Protective Effects of Velvet Antler Methanol Extracts on Hypoxia-Induced Damage in Caenorhabditis elegans through HIF-1 and ECH-8 Mediated Lipid Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Antler Velvet Extracts
2.2. Hypoxic and Hypoxia–Reoxygenation (HR) Incubation
2.3. Survival and Lifespan Analysis
2.4. Locomotion and Feeding Behavior Analysis
2.5. Mitochondrial Morphology
2.6. ATP Measurement
2.7. RNA Isolation and Quantitative PCR (qPCR)
2.8. Oil Red O-Based Lipid Staining
2.9. RNA Interference (RNAi) Assay
2.10. Statistical Analysis
3. Results
3.1. MEs Attenuate Damage Induced by Hypoxia in C. elegans
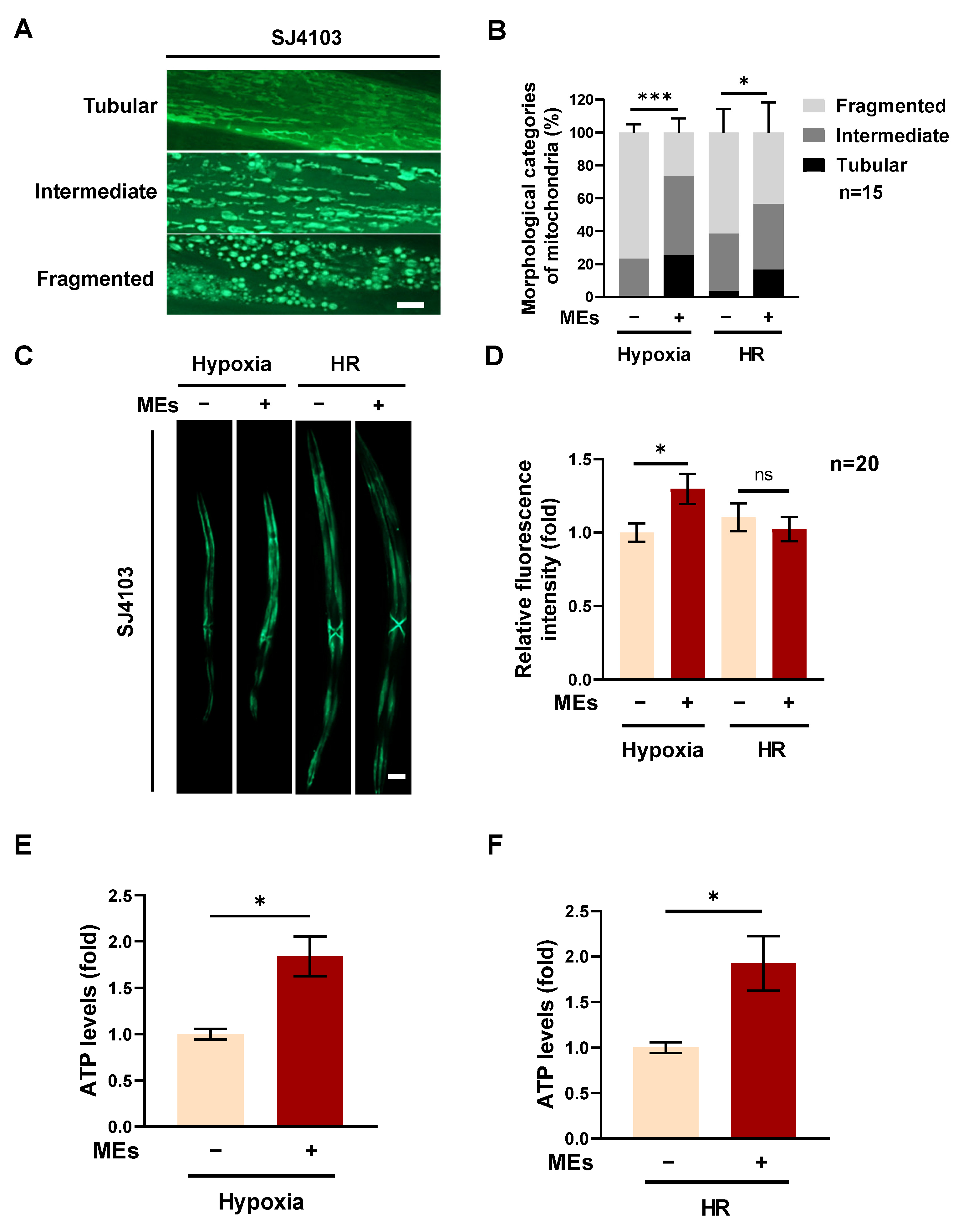
3.2. MEs Improve the Function of Mitochondria in Hypoxia-Treated C. elegans
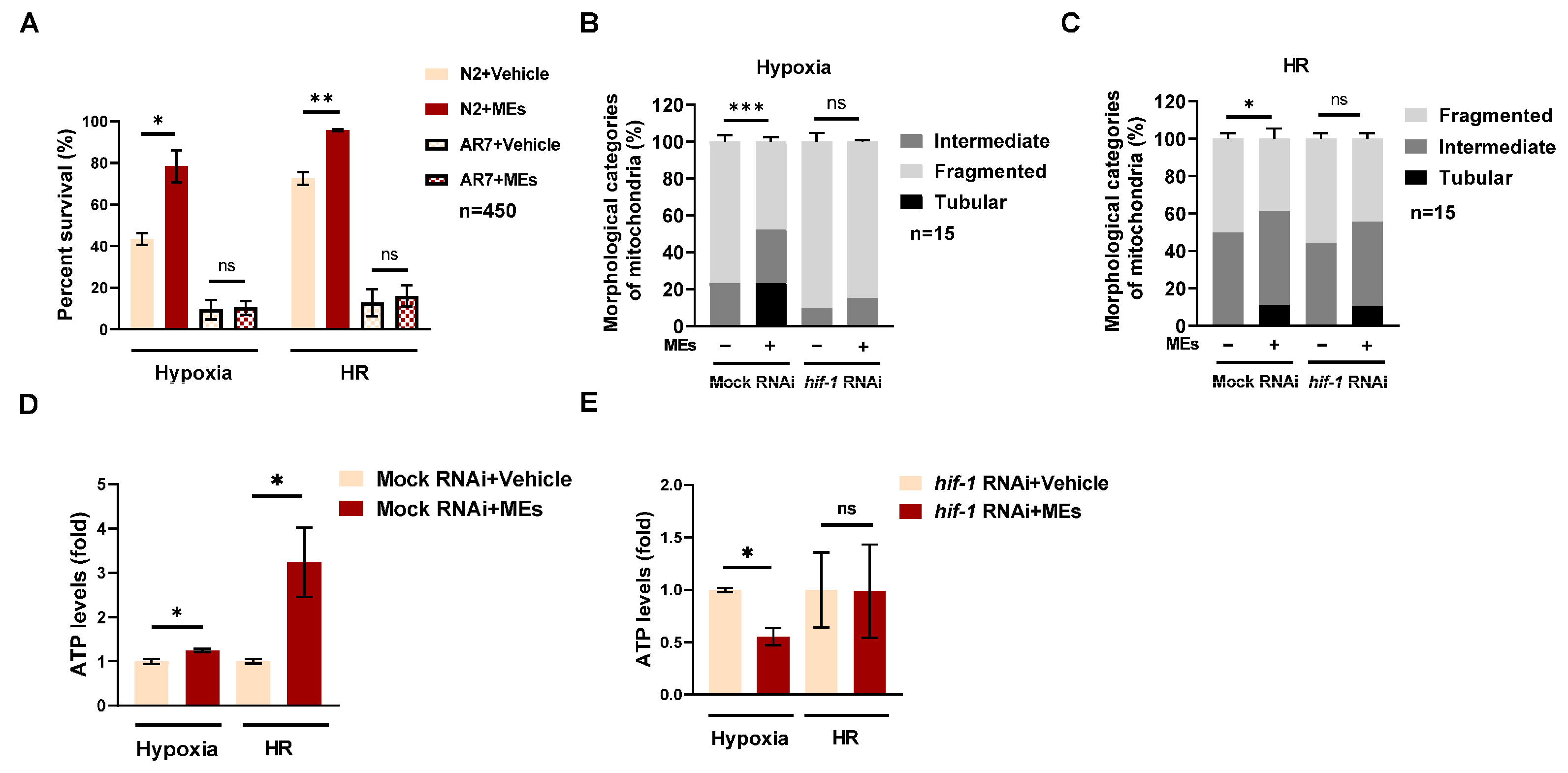
3.3. MEs Protect against Hypoxia Damage by HIF-1 in C. elegans
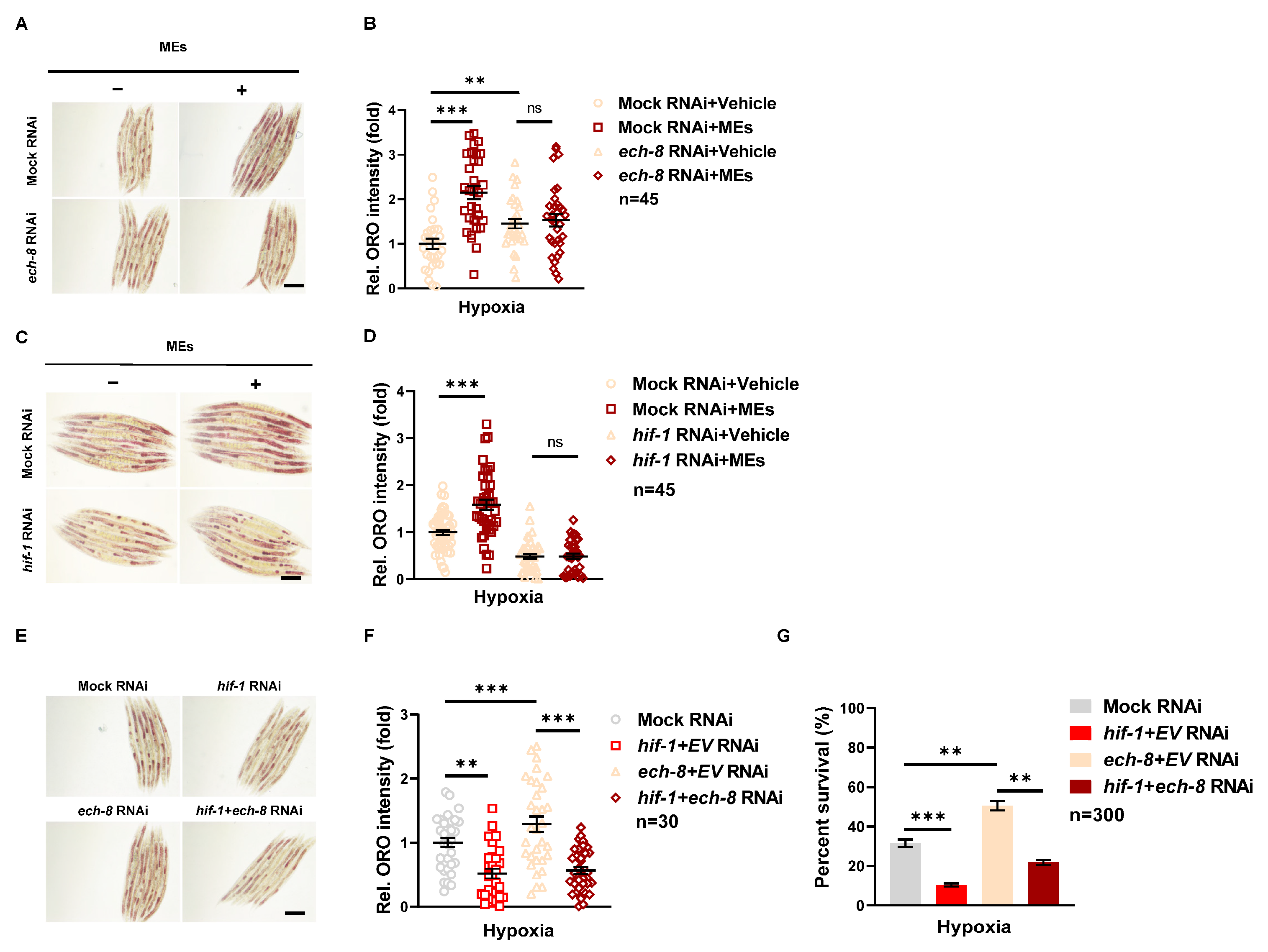
3.4. MEs Protect against Hypoxia-Induced Damage via Promoting Lipid Storage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Barneo, J.; Simon, M.C. Cellular adaptation to oxygen deficiency beyond the Nobel award. Nat. Commun. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Lodge, K.M.; Vassallo, A.; Liu, B.; Long, M.; Tong, Z.; Newby, P.R.; Agha-Jaffar, D.; Paschalaki, K.; Green, C.E.; Belchamber, K.B.R.; et al. Hypoxia Increases the Potential for Neutrophil-mediated Endothelial Damage in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 205, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Silagi, E.S.; Schipani, E.; Shapiro, I.M.; Risbud, M.V. The role of HIF proteins in maintaining the metabolic health of the intervertebral disc. Nat. Rev. Rheumatol. 2021, 17, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Yun, J.-E.; Kim, S.J.; Chun, Y.