A Randomized, Crossover Trial Assessing Appetite, Energy Metabolism, Blood Biomarkers, and Ad Libitum Food Intake Responses to a Mid-Morning Pecan Snack vs. an Equicaloric High-Carbohydrate Snack in Healthy Volunteers with Overweight/Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Protocol
2.3.1. Screening Visit
2.3.2. Testing Visits
2.4. Outcome Measures
2.5. Statistical Analysis
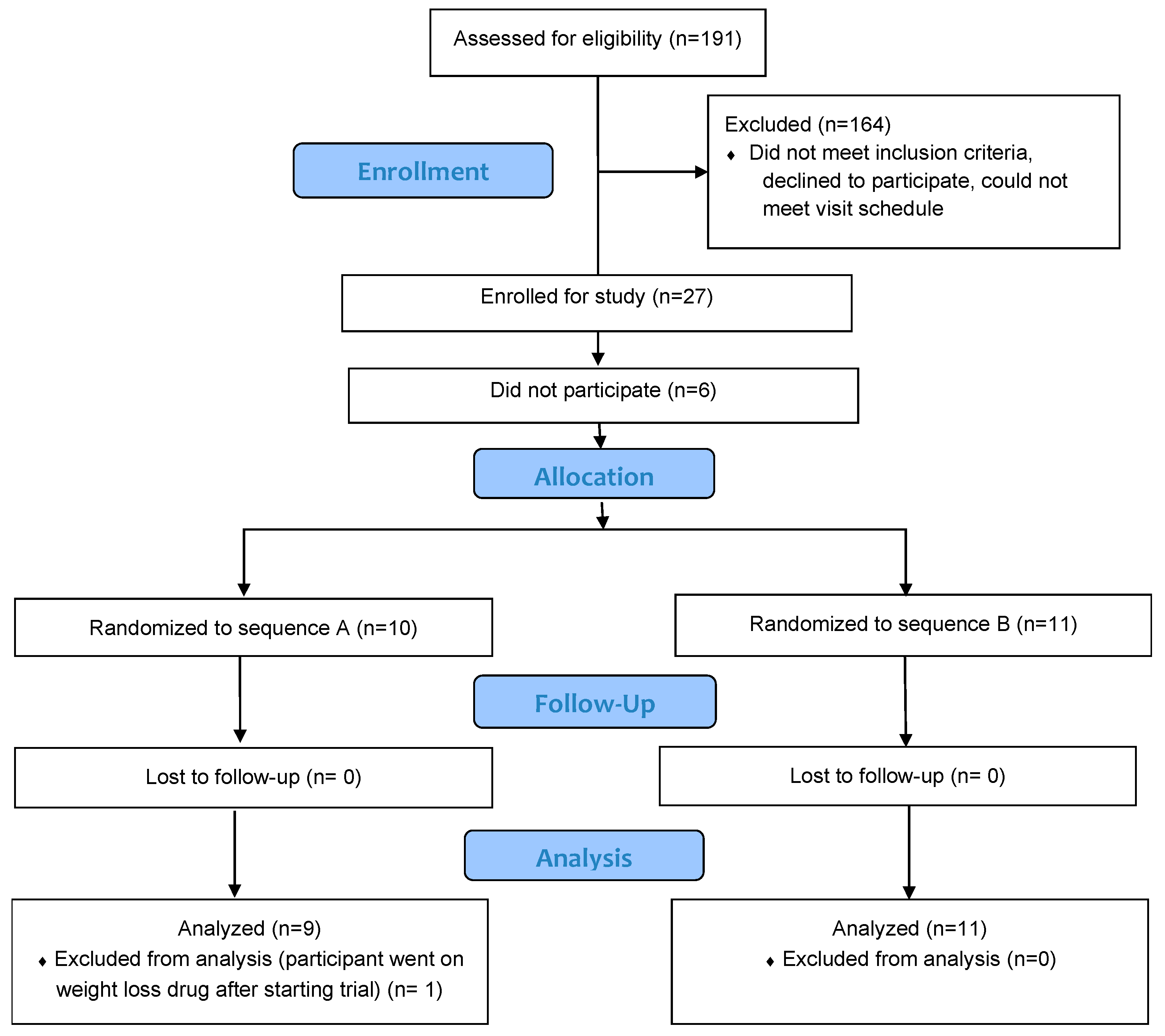
3. Results
3.1. Participant Characteristics
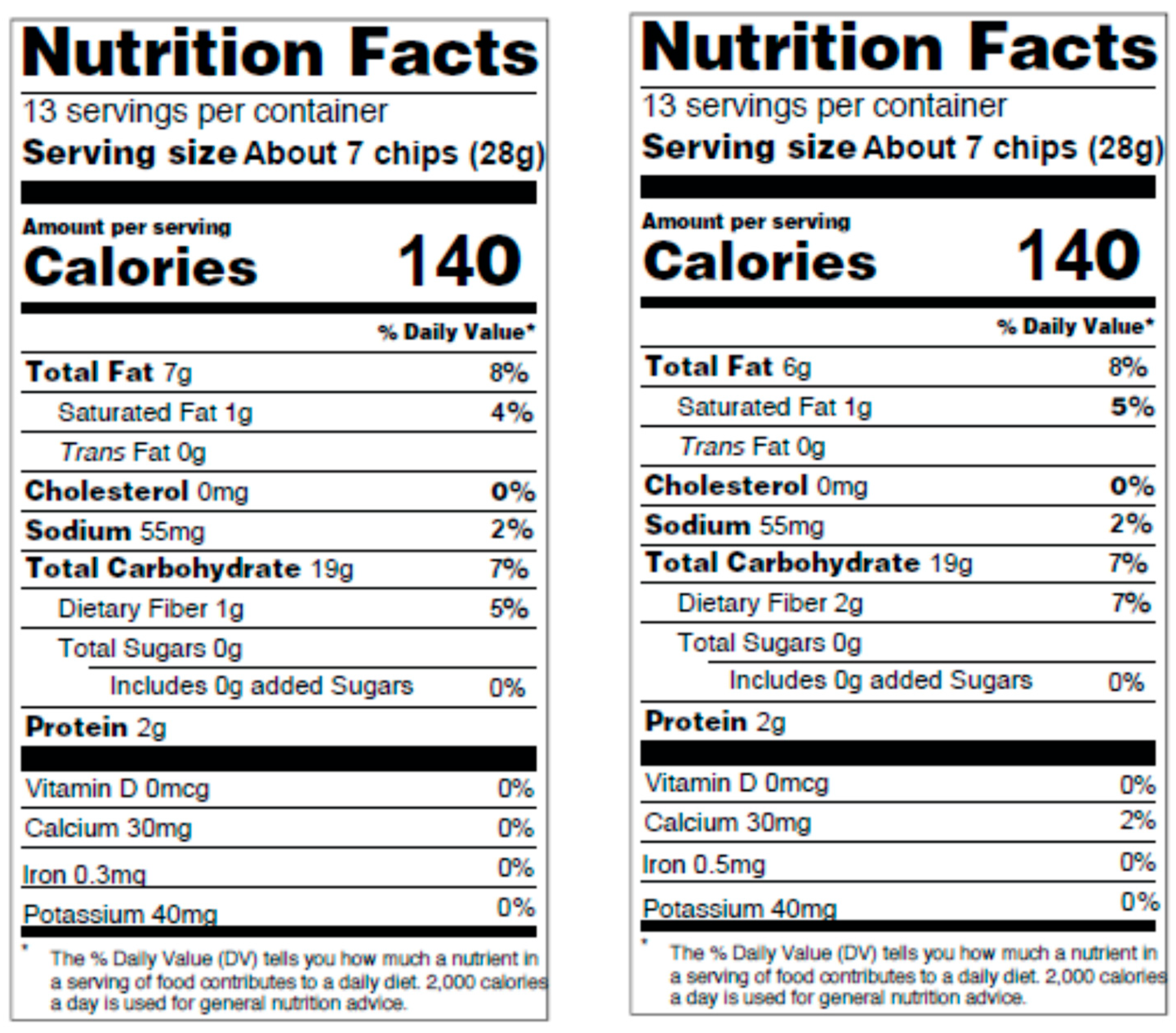
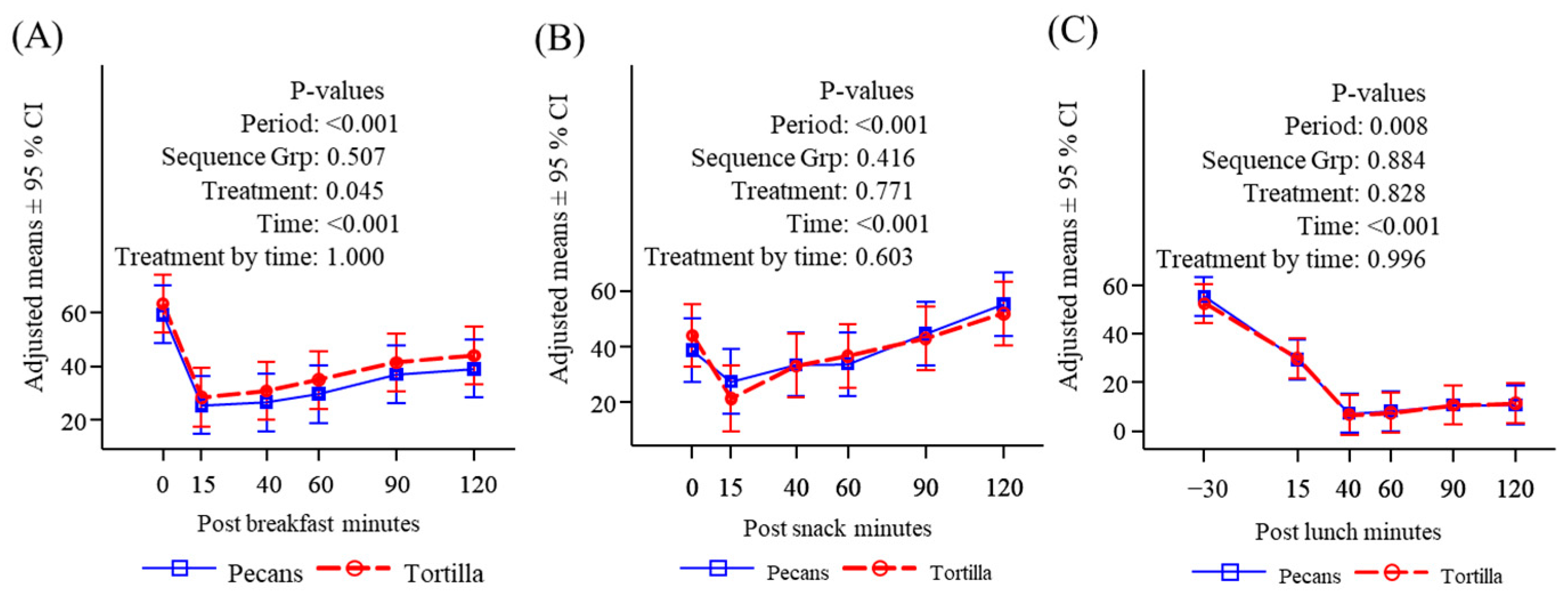
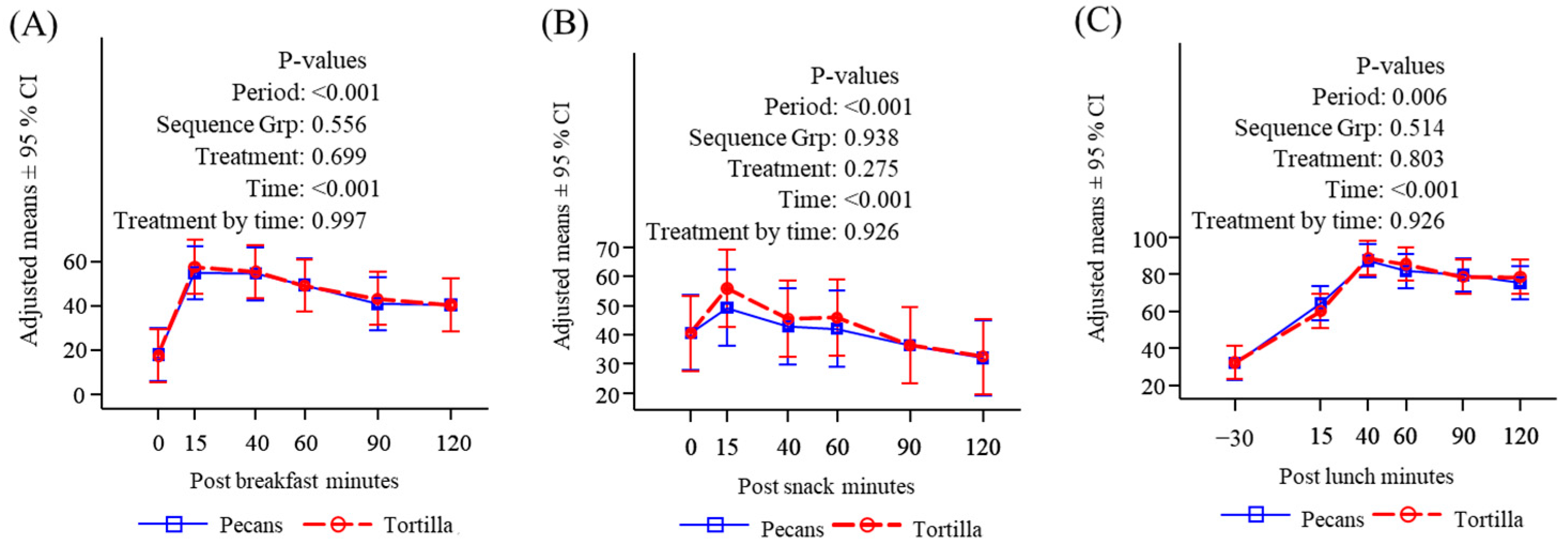
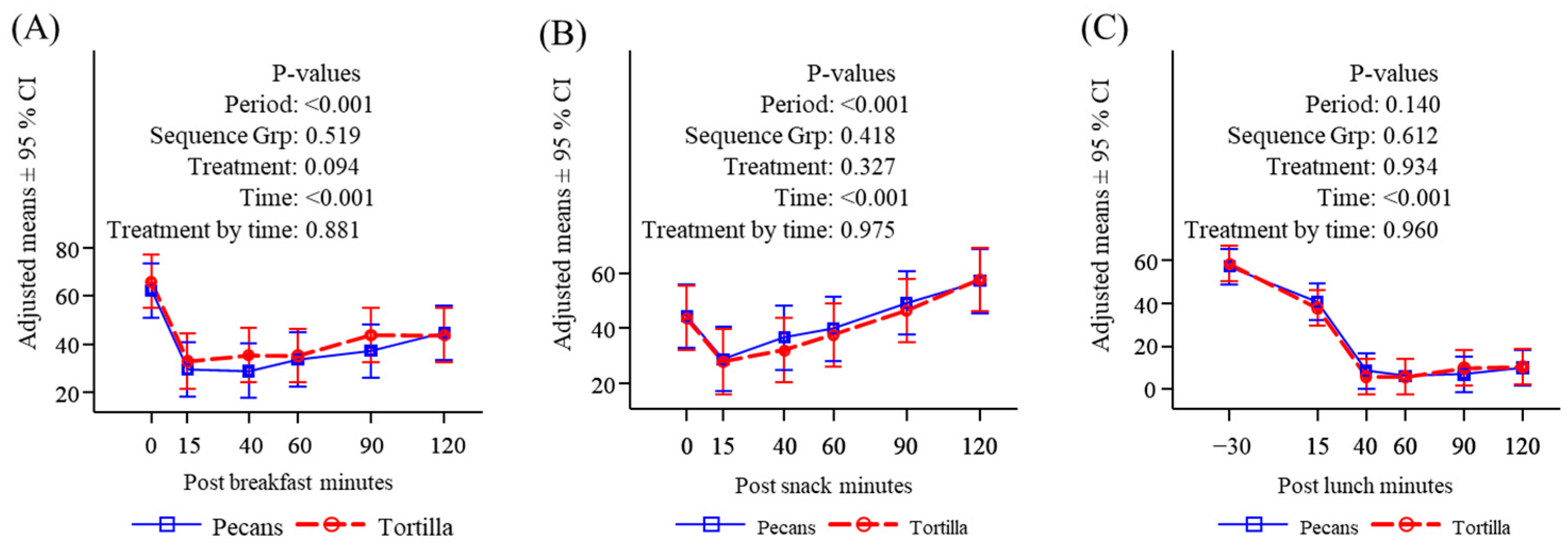
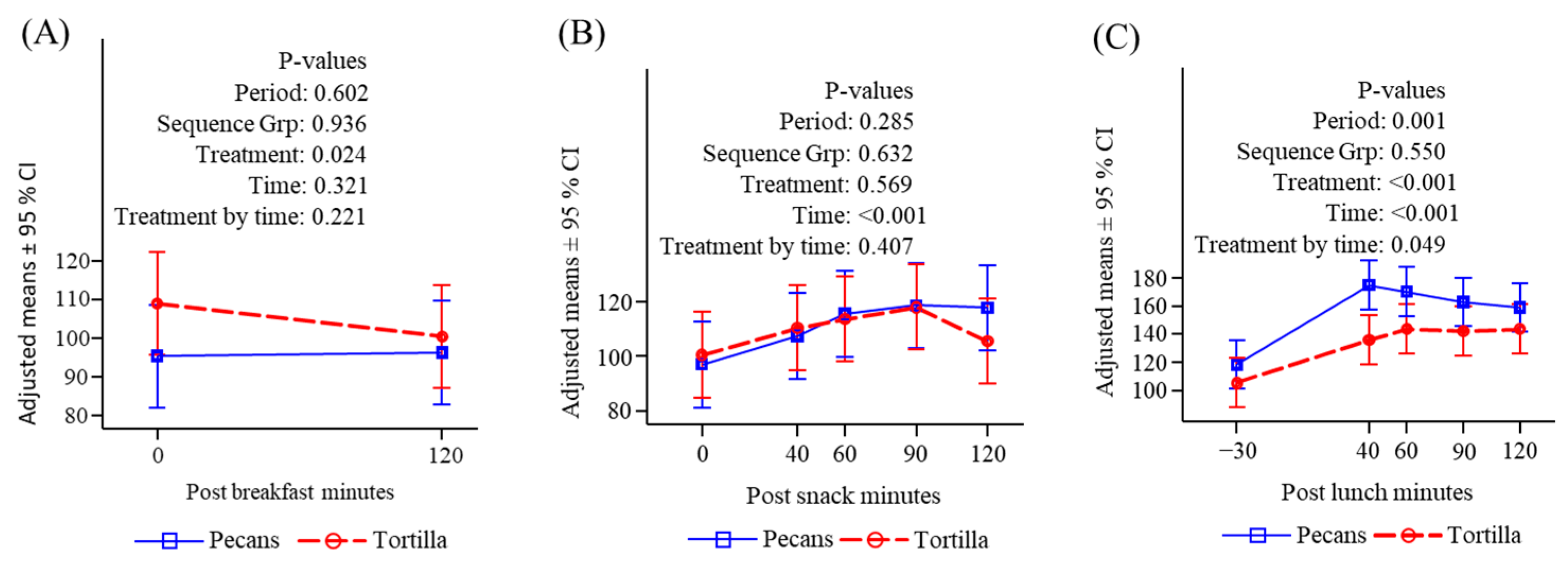
3.2. Subjective Appetite Measures
3.3. Breakfast, Snack, and Self-Selected Lunch Consumption
3.4. Plasma Metabolites and Appetite Hormones
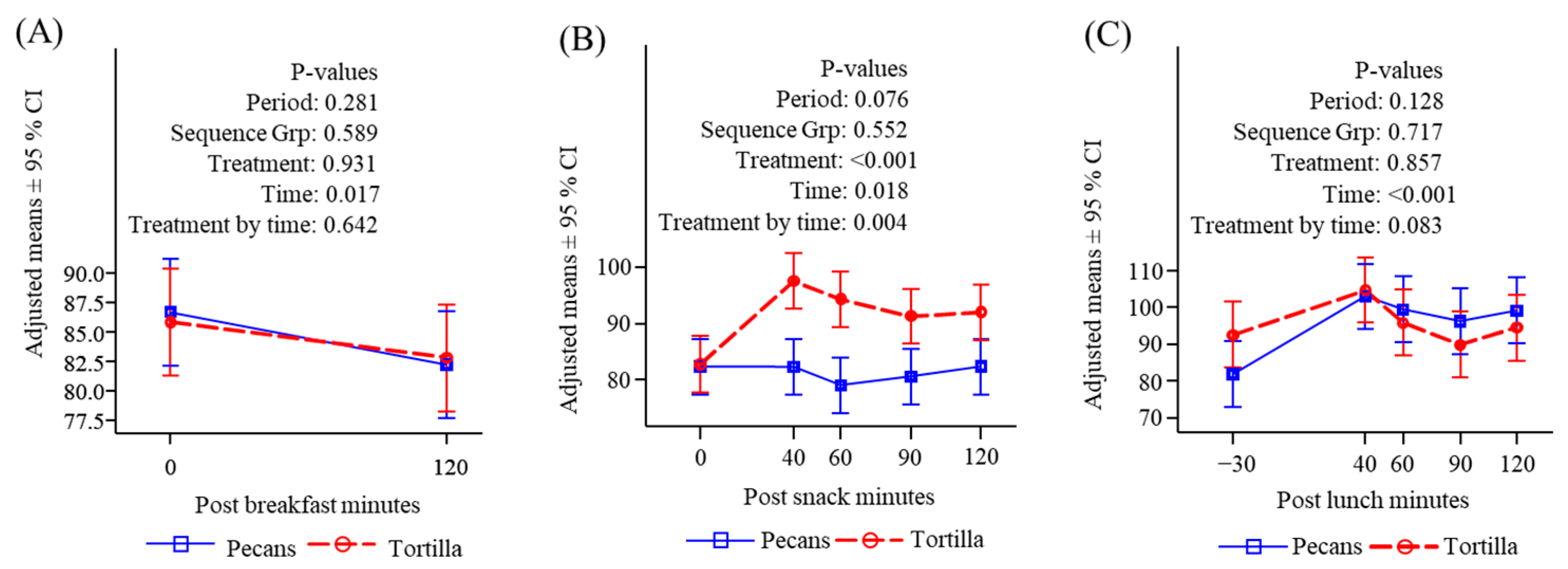
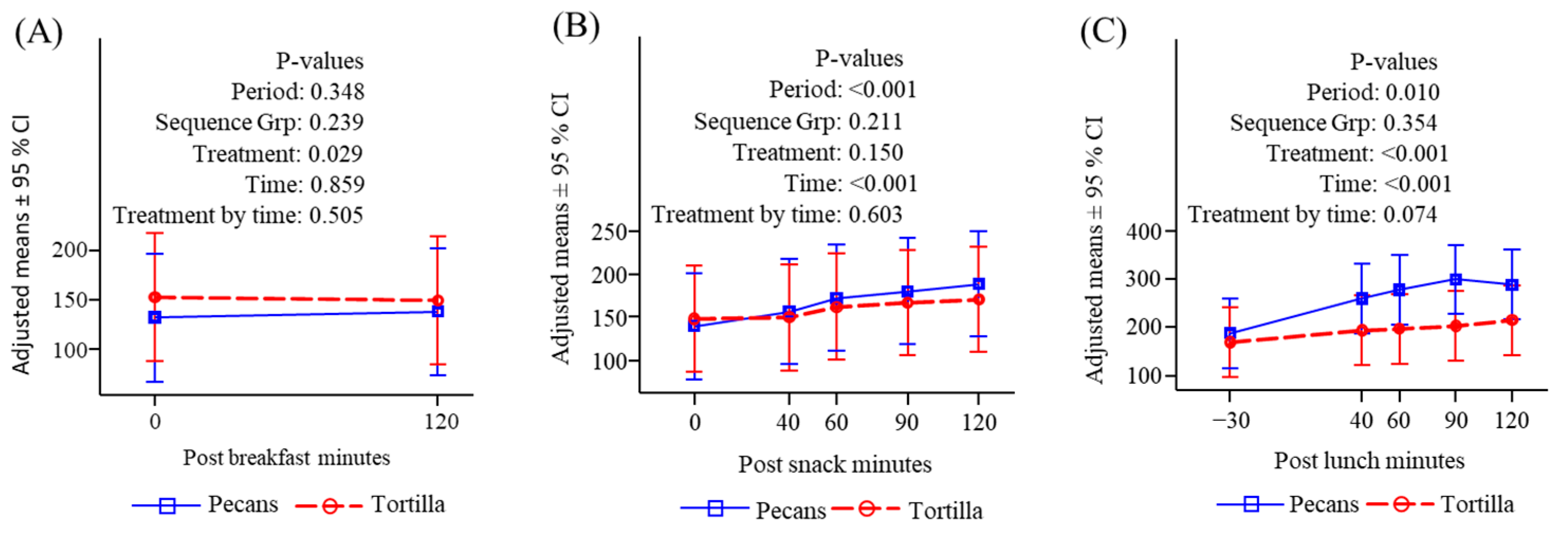
3.4.1. Glucose
3.4.2. Insulin
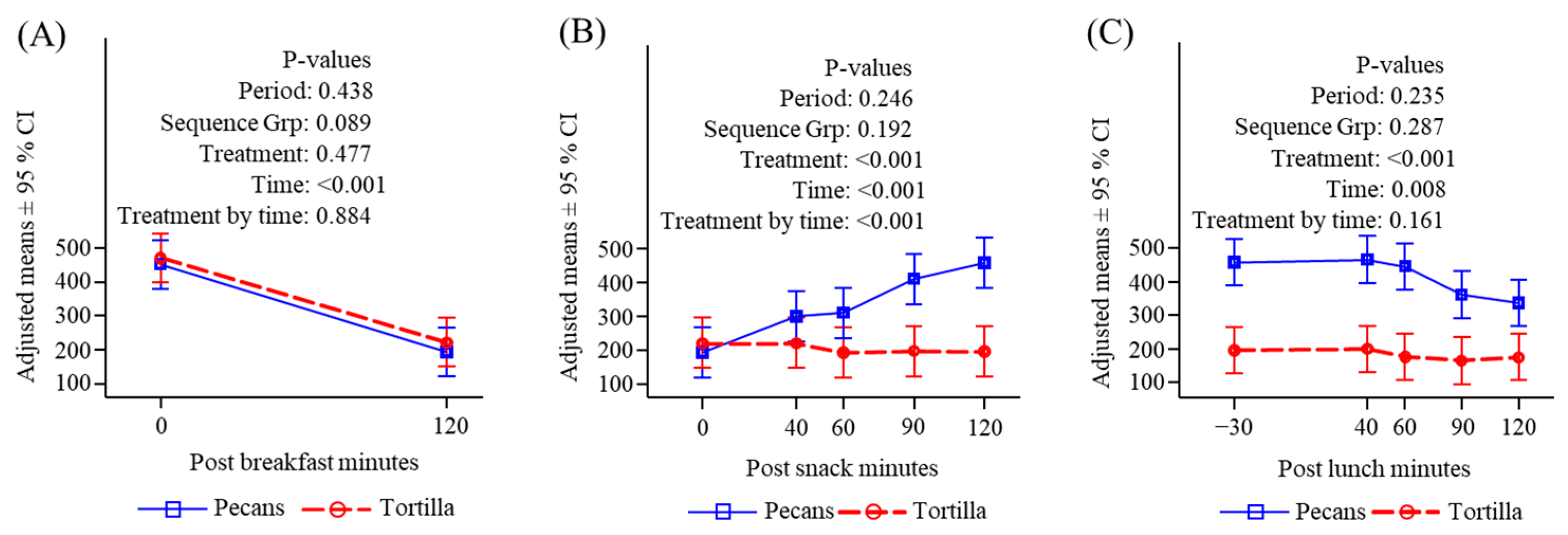
3.4.3. Free Fatty Acids
3.4.4. Triglycerides
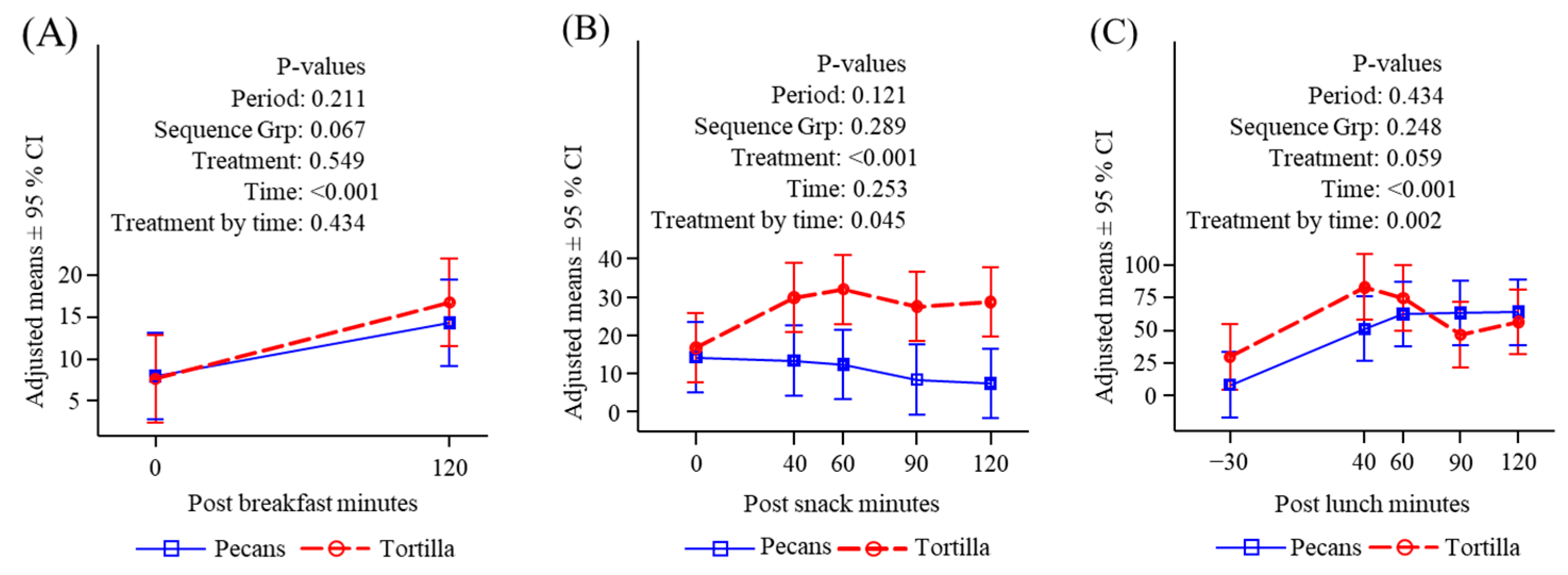
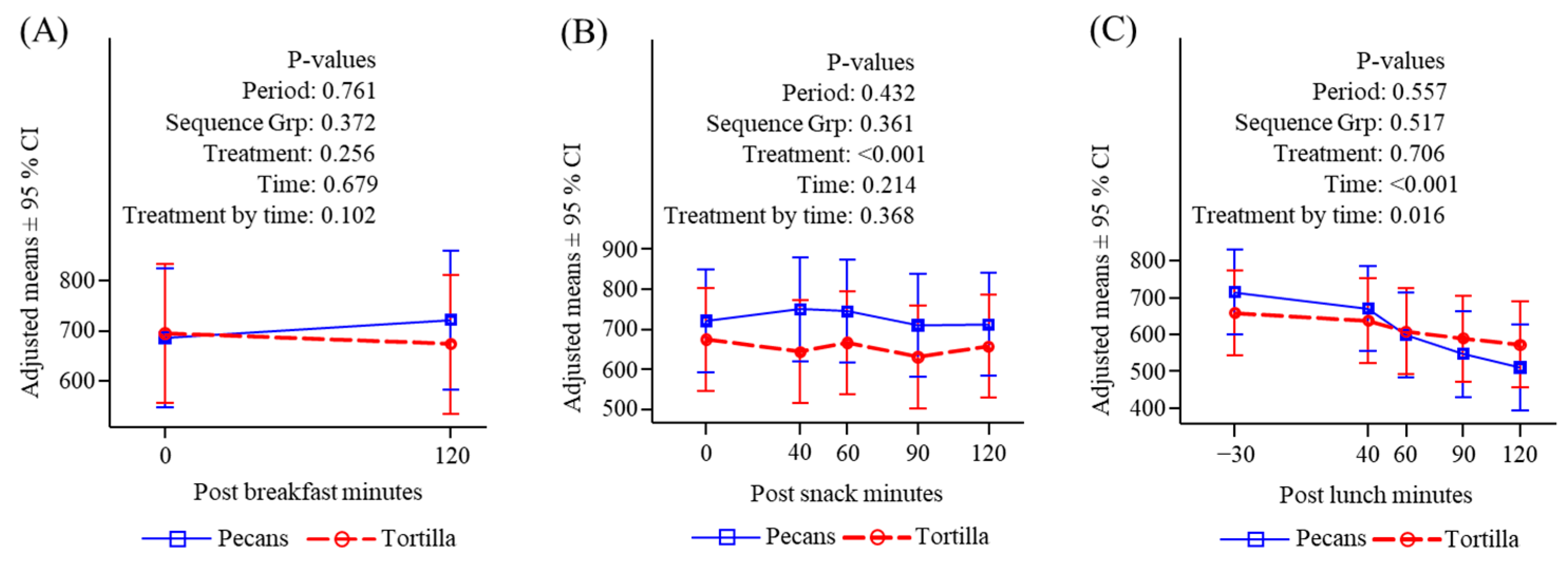
3.4.5. Ghrelin
3.4.6. Peptide YY
3.4.7. GLP-1
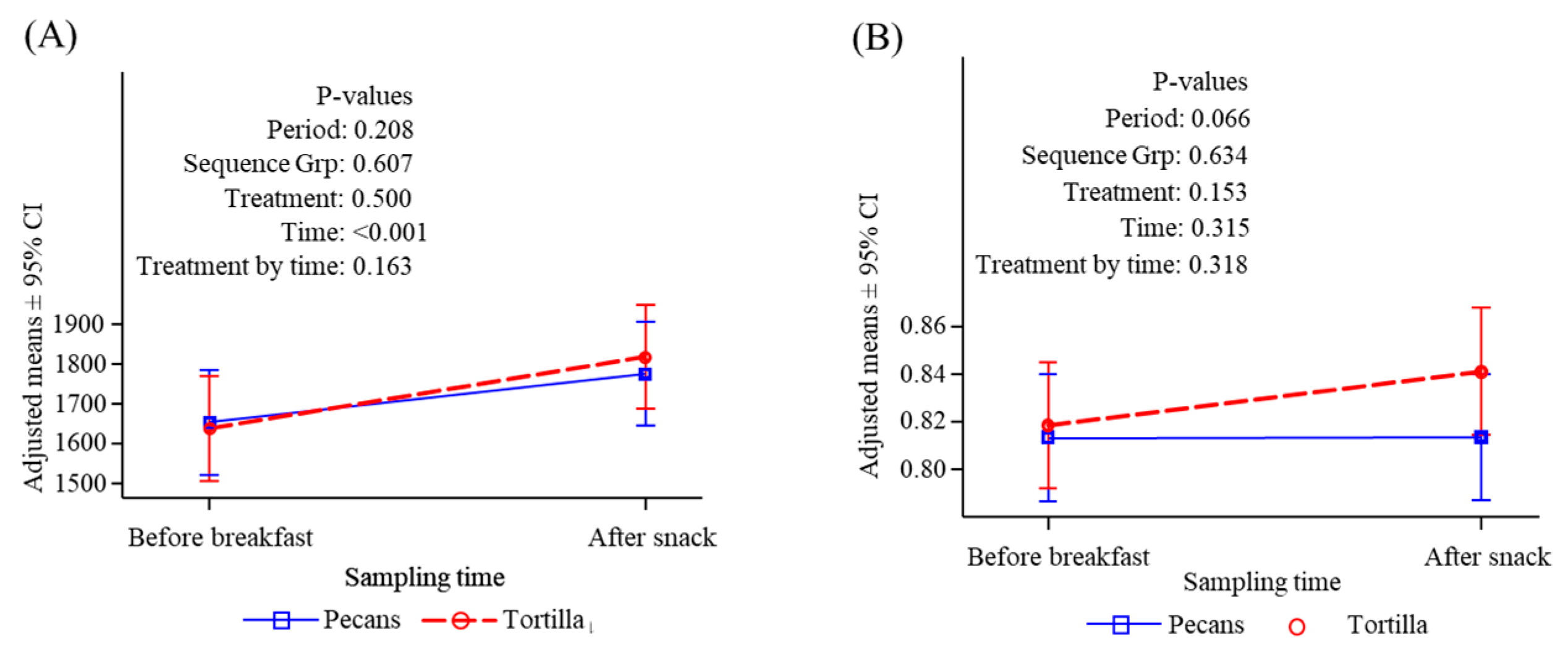
3.5. Resting and Postprandial Energy Expenditure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.; Zidan, S.; Badrasawi, M. Effect of Tree Nuts Consumption on Serum Lipid Profile in Hyperlipidemic Individuals: A Systematic Review. Nutr. Metab. Insights 2020, 13, 1178638820926521. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hu, F.B.; Ros, E.; Sabate, J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J. Nutr. 2008, 138, 1746S–1751S. [Google Scholar] [CrossRef] [PubMed]
- Guarneiri, L.L.; Paton, C.M.; Cooper, J.A. Appetite responses to pecan-enriched diets. Appetite 2022, 173, 106003. [Google Scholar] [CrossRef]
- Cogan, B.; Pearson, R.C.; Jenkins, N.T.; Paton, C.M.; Cooper, J.A. A pecan-enriched diet reduced postprandial appetite intensity and enhanced peptide YY secretion: A randomized control trial. Clin. Nutr. ESPEN 2023, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Guarneiri, L.L.; Cooper, J.A. Intake of Nuts or Nut Products Does Not Lead to Weight Gain, Independent of Dietary Substitution Instructions: A Systematic Review and Meta-Analysis of Randomized Trials. Adv. Nutr. 2020, 12, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Guarneiri, L.L.; Paton, C.M.; Cooper, J.A. Changes in body weight in response to pecan-enriched diets with and without substitution instructions: A randomised, controlled trial. J. Nutr. Sci. 2022, 11, e16. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Torre-Villalvazo, I.; Noriega, L.G.; Rodríguez-López, L.A.; Alemán, G.; Torre-Anaya, E.A.; Cariño-Cervantes, Y.Y.; Palacios-Gonzalez, B.; Furuzawa-Carballeda, J.; Tovar, A.R.; et al. Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 2591. [Google Scholar] [CrossRef]
- Woźniak, M.; Waśkiewicz, A.; Ratajczak, I. The Content of Phenolic Compounds and Mineral Elements in Edible Nuts. Molecules 2022, 27, 4326. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; Urrea-López, R.; de la Rosa, L.A. Bioactive components and health effects of pecan nuts and their byproducts: A review. J. Food Bioact. 2018, 1, 56–92. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.Y.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Sabharanjak, S.M.; Zengin, G.; Mollica, A.; Szostak, A.; Simirgiotis, M.; Huminiecki, Ł.; Horbanczuk, O.K.; Nabavi, S.M.; Mocan, A. Pecan nuts: A review of reported bioactivities and health effects. Trends Food Sci. Technol. 2018, 71, 246–257. [Google Scholar] [CrossRef]
- Villarreal-Lozoya, J.E.; Lombardini, L.; Cisneros-Zevallos, L. Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chem. 2007, 102, 1241–1249. [Google Scholar] [CrossRef]
- Sabate, J.; Wien, M. Nuts, blood lipids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2010, 19, 131–136. [Google Scholar] [PubMed]
- Casas-Agustench, P.; Lopez-Uriarte, P.; Bullo, M.; Ros, E.; Cabre-Vila, J.J.; Salas-Salvado, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.; Clifton, P.M. Nuts and Cardio-Metabolic Disease: A Review of Meta-Analyses. Nutrients 2018, 10, 1935. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xia, J.; Ke, Y.; Cheng, J.; Yuan, J.; Wu, S.; Lv, Z.; Huang, S.; Kim, J.H.; Wong, S.Y.; et al. Effects of nut consumption on selected inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2018, 54, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, S.; Hill, A.M.; Coates, A.M. The Effects of Nut Consumption on Vascular Function. Nutrients 2019, 11, 116. [Google Scholar] [CrossRef]
