Abstract
The outcome of total hip arthroplasty (THA) in patients with end-stage arthritis of the hip is associated with preoperative physical status. This study was performed to examine the relationship between the preoperative severity of sarcopenia and clinical outcomes after THA. This retrospective cohort study was performed among 306 consecutive patients (mean age: 63.7 ± 12.9 years, 222 women) undergoing THA at a university hospital. The severity of sarcopenia was determined based on the skeletal muscle mass index (SMI), handgrip strength, and gait speed according to the criteria of the Asian Working Group for Sarcopenia 2019. The severe sarcopenia prevalence rate was 10.6%. Severe sarcopenia was significantly associated with the risk of delayed functional recovery (adjusted odds ratio, 2.82; 95% confidence interval, 1.03–7.72; p = 0.043) compared with the non-sarcopenia group after adjusting for pre-existing risk factors, including preoperative hip function and physical activity. The addition of SMI, handgrip strength, and gait speed to the model for risk of functional recovery delay significantly increased the area under the receiver operating characteristic curve (p = 0.038). Severe sarcopenia was significantly associated with poorer hip function and patient-reported outcomes at 6 months after surgery compared with the non-sarcopenia group. Severe sarcopenia was adversely associated with postoperative clinical outcomes in patients undergoing THA.
1. Introduction
Total hip arthroplasty (THA) is commonly performed for the treatment of end-stage osteoarthritis of the hip [1], a leading cause of disability affecting an estimated 40 million people worldwide [2,3]. As persistent pain and functional decline are major indicators of THA, patients frequently have limited physical function and mobility prior to surgery. THA has been shown to provide pain relief, mobility enhancement, and restoration of function. It has been reported that the postoperative clinical outcomes of THA, including patient satisfaction, can be predicted by preoperative physical status [4]. Studies of the risk factors associated with the adverse outcomes of THA are required to identify possible interventions that may lead to improvements in patient satisfaction and functional recovery.
Sarcopenia is a progressive generalized skeletal muscle disorder associated with accelerated loss of muscle mass and strength, the incidence of which increases with advanced age but can also occur in younger patients in the context of chronic disease [5]. Sarcopenia is associated with adverse outcomes, including falls, frailty, and disability, with poor prognosis and reduced quality of life (QoL) [5]. In addition, preoperative sarcopenia was reported to be associated with increased postoperative complications and mortality as well as greater medical costs in patients undergoing THA [6,7]. Although sarcopenia has mostly been defined based on reduced muscle mass and/or strength, the current guidelines include an assessment of physical performance for determination of its severity [5,8]. Severe sarcopenia with markedly reduced muscle mass, strength, and physical performance is associated with poor cognitive function, greater risk of falls, and increased mortality risk in community-dwelling adults [9,10,11]. However, there have been no previous reports regarding the association between severe sarcopenia and post-THA outcomes.
We postulated that patients with severe sarcopenia would have poorer clinical out-comes than those without sarcopenia and examined the association between preoperative sarcopenia severity and clinical outcomes following THA.
2. Materials and Methods
2.1. Study Population
This retrospective cohort study was performed among all consecutive patients receiving rehabilitation following THA at Nagoya University Hospital between June 2016 and June 2020. Patients without a preoperative assessment of sarcopenic status were excluded. A standard rehabilitation program consisting of progressively improving walking ability, other functional activities, and walking stairs was begun on postoperative day (POD) 1. Patients also participated in a progressive program involving exercises to improve range of motion (ROM), muscle strength, and function. This study was approved by the Institutional Review Board of Nagoya University Hospital and performed in accordance with the tenets of the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Information regarding this study, including the objectives and inclusion and exclusion criteria, was published on a website, and the participants were free to opt out of this study at any time.
2.2. Data Collection
Details regarding clinical presentation and demographic and biochemical information for all patients were obtained from their electronic medical records. Hip function was classified preoperatively and at a 6-month follow-up based on the Japanese Orthopaedic Association (JOA) hip score, which allocates 40 points for pain, 20 points for ROM, 20 points for walking ability, and 20 points for activities of daily living (ADL) with a maximum total score of 100 points, where 100 indicates best performance [12,13]. The University of California Los Angeles (UCLA) activity score, with scores ranging from 1 (very low) to 10 (very high), was used to assess preoperative physical activity [14,15]. Orthopedic surgeons performed postoperative follow-ups by routine physical examination, pelvic radiography, and laboratory blood analyses.
2.3. Evaluation of Sarcopenia
Appendicular skeletal muscle mass, handgrip strength, and gait speed were measured by trained physiotherapists to determine the severity of sarcopenia. Sarcopenia was defined as low muscle mass and strength or low physical performance, while severe sarcopenia was characterized by the presence of all three conditions in accordance with the Asian Working Group for Sarcopenia 2019 (AWGS2019) [8].
The skeletal muscle mass index (SMI) was calculated by dividing the appendicular skeletal muscle mass measured by bioelectrical impedance analysis (InBody 720; InBody Inc., Tokyo, Japan) by the square of height (m2). Low appendicular skeletal muscle mass was defined as SMI < 7.0 kg/m2 for men and <5.7 kg/m2 for women [8]. Muscle strength was evaluated by measuring handgrip strength using a digital dynamometer (TKK 5101 Grip-D; Takei, Tokyo, Japan) with the patient in the standing position. Each patient performed two maximal isometric voluntary contractions of both hands for 3 s each, and the greatest strength expressed as an absolute value (in kg) was used in the analyses. Low muscle strength was defined as handgrip strength <28 kg for men and <18 kg for women [8]. Physical performance was evaluated based on the usual gait speed measured over the middle 10 m of a 16 m walkway with the use of any required assistive devices. Low physical performance was defined as gait speed <1.0 m/s [8].
