The Evolution in Non-Alcoholic Fatty Liver Disease Patients’ Profile and the Associated Sustainable Challenges: A Multidisciplinary Perspective
Abstract
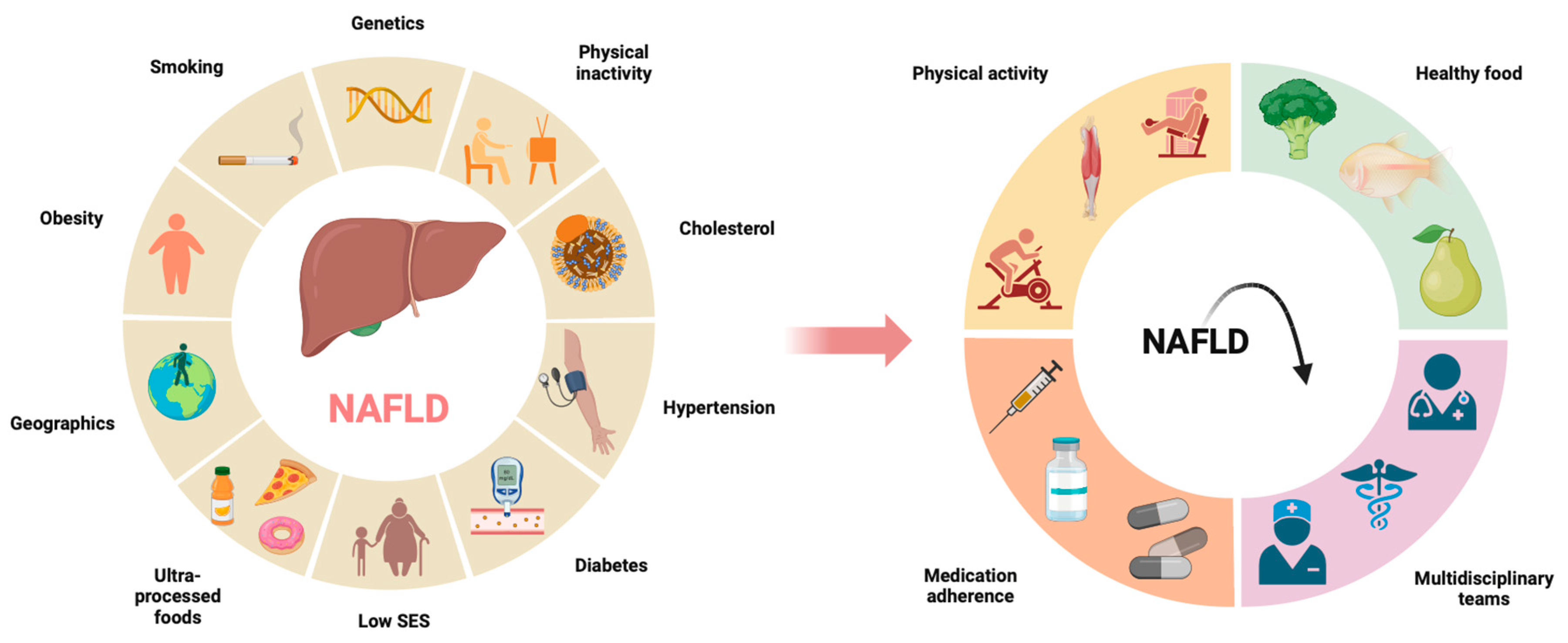
1. Introduction
2. Methods
3. Chronic Liver Disease
4. Lifestyle
4.1. Nutrition and Profile
4.2. Socioeconomic
4.3. Exercise
4.4. Smoking
4.5. Sleep
4.6. Patient Empowerment
5. Science Implementation
6. Interventions and Their Success Rates
6.1. Lifestyle
6.2. Pharmacological
7. Sustainability and Food Waste
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.-I. Association of regular walking and body mass index on metabolic syndrome among an elderly Korean population. Exp. Gerontol. 2018, 106, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.-C.; Batal, M.; Louzada, M.L.; Steele, E.M.; Monteiro, C.A. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017, 108, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Moubarac, J.-C.; Levy, R.B.; Canella, D.S.; da Costa Louzada, M.L.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018, 21, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Hemmingsson, E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr. 2015, 18, 3096–3107. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Peta, V.; Alfieri, F.; De Lorenzo, A.; Bellentani, S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J. Gastroenterol. 2014, 20, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Kummu, M.; de Moel, H.; Porkka, M.; Siebert, S.; Varis, O.; Ward, P. Lost food, wasted resources: Global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci. Total Environ. 2012, 438, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Peydayesh, M.; Bagnani, M.; Bagnani, M.; Soon, W.L.; Soon, W.L.; Mezzenga, R.; Mezzenga, R. Turning Food Protein Waste into Sustainable Technologies. Chem. Rev. 2023, 123, 2112–2154. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.Y.; Kim, S.U.; Ahn, S.H.; Jang, Y. Self-Management and Associated Factors among Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 667. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, J.Y.; Kim, S.U.; Kim, B. A qualitative study of self-management experiences in people with non-alcoholic fatty liver disease. Nurs. Open 2021, 8, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; E Underwood, F.; A King, J.; Afshar, E.E.; Swain, M.G.; E Congly, S.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Sweeny, K.F.; Lee, C.K. Nonalcoholic Fatty Liver Disease in Children. Gastroenterol. Hepatol. 2021, 17, 579. Available online: https://pubmed.ncbi.nlm.nih.gov/35465068/ (accessed on 10 May 2024).
- Bellentani, S.; Saccoccio, G.; Masutti, F.; Crocè, L.S.; Brandi, G.; Sasso, F.; Cristanini, G.; Tiribelli, C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. 2000, 132, 112–117. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Dolce, A.; Della Torre, S. Sex, Nutrition, and NAFLD: Relevance of Environmental Pollution. Nutrients 2023, 15, 2335. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, M.C.; Stableforth, W.; Krag, A.; Reuben, A. The Negative Bidirectional Interaction Between Climate Change and the Prevalence and Care of Liver Disease: A Joint BSG, BASL, EASL, and AASLD Commentary. Gastroenterology 2022, 162, 1561–1567. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schuetz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, K. The Role of Life Style Modifications in Comprehensive Non-Alcoholic Fatty Liver Disease Treatment. Clin. Liver Dis. 2021, 17, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schuetz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, X.; Grammatikopoulou, M.G.; Petalidou, A.; Kontonika, S.; Potamianos, S.P.; Bogdanos, D.P. A Systematic Review of Medical Nutrition Therapy Guidelines for Liver Cirrhosis: Do We Agree? Nutr. Clin. Pract. 2020, 35, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, K.M.; Horkan, C.M.; Purtle, S.W.; Moromizato, T.; Rawn, J.D.; Robinson, M.K.; Christopher, K.B. Malnutrition, Critical Illness Survivors, and Postdischarge Outcomes: A Cohort Study. J. Parenter. Enter. Nutr. 2018, 42, 557–565. [Google Scholar] [CrossRef]
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.J.; Buitrago, G.; Rodriguez, N.; Gomez, G.; Sulo, S.; Gomez, C.; Partridge, J.; Misas, J.; Dennis, R.; Alba, M.J.; et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin. Nutr. 2019, 38, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Guenter, P.; Abdelhadi, R.; Anthony, P.; Blackmer, A.; Malone, A.; Mirtallo, J.M.; Phillips, W.; Resnick, H.E. Malnutrition diagnoses and associated outcomes in hospitalized patients: United States, 2018. Nutr. Clin. Pract. 2021, 36, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.; Yannakoulia, M.; Papatheodoridis, G.V.; Deutsch, M.; Alexopoulou, A.; Vlachogiannakos, J.; Ioannidou, P.; Papageorgiou, M.-V.; Voulgaris, T.; Papadopoulos, N.; et al. Assessment of dietary habits and the adequacy of dietary intake of patients with cirrhosis-the KIRRHOS study. Clin. Nutr. 2021, 40, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.E.; Oji, S.; Mufti, A.R.; Browning, J.D.; Parikh, N.D.; Odewole, M.; Mayo, H.; Singal, A.G. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 198–210.e2. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Kopka, C.J.; Younossi, Z.M.; Allen, A.M. It is time to expand the fatty liver disease community of practice. Hepatology 2023, 78, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Kobylinska, M.; Antosik, K.; Decyk, A.; Kurowska, K. Malnutrition in Obesity: Is It Possible? Obes. Facts 2022, 15, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.-Y.; Zhai, Z.-Z.; Li, Z.-F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef] [PubMed]
- Raffensperger, S.; Kuczmarski, M.F.; Hotchkiss, L.; Cotugna, N.; Evans, M.K.; Zonderman, A.B. The effect of race and predictors of socioeconomic status on diet quality in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study sample NIH Public Access. J. Natl. Med. Assoc. 2010, 102, 923–930. [Google Scholar] [PubMed]
- Cho, J.; Lee, I.; Park, D.-H.; Kwak, H.-B.; Min, K. Relationships between socioeconomic status, handgrip strength, and non-alcoholic fatty liver disease in middle-aged adults. Int. J. Environ. Res. Public Health 2021, 18, 1892. [Google Scholar] [CrossRef] [PubMed]
- A Gilbert, P.; Khokhar, S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr. Rev. 2008, 66, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2020, 125, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Pikul, J.; Keller, H.; Yeung, C.Y.E.; Rahman, A.; Heyland, D.K.; Mourtzakis, M. Adequacy of Protein and Energy Intake in Critically Ill Adults Following Liberation from Mechanical Ventilation Is Dependent on Route of Nutrition Delivery. Nutr. Clin. Pract. 