- Neale, E.P.; Tapsell, L.C.; Guan, V.; Batterham, M.J. The effect of nut consumption on markers of inflammation and endothelial function: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e016863. [Google Scholar] [CrossRef]
- McKay, D.L.; Eliasziw, M.; Chen, C.Y.O.; Blumberg, J.B. A Pecan-Rich Diet Improves Cardiometabolic Risk Factors in Overweight and Obese Adults: A Randomized Controlled Trial. Nutrients 2018, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Burke, K.; Connell, B.; Myint, T.; Sabaté, J. A Monounsaturated Fatty Acid–Rich Pecan-Enriched Diet Favorably Alters the Serum Lipid Profile of Healthy Men and Women. J. Nutr. 2001, 131, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Gebauer, S.K.; Baer, D.J. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am. J. Clin. Nutr. 2012, 96, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Walnuts Consumed by Healthy Adults Provide Less Available Energy than Predicted by the Atwater Factors. J. Nutr. 2016, 146, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Re, R.; Chambers, L.; Echaniz, A.; Wickham, M.S.J. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur. J. Nutr. 2015, 54, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Sayer, R.D.; Dhillon, J.; Tamer, G.G.; Cornier, M.-A.; Chen, N.; Wright, A.J.; Campbell, W.W.; Mattes, R.D. Consuming Almonds vs. Isoenergetic Baked Food Does Not Differentially Influence Postprandial Appetite or Neural Reward Responses to Visual Food Stimuli. Nutrients 2017, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Dreher, M.L. Nuts and healthy body weight maintenance mechanisms. Asia Pac. J. Clin. Nutr. 2010, 19, 137–141. [Google Scholar] [PubMed]
- Tan, S.Y.; Dhillon, J.; Mattes, R.D. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 412S–422S. [Google Scholar] [CrossRef] [PubMed]
- Lawton, C.L.; Delargy, H.J.; Brockman, J.; Smith, F.C.; Blundell, J.E. The degree of saturation of fatty acids influences post-ingestive satiety. Br. J. Nutr. 2000, 83, 473–482. [Google Scholar] [CrossRef]
- Polley, K.R.; Kamal, F.; Paton, C.M.; Cooper, J.A. Appetite responses to high-fat diets rich in mono-unsaturated versus poly-unsaturated fats. Appetite 2019, 134, 172–181. [Google Scholar] [CrossRef]