2.4. Computed Tomography, Physical Function, and Mental Health Evaluations
Thigh muscle area, subcutaneous fat area, and circumference were determined semi-automatically (SYNAPSE VINCENT™; Fujifilm Medical Co., Ltd., Tokyo, Japan) on preoperative pelvic computed tomography (CT) scans obtained within 1 month before surgery at the mid-thigh level between the anterior superior iliac crest and the patella [16]. Muscle and fat areas were quantified based on thresholds of −29 to +150 and −190 to −30 Hounsfield units, respectively. Image analysis was performed in a blinded manner with regard to clinical information to minimize bias in measurements and calculations.
Calf circumference was measured to the nearest 1 mm at the point of the greatest circumference using a plastic tape measure with the patient in the supine position. Leg strength was evaluated by measuring maximal hip abductor strength and knee extensor strength using a handheld dynamometer (m-Tas; ANIMA, Tokyo, Japan). Hip abductor strength was assessed with the patient in the supine position with neutral hip adduction/abduction. Knee extensor strength was measured with the patient in the sitting position with the knee joint fixed at 60°. Maximal isometric voluntary contractions for 5 s each time were determined twice successively for both legs, and the length (m) of the lever arm was measured from the estimated joint center of rotation to the center of the dynamometer. The greatest values of strength on the right and left sides were used for the analyses, and the dynamometer variable (kgf) and lever arm length (m) were multiplied to obtain torque (kgf*m). ADL was determined based on the Barthel Index, routinely recorded in nursing and rehabilitation summaries, with scores ranging from 0 to 100, where 100 indicates independent in basic ADL.
Anxiety and depression were measured using the 14-item Hospital Anxiety and Depression Scale (HADS) [17], which has seven items for each of anxiety and depression scored from 0 to 3 with subscale scores ranging from 0 to 21, where higher scores indicate greater anxiety or depression. Patient-reported outcomes were evaluated preoperatively and at a 6-month follow-up after surgery using the Japanese Orthopaedic Association Hip-Disease Evaluation Questionnaire (JHEQ) [18], a validated self-administered questionnaire to determine the QoL of Asian patients with hip disease consisting of items on pain (0–28 points), function (0–28 points), and mental (0–28 points), with a total score ranging from 0 (worst) to 84 (best) [19]. Patient satisfaction with a hip condition was evaluated using a visual analog scale (VAS) ranging from 0 mm (greatest satisfaction) to 100 mm (greatest dissatisfaction).
2.5. Clinical Outcomes
Functional recovery, defined as the ability to walk over a distance of 100 m regardless of the use of a walking aid, was evaluated daily, and the primary end point of this study was functional recovery on POD7. The secondary end points of this study were the Barthel Index at hospital discharge, the incidence of hospital-acquired disability, length of hospital stay, non-home discharge, and the occurrence of adverse events within 6 months after surgery. A decrease in the Barthel Index of at least 5 points on the day before hospital discharge compared with admission was taken to indicate hospital-acquired disability [20]. Adverse events included wound complications, infection, dislocation, loosening, periprosthetic fracture, reoperation, and all-cause unplanned readmission and mortality.
2.6. Statistical Analysis
Continuous variables with a normal distribution are shown as the mean ± standard deviation, while those with a non-normal distribution are shown as the median and interquartile range. Categorical variables are expressed as numbers and percentages. Differences between the three groups classified by sarcopenia status (i.e., the non-sarcopenia group, sarcopenia group, and severe sarcopenia group) were evaluated by one-way analysis of variance or the Kruskal–Wallis test for continuous variables and the chi-squared or Fisher’s exact test for dichotomous variables, as appropriate. Differences between the severe sarcopenia and no sarcopenia assessment groups were evaluated by the unpaired Student’s t test, Mann–Whitney U test, chi-squared test, or Fisher’s exact test as appropriate.
Functional recovery delay on POD7 and non-home discharge risks were examined by logistic regression analysis of sarcopenia status with adjustment for age, sex, body mass index (BMI), JOA hip score, living alone, and UCLA activity score as potential confounders based on their clinical importance and the results of previous studies. Adjusted odds ratios (ORs) are reported with the corresponding 95% confidence intervals (CIs). Receiver operating characteristic (ROC) curve analysis of the study end points, i.e., functional recovery delay on POD7 and non-home discharge, was performed to compare the incremental prognostic values of adding assessments of sarcopenia to the baseline model incorporating risk factors, including all variables used for adjustment (i.e., age, sex, BMI, JOA hip score, living alone, and UCLA activity score), and the areas under the curves (AUCs) were compared according to the method of DeLong et al. [21]. Decision curve analysis was performed to quantify the net benefits at different threshold probabilities to assess the clinical usefulness of the model [22].
In all analyses, two-tailed p < 0.05 was taken to indicate statistical significance, and the Bonferroni correction was applied for multiple comparisons. Statistical analyses were performed using R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
After excluding 32 of the 338 THA patients treated during the study period because of a lack of preoperative assessment, a total of 306 patients including 52 for whom sarcopenia assessment could not be completed because of severe physical function decline or pain (no sarcopenia assessment group; n = 52) were included in this study. The study population consisted of 222 women (72.5%) and 84 men (27.5%) with a mean age of 63.7 ± 12.9 years. Of the study population, 78.1% had osteoarthritis and 17.3% were undergoing revision surgeries.
The patients for whom preoperative sarcopenia assessments were available (n = 254) were divided into the non-sarcopenia group (n = 195, 76.8%), sarcopenia group (n = 32, 12.6%), and severe sarcopenia group (n = 27, 10.6%). These patients had a mean SMI of 7.4 ± 1.0 cm2/m2 for men and 6.1 ± 1.0 cm2/m2 for women, a mean handgrip strength of 35.2 ± 8.4 kg for men and 22.2 ± 5.7 kg for women, and a mean usual gait speed of 0.83 ± 0.38 m/s. Low muscle mass, low muscle strength, and low physical performance had prevalence rates of 32%, 21%, and 63%, respectively, with the highest percentages of low physical performance across various subgroups except in patients with BMI < 18.5 kg/m2 (Figure A1). Euler diagrams demonstrated a significant overlap in the assessments of sarcopenia (Figure 1).
