New Perspectives for Low Muscle Mass Quantity/Quality Assessment in Probable Sarcopenic Older Adults: An Exploratory Analysis Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Assessment of Sarcopenia Parameters
2.4. Assessment of Isokinetic Parameters
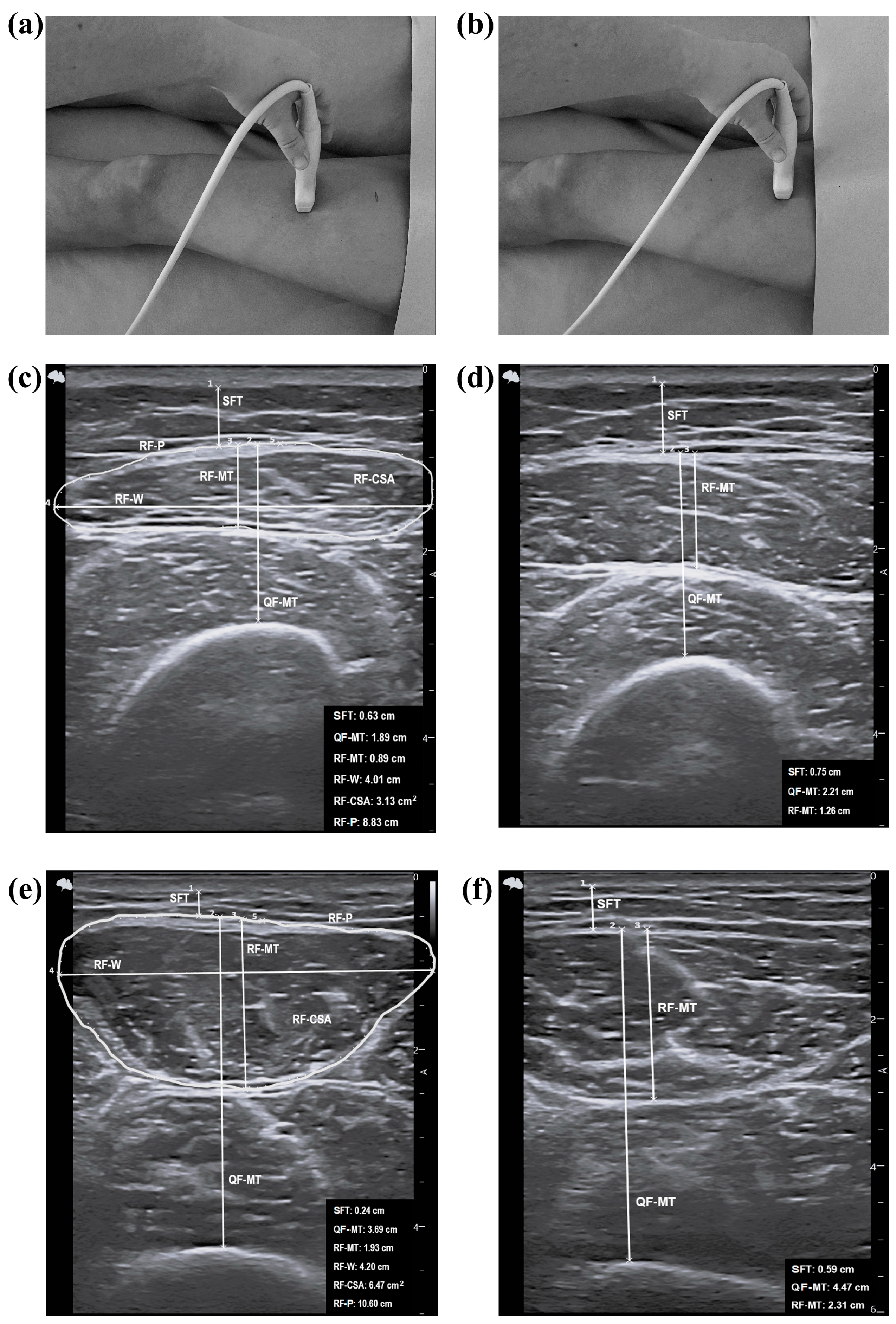
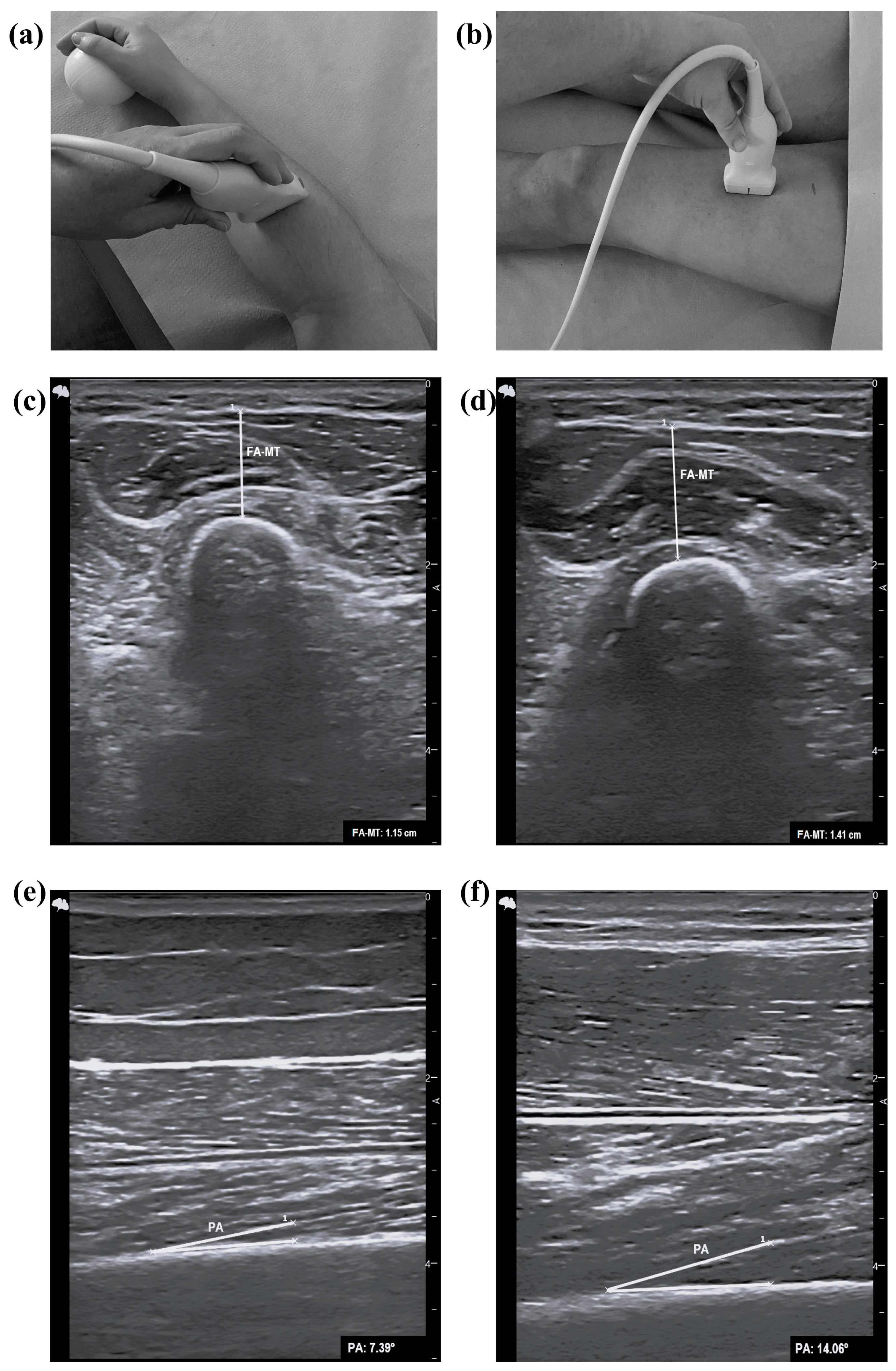
2.5. Assessment of Ultrasound Parameters
2.6. Statistical Methods
3. Results
3.1. Muscle Mass Assessed by BIA
3.2. Muscle Mass Assessed by Isokinetic Parameters
3.3. Muscle Mass Assessed by Ultrasound
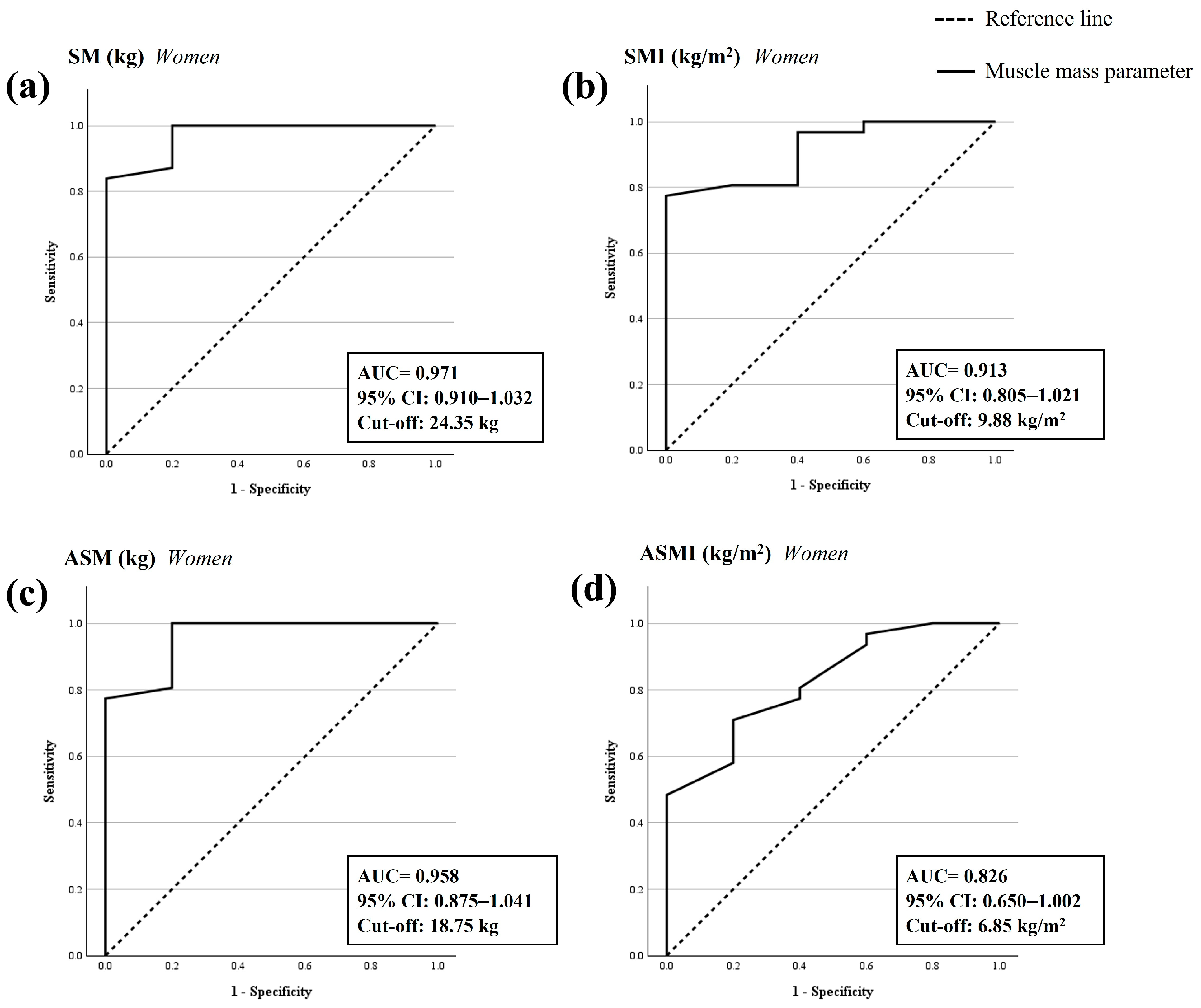
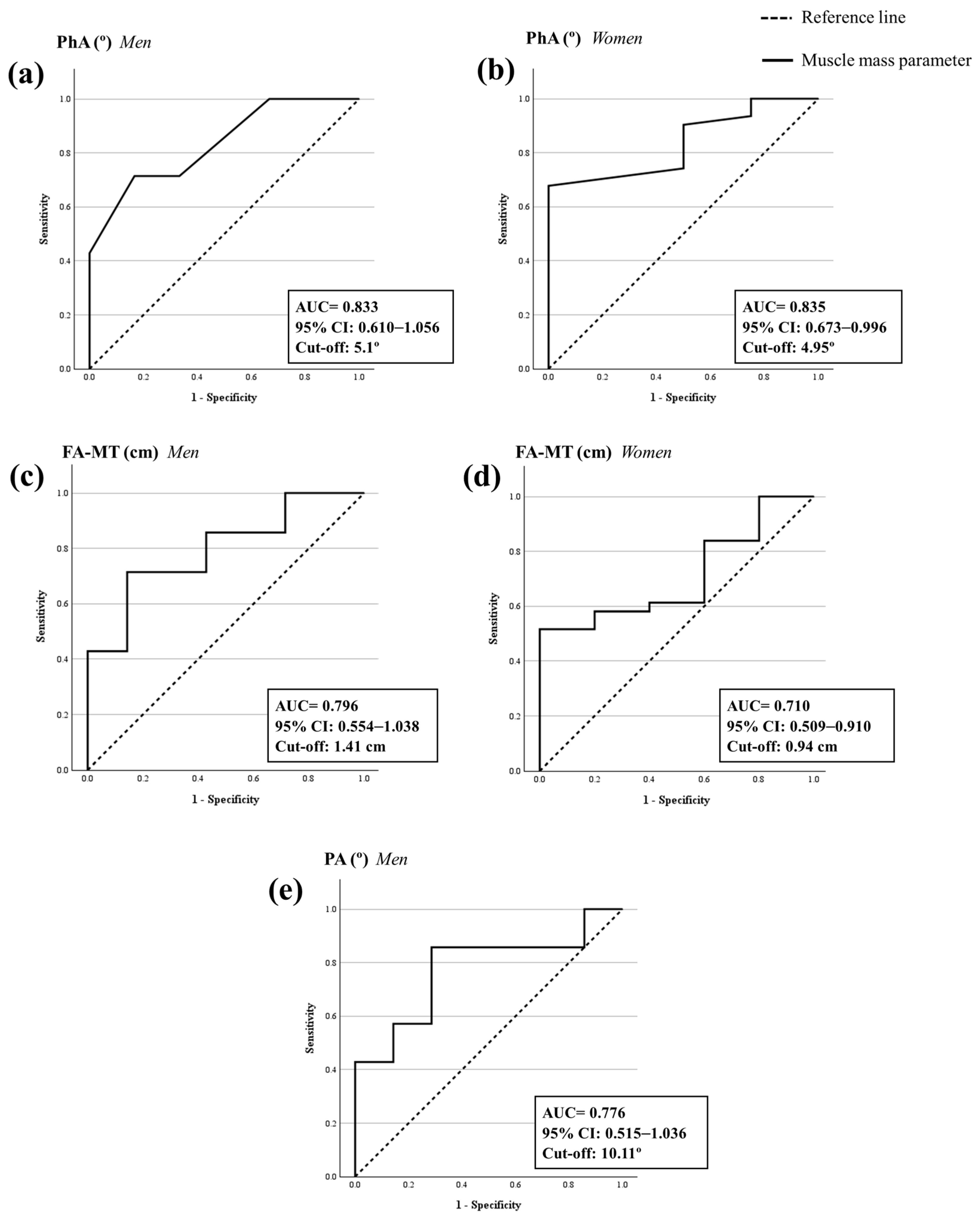
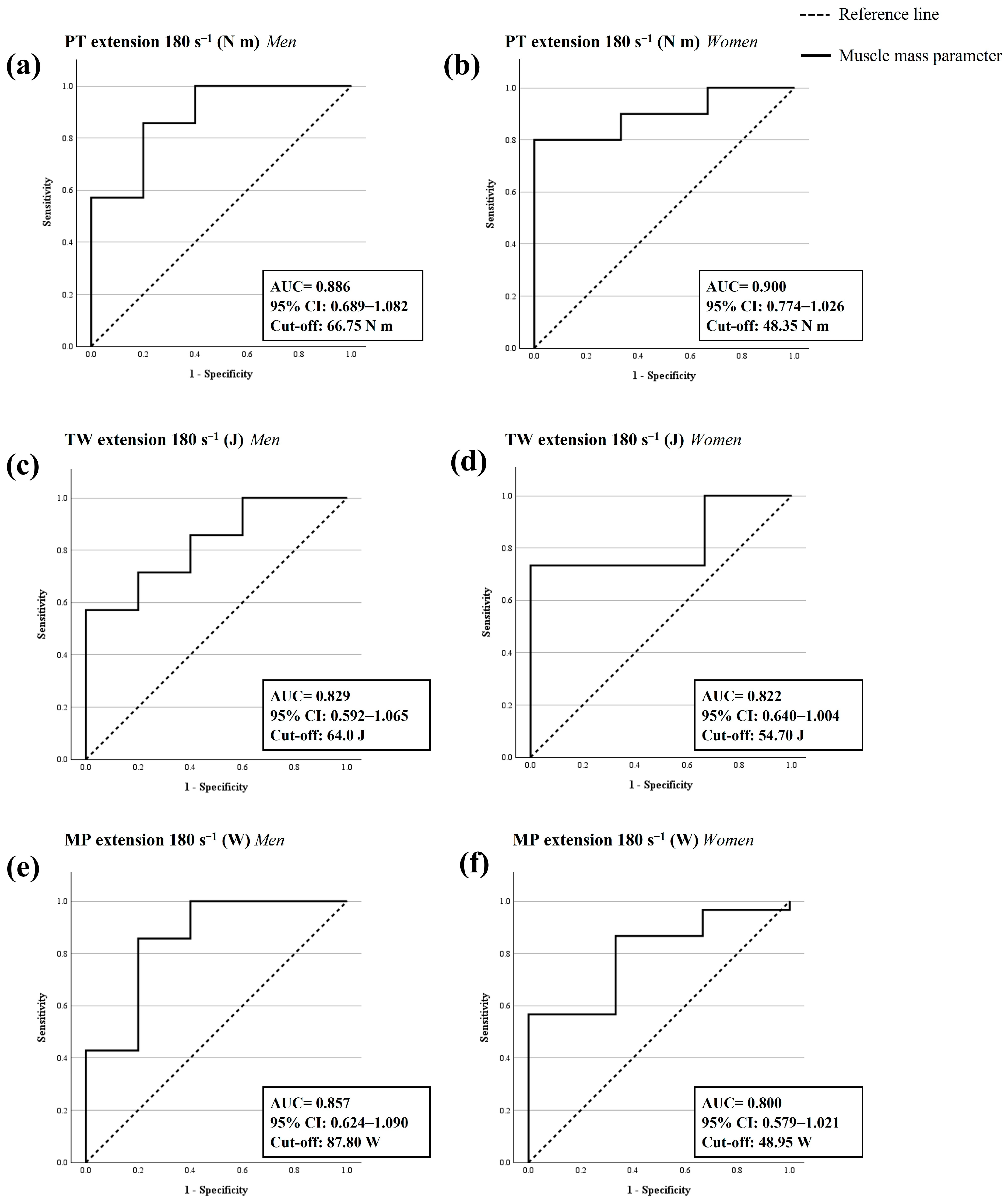
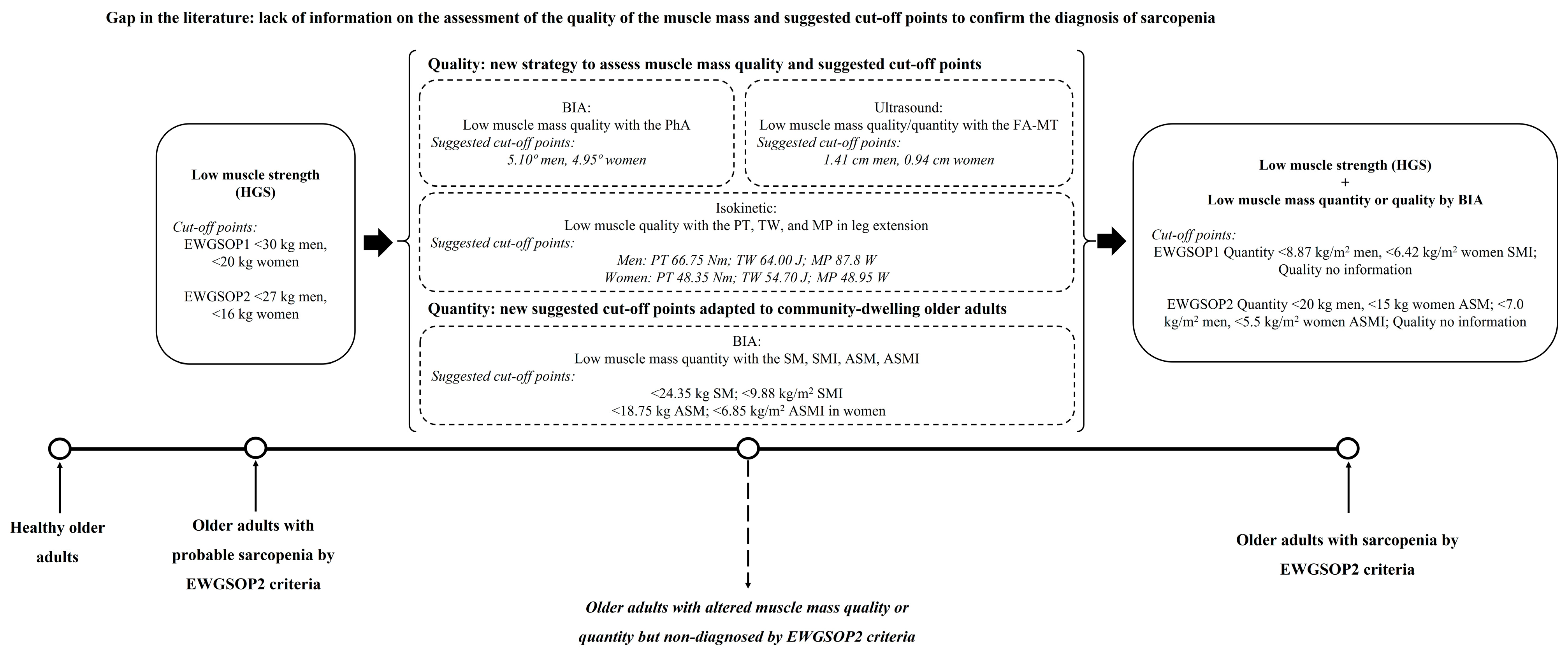
3.4. ROC Analysis and Cut-Off Points to Confirm the Sarcopenia Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| AUC | 95% CI | p-Value | Sensitivity | Specificity | Youden’s Index | Cut-Off | |
|---|---|---|---|---|---|---|---|
| Upper leg at 30% | |||||||
| SFT (cm) | |||||||
| Men | 0.571 | 0.253–0.890 | 0.660 | - | - | - | - |
| Women | 0.680 | 0.436–0.924 | 0.148 | - | - | - | - |
| QF-MT (cm) | |||||||
| Men | 0.592 | 0.262–0.922 | 0.586 | - | - | - | - |
| Women | 0.580 | 0.397–0.763 | 0.392 | - | - | - | - |
| RF-MT (cm) | |||||||
| Men | 0.469 | 0.144–0.795 | 0.854 | - | - | - | - |
| Women | 0.430 | 0.230–0.630 | 0.493 | - | - | - | - |
| RF-W (cm) | |||||||
| Men | 0.204 | –0.049–0.457 | 0.022 | - | - | - | - |
| Women | 0.630 | 0.395–0.865 | 0.279 | - | - | - | - |
| RF-CSA (cm2) | |||||||
| Men | 0.449 | 0.131–0.767 | 0.753 | - | - | - | - |
| Women | 0.527 | 0.294–0.759 | 0.822 | - | - | - | - |
| RF-P (cm) | |||||||
| Men | 0.429 | 0.102–0.756 | 0.669 | - | - | - | - |
| Women | 0.720 | 0.442–0.998 | 0.121 | - | - | - | - |
| Upper leg at 50% | |||||||
| SFT (cm) | |||||||
| Men | 0.347 | 0.015–0.679 | 0.366 | - | - | - | - |
| Women | 0.497 | 0.221–0.773 | 0.981 | - | - | - | - |
| QF-MT (cm) | |||||||
| Men | 0.633 | 0.330–0.935 | 0.390 | - | - | - | - |
| Women | 0.327 | 0.103–0.551 | 0.129 | - | - | - | - |
| RF-MT (cm) | |||||||
| Men | 0.531 | 0.210–0.852 | 0.852 | - | - | - | - |
| Women | 0.387 | 0.075–0.698 | 0.476 | - | - | - | - |
| Upper leg PA (°) | |||||||
| Men | 0.776 | 0.515–1.036 | 0.038 | 0.857 | 0.286 | 0.571 | 10.11 |
