nutrient intake in Japanese children and adolescents: A cross-sectional observational study. Environ. Health Prev. Med. 2018, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Jusienė, R.; Urbonas, V.; Laurinaitytė, I.; Rakickienė, L.; Breidokienė, R.; Kuzminskaitė, M.; Praninskienė, R. Screen Use During Meals Among Young Children: Exploration of Associated Variables. Medicina 2019, 55, 688. [Google Scholar] [CrossRef]
- Lee, S.T.; Wong, J.E.; Shanita, S.N.; Ismail, M.N.; Deurenberg, P.; Poh, B.K. Daily physical activity and screen time, but not other sedentary activities, are associated with measures of obesity during childhood. Int. J. Environ. Res. Public Health 2015, 12, 146–161. [Google Scholar] [CrossRef]
- Wethington, H.; Pan, L.P.; Sherry, B. The Association of Screen Time, Television in the Bedroom, and Obesity Among School-Aged Youth: 2007 National Survey of Children’s Health. J. Sch. Health 2013, 83, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Gorely, T.; Biddle, S. A descriptive epidemiology of screen based media use among youth: A review and critique. J. Adoles. 2006, 29, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Ottevaere, C.; Huybrechts, I.; Benser, J.; De Bourdeaudhuij, I.; Cuenca-Garcia, M.; Dallongeville, J.; Zaccaria, M.; Gottrand, F.; Kersting, M.; Rey-Lopez, J.P. Clustering patterns of physical activity, sedentary and dietary behavior among European adolescents: The HELENA study. BMC Public Health 2011, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Bloom, M.J.; Broshous, W.T.; Chen, G.Y.; Jost, S.R.; Lang, A.S.; Neto, L.C.L.; Mankin, N.V.; McMahan, E.R.; Merheb, J.A.; et al. Can leading a physically active and healthy lifestyle prevent gaining the freshman 15? Hum. Mov. 2023, 24, 118–125. [Google Scholar] [CrossRef]
- Pagliaro, A.; Alioto, A.; Rossi, C.; Baldassano, S.; Proia, P. Performance enhancing strategies in sailing sports: Beyond training and nutrition. Hum. Mov. 2024, 25, 15–25. [Google Scholar] [CrossRef]

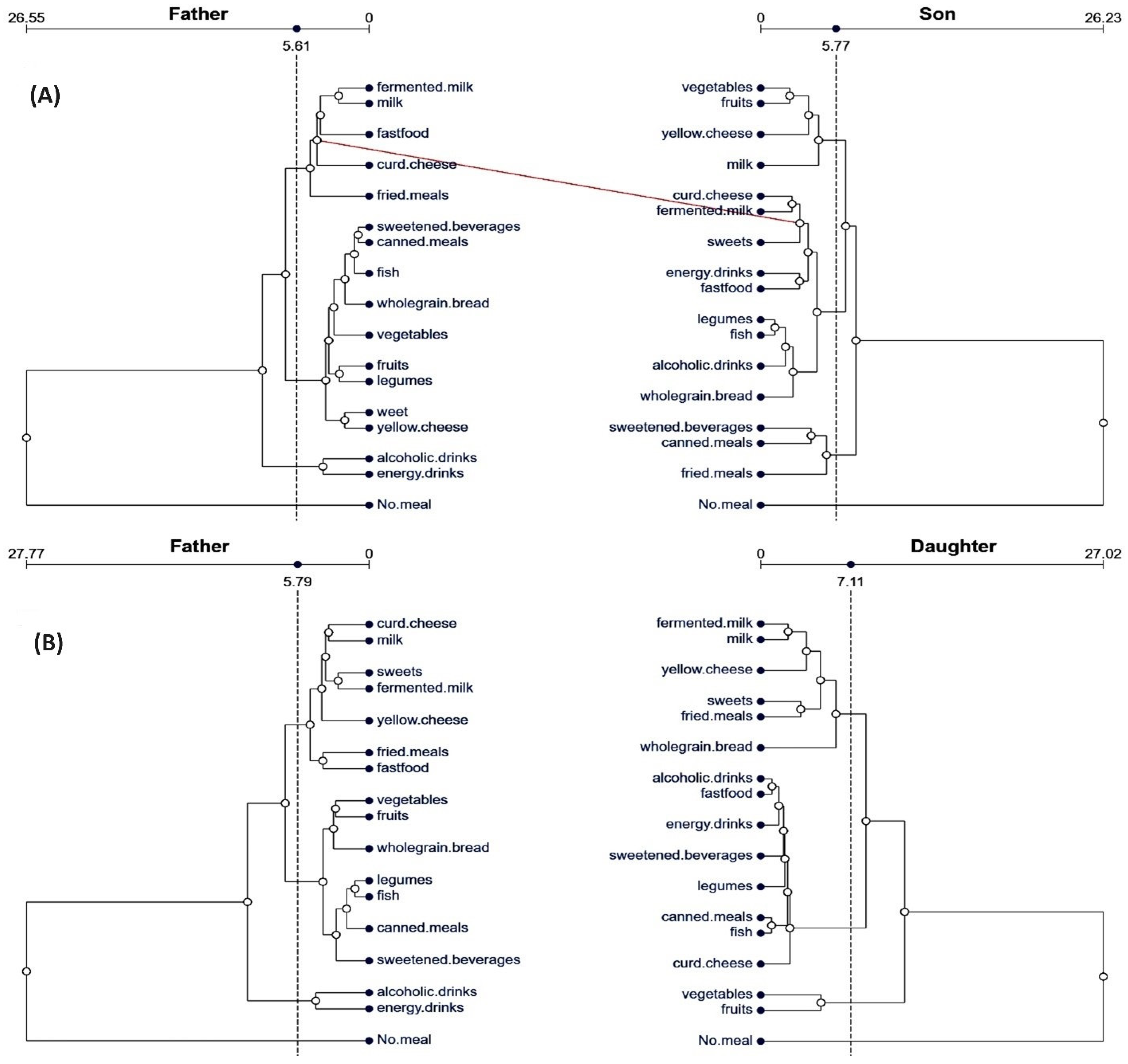
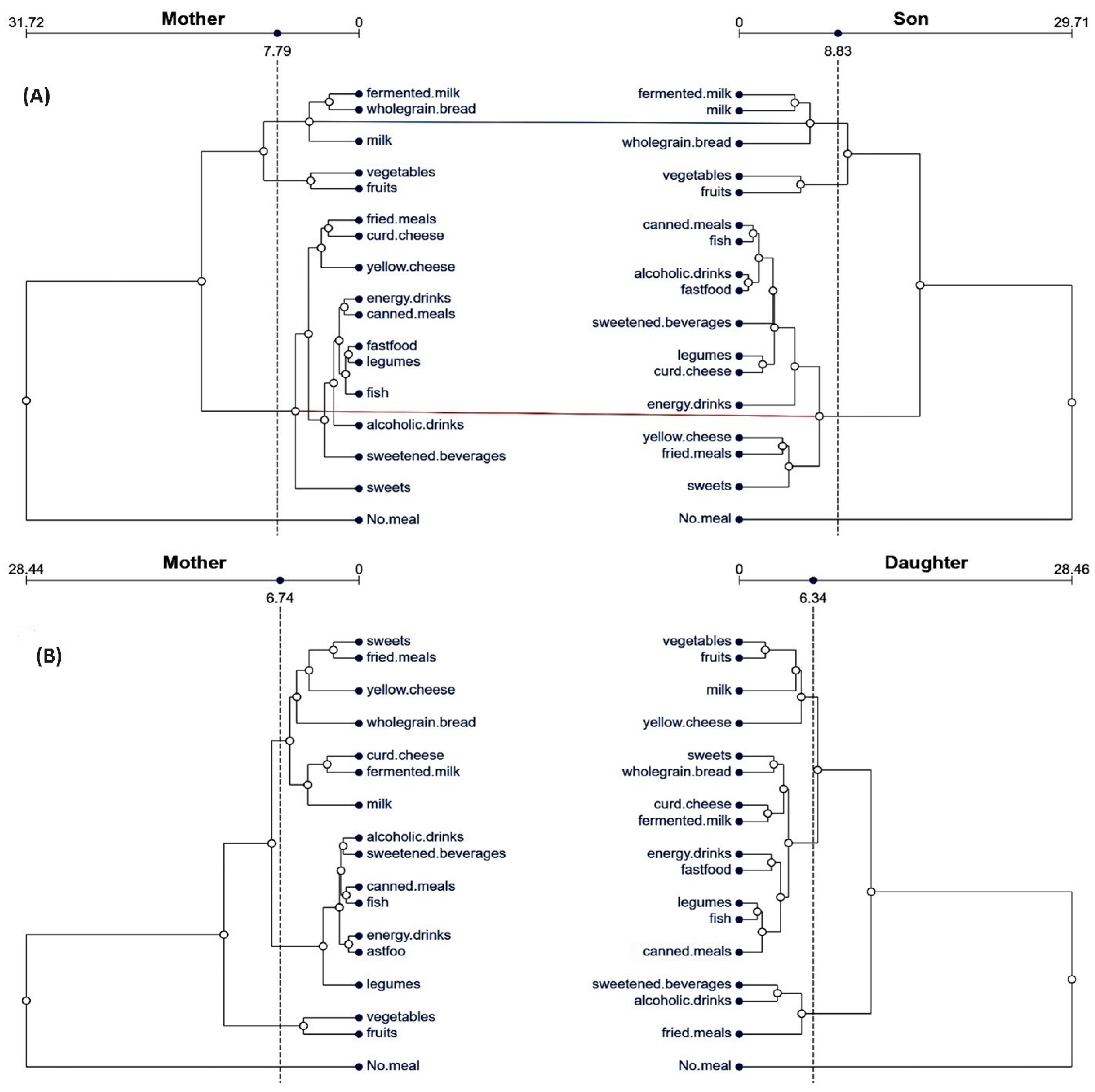
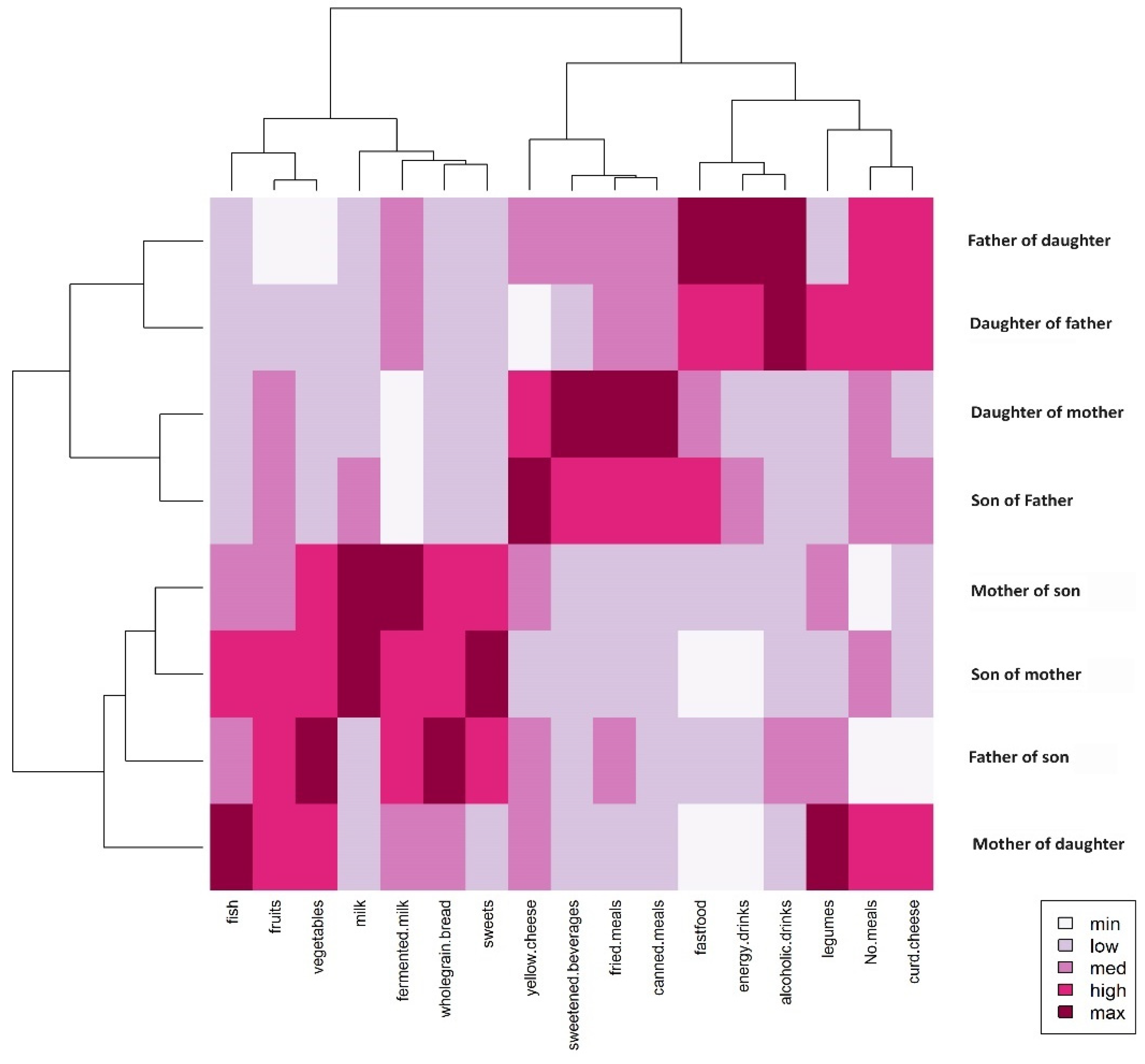
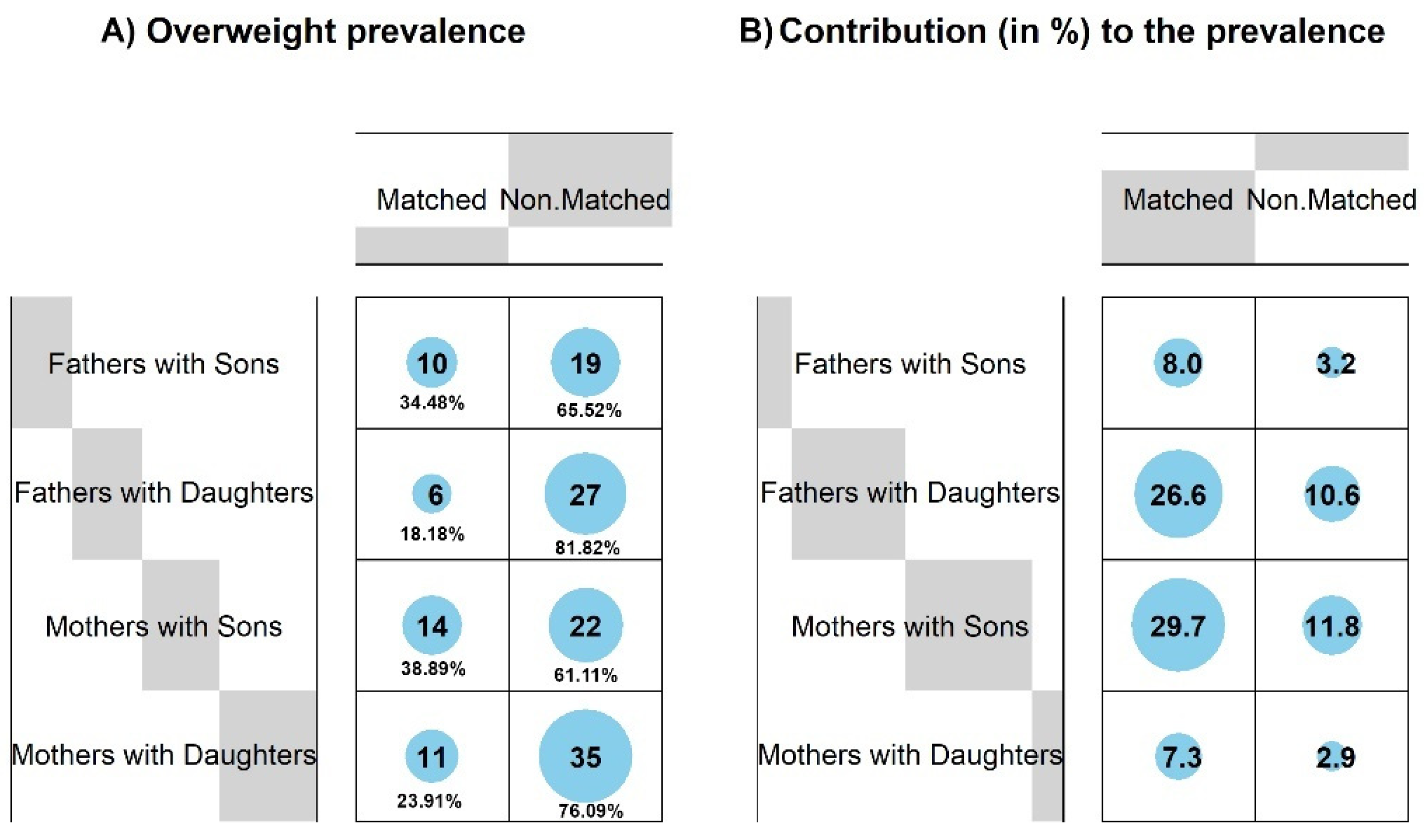

| Sons of Fathers | Daughters of Fathers | |||||||
| M | SD | −95% CI | +95% CI | M | SD | −95% CI | +95% CI | |
| Height [cm] | 183.3 | 6.4 | 180.8 | 185.7 | 167.8 | 5.5 | 165.8 | 169.7 |