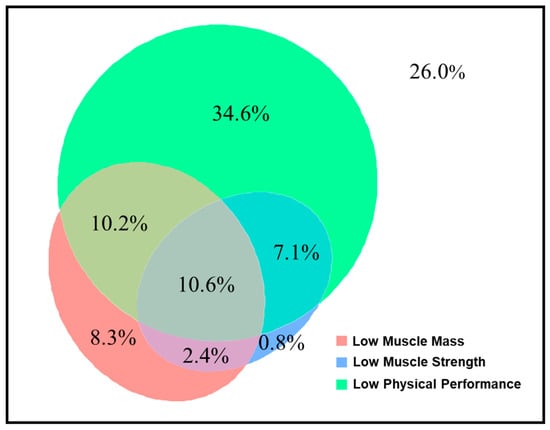
Figure 1.
Proportions of overlap and non-overlap among sarcopenia domains.
Table 1 presents the baseline characteristics of the total study population and each subgroup classified by preoperative sarcopenia status. Compared with the non-sarcopenia group, the severe sarcopenia group had significantly greater age, lower BMI, lower prevalence of living alone, lower UCLA activity score, and lower hemoglobin levels. There were no significant differences in sex, histological type, preoperative hip function, pain, comorbidities, smoking status, or surgery-related factors between the groups. The JOA hip score, UCLA activity score, and albumin level were significantly lower in the no sarcopenia assessment group than in the severe sarcopenia group (Table A1). Mid-thigh muscle area, limb circumferences, muscle strength, peak expiratory flow, and JHEQ function score were significantly lower in the severe sarcopenia group than in the non-sarcopenia group (Table 2). HADS anxiety and depression scores and the JHEQ mental score were significantly lower in the no sarcopenia assessment group than in the severe sarcopenia group (Table A2). Calf circumference was significantly correlated with mid-thigh muscle area assessed by CT and SMI in the total patient population (Figure A2).

Table 1.
Baseline patient characteristics.

Table 2.
Associations between preoperative sarcopenic status and physical and mental status.
A total of 210 patients achieved functional recovery on POD7 (82.7%), and 180 were discharged home (70.9%). The severe sarcopenia group had consistently lower daily functional recovery rates than the non-sarcopenia group (63% and 86% on POD7) (Figure 2).
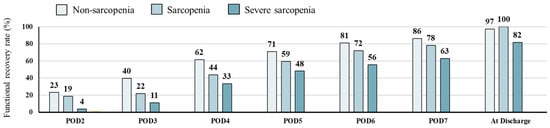
Figure 2.
Proportions of patients showing functional recovery after surgery.
The risks of functional recovery delay (adjusted OR, 2.82; 95% CI, 1.03–7.72; p = 0.043) and non-home discharge (adjusted OR, 2.72; 95% CI, 1.01–7.31; p = 0.047) were higher in the severe sarcopenia group than in the non-sarcopenia group based on the logistic regression analysis after adjusting for possible confounding factors (Table 3).

Table 3.
Logistic regression analyses for postoperative clinical outcomes.
The baseline logistic regression model and further models with the addition of sarcopenia assessment on ROC curve analysis are shown in Table 4. In the model with the addition of all sarcopenia assessments to the baseline model, the AUC for functional recovery delay on POD7 was significantly higher than in the baseline model (0.770; 95% CI 0.697–0.845; p = 0.038). Although the difference was not significant, the model with the addition of all sarcopenia assessments to the baseline model showed the greatest AUC for non-home discharge (0.753; 95% CI, 0.688–0.818; p = 0.371). However, there was a significant increase in AUC for non-home discharge with the inclusion of the number of days until functional recovery in the baseline model (0.807; 95% CI, 0.748–0.866; p < 0.001). The baseline model with the addition of all sarcopenia assessments showed a better predictive ability for functional recovery delay than the baseline model between threshold probabilities of 10% and 60% (Figure 3).

Table 4.
Predictive value analysis for postoperative outcomes.
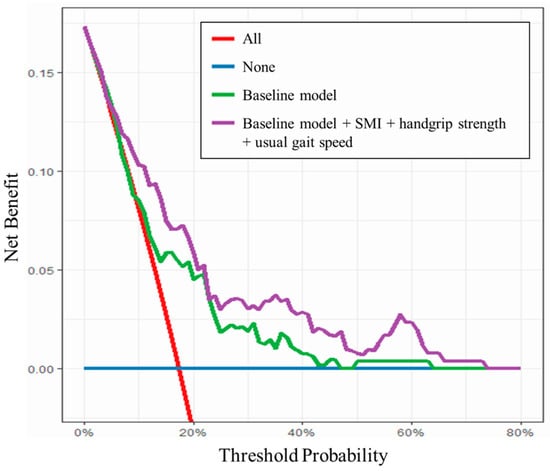
Figure 3.
Decision curve analysis for functional recovery delay.
The postoperative physical function and clinical outcomes are shown in Table A3. Muscle strength, physical function, and Barthel Index at discharge were significantly lower and hospital stays were significantly longer in the severe sarcopenia group than in the non-sarcopenia group. However, there were no significant differences in pain or changes in these parameters between the groups. Postoperative clinical outcomes, including functional recovery delay and non-home discharge, were not significantly different between the severe sarcopenia group and no sarcopenia assessment group (Table A4).
The clinical outcomes at 6 months after surgery are shown in Table 5. The JOA hip score and JHEQ total score were significantly lower, while the JHEQ VAS dissatisfaction scale score was significantly higher, in the severe sarcopenia group than in the non-sarcopenia group. However, the incidence of adverse events within 6 months after surgery was not significantly different between these two groups. Clinical outcomes at 6 months after surgery were not significantly different between the severe sarcopenia group and no sarcopenia assessment group (Table A5).