| Women | 0.453 | 0.259–0.648 | 0.639 | - | - | - | - |
| Forearm | |||||||
| FA-MT (cm) | |||||||
| Men | 0.796 | 0.554–1.038 | 0.017 | 0.714 | 0.143 | 0.571 | 1.41 |
| Women | 0.710 | 0.509–0.910 | 0.041 | 0.516 | 0.000 | 0.516 | 0.94 |
| Isokinetic | |||||||
| PT in leg extension at 180° s−1 (N m) | |||||||
| Men | 0.886 | 0.689–1.082 | <0.001 | 0.857 | 0.200 | 0.657 | 66.75 |
| Women | 0.900 | 0.774–1.026 | <0.001 | 0.800 | 0.000 | 0.800 | 48.35 |
| TW in leg extension at 180° s−1 (J) | |||||||
| Men | 0.829 | 0.592–1.065 | 0.007 | 0.571 | 0.000 | 0.571 | 64.00 |
| Women | 0.822 | 0.640–1.004 | 0.001 | 0.733 | 0.000 | 0.733 | 54.70 |
| MP in leg extension at 180° s−1 (W) | |||||||
| Men | 0.857 | 0.624–1.090 | 0.003 | 0.857 | 0.200 | 0.657 | 87.8 |
| Women | 0.800 | 0.579–1.021 | 0.008 | 0.567 | 0.000 | 0.567 | 48.95 |
| PT in leg flexion at 180° s−1 (N m) | |||||||
| Men | 0.629 | 0.284–0.973 | 0.465 | - | - | - | - |
| Women | 0.717 | 0.402–1.031 | 0.177 | - | - | - | - |
| TW in leg flexion at 180° s−1 (J) | |||||||
| Men | 0.686 | 0.366–1.006 | 0.256 | - | - | - | - |
| Women | 0.711 | 0.433–0.989 | 0.137 | - | - | - | - |
| MP in leg flexion at 180° s−1 (W) | |||||||
| Men | 0.743 | 0.417–1.068 | 0.144 | - | - | - | - |
| Women | 0.678 | 0.436–0.920 | 0.150 | - | - | - | - |
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.V.; Paiva, A.E.G.; Silva, A.C.B.; de Castro, I.C.; Santiago, A.F.; de Oliveira, E.P.; Porto, L.C.J. Prevalence of Sarcopenia According to EWGSOP1 and EWGSOP2 in Older Adults and Their Associations with Unfavorable Health Outcomes: A Systematic Review. Aging Clin. Exp. Res. 2022, 34, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Fielding, R.A.; Bens, C.; Bernabei, R.; Cawthon, P.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Del Signore, S.; Donahue, S.; Morley, J.; et al. Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J. Frailty Aging 2018, 7, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Salini, S.; Russo, A.; Calvani, R.; Covino, M.; Martone, A.M.; Tosato, M.; Damiano, F.P.; Picca, A.; Marzetti, E.; Landi, F. Self-Reported Difficulty in Walking 400 Meters: The “Red Flag” for Probable Sarcopenia. BMC Geriatr. 2022, 22, 530. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Zarco, R.; de León, A.O.