Circulatory Metabolite Ratios as Indicators of Lifestyle Risk Factors Based on a Greek NAFLD Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
MASLD Diagnosis
2.2. Data Collection
2.3. Construction of Lifestyle Risk Score
2.4. Univariate Analysis of the Anthropometrics, Physiological Parameters and LS Score
2.5. Circulatory Metabolome Analysis
NMR-Metabolomics Pipeline
2.6. Multivariate Data Analysis
2.6.1. Post Processing of NMR Spectral Data
2.6.2. Identification of Important Features
2.6.3. Model Validation
2.6.4. Metabolic Markers and Associated Metabolic Pathways
3. Results and Discussion
3.1. Participant Characteristics
3.2. The NMR Metabolic Patterns Interpret Lifestyle Trends
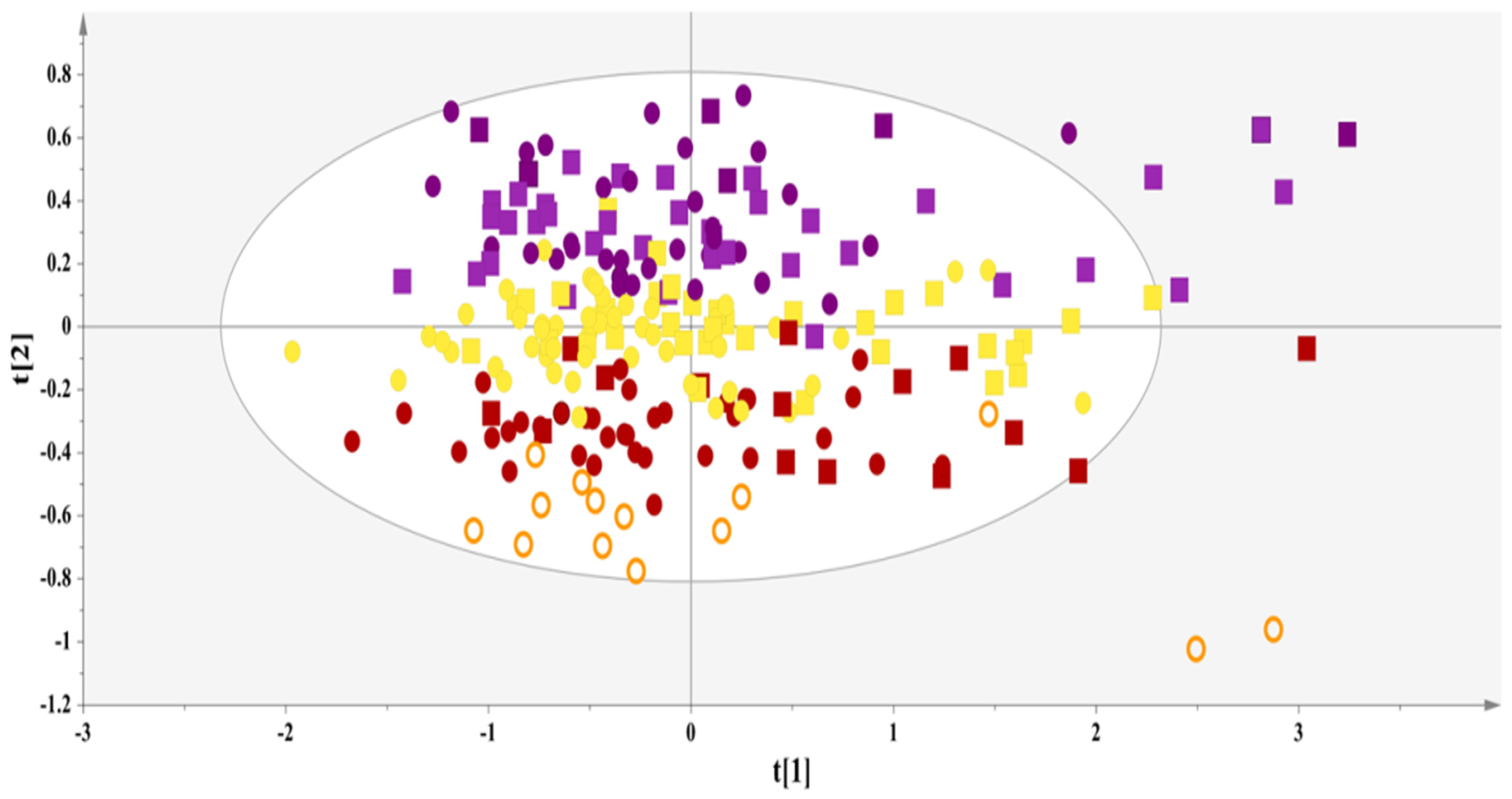
3.2.1. Exploratory Analysis
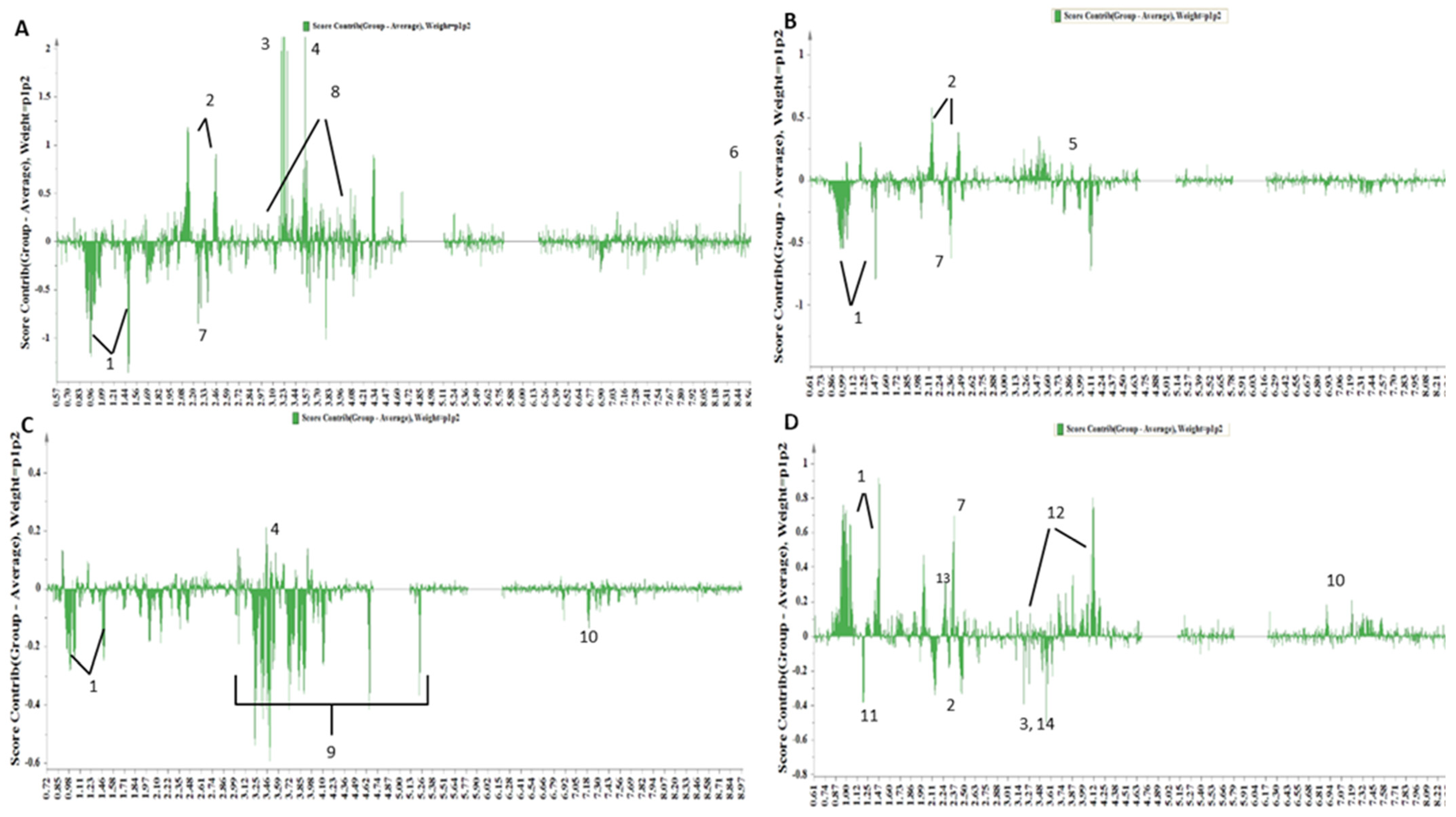
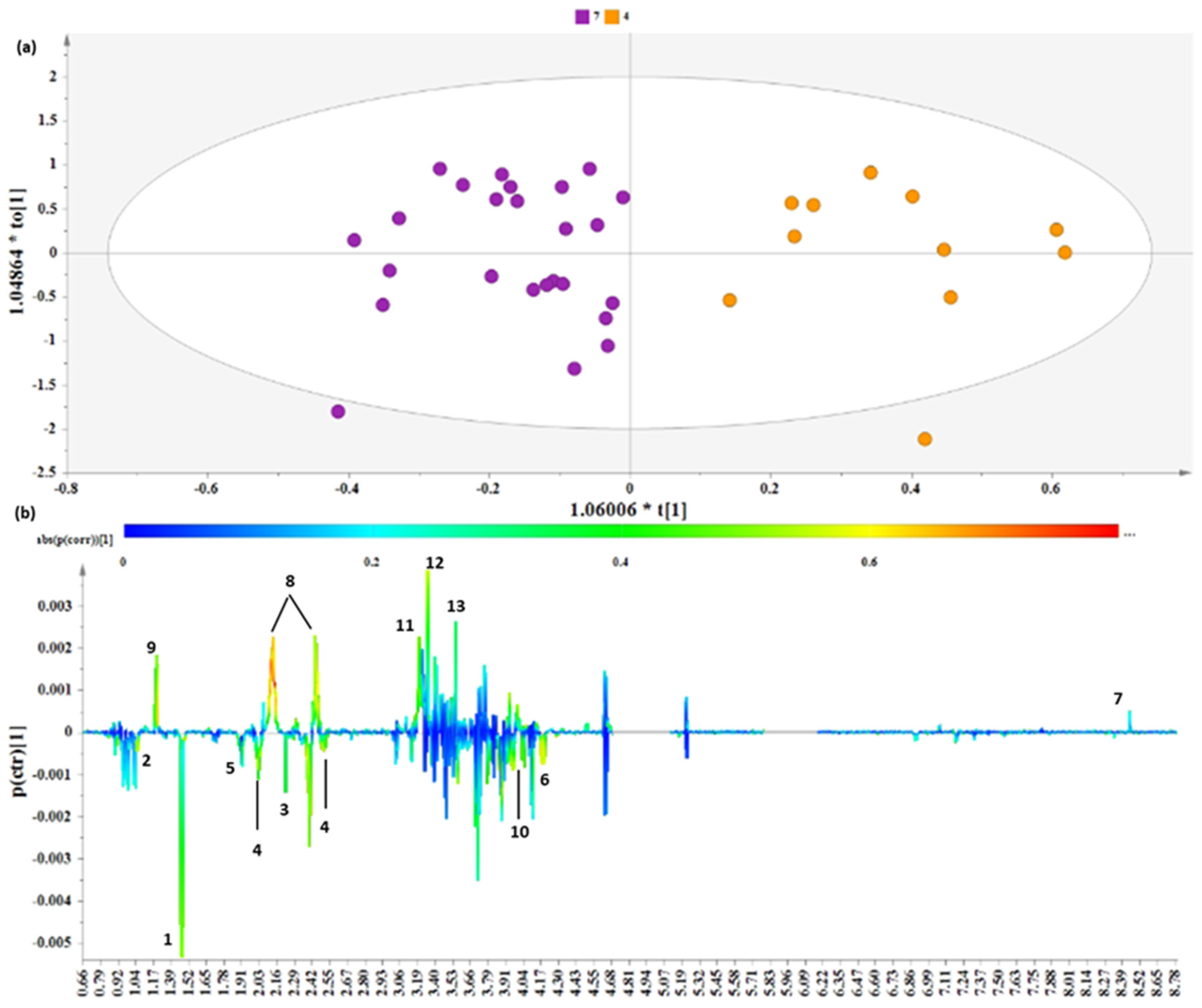
3.2.2. Distinct Metabolite Markers
3.2.3. Receiver Operating Characteristic (ROC) Curve Analysis for Metabolite Markers
3.2.4. Metabolite Pathway Analysis
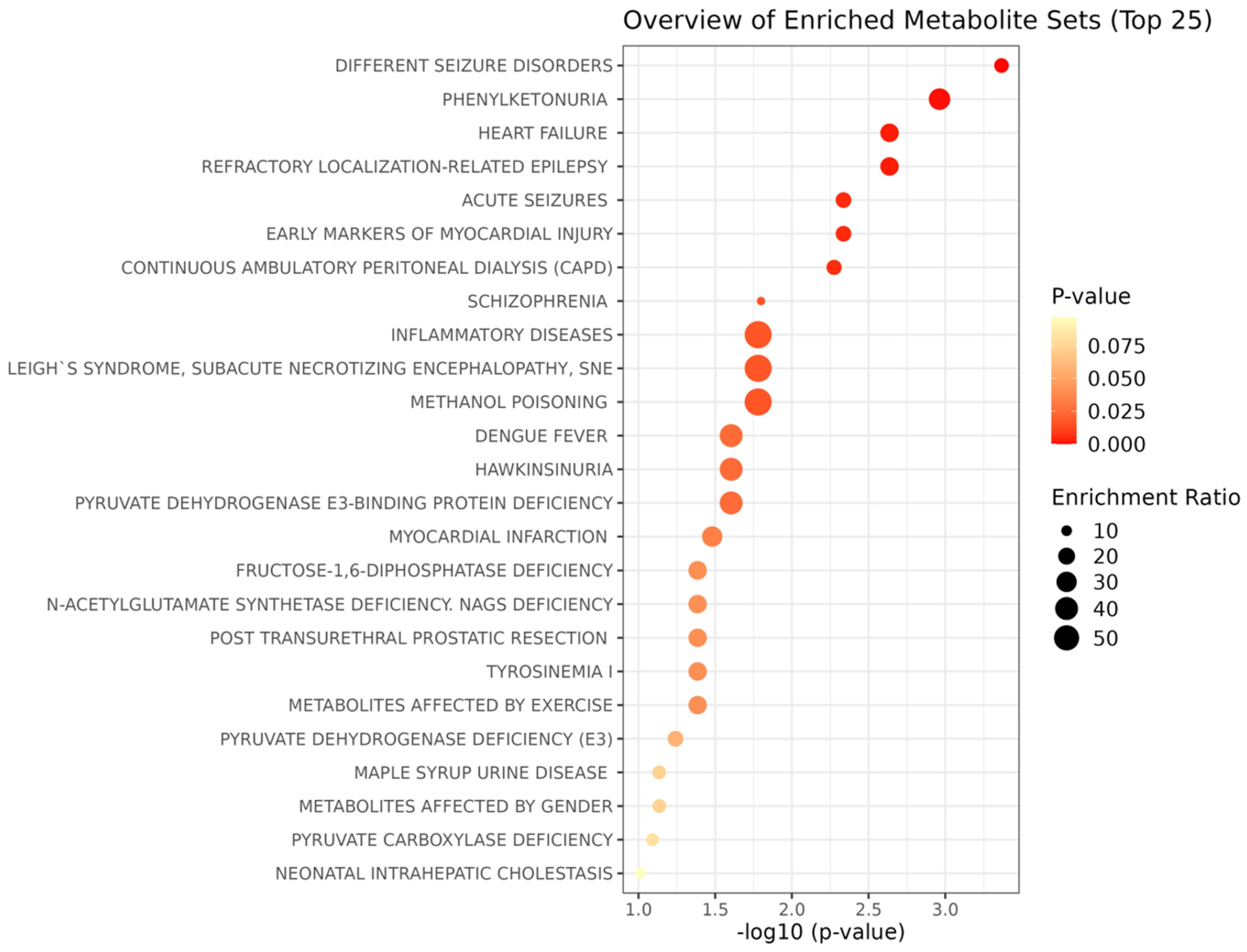
3.2.5. Metabolite Enrichment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, M.B.; Fullman, N.; Ng, M.; Salama, J.S.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abera, S.F.; Abraham, B.; Abyu, G.Y.; et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the global burden of disease study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, S.M.; Jonnalagadda, S.S.; Pisegna, J.L.; Kelly, O.J.; Krok-Schoen, J.L.; Taylor, C.A. Poorer diet quality observed among US adults with a greater number of clinical chronic disease risk factors. J. Prim. Care Community Health 2020, 11, 2150132720945898. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary lifestyle: Overview of updated evidence of potential health risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, J.J. The world health report 2002—Reducing risks, promoting healthy life. Educ. Health Chang. Learn. Pr. 2003, 16, 230. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Ezzati, M.S.; Vander Hoorn, A.D.; Lopez, G.; Danaei, A.; Rodgers, C.; Mathers, D.; Murray, C.J. Comparative quantification of mortality and burden of disease attributable to selected risk factors. Glob. Burd. Dis. Risk Factors 2006, 2, 241–396. [Google Scholar]