Table 5.
Clinical outcomes at 6 months after surgery.
4. Discussion
This study was performed to investigate the association between preoperative sarcopenic status and clinical outcomes following THA. Approximately 25% of patients in the present study had preoperative sarcopenia. The rate of severe sarcopenia was 10.6%, which was associated with increased incidence rates of functional recovery delay and non-home discharge, lower ADL level at discharge, and longer hospital stay in comparison with the non-sarcopenia group. SMI, handgrip strength, and usual gait speed as indicators of sarcopenia assessed in the present study had complementary predictive capability to known risk factors, such as preoperative hip function and physical activity, for risk of functional recovery delay after THA. Hip function and patient-reported QoL outcomes, including patient satisfaction, were significantly poorer in the severe sarcopenia group than in the non-sarcopenia group at 6 months after THA. These observations indicate the need for comprehensive evaluation taking preoperative sarcopenia status into consideration in THA.
Consistent with previous reports, our study population had a preoperative sarcopenia prevalence rate of 23.2%, which was reported to be associated with unfavorable short- and long-term clinical outcomes following THA [7,23]. In these previous studies, however, sarcopenia was evaluated based only on muscle mass and handgrip strength, while we defined sarcopenia using the criteria of the AWGS2019, involving a comprehensive assessment of muscle mass, muscle strength (monitored by handgrip strength), and physical performance (monitored by gait speed) [8]. Walking ability is limited by diseases of the hip, and preoperative gait speed was reported previously to be a good prognostic indicator for functional recovery after THA [24]. Measures of physical performance are recommended for assessment of the severity of sarcopenia [5,8]. The highest prevalence rate of low physical performance was 63% in the present study, and severe sarcopenia, but not sarcopenia, was related to poor outcomes, including functional recovery delay in the short term, poor hip function, and patient-reported outcomes at 6 months after surgery. The results presented here indicated the benefit of examining the severity of sarcopenia preoperatively in patients prior to THA. Previous large observational studies showed the association between frailty, which is closely related to sarcopenia, and increased rates of complications and mortality after THA [25,26], although the present study showed no significant differences in rates of adverse events within 6 months postoperatively according to sarcopenia status. As the statistical power of the present study may have been too low to detect such associations because of the small sample size and limited number of recorded events, further studies are required in larger numbers of patients undergoing THA. The gait speed of patients with specific diseases, such as hip fracture, cannot be assessed before surgery, as they are unable to walk. Preoperative mental status and ADL level were poorer in our no sarcopenia assessment group compared with the severe sarcopenia group, and these groups had no significant differences in postoperative clinical outcomes. The results presented here suggested that greater attention may be required in the management of patients in whom sarcopenia cannot be assessed in addition to those with severe sarcopenia.
Even in the logistic regression model adjusted for potential confounding factors, severe sarcopenia was shown to be a significant independent predictor of functional recovery delay and non-home discharge after THA. The model with the addition of all sarcopenia assessments to the previously identified risk factors, but not any individual factor, had the highest AUC for functional recovery delay (AUC, 0.770), suggesting that a multi-item composite measure would outperform the assessment of individual items in patients undergoing THA. The ability to predict functional recovery delay regardless of risk was consistently increased by the addition of sarcopenia assessments to previously identified risk factors (threshold probabilities, 10–60%). To our knowledge, this is the first report of the additional predictive capability of preoperative sarcopenia assessments consisting of multiple objectively measured items in THA patients. There was no improvement in the AUC for non-home discharge by the addition of sarcopenia assessments to the baseline model in the present study. The significant increase in AUC for non-home discharge by the inclusion of the number of days to functional recovery in the baseline model (AUC, 0.807) suggested the importance of postoperative rehabilitation for improvement of the postoperative clinical course in patients undergoing THA.
The results of the present study have implications for both clinical practice and the design of future clinical studies regarding THA. For medical treatment planning and to improve clinical outcomes in vulnerable populations, it is necessary to develop means of accurately stratifying patient risk. The accurate assessment of preoperative sarcopenia is necessary because suitable interventions, including nutritional recommendations and rehabilitation, can be implemented both pre- and postoperatively [27,28,29]. Exercise represents a nonpharmacological intervention that can improve the pain, physical function, QoL, and mental health of patients with hip osteoarthritis [30]. Resistance training and the use of nutritional supplements, including branched-chain amino acids, vitamin D, whey protein, and hydroxymethylbutyrate-enriched milk, can play important roles in ameliorating sarcopenia [8], which may improve the outcomes of frail patients following THA. However, the effects of these interventions on severe sarcopenia have yet to be determined, and whether such amelioration of sarcopenia can improve clinical outcomes remains to be determined. Further studies are therefore required to facilitate clinical decision-making processes in patients requiring THA.
This study had several limitations. First, this was a single-center observational study in a small population of Asian patients, and the small number of events may have increased the risk of type I errors. Second, substantially longer hospital stays in Japan compared with Western countries may have affected the results of this study, especially with regard to non-home discharge. Further studies in larger cohorts and other populations are required to validate the prognostic effects of preoperative sarcopenic status assessment. Third, the retrospective nature of this study meant that the accuracy of some variables was dependent on the quality of the medical records. Finally, although multivariate analysis may mitigate bias after adjustment for previously identified risk factors, other factors that were not measured or for which the models were not adjusted, such as cognitive function and changes in baseline variables, can also result in residual bias. Further studies are therefore required to confirm the findings presented here.
5. Conclusions
This study showed that preoperative severe sarcopenia was associated with the risks of functional recovery delay and non-home discharge in patients undergoing THA, and this condition adversely affected hip function and patient-reported outcomes, including patient satisfaction with hip condition, at 6 months postoperatively. This study suggested the beneficial effects of determining preoperative sarcopenia status for accurate risk stratification in patients requiring THA.