- Stevenson, J.L.; Clevenger, H.C.; Cooper, J.A. Hunger and satiety responses to high-fat meals of varying fatty acid composition in women with obesity. Obesity 2015, 23, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, H.C.; Kozimor, A.L.; Paton, C.M.; Cooper, J.A. Acute effect of dietary fatty acid composition on postprandial metabolism in women. Exp. Physiol. 2014, 99, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Lopez-Uriarte, P.; Bullo, M.; Ros, E.; Gomez-Flores, A.; Salas-Salvado, J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin. Nutr. 2009, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.G.; Otieno, D.; Ha, K. Flavonoids, Potential Bioactive Compounds, and Non-Shivering Thermogenesis. Nutrients 2018, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Rojas-Rueda, D.; Basora, J.; Ros, E.; Salas-Salvadó, J. Nut intake and adiposity: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2013, 97, 1346–1355. [Google Scholar] [CrossRef]
- Sabate, J. Nut consumption and body weight. Am. J. Clin. Nutr. 2003, 78, 647s–650s. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Sabate, J. Nuts, body weight and insulin resistance. Br. J. Nutr. 2006, 96 (Suppl. S2), S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.A.; Sabate, J.M.; Ikle, D.N.; Cole, S.E.; Kandeel, F.R. Almonds vs complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1365–1372. [Google Scholar] [CrossRef]
- Fraser, G.E.; Bennett, H.W.; Jaceldo, K.B.; Sabate, J. Effect on body weight of a free 76 Kilojoule (320 calorie) daily supplement of almonds for six months. J. Am. Coll. Nutr. 2002, 21, 275–283. [Google Scholar] [CrossRef]
- Baer, D.J.; Dalton, M.; Blundell, J.; Finlayson, G.; Hu, F.B. Nuts, Energy Balance and Body Weight. Nutrients 2023, 15, 1162. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Grunwald, G.K.; Johnson, S.L.; Bessesen, D.H. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite 2004, 43, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Sama, S.; Jain, G.; Kant, R.; Bhadoria, A.S.; Naithani, M.; Kumar, A. Quantifying the Homeostatic Model Assessment of Insulin Resistance to Predict Mortality in Multi-organ Dysfunction Syndrome. Indian J. Crit. Care Med. 2021, 25, 1364–1369. [Google Scholar] [CrossRef]
- Wang, B.-S.; Wang, X.-J.; Gong, L.-K. The Construction of a Williams Design and Randomization in Cross-Over Clinical Trials Using SAS. J. Stat. Softw. Code Snippets 2009, 29, 1–10. [Google Scholar] [CrossRef]