-G. Muscle Quality an Evolving Concept. J. Frailty Sarcopenia Falls 2023, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C.; Brantlov, S. Bioimpedance Basics and Phase Angle Fundamentals. Rev. Endocr. Metab. Disord. 2023, 24, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of Ultrasound for Muscle Assessment in Sarcopenia: 2020 SARCUS Update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Alfred, L. Fisher 81—Models of Sarcopenia. In Handbook of Models for Human Aging; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 977–991. [Google Scholar]
- Stringer, H.J.; Wilson, D. The Role of Ultrasound as a Diagnostic Tool for Sarcopenia. J. Frailty Aging 2018, 7, 258–261. [Google Scholar] [CrossRef]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; van der Schans, C.P. The Reliability and Validity of Ultrasound to Quantify Muscles in Older Adults: A Systematic Review. J. Cachexia Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The Current Use of Ultrasound to Measure Skeletal Muscle and Its Ability to Predict Clinical Outcomes: A Systematic Review. J. Cachexia Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Nies, I.; Ackermans, L.L.G.C.; Poeze, M.; Blokhuis, T.J.; Ten Bosch, J.A. The Diagnostic Value of Ultrasound of the Rectus Femoris for the Diagnosis of Sarcopenia in Adults: A Systematic Review. Injury 2022, 53 (Suppl. S3), S23–S29. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Mccleary, R.W.; Andersen, A.J.C. Test-Retest Reliability of Reciprocal Isokinetic Knee Extension and Flexion Peak Torque Measurements. J. Athl. Train 1992, 27, 362. [Google Scholar] [PubMed]
- Martinez-Puig, D.; Möller, I.; Fernández, C.; Chetrit, C. Efficacy of Oral Administration of Yoghurt Supplemented with a Preparation Containing Hyaluronic Acid (MobileeTM) in Adults with Mild Joint Discomfort: A Randomized, Double-Blind, Placebo-Controlled Intervention Study. Med. J. Nutr. Metab. 2012, 6, 63–68. [Google Scholar] [CrossRef]