2021, 36, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Dhaliwal, R.; Jurewitsch, B.; Stapleton, R.D.; Jeejeebhoy, K.N.; Heyland, D.K. Parenteral fish oil lipid emulsions in the critically ill. J. Parenter. Enter. Nutr. 2014, 38, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2016, 66, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lu, J.; Liu, X.; Liu, J.; Ma, Q.; Shi, Y.; Su, H. Effect of Long-Term Exercise on Liver Lipid Metabolism in Chinese Patients With NAFLD: A Systematic Review and Meta-Analysis. Front. Physiol. 2021, 12, 748517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, W.; Li, J.; Ossowski, Z. Effects of aerobic exercise on metabolic indicators and physical performance in adult NAFLD patients: A systematic review and network meta-analysis. Medicine 2023, 102, e33147. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.F.; Csader, S.; Lok, J.; Gómez-Gallego, C.; Hanhineva, K.; El-Nezami, H.; Schwab, U. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: A systematic review and meta-analysis. Nutrients 2021, 13, 3135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Z.; Yang, Q.; Hu, Z.; Zhou, W.; Ji, G.; Dang, Y. Impact of smoking cessation on non-alcoholic fatty liver disease prevalence: A systematic review and meta-analysis. BMJ Open 2023, 13, e074216. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Joo, H.J.; Park, Y.S.; Park, E.C.; Jang, S.I. REVIEWED BY Association between smoking cessation and non-alcoholic fatty liver disease using NAFLD liver fat score. Front. Public Health 2023, 11, 1015919. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.K.; Zopey, M.; Friedman, T.C. Erratum: Metabolic effects of smoking cessation. Nat. Rev. Endocrinol. 2016, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Fullick, S.; Grindey, C.; Maclaren, D. Exercise, Energy Balance and the Shift Worker. Sports Med. 2008, 38, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, H.; Takahashi, A.; Tanji, N.; Abe, K.; Sato, Y.; Anzai, Y.; Watanabe, H.; Ohira, H. The Association between Sleep Duration and Non-Alcoholic Fatty Liver Disease among Japanese Men and Women. Obes. Facts 2015, 8, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, K.; Xi, Z.; Ma, Y.; Shao, C.; Wang, W.; Tang, Y.-D. Short sleep duration and the risk of nonalcoholic fatty liver disease/metabolic associated fatty liver disease: A systematic review and meta-analysis. Sleep Breath. 2023, 27, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Short sleep duration is a risk of incident nonalcoholic fatty liver disease: A population-based longitudinal study. J. Gastrointest. Liver Dis. 2019, 28, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, P.G.; Juvé-Udina, M.-E.; Delgado-Hito, P. Concepts and measures of patient empowerment: A comprehensive review. Rev. Esc. Enferm. USP 2016, 50, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Z.M.; Bloom, P.P.; Grzyb, K.; Tapper, E.B. How Do Patients With Cirrhosis and Their Caregivers Learn About and Manage Their Health? A Review and Qualitative Study. Hepatol. Commun. 2021, 5, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Curtis, S.; Shariff, M. Patient education and its effect on self-management in cirrhosis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 582–587. [Google Scholar] [CrossRef] [PubMed]