| Weight [kg] | 78.7 | 8.2 | 75.6 | 81.8 | 61.4 | 9.0 | 58.2 | 64.6 |
| BMI [kg/m2] | 23.4 | 2.6 | 22.4 | 24.3 | 21.8 | 3.1 | 20.7 | 22.9 |
| Positive HDI | 6.1 | 1.8 | 5.5 | 6.8 | 7.3 | 2.0 | 6.6 | 8.0 |
| Negative HDI | 6.9 | 2.1 | 6.1 | 7.6 | 4.5 | 1.0 | 4.1 | 4.8 |
| IPAQ | 159.7 | 48.7 | 141.2 | 178.2 | 143.0 | 48.6 | 125.7 | 160.2 |
| Sitting time | 86.3 | 17.2 | 79.8 | 92.9 | 88.0 | 15.0 | 82.7 | 93.3 |
| Sons of Mothers | Daughters of Mothers | |||||||
| Height [cm] | 182.8 | 6.7 | 180.5 | 185.1 | 167.9 | 5.3 | 166.3 | 169.5 |
| Weight [kg] | 80.4 | 10.3 | 77.0 | 83.9 | 61.4 | 9.7 | 58.6 | 64.3 |
| BMI [kg/m2] | 24.0 | 2.6 | 23.1 | 24.9 | 21.8 | 2.9 | 20.9 | 22.6 |
| Positive HDI | 6.0 | 1.7 | 5.4 | 6.6 | 8.8 | 1.3 | 8.4 | 9.2 |
| Negative HDI | 7.7 | 1.4 | 7.2 | 8.2 | 4.8 | 1.6 | 4.3 | 5.3 |
| IPAQ | 172.3 | 50.0 | 155.3 | 189.2 | 132.8 | 30.0 | 123.9 | 141.7 |
| Sitting time | 88.4 | 20.4 | 81.5 | 95.3 | 88.2 | 18.2 | 82.8 | 93.6 |
| Significance F, p | Height: F = 76.40, p < 0.001; Weight: F = 44.96, p < 0.001; BMI: F = 5.81, p < 0.001; Positive HDI: F = 24.46, p < 0.001, Negative HID: F = 36.15, p < 0.001; IPAQ; F = 6.2, p < 0.001 | |||||||