- Purcell, S.A.; Legget, K.T.; Halliday, T.M.; Pan, Z.; Creasy, S.A.; Blankenship, J.M.; Hild, A.; Tregellas, J.R.; Melanson, E.L.; Cornier, M.A. Appetitive and Metabolic Responses to an Exercise versus Dietary Intervention in Adults with Obesity. Transl. J. Am. Coll. Sports Med. 2022, 7, e000211. [Google Scholar] [CrossRef] [PubMed]
- Sayer, R.D.; Peters, J.C.; Pan, Z.; Wyatt, H.R.; Hill, J.O. Hunger, Food Cravings, and Diet Satisfaction are Related to Changes in Body Weight during a 6-Month Behavioral Weight Loss Intervention: The Beef WISE Study. Nutrients 2018, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.M.; Byrne, N.M.; King, N.A. Reproducibility of subjective appetite ratings and ad libitum test meal energy intake in overweight and obese males. Appetite 2014, 81, 116–122. [Google Scholar] [CrossRef]
- Rolls, B.J. Dietary energy density: Applying behavioural science to weight management. Nutr. Bull. 2017, 42, 246–253. [Google Scholar] [CrossRef]
- Rolls, B.J. The relationship between dietary energy density and energy intake. Physiol. Behav. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Bell, E.A.; Castellanos, V.H.; Pelkman, C.L.; Thorwart, M.L.; Rolls, B.J. Energy density of foods affects energy intake in normal-weight women. Am. J. Clin. Nutr. 1998, 67, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.W.; French, S.J.; Robinson, T.M.; Yeomans, M.R. Dissociation of the effects of preload volume and energy content on subjective appetite and food intake. Physiol. Behav. 2002, 76, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Rolls, B.J. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am. J. Clin. Nutr. 2001, 73, 1010–1018. [Google Scholar] [CrossRef]
- Cornier, M.A.; Salzberg, A.K.; Endly, D.C.; Bessesen, D.H.; Rojas, D.C.; Tregellas, J.R. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE 2009, 4, e6310. [Google Scholar] [CrossRef]
- Cornier, M.A.; Von Kaenel, S.S.; Bessesen, D.H.; Tregellas, J.R. Effects of overfeeding on the neuronal response to visual food cues. Am. J. Clin. Nutr. 2007, 86, 965–971. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Gregersen, N.T.; Pedersen, S.D.; Arentoft, J.L.; Ritz, C.; Schwartz, T.W.; Holst, J.J.; Astrup, A.; Sjödin, A. Effects of PYY3–36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E1248–E1256. [Google Scholar] [CrossRef] [PubMed]