- Perkisas, S.; Baudry, S.; Bauer, J.; Beckwée, D.; De Cock, A.M.; Hobbelen, H.; Jager-Wittenaar, H.; Kasiukiewicz, A.; Landi, F.; Marco, E.; et al. Application of Ultrasound for Muscle Assessment in Sarcopenia: Towards Standardized Measurements. Eur. Geriatr. Med. 2018, 9, 739–757. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Sánchez- Rodríguez, D.; Perkisas, S.; Duran, X.; Bastijns, S.; Dávalos-Yerovi, V.; Da Costa, E.; Marco, E. The Feasibility and Reliability of Measuring Forearm Muscle Thickness by Ultrasound in a Geriatric Inpatient Setting: A Cross-Sectional Pilot Study. BMC Geriatr. 2022, 22, 137. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver Operating Characteristic Curve: Overview and Practical Use for Clinicians. Korean J. Anesthesiol. 2022, 75, 36. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Herpich, C.; Müller-Werdan, U. Role of Phase Angle in Older Adults with Focus on the Geriatric Syndromes Sarcopenia and Frailty. Rev. Endocr. Metab. Disord. 2023, 24, 429–437. [Google Scholar] [CrossRef]
- Thornley, I.; Hynd, J.; Stein, S.; Villada-Gómez, J.S.; González-Correa, C.H.; Pineda-Zuluaga, M.C. Electrical Bioimpedance Phase Angle and Sarcopenia Diagnostic in Functional Elderly. J. Phys. Conf. Ser. 2021, 2008, 012004. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Ohji, S.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Phase Angle Is a Useful Indicator for Muscle Function in Older Adults. J. Nutr. Health Aging 2019, 23, 251–255. [Google Scholar] [CrossRef] [PubMed]
- de Moura, T.G.; Nagata, C.A.; Garcia, P.A. The Influence of Isokinetic Peak Torque and Muscular Power on the Functional Performance of Active and Inactive Community-Dwelling Elderly: A Cross-Sectional Study. Braz. J. Phys. Ther. 2020, 24, 263. [Google Scholar] [CrossRef] [PubMed]
- Di Lenarda, L.; Buoite Stella, A.; Ratti, C.; Ruggiero, L.; Bernard, M.; Cavarzerani, L.P.; Canton, G.; Murena, L. Assessing Muscle Mass in the Orthopedic Clinical Setting: Application of the Ultrasound Sarcopenia Index in Elderly Subjects with a Recent Femoral Fracture. Nutrients 2024, 16, 711. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.; McPhee, J.; Conte, M.; Franchi, M.V.; Mitchell, K.; Tagliaferri, S.; Monti, E.; Marcolin, G.; Atherton, P.J.; Smith, K.; et al. Age-related Alterations in Muscle Architecture Are a Signature of Sarcopenia: The Ultrasound Sarcopenia Index. J. Cachexia Sarcopenia Muscle 2021, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kumon, Y.; Takamatsu, N.; Nozaki, T.; Inoue, M.; Nodera, H.; Albayda, J.; Izumi, Y. Ultrasound Assessment of Sarcopenia in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 2022, 32, 728–735. [Google Scholar] [CrossRef]