-S. Lipid metabolic reprogramming by hypoxia-inducible factor-1 in the hypoxic tumour microenvironment. Pflügers Arch. Eur. J. Physiol. 2022, 474, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Manuelli, V.; Pecorari, C.; Filomeni, G.; Zito, E. Regulation of redox signaling in HIF-1-dependent tumor angiogenesis. FEBS J. 2022, 289, 5413–5425. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Song, Y.; Xie, G.; Jiao, S.; Shi, L.; Cao, X.; Han, X.; Qu, A. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol. Ther. 2022, 238, 108186. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Wu, W.; Wang, X.; Fang, L.; Adam, V.; Nepovimova, E.; Wu, Q.; Kuca, K. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med. Res. Rev. 2021, 41, 1622–1643. [Google Scholar] [CrossRef] [PubMed]
- March-Diaz, R.; Lara-Ureña, N.; Romero-Molina, C.; Heras-Garvin, A.; Luis, C.O.-D.S.; Alvarez-Vergara, M.I.; Sanchez-Garcia, M.A.; Sanchez-Mejias, E.; Davila, J.C.; Rosales-Nieves, A.E.; et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat. Aging 2021, 1, 385–399. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Geng, L.; Sun, L.; Wang, Q.; Yu, Y.; Yan, P.; Liang, C.; Ren, J.; Song, M.; et al. Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor. Cell Discov. 2022, 8, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, Y.; Yang, M.; Wang, X.; Peng, Y. Velvet Antler Methanol Extracts Ameliorate Parkinson’s Disease by Inhibiting Oxidative Stress and Neuroinflammation: From C. elegans to Mice. Oxidative Med. Cell. Longev. 2021, 2021, 8864395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Liu, Y.; Wu, H.; Wang, H.; Jin, S.; Lu, Y.; Chang, S.; Liu, R.; Peng, Y.; et al. Velvet antler methanol extracts (MEs) protects against oxidative stress in Caenorhabditis elegans by SKN-1. Biomed. Pharmacother. 2020, 121, 109668. [Google Scholar] [CrossRef] [PubMed]