- Fiszman, S.; Tarrega, A. Expectations of food satiation and satiety reviewed with special focus on food properties. Food Funct. 2017, 8, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Flatt, S.W.; Barkai, H.S.; Pakiz, B.; Heath, D.D. A walnut-containing meal had similar effects on early satiety, CCK, and PYY, but attenuated the postprandial GLP-1 and insulin response compared to a nut-free control meal. Appetite 2017, 117, 51–57. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Kim, D. Chapter 14—Glycemic index. In Obesity; Mehrzad, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–189. [Google Scholar]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes1,2,3. Am. J. Clin. Nutr. 2002, 76, 274S–280S. [Google Scholar] [CrossRef]
- Laws, A.; Hoen, H.M.; Selby, J.V.; Saad, M.F.; Haffner, S.M.; Howard, B.V. Differences in Insulin Suppression of Free Fatty Acid Levels by Gender and Glucose Tolerance Status. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 64–71. [Google Scholar] [CrossRef]
- Campbell, P.J.; Carlson, M.G.; Hill, J.O.; Nurjhan, N. Regulation of free fatty acid metabolism by insulin in humans: Role of lipolysis and reesterification. Am. J. Physiol.-Endocrinol. Metab. 2006, 263, E1063–E1069. [Google Scholar] [CrossRef]
- Samson, C.E.; Galia, A.L.; Llave, K.I.; Zacarias, M.B.; Mercado-Asis, L.B. Postprandial Peaking and Plateauing of Triglycerides and VLDL in Patients with Underlying Cardiovascular Diseases Despite Treatment. Int. J. Endocrinol. Metab. 2012, 10, 587–593. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef]
- Tschöp, M.; Wawarta, R.; Riepl, R.L.; Friedrich, S.; Bidlingmaier, M.; Landgraf, R.; Folwaczny, C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Investig. 2001, 24, Rc19–Rc21. [Google Scholar] [CrossRef]
- Monteleone, P.; Bencivenga, R.; Longobardi, N.; Serritella, C.; Maj, M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 5510–5514. [Google Scholar] [CrossRef]
- Thomas, E.A.; Bechtell, J.L.; Vestal, B.E.; Johnson, S.L.; Bessesen, D.H.; Tregellas, J.R.; Cornier, M.A. Eating-related behaviors and appetite during energy imbalance in obese-prone and obese-resistant individuals. Appetite 2013, 65, 96–102. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Stanley, S.; Wynne, K.; Bloom, S. Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G693–G697. [Google Scholar] [CrossRef]