- Fu, H.; Wang, L.; Zhang, W.; Lu, J.; Yang, M. Diagnostic Test Accuracy of Ultrasound for Sarcopenia Diagnosis: A Systematic Review and Meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 70. [Google Scholar] [CrossRef]
| Probable Sarcopenia (n = 38) | Non-Sarcopenic (n = 12) | p-Value | |
|---|---|---|---|
| Age (years) | 69.6 ± 4.1 | 67.6 ± 4.5 | 0.151 a |
| Sex n (%) | |||
| Men | 7 (18.4) | 7 (58.3) | 0.026 a |
| Women | 31 (81.6) | 5 (41.7) | |
| Weight (kg) | 67.4 ± 13.1 | 82.6 ± 9.7 | <0.001 b |
| Height (m) | 1.56 (0.08) | 1.69 (0.08) | <0.001 b |
| BMI (kg/m2) | 27.27 ± 4.21 | 29.46 ± 2.89 | 0.032 b |
| Probable Sarcopenia (n = 38) a | Non-Sarcopenic (n = 12) b | p-Value c | Effect Size | ||
|---|---|---|---|---|---|
| Muscle strength and physical performance | |||||
| HGS (kg) | All | 17.68 (3.82) | 35.38 (13.04) | <0.001 | 0.015 |
| Men | 26.90 (10.10) | 40.15 (10.80) | 0.002 | ||
| Women | 17.15 (3.75) | 28.90 (5.75) | <0.001 | ||
| GS (m/s) | All | 1.03 ± 0.18 | 1.12 ± 0.16 | 0.266 | 0.393 |
| Men | 1.10 ± 0.08 | 1.20 ± 0.15 | 0.142 | ||
| Women | 1.02 ± 0.20 | 1.00 ± 0.10 | 0.631 | ||
| Quantity of muscle mass | |||||
| SM (kg) | All | 22.95 (3.76) | 33.50 (7.60) | <0.001 | 0.122 |
| Men | 33.50 (5.20) | 34.95 (2.85) | 0.224 | ||
| Women | 22.30 (2.40) | 27.40 (2.10) | <0.001 | ||
| SMI (kg/m2) | All | 9.64 (0.88) | 11.27 (1.75) | <0.001 | 0.165 |
| Men | 12.05 (2.16) | 11.86 (1.09) | 0.830 | ||
| Women | 9.49 (0.78) | 10.22 (0.70) | 0.003 | ||
| ASM (kg) | All | 16.25 (2.43) | 24.80 (7.20) | <0.001 | 0.132 |
| Men | 24.80 (5.00) | 26.15 (2.80) | 0.283 | ||
| Women | 15.80 (1.80) | 19.10 (1.25) | 0.001 | ||
| ASM (%) | All | 26.25 (4.58) | 29.20 (4.90) | 0.098 | 0.335 |
| Men | 29.70 (2.20) | 29.90 (1.45) | 0.475 | ||
| Women | 25.00 (3.40) | 25.10 (2.10) | 0.982 | ||
| ASMI (kg/m2) | All | 6.80 (0.63) | 8.40 (1.90) | 0.002 | 0.189 |
| Men | 8.70 (2.00) | 8.95 (1.00) | 0.774 | ||
| Women | 6.70 (0.50) | 7.10 (0.60) | 0.020 | ||
| Quality of muscle mass | |||||
| PhA (°) | All | 4.76 ± 0.51 | 5.38 ± 0.33 | 0.003 | 0.208 |
| Men | 4.86 ± 0.40 | 5.38 ± 0.31 | 0.041 | ||
| Women | 4.74 ± 0.54 | 5.38 ± 0.41 | 0.124 | ||