- Torossian, M.; Jacelon, C.S.P. Chronic illness and fatigue in older individuals: A systematic review. Rehabil. Nurs. 2020, 46, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic dysfunction-associated steatotic liver disease (MASLD): A state-of-the-art review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Goh, G.B.-B.; Pagadala, M.R.; Dasarathy, J.; Unalp-Arida, A.; Sargent, R.; Hawkins, C.; Sourianarayanane, A.; Khiyami, A.; Yerian, L.; Pai, R.K.; et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2015, 3, 141–145. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006, 26, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Speliotes, E.K.; Yerges-Armstrong, L.M.; Wu, J.; Hernaez, R.; Kim, L.J.; Palmer, C.D.; Gudnason, V.; Eiriksdottir, G.; Garcia, M.E.; Launer, L.J.; et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011, 7, e1001324. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Romano, C.; Chirico, V.; Filippelli, M.; Cuppari, C.; Loddo, I.; Salpietro, C.; Arrigo, T. Nonalcoholic fatty liver disease/non-alcoholic steatohepatitis in childhood: Endocrine-metabolic “mal-programming”. Hepat. Mon. 2014, 14, e17641. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Andres Lacueva, C.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An integrative action for metabolomic analyses in human nutritional studies. Mol. Nutr. Food Res. 2019, 63, e1800384. [Google Scholar] [CrossRef] [PubMed]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite concentration changes in humans after a bout of exercise: A systematic review of exercise metabolomics studies. Sports Med.-Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, Q.; Liang, J.; Weng, Z.; Li, W.; Xu, J.; Zhang, X.; Xu, C.; Gu, A. Association between NMR metabolomic signatures of healthy lifestyle and incident coronary artery disease. Eur. J. Prev. Cardiol. 2022, 30, 243–253. [Google Scholar] [CrossRef]
- Kalafati, I.P.; Borsa, D.; Dimitriou, M.; Revenas, K.; Kokkinos, A.; Dedoussis, G.V. Dietary patterns and non-alcoholic fatty liver disease in a Greek case–control study. Nutrition 2019, 61, 105–110. [Google Scholar] [CrossRef]