- Spreckley, E.; Murphy, K.G. The L-Cell in Nutritional Sensing and the Regulation of Appetite. Front. Nutr. 2015, 2, 23. [Google Scholar] [CrossRef]
- Cooper, J.A. Factors affecting circulating levels of peptide YY in humans: A comprehensive review. Nutr. Res. Rev. 2014, 27, 186–197. [Google Scholar] [CrossRef]
- Helou, N.; Obeid, O.; Azar, S.T.; Hwalla, N. Variation of postprandial PYY3–36Response following ingestion of differing macronutrient meals in obese females. Ann. Nutr. Metab. 2008, 52, 188–195. [Google Scholar] [CrossRef]
- Essah, P.A.; Levy, J.R.; Sistrun, S.N.; Kelly, S.M.; Nestler, J.E. Effect of macronutrient composition on postprandial peptide YY levels. J. Clin. Endocrinol. Metab. 2007, 92, 4052–4055. [Google Scholar] [CrossRef]
- Brubaker, P.L. The glucagon-like peptides: Pleiotropic regulators of nutrient homeostasis. Ann. N. Y. Acad. Sci. 2006, 1070, 10–26. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef]
- Guarneiri, L.L.; Paton, C.M.; Cooper, J.A. Pecan-enriched diets increase energy expenditure and fat oxidation in adults at-risk for cardiovascular disease in a randomised, controlled trial. J. Hum. Nutr. Diet. 2022, 35, 774–785. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]


| Time (min) | −30 | 0 | 15 | 40 | 60 | 90 | 120 | 15 | 40 | 60 | 90 | 120 | 15 | 40 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meal | B’fast | Snack | Lunch | ||||||||||||||
| Glucose | X | X | X | X | X | X | X | X | X | X | |||||||
| Insulin | X | X | X | X | X | X | X | X | X | X | |||||||
| FFA | X | X | X | X | X | X | X | X | X | X | |||||||
| TG | X | X | X | X | X | X | X | X | X | X | |||||||
| Leptin | X | ||||||||||||||||
| Ghrelin, PYY, GLP1 | X | X | X | X | X | X | X | X | X | X | |||||||
| Appetite ratings | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Calorimetry | X | X |
| Characteristic | (N = 20) |
|---|---|
| Sex n, (%) | Female 14 Male 6 |
| Age, mean (SD 1, range) Age, median | 35.8 (8.6; 24–51) 38.5 |
| Ethnicity n, (%) | Hispanic/Latino 5 (25) Non-Hispanic/Latino 15 (75) |
| Race n, (%) | White 13 (65) Black or AA 2 (10) Asian 3 (15) Other 2 (10) |
| Height (cm) mean (SD) | 165.0 (9.3) |
| Body weight (kg) mean (SD) | 84.7 (15.9) |