- A Goldsworthy, M.; Fateen, W.; Thygesen, H.; A Aldersley, M.; A Rowe, I.; Jones, R.L. Patient understanding of liver cirrhosis and improvement using multimedia education. Front. Gastroenterol. 2017, 8, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.L.; Horsfall, L.U.; Ruffin, B.J.; Cottrell, W.N.; Chachay, V.S.; Irvine, K.M.; Martin, J.H.; Powell, E.E.; Valery, P.C. Optimising care of patients with chronic disease: Patient-oriented education may improve disease knowledge and self-management. Intern. Med. J. 2017, 47, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Volk, M.L.; Fisher, N.; Fontana, R.J. Patient knowledge about disease self-management in cirrhosis. Am. J. Gastroenterol. 2013, 108, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M.; Adib-Hajbagheri, M.; Memarian, R.; Nejhad, A.K.; Alavian, S.M. Effects of a self-care program on quality of life of cirrhotic patients referring to Tehran Hepatitis Center. Health Qual. Life Outcomes 2005, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Wang, H.; Zhou, N.; Zhang, Y.-D.; Lin, Y.; Wu, L.-Y.; Wei, S.-F.; Ma, Y.-Y.; Wang, C.-X. A multidisciplinary team approach to the treatment of liver cirrhosis. J. Inflamm. Res. 2021, 14, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- Tumilowicz, A.; Ruel, M.T.; Pelto, G.; Pelletier, D.; Monterrosa, E.C.; Lapping, K.; Kraemer, K.; De Regil, L.M.; Bergeron, G.; Arabi, M.; et al. Implementation Science in Nutrition: Concepts and Frameworks for an Emerging Field of Science and Practice. Curr. Dev. Nutr. 2018, 3, nzy080. [Google Scholar] [CrossRef] [PubMed]
- Traub, J.; Reiss, L.; Aliwa, B.; Stadlbauer, V. Malnutrition in Patients with Liver Cirrhosis. Nutrients 2021, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Hyde, A.M.; Johnson, E.; Luig, T.; Schroeder, D.; Carbonneau, M.; Campbell-Scherer, D.; Tandon, P. Implementing a cirrhosis order set in a tertiary healthcare system: A theory-informed formative evaluation. BMC Health Serv. Res. 2023, 23, 636. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.J.; Ogola, G.; Fleming, N.S.; Stauffer, B.D.; Leonard, B.M.; Khetan, R.; Yancy, C.W. Impact of a standardized heart failure order set on mortality, readmission, and quality and costs of care. Int. J. Qual. Health Care 2010, 22, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Bright, T.J.; Wong, A.; Dhurjati, R.; Bristow, E.; Bastian, L.; Coeytaux, R.R.; Samsa, G.; Hasselblad, V.; Williams, J.W.; Musty, M.D.; et al. Effect of Clinical Decision-Support Systems A Systematic Review. Ann. Intern. Med. 2012, 157, 29–43. Available online: https://www.acpjournals.org/doi/full/10.7326/0003-4819-157-1-201207030-00450 (accessed on 15 January 2024). [CrossRef] [PubMed]
- Neilson, L.J.; Macdougall, L.; Lee, P.S.; Hardy, T.; Beaton, D.; Chandrapalan, S.; Ebraheem, A.; Hussien, M.; Galbraith, S.; Looi, S.; et al. Implementation of a care bundle improves the management of patients with non-Alcoholic fatty liver disease. Front. Gastroenterol. 2021, 12, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.A.; Rizvi, S.; Saeian, K. Assessment of Adherence to Baseline Quality Measures for Cirrhosis and the Impact of Performance Feedback in a Regional VA Medical Center. Am. J. Med. Qual. 2017, 33, 262–268. [Google Scholar] [CrossRef]