- Kavouras, S.; Maraki, M.; Kollia, M.; Gioxari, A.; Jansen, L.; Sidossis, L. Development, reliability and validity of a physical activity questionnaire for estimating energy expenditure in Greek adults. Sci. Sports 2016, 31, e47–e53. [Google Scholar] [CrossRef]
- Dimitriou, M.; Rallidis, L.S.; Theodoraki, E.V.; Kalafati, I.P.; Kolovou, G.; Dedoussis, G.V. Exclusive olive oil consumption has a protective effect on coronary artery disease; overview of the THISEAS study. Public Health Nutr. 2016, 19, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Kalafati, I.-P.; Amanatidou, A.I.; Andreou, V.; Matzapetakis, M.; Kafyra, M.; Varlamis, I.; Zervou, M.; Dedoussis, G.V. Serum metabolomic profiling unveils distinct sex-related metabolic patterns in NAFLD. Front. Endocrinol. 2023, 14, 1230457. [Google Scholar] [CrossRef] [PubMed]
- Filntisi, A.; Fotakis, C.; Asvestas, P.; Matsopoulos, G.K.; Zoumpoulakis, P.; Cavouras, D. Automated metabolite identification from biological fluid 1H NMR spectra. Metabolomics 2017, 13, 146. [Google Scholar] [CrossRef]
- Pietzke, M.; Meiser, J.; Vazquez, A. Formate metabolism in health and disease. Mol. Metab. 2020, 33, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Harnett, W.; Harnett, M.M. Phosphorylcholine: Friend or foe of the immune system? Immunol. Today 1999, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Aguilera, E.; Brunius, C.; Bernal-Lopez, M.R.; Garcia-Aloy, M.; Madrid-Gambin, F.; Tinahones, F.J.; Gómez-Huelgas, R.; Landberg, R.; Andres-Lacueva, C. Impact in plasma metabolome as effect of lifestyle intervention for weight-loss reveals metabolic benefits in metabolically healthy obese women. J. Proteome Res. 2018, 17, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Murphy, N.; Bešević, J.; Kliemann, N.; Jenab, M.; Ferrari, P.; Achaintre, D.; Gicquiau, A.; Vozar, B.; Scalbert, A.; et al. Metabolic signatures of healthy lifestyle patterns and colorectal cancer risk in a european cohort. Clin. Gastroenterol. Hepatol. 2020, 20, e1061–e1082. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Iino, C.; Endo, T.; Mikami, K.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Changed Amino Acids in NAFLD and liver fibrosis: A large cross-sectional study without influence of insulin resistance. Nutrients 2020, 12, 1450. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, L.; Stanley, W.C.; Cabrera, M.E.; Saidel, G.M.; Yu, X. Role of the malate–aspartate shuttle on the metabolic response to myocardial ischemia. J. Theor. Biol. 2008, 254, 466–475. [Google Scholar] [CrossRef]