| BMI 2 (kg/m2) mean (SD, range) BMI median | 30.9 (3.3, 27.2–39.2) 29.8 |
| Leptin (ng/mL) mean (SD); Visit 1; Visit 2 | 37.4 (27.5) 34.9 (22.3) |
| Glucose (mg/dL) mean (SD); Visit 1 Visit 2 | 85.8 (6.6) 87.3 (12.7) |
| Insulin (µU/mL) mean (SD); Visit 1 Visit 2 | 7.9 (6.0) 6.9 (5.3) |
| HOMA-IR mean (SD); Visit 1 Visit 2 | 1.7 (1.4) 1.6 (1.4) |
| FFA (µmol/L) mean (SD); Visit 1 Visit 2 | 443.0 (191.0) 470.8 (161.8) |
| Triglycerides (mg/dL) mean (SD); Visit 1, Visit 2 | 143.9 (170.2) 133.9 (118.8) |
| Ghrelin (pg/mL) mean (SD); Visit 1 Visit 2 | 697.7 (289.2) 694.7 (319.0) |
| Peptide YY (pg/mL) mean (SD); Visit 1, Visit 2 | 105.9 (33.1) 98.5 (28.8) |
| GLP-1 (pmol/L) mean (SD); Visit 1 Visit 2 | 4.6 (4.3) 8.1 (19.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, J.C.; Breen, J.A.; Pan, Z.; Nicklas, J.; Cornier, M.-A. A Randomized, Crossover Trial Assessing Appetite, Energy Metabolism, Blood Biomarkers, and Ad Libitum Food Intake Responses to a Mid-Morning Pecan Snack vs. an Equicaloric High-Carbohydrate Snack in Healthy Volunteers with Overweight/Obesity. Nutrients 2024, 16, 2084. https://doi.org/10.3390/nu16132084
Peters JC, Breen JA, Pan Z, Nicklas J, Cornier M-A. A Randomized, Crossover Trial Assessing Appetite, Energy Metabolism, Blood Biomarkers, and Ad Libitum Food Intake Responses to a Mid-Morning Pecan Snack vs. an Equicaloric High-Carbohydrate Snack in Healthy Volunteers with Overweight/Obesity. Nutrients. 2024; 16(13):2084. https://doi.org/10.3390/nu16132084
Chicago/Turabian StylePeters, John C., Jeanne Anne Breen, Zhaoxing Pan, Jacinda Nicklas, and Marc-Andre Cornier. 2024. "A Randomized, Crossover Trial Assessing Appetite, Energy Metabolism, Blood Biomarkers, and Ad Libitum Food Intake Responses to a Mid-Morning Pecan Snack vs. an Equicaloric High-Carbohydrate Snack in Healthy Volunteers with Overweight/Obesity" Nutrients 16, no. 13: 2084. https://doi.org/10.3390/nu16132084
APA StylePeters, J. C., Breen, J. A., Pan, Z., Nicklas, J., & Cornier, M.-A. (2024). A Randomized, Crossover Trial Assessing Appetite, Energy Metabolism, Blood Biomarkers, and Ad Libitum Food Intake Responses to a Mid-Morning Pecan Snack vs. an Equicaloric High-Carbohydrate Snack in Healthy Volunteers with Overweight/Obesity. Nutrients, 16(13), 2084. https://doi.org/10.3390/nu16132084