Author Contributions
Conceptualization, S.T. and Y.N.; methodology, S.T., Y.T. and Y.N.; validation, S.T., S.N. and Y.N.; formal analysis, S.T.; investigation, A.K., C.T., S.N., Y.F., T.M., M.N., M.K., H.I. and Y.O.; data curation, A.K., C.T., S.N., Y.F., T.M., M.N., M.K., H.I. and Y.O.; writing—original draft preparation, S.T.; writing—review and editing, Y.O., Y.T. and Y.N.; visualization, S.T.; supervision, Y.T. and Y.N.; project administration, S.T. and M.K.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Grant from the Aichi Society for Physical Therapy to promote research and the Japan Society for the Promotion of Science Grant-in-Aid (JSPS KAKENHI, Grant No. 24K20527).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Nagoya University Hospital and performed in accordance with the tenets of the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects (approval number: 2020-0232/16.05.2022).
Informed Consent Statement
Patient consent was waived, but information on this study, including the objectives and inclusion and exclusion criteria, was provided on the institutional website, and all participants were free to opt out of this study at any time. This method was approved by the ethics committee because of the nature of this study in which data were obtained as clinically necessary.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available because of privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
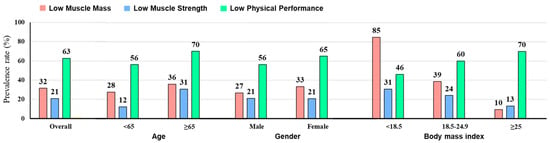
Figure A1.
Prevalence rates of sarcopenia domains.
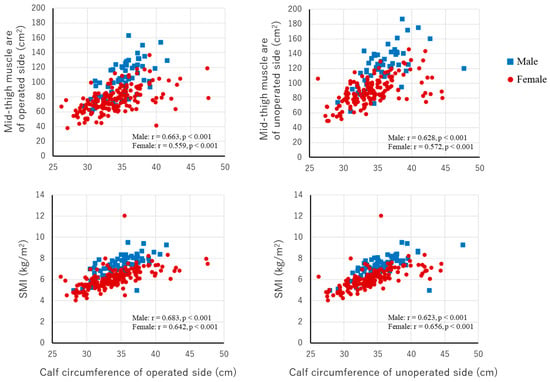
Figure A2.
Associations between calf circumference and mid-thigh muscle area and SMI. SMI, skeletal muscle index.
Appendix B

Table A1.
Baseline patient characteristics between two groups.
Table A1.
Baseline patient characteristics between two groups.
| Severe Sarcopenia | No Sarcopenia Assessment | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 27) | (n = 52) | |||||||
| Age (years) | 70.8 | ± | 10.6 | 69.7 | ± | 14.6 | 0.720 | |
| ≥65 | 20 (74.1) | 33 (63.5) | 0.451 | |||||
| Male | 7 (25.9) | 13 (25.0) | 1.000 | |||||
| BMI (kg/m2) | 22.1 | ± | 2.8 | 23.4 | ± | 4.3 | 0.166 | |
| BMI group | 0.073 | |||||||
| <18.5 | 2 (7.4) | 7 (13.5) | ||||||
| 18.5–24.9 | 22 (81.5) | 29 (55.8) | ||||||
| ≥25 | 3 (11.1) | 16 (30.8) | ||||||
| Etiology | 0.316 | |||||||
| Osteoarthritis | 18 (66.7) | 36 (69.2) | ||||||
| Osteonecrosis of the femoral head | 5 (18.5) | 4 (7.7) | ||||||
| Other | 4 (14.8) | 12 (23.1) | ||||||
| JOA hip score (points) | 56 | ± | 18 | 46 | ± | 19 | 0.034 | |
| Hip flexion ROM (°) | ||||||||
| Operated side | 75 | ± | 22 | 68 | ± | 26 | 0.273 | |
| Contralateral side | 93 | ± | 24 | 90 | ± | 33 | 0.783 | |
| VAS for pain (mm) | ||||||||
| Average | 42 [20, 55] | 45 [24, 65] | 0.526 | |||||
| Maximum | 53 [35, 79] | 64 [30, 86] | 0.261 | |||||
| Living alone | 0 (0.0) | 12 (23.1) | 0.006 | |||||
| Diabetes | 4 (14.8) | 8 (15.4) | 1.000 | |||||
| Charlson comorbidity index | 0.89 | ± | 0.85 | 0.89 | ± | 1.29 | 0.957 | |
| Never a smoker | 23 (85.2) | 39 (75.0) | 0.392 | |||||
| UCLA activity score | 3.6 | ± | 1.4 | 2.8 | ± | 1.4 | 0.017 | |
| Laboratory findings | ||||||||
| Albumin (g/dL) | 4.0 | ± | 0.3 | 3.7 | ± | 0.6 | 0.023 | |
| Hemoglobin (g/dL) | 12.4 | ± | 1.3 | 12.1 | ± | 1.6 | 0.309 | |
| Creatinine (mg/dL) | 0.7 | ± | 0.3 | 1.0 | ± | 1.2 | 0.257 | |
| eGFR (mL/min/1.73 m2) | 74.2 | ± | 22.0 | 74.7 | ± | 34.4 | 0.947 | |
| CRP (mg/L) | 0.11 [0.08, 0.18] | 0.22 [0.07, 0.75] | 0.091 | |||||
| Type of surgery | 0.583 | |||||||
| Primary | 22 (81.5) | 39 (75.0) | ||||||
| Revision | 5 (18.5) | 13 (25.0) | ||||||
| Operation time (min) | 122 [80, 186] | 146 [113, 206] | 0.075 | |||||
| Blood loss during surgery (g) | 416 [195, 674] | 501 [279, 206] | 0.075 | |||||
BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; JOA, Japanese Orthopaedic Association; ROM, range of motion; UCLA, University of California Los Angeles; VAS, visual analog scale.