| Probable Sarcopenia (n = 38) a | Non-Sarcopenic (n = 12) b | p-Value c | p-Value Adjusted d | Effect Size | ||
|---|---|---|---|---|---|---|
| Ultrasound | ||||||
| Upper leg at 30% | ||||||
| SFT (cm) | All | 1.28 ± 0.57 | 0.80 ± 0.39 | 0.004 | <0.001 | 0.221 |
| Men | 0.75 ± 0.58 | 0.54 ± 0.18 | 0.654 | |||
| Women | 1.41 ± 0.50 | 1.16 ± 0.29 | 0.203 | |||
| QF-MT (cm) | All | 2.31 (0.89) | 2.81 (1.35) | 0.058 | <0.001 | 0.316 |
| Men | 2.97 (0.30) | 3.20 (1.20) | 0.565 | |||
| Women | 2.14 (0.74) | 2.20 (0.38) | 0.572 | |||
| RF-MT (cm) | All | 1.32 (0.45) | 1.33 (0.82) | 0.205 | <0.001 | 0.377 |
| Men | 1.79 (0.75) | 1.90 (0.75) | 0.848 | |||
| Women | 1.15 (0.43) | 1.10 (0.24) | 0.621 | |||
| RF-W (cm) | All | 3.89 ± 0.38 | 4.05 ± 0.22 | 0.109 | <0.001 | 0.345 |
| Men | 4.37 ± 0.22 | 4.15 ± 0.17 | 0.064 | |||
| Women | 3.77 ± 0.32 | 3.91 ± 0.22 | 0.346 | |||
| RF-CSA (cm2) | All | 4.06 (1.60) | 4.67 (3.10) | 0.085 | <0.001 | 0.333 |
| Men | 7.02 (3.47) | 6.61 (2.33) | 0.749 | |||
| Women | 3.62 (1.38) | 3.90 (0.91) | 0.850 | |||
| RF-P (cm) | All | 8.97 (0.92) | 9.75 (1.69) | 0.014 | <0.001 | 0.261 |
| Men | 11.12 (1.91) | 10.60 (0.85) | 0.655 | |||
| Women | 8.82 (0.73) | 9.28 (0.76) | 0.120 | |||
| Upper leg at 50% | ||||||
| SFT (cm) | All | 1.45 ± 0.59 | 1.08 ± 0.47 | 0.061 | <0.001 | 0.319 |
| Men | 0.92 ± 0.71 | 0.76 ± 0.17 | 0.337 | |||
| Women | 1.57 ± 0.49 | 1.52 ± 0.36 | 0.981 | |||
| QF-MT (cm) | All | 3.29 ± 0.71 | 3.80 ± 1.14 | 0.264 | <0.001 | 0.392 |
| Men | 4.04 ± 0.71 | 4.49 ± 0.97 | 0.406 | |||
| Women | 3.12 ± 0.59 | 2.82 ± 0.37 | 0.220 | |||
| RF-MT (cm) | All | 1.64 (0.33) | 1.93 (0.95) | 0.114 | <0.001 | 0.347 |
| Men | 2.25 (0.89) | 2.32 (0.55) | 0.848 | |||
| Women | 1.62 (0.28) | 1.53 (0.47) | 0.423 | |||
| Upper leg | ||||||
| PA (°) | All | 9.63 ± 3.32 | 10.56 ± 2.26 | 0.317 | 0.597 | 0.403 |
| Men | 8.14 ± 3.20 | 11.40 ± 2.52 | 0.085 | |||
| Women | 9.98 ± 3.30 | 9.38 ± 1.21 | 0.741 | |||
| Forearm | ||||||
| FA-MT (cm) | All | 1.02 ± 0.33 | 1.48 ± 0.40 | 0.001 | <0.001 | 0.184 |
| Men | 1.36 ± 0.30 | 1.69 ± 0.33 | 0.064 | |||
| Women | 0.94 ± 0.29 | 1.17 ± 0.27 | 0.137 | |||
| Isokinetic | ||||||
| Leg extension at 180° s−1 | ||||||
| PT (N-m) | All | 43.40 (16.55) | 65.55 (34.65) | <0.001 | <0.001 | 0.100 |