- Vora, M.; Pyonteck, S.M.; Popovitchenko, T.; Matlack, T.L.; Prashar, A.; Kane, N.S.; Favate, J.; Shah, P.; Rongo, C. The hypoxia response pathway promotes PEP carboxykinase and gluconeogenesis in C. elegans. Nat. Commun. 2022, 13, 6168. [Google Scholar] [CrossRef] [PubMed]
- Salceda, S.; Caro, J. Hypoxia-inducible Factor 1α (HIF-1α) Protein Is Rapidly Degraded by the Ubiquitin-Proteasome System under Normoxic Conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 1997, 272, 22642–22647. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Kakeya, H. Targeting hypoxia-inducible factor 1 (HIF-1) signaling with natural products toward cancer chemotherapy. J. Antibiot. 2021, 74, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Vianey-Saban, C.; Fouilhoux, A.; Vockley, J.; Acquaviva-Bourdain, C.; Guffon, N. Improving diagnosis of mitochondrial fatty-acid oxidation disorders. Eur. J. Hum. Genet. 2023, 31, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Spanier, B.; Lasch, K.; Marsch, S.; Benner, J.; Liao, W.; Hu, H.; Kienberger, H.; Eisenreich, W.; Daniel, H. How the Intestinal Peptide Transporter PEPT-1 Contributes to an Obesity Phenotype in Caenorhabditits elegans. PLoS ONE 2009, 4, e6279. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Chang, C.H.; Lin, W.S.; Nagabhushanam, K.; Ho, C.; Pan, M. S-Allylcysteine Ameliorates Aging Features via Regulating Mitochondrial Dynamics in Naturally Aged C57BL/6J Mice. Mol. Nutr. Food Res. 2022, 66, 2101077. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, W.; Yuan, J.; Bullova, P.; Wu, J.; Zhang, X.; Liu, Y.; Plescher, M.; Rodriguez, J.; Bedoya-Reina, O.C.; et al. Impaired oxygen-sensitive regulation of mitochondrial biogenesis within the von Hippel–Lindau syndrome. Nat. Metab. 2022, 4, 739–758. [Google Scholar] [CrossRef]