Table A2.
Associations between preoperative sarcopenic status and physical and mental status between two groups.
Table A2.
Associations between preoperative sarcopenic status and physical and mental status between two groups.
| Severe Sarcopenia | No Sarcopenia Assessment | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 27) | (n = 52) | |||||||
| Mid-thigh muscle area (cm2) | ||||||||
| Operated side | 66.6 | ± | 13.0 | 73.0 | ± | 21.6 | 0.170 | |
| Contralateral side | 77.8 | ± | 19.3 | 82.2 | ± | 23.2 | 0.417 | |
| Mid-thigh muscle attenuation (HU) | ||||||||
| Operated side | 39.1 | ± | 8.2 | 35.2 | ± | 8.2 | 0.051 | |
| Contralateral side | 44.1 | ± | 8.0 | 39.5 | ± | 8.4 | 0.027 | |
| Mid-thigh subcutaneous fat area (cm2) | ||||||||
| Operated side | 50.4 | ± | 23.1 | 61.3 | ± | 31.6 | 0.126 | |
| Contralateral side | 45.7 | ± | 18.6 | 54.4 | ± | 28.9 | 0.175 | |
| Mid-thigh circumference (cm) | ||||||||
| Operated side | 36.6 | ± | 6.7 | 39.1 | ± | 5.4 | 0.132 | |
| Contralateral side | 38.6 | ± | 3.3 | 40.1 | ± | 5.4 | 0.257 | |
| Calf circumference (cm) | ||||||||
| Operated side | 31.5 | ± | 2.0 | 32.2 | ± | 4.4 | 0.493 | |
| Contralateral side | 31.7 | ± | 2.2 | 33.0 | ± | 4.6 | 0.196 | |
| Pulmonary function | ||||||||
| VC (%predicted) | 102.2 | ± | 17.4 | 99.4 | ± | 19.2 | 0.517 | |
| FEV1 (%predicted) | 95.1 | ± | 17.3 | 98.8 | ± | 22.5 | 0.454 | |
| FEV1/FVC | 105.9 | ± | 19.5 | 102.4 | ± | 21.4 | 0.479 | |
| PEF (%predicted) | 89.6 | ± | 16.4 | 90.6 | ± | 23.8 | 0.839 | |
| Barthel Index (points) | 100 [90, 100] | 85 [70, 100] | 0.002 | |||||
| HADS | ||||||||
| Anxiety | 5 | ± | 3 | 9 | ± | 5 | 0.001 | |
| Depression | 5 | ± | 4 | 9 | ± | 5 | 0.004 | |
| JHEQ score (points) | ||||||||
| Total | 26 | ± | 12 | 20 | ± | 13 | 0.080 | |
| Pain | 10 | ± | 8 | 9 | ± | 6 | 0.342 | |
| Function | 4 | ± | 4 | 3 | ± | 4 | 0.898 | |
| Mental | 12 | ± | 5 | 8 | ± | 6 | 0.010 | |
| JHEQ VAS dissatisfaction scale (mm) | 75 | ± | 31 | 77 | ± | 28 | 0.760 | |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HADS, hospital anxiety and depression scale; HU, Hounsfield units; JHEQ, Japanese Orthopaedic Association Hip-Disease Evaluation Questionnaire; PEF, peak expiratory flow; VC, vital capacity; VAS, visual analog scale.

Table A3.
Postoperative physical function and clinical outcomes.
Table A3.
Postoperative physical function and clinical outcomes.
| Overall (n = 254) | Non-Sarcopenia | Sarcopenia | Severe Sarcopenia | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 195; 76.8%) | (n = 32; 12.6%) | (n = 27; 10.6%) | ||||||||||||
| VAS for pain (mm) | ||||||||||||||
| Average | 8 [3, 15] | 7 [2, 14] | 11 [7, 18] | 8 [2, 13] | 0.072 | |||||||||
| Maximum | 15 [5, 30] | 15 [4, 31] | 26 [10, 38] | 14 [5, 26] | 0.090 | |||||||||
| Hip abductor strength (kgf*m) | ||||||||||||||
| Operated side | 2.5 | ± | 2.6 | 3.2 | ± | 3.2 | 1.8 | ± | 1.6 a | 1.6 | ± | 0.8 a | 0.003 | |
| Contralateral side | 3.3 | ± | 2.9 | 4.3 | ± | 4.1 | 2.5 | ± | 1.7 a | 2.0 | ± | 1.1 a | 0.002 | |
| Change in hip abductor strength (kgf*m) | ||||||||||||||
| Operated side | 0.0 | ± | 3.5 | 0.2 | ± | 3.9 | −0.7 | ± | 2.3 | −0.3 | ± | 1.3 | 0.450 | |
| Contralateral side | 1.0 | ± | 3.5 | 1.2 | ± | 4.0 | 0.4 | ± | 1.8 | 0.2 | ± | 1.2 | 0.272 | |
| Knee extensor strength (kgf*m) | ||||||||||||||
| Operated side | 5.4 | ± | 5.5 | 6.8 | ± | 6.3 | 3.4 | ± | 2.1 a | 2.6 | ± | 2.2 a | <0.001 | |