| Men | 61.80 (12.80) | 87.20 (25.30) | 0.028 | |||
| Women | 41.90 (13.65) | 52.00 (-) | 0.024 | |||
| TW (J) | All | 48.75 ± 17.84 | 76.56 ± 18.52 | <0.001 | <0.001 | 0.125 |
| Men | 63.76 ± 19.99 | 85.34 ± 16.54 | 0.062 | |||
| Women | 45.25 ± 15.67 | 61.93 ± 11.84 | 0.069 | |||
| MP (W) | All | 49.26 ± 19.92 | 87.25 ± 31.33 | 0.002 | <0.001 | 0.142 |
| Men | 65.69 ± 23.18 | 102.3 ± 29.92 | 0.042 | |||
| Women | 45.43 ± 17.35 | 62.17 ± 11.61 | 0.091 | |||
| Leg flexion at 180° s−1 | ||||||
| PT (N-m) | All | 27.20 (11.20) | 38.40 (23.08) | 0.007 | <0.001 | 0.191 |
| Men | 37.30 (18.70) | 51.90 (20.35) | 0.465 | |||
| Women | 26.25 (8.95) | 30.30 (-) | 0.222 | |||
| TW (J) | All | 22.53 ± 12.32 | 37.99 ± 14.68 | 0.007 | <0.001 | 0.191 |
| Men | 34.27 ± 16.85 | 45.70 ± 12.68 | 0.291 | |||
| Women | 19.79 ± 9.41 | 25.13 ± 6.01 | 0.234 | |||
| MP (W) | All | 20.60 (20.90) | 41.40 (36.25) | 0.011 | <0.001 | 0.209 |
| Men | 35.90 (15.50) | 57.70 (39.55) | 0.167 | |||
| Women | 18.40 (18.03) | 24.80 (-) | 0.316 | |||
| AUC | 95% CI | p-Value | Sensitivity | Specificity | Youden’s Index | Cut-Off | |
|---|---|---|---|---|---|---|---|
| BIA | |||||||
| SM (kg) | |||||||
| Men | 0.702 | 0.401–1.004 | 0.188 | - | - | - | - |
| Women | 0.971 | 0.910–1.032 | <0.001 | 0.839 | 0.000 | 0.839 | 24.35 |
| SMI (kg/m2) | |||||||
| Men | 0.464 | 0.135–0.794 | 0.832 | - | - | - | - |
| Women | 0.913 | 0.805–1.021 | <0.001 | 0.774 | 0.000 | 0.774 | 9.88 |
| ASM (kg) | |||||||
| Men | 0.679 | 0.370–0.987 | 0.256 | - | - | - | - |
| Women | 0.958 | 0.875–1.041 | <0.001 | 1.000 | 0.200 | 0.800 | 18.75 |
| ASM (%) | |||||||
| Men | 0.619 | 0.298–0.940 | 0.468 | - | - | - | - |
| Women | 0.497 | 0.301–0.693 | 0.974 | - | - | - | - |
| ASMI (kg/m2) | |||||||
| Men | 0.548 | 0.219–0.876 | 0.776 | - | - | - | - |
| Women | 0.826 | 0.650–1.002 | <0.001 | 0.710 | 0.200 | 0.510 | 6.85 |
| PhA (°) | |||||||
| Men | 0.833 | 0.610–1.056 | 0.003 | 0.714 | 0.167 | 0.548 | 5.10 |
| Women | 0.835 | 0.673–0.996 | <0.001 | 0.677 | 0.000 | 0.677 | 4.95 |
| Ultrasound | |||||||
| Upper leg PA (°) | |||||||
| Men | 0.776 | 0.515–1.036 | 0.038 | 0.857 | 0.286 | 0.571 | 10.11 |
| Women | 0.453 | 0.259–0.648 | 0.639 | - | - | - | - |
| FA-MT (cm) | |||||||
| Men | 0.796 | 0.554–1.038 | 0.017 | 0.714 | 0.143 | 0.571 | 1.41 |