- Amiel, J.M.; Mathew, S.J. Glutamate and anxiety disorders. Curr. Psychiatry Rep. 2007, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.M.; Phan, K.L. The role of glutamate in anxiety and related disorders. CNS Spectrums 2005, 10, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Assi, N.; Gunter, M.J.; Thomas, D.C.; Leitzmann, M.; Stepien, M.; Chajès, V.; Philip, T.; Vineis, P.; Bamia, C.; Boutron-Ruault, M.-C.; et al. Metabolic signature of healthy lifestyle and its relation with risk of hepatocellular carcinoma in a large European cohort. Am. J. Clin. Nutr. 2018, 108, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Ingwall, J.S.; Brewster, L.M.; Mairuhu, G.; Bindraban, N.R.; Koopmans, R.P.; Clark, J.F.; van Montfrans, G.A.; Siddiqui, M.K.; Veluchamy, A.; et al. Creatine—A Dispensable Metabolite? Circ. Res. 2013, 112, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; de Castro, G.S.; Brosnan, M.E.; Brosnan, J.T. Creatine supplementation as a possible new therapeutic approach for fatty liver disease: Early findings. Amino Acids 2016, 48, 1983–1991. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, J.; Feng, Y.-H.; Su, X.; Chen, S.-Y.; Liu, J.-Z.; Li, W.-L.; Ouyang, R.-Q.; Ma, J.-R.; Cheng, C.; et al. The association between dietary branched-chain amino acids and the risk of cardiovascular diseases in Chinese patients with type 2 diabetes: A hospital-based case–control study. Front. Nutr. 2022, 9, 999189. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef] [PubMed]
- Rios, S.; García-Gavilán, J.F.; Babio, N.; Paz-Graniel, I.; Ruiz-Canela, M.; Liang, L.; Clish, C.B.; Toledo, E.; Corella, D.; Estruch, R.; et al. Plasma Metabolite Profiles Associated with the World Cancer Research Fund/American Institute for Cancer Research Lifestyle Score and Future Risk of Cardiovascular Disease and Type 2 Diabetes. Cardiovasc. Diabetol. 2023, 22, 252. [Google Scholar] [CrossRef]