| Fathers of Sons | Fathers of Daughters | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | −95% CI | +95% CI | M | SD | −95% CI | +95% CI | |
| Height [cm] | 180.0 | 8.2 | 176.9 | 183.2 | 177.8 | 5.6 | 175.8 | 179.8 |
| Weight [kg] | 85.6 | 12.1 | 81.0 | 90.2 | 79.5 | 11.5 | 75.5 | 83.6 |
| BMI [kg/m2] | 26.4 | 3.4 | 25.1 | 27.7 | 25.1 | 2.9 | 24.1 | 26.1 |
| Positive HDI | 5.1 | 1.9 | 4.4 | 5.8 | 4.9 | 1.7 | 4.3 | 5.5 |
| Negative HDI | 6.9 | 2.0 | 6.1 | 7.7 | 7.5 | 2.2 | 6.7 | 8.3 |
| IPAQ | 160.4 | 46.6 | 142.7 | 178.2 | 162.6 | 53.1 | 143.8 | 181.4 |
| Sitting time | 89.1 | 15.2 | 83.4 | 94.9 | 92.2 | 17.2 | 86.1 | 98.3 |
| Mothers of Sons | Mothers of Daughters | |||||||
| Height [cm] | 169.0 | 5.0 | 167.3 | 170.7 | 167.5 | 5.5 | 165.8 | 169.1 |
| Weight [kg] | 70.7 | 11.7 | 66.7 | 74.7 | 70.2 | 12.0 | 66.6 | 73.8 |
| BMI [kg/m2] | 24.7 | 3.8 | 23.5 | 26.0 | 25.0 | 4.1 | 23.8 | 26.3 |
| Positive HDI | 8.3 | 1.7 | 7.7 | 8.9 | 8.4 | 2.0 | 7.8 | 9.0 |
| Negative HDI | 4.1 | 1.9 | 3.5 | 4.8 | 4.4 | 1.4 | 4.0 | 4.8 |
| IPAQ | 139.4 | 35.5 | 127.4 | 151.4 | 144.7 | 33.6 | 134.7 | 154.7 |
| Sitting time | 88.3 | 19.9 | 81.6 | 95.1 | 86.4 | 15.1 | 81.9 | 90.9 |