- Qin, X.; Li, H.; Zhao, H.; Fang, L.; Wang, X. Enhancing healthy aging with small molecules: A mitochondrial perspective. Med. Res. Rev. 2024, 44, 1904–1922. [Google Scholar] [CrossRef]
- Pocock, R.; Hobert, O. Oxygen levels affect axon guidance and neuronal migration in Caenorhabditis elegans. Nat. Neurosci. 2008, 11, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Doering, K.R.S.; Cheng, X.; Milburn, L.; Ratnappan, R.; Ghazi, A.; Miller, D.L.; Taubert, S. Nuclear hormone receptor NHR-49 acts in parallel with HIF-1 to promote hypoxia adaptation in Caenorhabditis elegans. eLife 2022, 11, e67911. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Powell-Coffman, J.A. Genetic Analysis of Hypoxia Signaling and Response in C. elegans. Ann. N. Y. Acad. Sci. 2003, 995, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, T.; Sang, H. The role of hypoxia-inducible factor 1α in hepatic lipid metabolism. J. Mol. Med. 2023, 101, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.A.d.l.R.; Deng, L.; Gemmink, A.; van Weeghel, M.; Aoun, M.L.; Warnecke, C.; Singh, R.; Borst, J.W.; Kersten, S. Hypoxia-inducible lipid droplet-associated induces DGAT1 and promotes lipid storage in hepatocytes. Mol. Metab. 2021, 47, 101168. [Google Scholar] [CrossRef]
- Kishore, R.; Arnaboldi, V.; Van Slyke, C.E.; Chan, J.; Nash, R.S.; Urbano, J.M.; Dolan, M.E.; Engel, S.R.; Shimoyama, M.; Sternberg, P.W.; et al. Automated generation of gene summaries at the Alliance of Genome Resources. Database 2020, 2020, baaa037. [Google Scholar] [CrossRef]