- Tokarz, J.; Möller, G.; Artati, A.; Huber, S.; Zeigerer, A.; Blaauw, B.; Adamski, J.; Dyar, K.A. Common Muscle Metabolic Signatures Highlight Arginine and Lysine Metabolism as Potential Therapeutic Targets to Combat Unhealthy Aging. Int. J. Mol. Sci. 2021, 22, 7958. [Google Scholar] [CrossRef]
- Wu, T.; Giovannucci, E.; Pischon, T.; Hankinson, S.E.; Ma, J.; Rifai, N.; Rimm, E.B. Fructose, Glycemic Load, and Quantity and Quality of Carbohydrate in Relation to Plasma C-Peptide Concentrations in US Women. Am. J. Clin. Nutr. 2004, 80, 1043–1049. [Google Scholar] [CrossRef]
- True, M.W. Circulating biomarkers of glycemia in diabetes management and implications for personalized medicine. J. Diabetes Sci. Technol. 2009, 3, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Bugianesi, E.; Gastaldelli, A.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.H.v.D.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-alcoholic fatty liver disease and risk of incident type 2 diabetes: Role of circulating branched-chain amino acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Witt, H.; Engeli, S.; Kaminski, J.; Utz, W.; Fuhrmann, J.; Rein, D.; Schulz-Menger, J.; Luft, F.; Boschmann, M.; et al. Branched-chain and aromatic amino acids, insulin resistance and liver specific ectopic fat storage in overweight to obese subjects. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metab. Clin. Exp. 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- de Mello, V.D.; Sehgal, R.; Männistö, V.; Klåvus, A.; Nilsson, E.; Perfilyev, A.; Kaminska, D.; Miao, Z.; Pajukanta, P.; Ling, C.; et al. Serum aromatic and branched-chain amino acids associated with NASH demonstrate divergent associations with serum lipids. Liver Int. 2021, 41, 754–763. [Google Scholar] [CrossRef]
- Swierczynski, J.; Sledzinski, T.; Slominska, E.; Smolenski, R.; Sledzinski, Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes. Surg. 2009, 19, 883–889. [Google Scholar] [CrossRef]