| Women | 0.710 | 0.509–0.910 | 0.041 | 0.516 | 0.000 | 0.516 | 0.94 |
| Isokinetic | |||||||
| PT in leg extension at 180° s−1 (N-m) | |||||||
| Men | 0.886 | 0.689–1.082 | <0.001 | 0.857 | 0.200 | 0.657 | 66.75 |
| Women | 0.900 | 0.774–1.026 | <0.001 | 0.800 | 0.000 | 0.800 | 48.35 |
| TW in leg extension at 180° s−1 (J) | |||||||
| Men | 0.829 | 0.592–1.065 | 0.007 | 0.571 | 0.000 | 0.571 | 64.00 |
| Women | 0.822 | 0.640–1.004 | 0.001 | 0.733 | 0.000 | 0.733 | 54.70 |
| MP in leg extension at 180° s−1 (W) | |||||||
| Men | 0.857 | 0.624–1.090 | 0.003 | 0.857 | 0.200 | 0.657 | 87.8 |
| Women | 0.800 | 0.579–1.021 | 0.008 | 0.567 | 0.000 | 0.567 | 48.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besora-Moreno, M.; Llauradó, E.; Jiménez-ten Hoevel, C.; Sepúlveda, C.; Queral, J.; Bernal, G.; Pérez-Merino, L.; Martinez-Hervas, S.; Alabadi, B.; Ortega, Y.; et al. New Perspectives for Low Muscle Mass Quantity/Quality Assessment in Probable Sarcopenic Older Adults: An Exploratory Analysis Study. Nutrients 2024, 16, 1496. https://doi.org/10.3390/nu16101496
Besora-Moreno M, Llauradó E, Jiménez-ten Hoevel C, Sepúlveda C, Queral J, Bernal G, Pérez-Merino L, Martinez-Hervas S, Alabadi B, Ortega Y, et al. New Perspectives for Low Muscle Mass Quantity/Quality Assessment in Probable Sarcopenic Older Adults: An Exploratory Analysis Study. Nutrients. 2024; 16(10):1496. https://doi.org/10.3390/nu16101496
Chicago/Turabian StyleBesora-Moreno, Maria, Elisabet Llauradó, Claudia Jiménez-ten Hoevel, Cristina Sepúlveda, Judit Queral, Glòria Bernal, Laura Pérez-Merino, Sergio Martinez-Hervas, Blanca Alabadi, Yolanda Ortega, and et al. 2024. "New Perspectives for Low Muscle Mass Quantity/Quality Assessment in Probable Sarcopenic Older Adults: An Exploratory Analysis Study" Nutrients 16, no. 10: 1496. https://doi.org/10.3390/nu16101496
APA StyleBesora-Moreno, M., Llauradó, E., Jiménez-ten Hoevel, C., Sepúlveda, C., Queral, J., Bernal, G., Pérez-Merino, L., Martinez-Hervas, S., Alabadi, B., Ortega, Y., Valls, R. M., Solà, R., & Pedret, A. (2024). New Perspectives for Low Muscle Mass Quantity/Quality Assessment in Probable Sarcopenic Older Adults: An Exploratory Analysis Study. Nutrients, 16(10), 1496. https://doi.org/10.3390/nu16101496