- Gao, C.; Li, Q.; Yu, J.; Li, S.; Cui, Q.; Hu, X.; Chen, L.; Zhang, S.O. Endocrine pheromones couple fat rationing to dauer diapause through HNF4α nuclear receptors. Sci. China Life Sci. 2021, 64, 2153–2174. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhang, J.; Xu, X.; Fu, L.; Xu, J.; Zhu, H.; Hu, Y.; Li, C.; Wang, M.; et al. Polyunsaturated fatty acids promote the rapid fusion of lipid droplets in Caenorhabditis elegans. J. Biol. Chem. 2022, 298, 102179. [Google Scholar] [CrossRef]
- Pei, H.; Du, R.; He, Z.; Yang, Y.; Wu, S.; Li, W.; Sheng, J.; Lv, Y.; Han, C. Protection of a novel velvet antler polypeptide PNP1 against cerebral ischemia-reperfusion injury. Int. J. Biol. Macromol. 2023, 247, 125815. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Yang, H.-T.; Chiang, C.-C.; Lai, K.-H.; Chen, Y.-L.; Shih, H.-L.; Kuo, J.-J.; Hwang, T.-L.; Lin, C.-C. Deer Velvet Antler Extracts Exert Anti-Inflammatory and Anti-Arthritic Effects on Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes and Distinct Mouse Arthritis. Am. J. Chin. Med. 2022, 50, 1617–1643. [Google Scholar] [CrossRef]
- Baumeister, R.; Murphy, C.T.; Heimbucher, T. Metabolic adaptation to hypoxia: Do worms and cancer cells share common metabolic responses to hypoxic stress? Cell Death Differ 2021, 28, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Snoek, L.B.; De Bono, M.; Kammenga, J.E. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends Genet. 2013, 29, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.-L.; et al. Fatty Acid Uptake and Lipid Storage Induced by HIF-1α Contribute to Cell Growth and Survival after Hypoxia-Reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Lee, J.H.; Kong, J.; Ji, Y.; Kim, J.; Choe, S.S.; Kim, J.B. Hypoxia Restrains Lipid Utilization via Protein Kinase A and Adipose Triglyceride Lipase Downregulation through Hypoxia-Inducible Factor. Mol. Cell. Biol. 2019, 39, e00390-18. [Google Scholar] [CrossRef] [PubMed]