- Kawanaka, M.M.; Nishino, K.; Oka, T.; Urata, N.; Nakamura, J.; Suehiro, M.; Kawamoto, H.; Chiba, Y.; Yamada, G. Tyrosine levels are associated with insulin resistance in patients with nonalcoholic fatty liver disease. Hepatic Med. Evid. Res. 2015, 7, 29–35. [Google Scholar] [CrossRef]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.J.; Rehkopf, D.H.; Leppert, J.T.; Bortz, W.M.; Cullen, M.R.; Chertow, G.M.; Ioannidis, J.P. Systematic evaluation of environmental and behavioural factors associated with all-cause mortality in the united states national health and nutrition examination survey. Int. J. Epidemiol. 2013, 42, 1795–1810. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Zeimbekis, A.; Kastorini, C.-M.; Stefanadis, C. Adherence to the mediterranean diet is associated with renal function among healthy adults: The ATTICA study. J. Ren. Nutr. 2010, 20, 176–184. [Google Scholar] [CrossRef]
- Choi, J.; Joe, H.; Oh, J.-E.; Cho, Y.-J.; Shin, H.-S.; Heo, N.H. The correlation between NAFLD and serum uric acid to serum creatinine ratio. PLoS ONE 2023, 18, e0288666. [Google Scholar] [CrossRef]
- Mahendran, Y.; Vangipurapu, J.; Cederberg, H.; Stančáková, A.; Pihlajamäki, J.; Soininen, P.; Kangas, A.J.; Paananen, J.; Civelek, M.; Saleem, N.K.; et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes 2013, 62, 3618–3626. [Google Scholar] [CrossRef]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, J.; Li, R.; Wang, Y.; Tu, Z.; Geng, T.; Liu, L.; Pan, A.; Liu, G. Healthy lifestyle, plasma metabolites, and risk of cardiovascular disease among individuals with diabetes. Atherosclerosis 2022, 367, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, Y.; Li, L. Metabolic pathway genes associated with susceptibility genes to coronary artery disease. Int. J. Genom. 2018, 2018, 9025841. [Google Scholar] [CrossRef]
- Chowdhury, C.R.; Kavitake, D.; Jaiswal, K.K.; Jaiswal, K.S.; Reddy, G.B.; Agarwal, V.; Shetty, P.H. NMR-based metabolomics as a significant tool for human nutritional research and health applications. Food Biosci. 2023, 53, 102538. [Google Scholar] [CrossRef]
- Aggarwal, M.; Bozkurt, B.; Panjrath, G.; Aggarwal, B.; Ostfeld, R.J.; Barnard, N.D.; Gaggin, H.; Freeman, A.M.; Allen, K.; Madan, S.; et al. Lifestyle modifications for preventing and treating heart failure. J. Am. Coll. Cardiol. 2018, 72, 2391–2405. [Google Scholar] [CrossRef]
- Ma, Q.; Ye, J.; Shao, C.; Lin, Y.; Wu, T.; Zhong, B. Metabolic benefits of changing sedentary lifestyles in nonalcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221122426. [Google Scholar] [CrossRef] [PubMed]