- Xiol, X.; Salord, S.; Amador, A.; Baliellas, C.; Cachero, A.; Roca, R.R.; Campuzano, V.P.; Castellote, J. Quality of care provided to outpatients with hepatic cirrhosis in a teaching hospital. Rev. Esp. Enfermedades Dig. 2020, 112, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Spelman, T.; Chong, C.-P.; Ha, P.; Sahhar, L.; Lim, J.; He, T.; Heerasing, N.; Sievert, W. Could Adherence to Quality of Care Indicators for Hospitalized Patients with Cirrhosis-Related Ascites Improve Clinical Outcomes? Am. J. Gastroenterol. 2016, 111, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Sinclair, M.; Gow, P.J.; Testro, A.G. Malnutrition in cirrhosis: More food for thought. World J. Hepatol. 2020, 12, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Mokhtare, M.; Abdi, A.; Sadeghian, A.M.; Sotoudeheian, M.; Namazi, A.; Sikaroudi, M.K. Investigation about the correlation between the severity of metabolic-associated fatty liver disease and adherence to the Mediterranean diet. Clin. Nutr. ESPEN 2023, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.-Y.; Hsieh, M.-L.; Chen, J.-A.; Hsieh, V.C.-R. Association between medication adherence and disease outcomes in patients with hepatitis B-related cirrhosis: A population-based case–control study. BMJ Open 2022, 12, e059856. [Google Scholar] [CrossRef] [PubMed]
- Polis, S.; Zang, L.; Mainali, B.; Pons, R.; Pavendranathan, G.; Zekry, A.; Fernandez, R. Factors associated with medication adherence in patients living with cirrhosis. J. Clin. Nurs. 2016, 25, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.L.; Valery, P.C.; Martin, J.H.; Karmakar, A.; Patel, P.J.; Horsfall, L.U.; Tallis, C.J.; A Stuart, K.; Wright, P.L.; Smith, D.D.; et al. Medication beliefs predict medication adherence in ambulatory patients with decompensated cirrhosis. World J. Gastroenterol. 2017, 23, 7321–7331. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Vojjala, N.; Mishra, S.; Valsan, A.; Kaur, R.; Kaur, T.; De, A.; Premkumar, M.; Taneja, S.; Duseja, A.; et al. Machine learning can guide suitability of consultation and patient referral through telemedicine for hepatobiliary diseases. J. Gastroenterol. Hepatol. 2023, 38, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Maccauro, V.; Cintoni, M.; Cambieri, A.; Fiore, A.; Zega, M.; Gasbarrini, A.; Mele, M.C. Hospital Services to Improve Nutritional Intake and Reduce Food Waste: A Systematic Review. Nutrients 2023, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Berardy, A.; Egan, B.; Birchfield, N.; Sabaté, J.; Lynch, H. Comparison of Plate Waste between Vegetarian and Meat-Containing Meals in a Hospital Setting: Environmental and Nutritional Considerations. Nutrients 2022, 14, 1174. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aerts, M.; Rosseel, Z.; De Waele, E. The Evolution in Non-Alcoholic Fatty Liver Disease Patients’ Profile and the Associated Sustainable Challenges: A Multidisciplinary Perspective. Nutrients 2024, 16, 1584. https://doi.org/10.3390/nu16111584
Aerts M, Rosseel Z, De Waele E. The Evolution in Non-Alcoholic Fatty Liver Disease Patients’ Profile and the Associated Sustainable Challenges: A Multidisciplinary Perspective. Nutrients. 2024; 16(11):1584. https://doi.org/10.3390/nu16111584
Chicago/Turabian StyleAerts, Maridi, Zenzi Rosseel, and Elisabeth De Waele. 2024. "The Evolution in Non-Alcoholic Fatty Liver Disease Patients’ Profile and the Associated Sustainable Challenges: A Multidisciplinary Perspective" Nutrients 16, no. 11: 1584. https://doi.org/10.3390/nu16111584
APA StyleAerts, M., Rosseel, Z., & De Waele, E. (2024). The Evolution in Non-Alcoholic Fatty Liver Disease Patients’ Profile and the Associated Sustainable Challenges: A Multidisciplinary Perspective. Nutrients, 16(11), 1584. https://doi.org/10.3390/nu16111584