| Contralateral side | 6.9 | ± | 6.4 | 9.0 | ± | 7.7 | 4.8 | ± | 3.6 a | 3.7 | ± | 2.7 a | <0.001 | |
| Change in knee extensor strength (kgf*m) | ||||||||||||||
| Operated side | 1.3 | ± | 5.3 | 1.8 | ± | 5.9 | 0.0 | ± | 2.2 | −0.1 | ± | 2.4 | 0.087 | |
| Contralateral side | 2.3 | ± | 6.8 | 2.8 | ± | 7.7 | 1.2 | ± | 3.2 | 0.3 | ± | 2.7 | 0.138 | |
| Handgrip strength (kg) | ||||||||||||||
| Male | 33.2 | ± | 7.9 | 35.2 | ± | 6.6 | 25.8 | ± | 11.2 a | 22.9 | ± | 2.8 a | <0.001 | |
| Female | 21.9 | ± | 5.6 | 23.6 | ± | 4.9 | 21.2 | ± | 3.6 a | 13.6 | ± | 3.8 a,b | <0.001 | |
| Change in handgrip strength (kg) | ||||||||||||||
| Male | −0.6 | ± | 3.6 | −0.5 | ± | 3.2 | −0.5 | ± | 7.5 | −1.4 | ± | 3.5 | 0.855 | |
| Female | 0.0 | ± | 3.2 | −0.1 | ± | 3.4 | 0.0 | ± | 3.2 | 0.2 | ± | 1.8 | 0.938 | |
| Usual gait speed (m/s) | 0.84 | ± | 0.24 | 0.92 | ± | 0.25 | 0.80 | ± | 0.19 | 0.71 | ± | 0.25 a | <0.001 | |
| Change in usual gait speed (m/s) | 0.06 | ± | 0.35 | 0.04 | ± | 0.36 | 0.11 | ± | 0.36 | 0.09 | ± | 0.23 | 0.520 | |
| Maximal gait speed (m/s) | 1.07 | ± | 0.25 | 1.17 | ± | 0.29 | 0.99 | ± | 0.25 a | 0.95 | ± | 0.24 a | <0.001 | |
| Change in maximal gait speed (m/s) | −0.12 | ± | 0.30 | −0.14 | ± | 0.31 | −0.09 | ± | 0.29 | 0.03 | ± | 0.23 | 0.096 | |
| Barthel Index at discharge (points) | 100 [95, 100] | 100 [95, 100] | 100 [95, 100] | 95 [83, 100] a | 0.022 | |||||||||
| Change in Barthel Index (points) | 0 [−5, 0] | 0 [−5, 0] | 0 [−5, 0] | 0 [−6.3, 0] | 0.920 | |||||||||
| Hospital-acquired disability | 72 (28.3) | 52 (26.7) | 10 (31.2) | 10 (37.0) | 0.495 | |||||||||
| Length of hospital stay (day) | 17 | [15–22] | 17 | [14–21] | 17 | [14–24] | 21 | [17–29] a | 0.004 | |||||
VAS, visual analogue scale. a Significant difference from the non-sarcopenia group. b Significant difference from the sarcopenia group.

Table A4.
Postoperative clinical outcomes.
Table A4.
Postoperative clinical outcomes.
| Severe Sarcopenia | No Sarcopenia Assessment | p Value | |
|---|---|---|---|
| (n = 27) | (n = 52) | ||
| Functional recovery delay on POD7 | 10 (37.0) | 27 (51.9) | 0.241 |
| Barthel Index at discharge (points) | 95 [85, 100] | 80 [75, 100] | 0.054 |
| Change in Barthel Index at discharge (points) | 0 [−5, 0] | 0 [−5, 10] | 0.251 |
| Hospital-acquired disability | 10 (37.0) | 18 (34.6) | 1.000 |
| Length of hospital stay (day) | 21 [17–29] | 21 [16–26] | 0.431 |
| Non-home discharge | 14 (51.9) | 37 (71.2) | 0.136 |
POD, postoperative day.

Table A5.
Clinical outcomes at 6 months after surgery between two groups.
Table A5.
Clinical outcomes at 6 months after surgery between two groups.
| Severe Sarcopenia | No Sarcopenia Assessment | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 27) | (n = 52) | |||||||
| JOA hip score (points) | 76 | ± | 17 | 74 | ± | 17 | 0.642 | |
| JHEQ score (points) | ||||||||
| Total | 50 | ± | 17 | 51 | ± | 19 | 0.910 | |
| Pain | 23 | ± | 7 | 23 | ± | 7 | 0.869 | |
| Function | 8 | ± | 7 | 10 | ± | 7 | 0.449 | |
| Mental | 19 | ± | 7 | 18 | ± | 8 | 0.796 | |
| JHEQ VAS dissatisfaction scale (mm) | 35 | ± | 38 | 23 | ± | 28 | 0.232 | |
| Adverse events within 180 days after surgery | 4 (14.8) | 13 (25.0) | 0.392 | |||||
JOA, Japanese Orthopaedic Association; JHEQ, Japanese Orthopaedic Association Hip-Disease Evaluation Questionnaire; VAS, visual analog scale.