- Panahi, S.; Tremblay, A. Sedentariness and health: Is sedentary behavior more than just physical inactivity? Front. Public Health 2018, 6, 258. [Google Scholar] [CrossRef] [PubMed]
- Engel, J. Approaches to refractory epilepsy. Ann. Indian Acad. Neurol. 2014, 17, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, S.E.; Dhaher, R.; Rapuano, A.B.; Zaveri, H.P.; Tang, A.; de Lanerolle, N.; Eid, T. Effects of branched-chain amino acid supplementation on spontaneous seizures and neuronal viability in a model of mesial temporal lobe epilepsy. J. Neurosurg. Anesthesiol. 2019, 31, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Verrier, R.L.; Pang, T.D.; Nearing, B.D.; Schachter, S.C. The epileptic heart: Concept and clinical evidence. Epilepsy Behav. 2020, 105, 106946. [Google Scholar] [CrossRef]
- Gama, M.I.; Pinto, A.; Daly, A.; Rocha, J.C.; MacDonald, A. The impact of the quality of nutrition and lifestyle in the reproductive years of women with PKU on the long-term health of their children. Nutrients 2022, 14, 1021. [Google Scholar] [CrossRef]



| LS 3–4 (N = 15) | LS 5 (N = 50) | LS 6 (N = 82) | LS 7–8 (N = 76) | p-Value | |
|---|---|---|---|---|---|
| Cases/Controls (89/134) | 0/15 | 15/35 | 32/50 | 42/34 | 2.15 × 10−4 |
| FLI | 8.34 ± 7.36 ** † ¶ | 30.90 ± 29.46 **,* | 38.2 ± 30.94 † # | 51.90 ± 28.38 ¶ * # | 3.24 × 10−7 |
| Age (years) | 43.85 ± 11.27 | 48 ± 10.75 | 46.20 ± 12.08 | 44.93 ± 12.13 | 0.413 |
| Sex (Females—%) | 73.3 | 62 | 65.8 | 46 | 0.04 |
| MedDietScore | 23.23 ± 1.54 † ¶ | 21.80 ± 2.42 § * | 19.41 ± 3.84 † § # | 17.46 ± 4.743 ¶ * # | 4.48 × 10−10 |
| PAL | 1.51 ± 0.10 ¶ | 1.52 ± 0.21 * | 1.45 ± 0.24 # | 1.27 ± 0.17 ¶ * # | 2.01 × 10−10 |
| Smoking status (current smoker—%) | 0 | 8 | 30.4 | 55.2 | 1.44 × 10−8 |
| BMI (kg/m2) | 22.42 ± 1.48 ** ✦ ¶ | 26.28 ± 5.03 **,* | 27.85 ± 5.10 ✦ | 29.41 ± 4.73 ¶ * | 9.49 × 10−7 |
| WHR | 0.77 ± 0.07 † ¶ ✦ | 0.86 ± 0.12 ✦ * | 0.85 ± 0.08 # | 0.90 ± 0.09 † ¶ * # | 2.74 × 10−6 |
| AST (U/L) | 20.40 ± 4.89 | 21.44 ± 4.78 | 21.94 ± 6.48 | 21.22 ± 7.28 | 0.641 |
| ALT (U/L) | 20.27 ± 11.19 | 22.40 ± 9.39 | 25.06 ± 13.19 | 26.04 ± 12.89 | 0.272 |