| Significance F, p | Height: F = 37.50, p < 0.001; Weight: F = 13.29, p < 0.001 Positive HDI: F = 39.45, p < 0.001, Negative HID: F = 29.29, p < 0.001 | |||||||
| Independent | b | 95%CI | p-Value |
|---|---|---|---|
| Intrinsic (children’s) | |||
| Sex | 2.31 | 1.34–3.28 | <0.01 |
| ferm.curd | −0.55 | −1.01–−0.07 | 0.024 |
| IPAQ | −0.02 | −0.03–−0.01 | <0.01 |
| Sitting (inactivity) | 0.02 | −0.00–0.05 | 0.082 |
| Accuracy: R2 = 0.18, RMSE = 2.85, MAE = 2.25, AIC = 279.84 | |||
| Extrinsic (parental) | |||
| vege.fruits | −0.29 | −0.66–0.068 | 0.111 |
| fast.fried | −0.44 | −0.88–0.00 | 0.049 |
| IPAQ | −0.01 | −0.02–0.00 | 0.112 |
| Accuracy: R2 = 0.10, RMSE = 2.90, MAE = 2.26, AIC = 311.97 | |||
| Variable | b | OR | 95%CI | p-Value |
|---|---|---|---|---|
| Males | ||||
| DI | −1.25 | 0.29 | 0.11–0.72 | 0.008 |
| PA | −0.04 | 0.96 | 0.94–0.98 | <0.001 |
| Sitting time | 0.03 | 1.03 | 1.00–1.08 | 0.076 |
| Females | ||||
| DI | −0.61 | 0.55 | 0.21–1.40 | 0.209 |
| PA | −0.04 | 0.96 | 0.94–0.99 | 0.005 |
| Sitting time | 0.01 | 1.00 | 0.95–1.05 | 0.948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaradzki, J.; Słowińska-Lisowska, M.R. Sex-Moderated Divergence between Adult Child