- Heimbucher, T.; Hog, J.; Gupta, P.; Murphy, C.T. PQM-1 controls hypoxic survival via regulation of lipid metabolism. Nat. Commun. 2020, 11, 4627. [Google Scholar] [CrossRef]
- Danielli, M.; Perne, L.; Jovičić, E.J.; Petan, T. Lipid droplets and polyunsaturated fatty acid trafficking: Balancing life and death. Front. Cell Dev. Biol. 2023, 11, 1104725. [Google Scholar] [CrossRef]

| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| cdc-42 | ctgctggacaggaagattacg | ctcggacattctcgaatgaag |
| y45f10d.4 | gtcgcttcaaatcagttcagc | gttcttgtcaagtgatccgaca |
| hif-1 | ttaacagtcccccgagttgc | gcttccgatgactgggttga |
| ech-8 | ttgaacgatcaggatgccgt | ctcatcggaccaccagtagc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, H.; Wang, X.; Peng, Y. Protective Effects of Velvet Antler Methanol Extracts on Hypoxia-Induced Damage in Caenorhabditis elegans through HIF-1 and ECH-8 Mediated Lipid Accumulation. Nutrients 2024, 16, 2257. https://doi.org/10.3390/nu16142257
Li R, Li H, Wang X, Peng Y. Protective Effects of Velvet Antler Methanol Extracts on Hypoxia-Induced Damage in Caenorhabditis elegans through HIF-1 and ECH-8 Mediated Lipid Accumulation. Nutrients. 2024; 16(14):2257. https://doi.org/10.3390/nu16142257
Chicago/Turabian StyleLi, Ru, Hongyuan Li, Xiaohui Wang, and Yinghua Peng. 2024. "Protective Effects of Velvet Antler Methanol Extracts on Hypoxia-Induced Damage in Caenorhabditis elegans through HIF-1 and ECH-8 Mediated Lipid Accumulation" Nutrients 16, no. 14: 2257. https://doi.org/10.3390/nu16142257
APA StyleLi, R., Li, H., Wang, X., & Peng, Y. (2024). Protective Effects of Velvet Antler Methanol Extracts on Hypoxia-Induced Damage in Caenorhabditis elegans through HIF-1 and ECH-8 Mediated Lipid Accumulation. Nutrients, 16(14), 2257. https://doi.org/10.3390/nu16142257