| AST/ALT Ratio | 1.14 ± 0.36 † | 1.06 ± 0.33 | 0.98 ± 0.29 | 0.90 ± 0.35 † | 0.005 |
| GammaGT (U/L) | 18.80 ± 15.63 | 20.54 ± 15.35 | 23.36 ± 19.99 | 23.37 ± 15.78 | 0.643 |
| FGlu (mg/dL) | 85.27 ± 8.92 | 85.12 ± 7.93 | 86.01 ± 9.80 | 89.49 ± 12.03 | 0.063 |
| FIns (μU/mL) | 9.56 ± 3.35 | 11.18 ± 5.04 | 10.87 ± 4.75 # | 14.14 ± 8.72 # | 0.003 |
| HOMA-IR | 2.01 ± 0.88 | 2.38 ± 1.67 * | 2.05 ± 1.37 # | 3.2 ± 1.88 * # | 0.003 |
| TC (mg/dL) | 186.2 ± 45.51 | 203.14 ± 41.36 | 203.23 ± 35.86 | 204.04 ± 34.85 | 0.393 |
| LDL (mg/dL) | 108.66 ± 36.30 | 128.81 ± 37.09 | 126.39 ± 33.32 | 126.67 ± 26.29 | 0.194 |
| HDL (mg/dL) | 64.38 ± 16.15 ¶ | 57.02 ± 12.39 * | 57.97 ± 14.05 # | 49.76 ± 11.16 ¶ * # | 2 × 10−5 |
| TG (mg/dL) | 66.40 ± 45.30 ¶ | 86.81 ± 43.38 * | 94.85 ± 54.06 # | 117.85 ± 62.63 ¶ * # | 0.001 |
| Hyperlipidemia (%) | 26.6 | 58 | 50 | 56.5 | 0.149 |
| DMII (%) | 0 | 0 | 4 | 5 | 0.33 |
| Hypertension (%) | 13.3 | 34 | 32.9 | 55.2 | 0.002 |
| MetS (%) | 6 | 28 | 26.8 | 42.1 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotakis, C.; Amanatidou, A.I.; Kafyra, M.; Andreou, V.; Kalafati, I.P.; Zervou, M.; Dedoussis, G.V. Circulatory Metabolite Ratios as Indicators of Lifestyle Risk Factors Based on a Greek NAFLD Case–Control Study. Nutrients 2024, 16, 1235. https://doi.org/10.3390/nu16081235
Fotakis C, Amanatidou AI, Kafyra M, Andreou V, Kalafati IP, Zervou M, Dedoussis GV. Circulatory Metabolite Ratios as Indicators of Lifestyle Risk Factors Based on a Greek NAFLD Case–Control Study. Nutrients. 2024; 16(8):1235. https://doi.org/10.3390/nu16081235
Chicago/Turabian StyleFotakis, Charalambos, Athina I. Amanatidou, Maria Kafyra, Vasiliki Andreou, Ioanna Panagiota Kalafati, Maria Zervou, and George V. Dedoussis. 2024. "Circulatory Metabolite Ratios as Indicators of Lifestyle Risk Factors Based on a Greek NAFLD Case–Control Study" Nutrients 16, no. 8: 1235. https://doi.org/10.3390/nu16081235
APA StyleFotakis, C., Amanatidou, A. I., Kafyra, M., Andreou, V., Kalafati, I. P., Zervou, M., & Dedoussis, G. V. (2024). Circulatory Metabolite Ratios as Indicators of Lifestyle Risk Factors Based on a Greek NAFLD Case–Control Study. Nutrients, 16(8), 1235. https://doi.org/10.3390/nu16081235