References
- Liu, X.W.; Zi, Y.; Xiang, L.B.; Wang, Y. Total hip arthroplasty: Areview of advances, advantages and limitations. Int. J. Clin. Exp. Med. 2015, 8, 27–36. [Google Scholar] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Rotonda, C.; Guillemin, F.; Rat, A.C. What Have We Learned About the Course of Clinical Outcomes After Total Knee or Hip Arthroplasty? Arthritis Care Res. 2020, 72, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Albright, J.A.; Testa, E.J.; Balboni, A.B.; Daniels, A.H.; Cohen, E. Sarcopenia Is Associated with an Increased Risk of Postoperative Complications Following Total Hip Arthroplasty for Osteoarthritis. Biology 2023, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Malafarina, C.; Biain Ugarte, A.; Martinez, J.A.; Abete Goñi, I.; Zulet, M.A. Factors Associated with Sarcopenia and 7-Year Mortality in Very Old Patients with Hip Fracture Admitted to Rehabilitation Units: A Pragmatic Study. Nutrients 2019, 11, 2243. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sasai, H.; Osuka, Y.; Kojima, N.; Abe, T.; Yamashita, M.; Obuchi, S.P.; Ishizaki, T.; Fujiwara, Y.; Awata, S.; et al. Age- and sex-specific associations between sarcopenia severity and poor cognitive function among community-dwelling older adults in Japan: The IRIDE Cohort Study. Front. Public Health 2023, 11, 1148404. [Google Scholar] [CrossRef]
- Gadelha, A.B.; Neri, S.G.R.; Oliveira, R.J.; Bottaro, M.; David, A.C.; Vainshelboim, B.; Lima, R.M. Severity of sarcopenia is associated with postural balance and risk of falls in community-dwelling older women. Exp. Aging Res. 2018, 44, 258–269. [Google Scholar] [CrossRef]
- Bachettini, N.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Menezes, A.M.B.; Tomasi, E.; Gonzalez, M.C. Sarcopenia as a mortality predictor in community-dwelling older adults: A comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur. J. Clin. Nutr. 2020, 74, 573–580. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Iwata, H.; Mizuno, M.; Genda, E.; Sato, S.; Miura, T. The natural course of osteoarthritis of the hip due to subluxation or acetabular dysplasia. Arch. Orthop. Trauma. Surg. 1992, 111, 187–191. [Google Scholar] [CrossRef]
- Kuribayashi, M.; Takahashi, K.A.; Fujioka, M.; Ueshima, K.; Inoue, S.; Kubo, T. Reliability and validity of the Japanese Orthopaedic Association hip score. J. Orthop. Sci. 2010, 15, 452–458. [Google Scholar] [CrossRef]
- Zahiri, C.A.; Schmalzried, T.P.; Szuszczewicz, E.S.; Amstutz, H.C. Assessing activity in joint replacement patients. J. Arthroplast. 1998, 13, 890–895. [Google Scholar] [CrossRef]
- Lübbeke, A.; Zimmermann-Sloutskis, D.; Stern, R.; Roussos, C.; Bonvin, A.; Perneger, T.; Peter, R.; Hoffmeyer, P. Physical activity before and after primary total hip arthroplasty: A registry-based study. Arthritis Care Res. 2014, 66, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, K.; Matsui, Y.; Suzuki, Y.; Mizuno, T.; Watanabe, T.; Takemura, M.; Ishizuka, S.; Yamashita, S.; Imagama, S.; Arai, H. Relationship between sarcopenia classification and thigh muscle mass, fat area, muscle CT value and osteoporosis in middle-aged and older Japanese adults. Bone 2022, 163, 116487. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kaneuji, A.; Hiejima, Y.; Sugiyama, H.; Akiyama, H.; Atsumi, T.; Ishii, M.; Izumi, K.; Ichiseki, T.; Ito, H.; et al. Japanese Orthopaedic Association Hip Disease Evaluation Questionnaire (JHEQ): A patient-based evaluation tool for hip-joint disease. The Subcommittee on Hip Disease Evaluation of the Clinical Outcome Committee of the Japanese Orthopaedic Association. J. Orthop. Sci. 2012, 17, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hasegawa, Y.; Ikeuchi, K.; Ishiguro, N.; Hiejima, Y. Reliability and validity of the Japanese Orthopaedic Association hip disease evaluation questionnaire (JHEQ) for patients with hip disease. J. Orthop. Sci. 2013, 18, 782–787. [Google Scholar] [CrossRef]
- Tanaka, S.; Imaizumi, T.; Morohashi, A.; Sato, K.; Shibata, A.; Fukuta, A.; Nakagawa, R.; Nagaya, M.; Nishida, Y.; Hara, K.; et al. In-Hospital Fall Risk Prediction by Objective Measurement of Lower Extremity Function in a High-Risk Population. J. Am. Med. Dir. Assoc. 2023, 24, 1861–1867.e2. [Google Scholar] [CrossRef]
- De Long, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.F.; Brown, M.D.; Zhu, K.; Janes, H. Assessing the Clinical Impact of Risk Prediction Models with Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J. Clin. Oncol. 2016, 34, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- González-Montalvo, J.I.; Alarcón, T.; Gotor, P.; Queipo, R.; Velasco, R.; Hoyos, R.; Pardo, A.; Otero, A. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr. Gerontol. Int. 2016, 16, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Nanri, Y.; Kamiya, K.; Fukushima, K.; Uchiyama, K.; Takahira, N.; Takaso, M.; Fukuda, M.; Matsunaga, A. The maximal gait speed is a simple and useful prognostic indicator for functional recovery after total hip arthroplasty. BMC Musculoskelet. Disord. 2020, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Traven, S.A.; Reeves, R.A.; Slone, H.S.; Walton, Z.J. Frailty Predicts Medical Complications, Length of Stay, Readmission, and Mortality in Revision Hip and Knee Arthroplasty. J. Arthroplast. 2019, 34, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Abdel, M.P.; Frank, R.D.; Chamberlain, A.M.; Habermann, E.B.; Mantilla, C.B. Impact of Frailty on Outcomes After Primary and Revision Total Hip Arthroplasty. J. Arthroplast. 2019, 34, 56–64.e55. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Widmer, P.; Oesch, P.; Bachmann, S. Effect of Prehabilitation in Form of Exercise and/or Education in Patients Undergoing Total Hip Arthroplasty on Postoperative Outcomes—A Systematic Review. Medicina 2022, 58, 742. [Google Scholar] [CrossRef]
- Ninomiya, K.; Takahira, N.; Ikeda, T.; Suzuki, K.; Sato, R.; Mihara, M. Effects of perioperative exercise therapy combined with nutritional supplementation on functional recovery after fast-track total hip arthroplasty. J. Orthop. Sci. 2022, 28, 1291–1297. [Google Scholar] [CrossRef]
- Hurley, M.; Dickson, K.; Hallett, R.; Grant, R.; Hauari, H.; Walsh, N.; Stansfield, C.; Oliver, S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: A mixed methods review. Cochrane Database Syst. Rev. 2018, 4, Cd010842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).