and Parental Dietary Behavior Patterns in Relation to Body Mass Condition—Evaluating the Mediating Role of Physical Activity: A Cross-Sectional Study. Nutrients 2024, 16, 2256. https://doi.org/10.3390/nu16142256
Domaradzki J, Słowińska-Lisowska MR. Sex-Moderated Divergence between Adult Child and Parental Dietary Behavior Patterns in Relation to Body Mass Condition—Evaluating the Mediating Role of Physical Activity: A Cross-Sectional Study. Nutrients. 2024; 16(14):2256. https://doi.org/10.3390/nu16142256
Chicago/Turabian StyleDomaradzki, Jarosław, and Małgorzata Renata Słowińska-Lisowska. 2024. "Sex-Moderated Divergence between Adult Child and Parental Dietary Behavior Patterns in Relation to Body Mass Condition—Evaluating the Mediating Role of Physical Activity: A Cross-Sectional Study" Nutrients 16, no. 14: 2256. https://doi.org/10.3390/nu16142256
APA StyleDomaradzki, J., & Słowińska-Lisowska, M. R. (2024). Sex-Moderated Divergence between Adult Child and Parental Dietary Behavior Patterns in Relation to Body Mass Condition—Evaluating the Mediating Role of Physical Activity: A Cross-Sectional Study. Nutrients, 16(14), 2256. https://doi.org/10.3390/nu16142256






