The Link between Abdominal Obesity Indices and the Progression of Liver Fibrosis: Insights from a Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
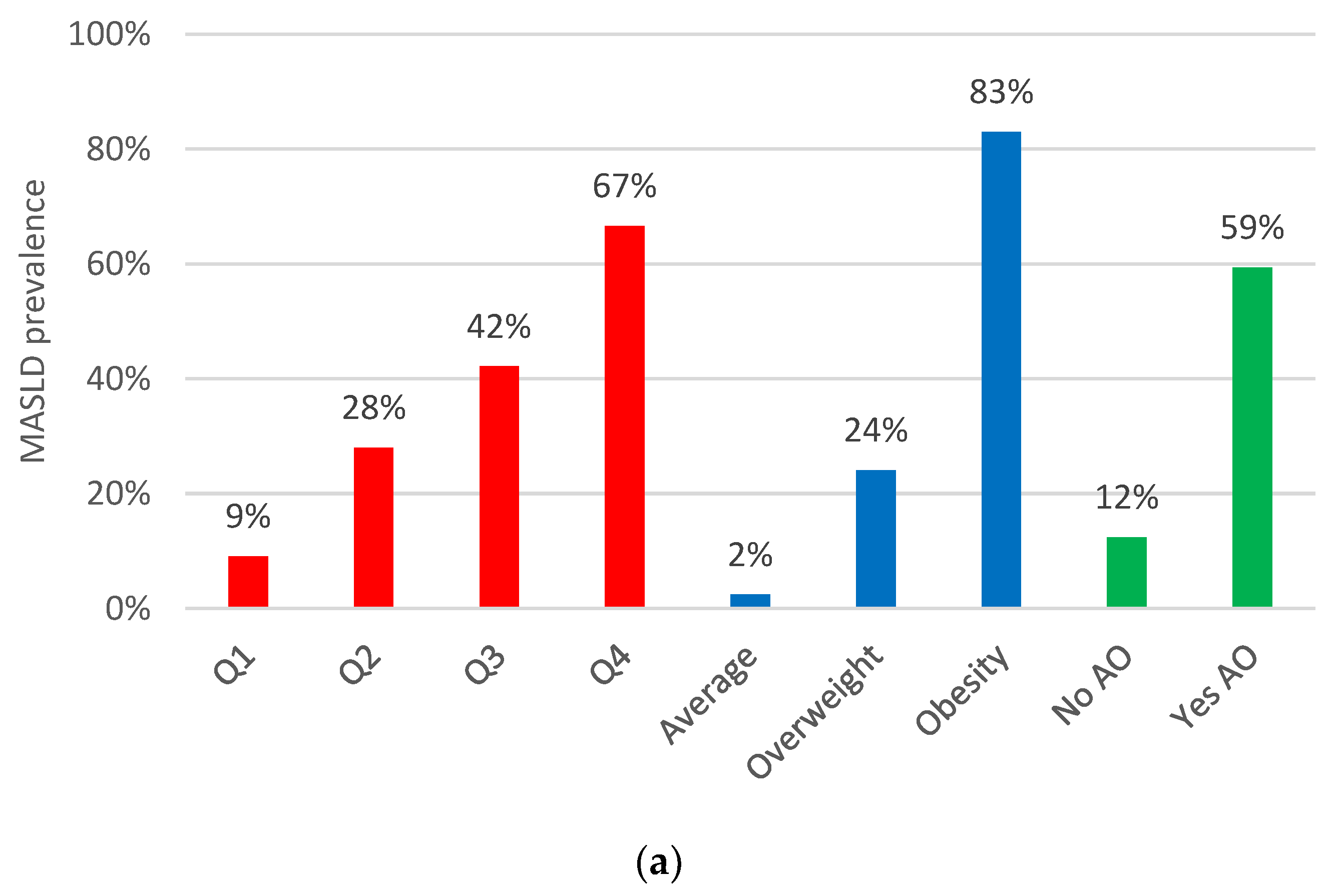
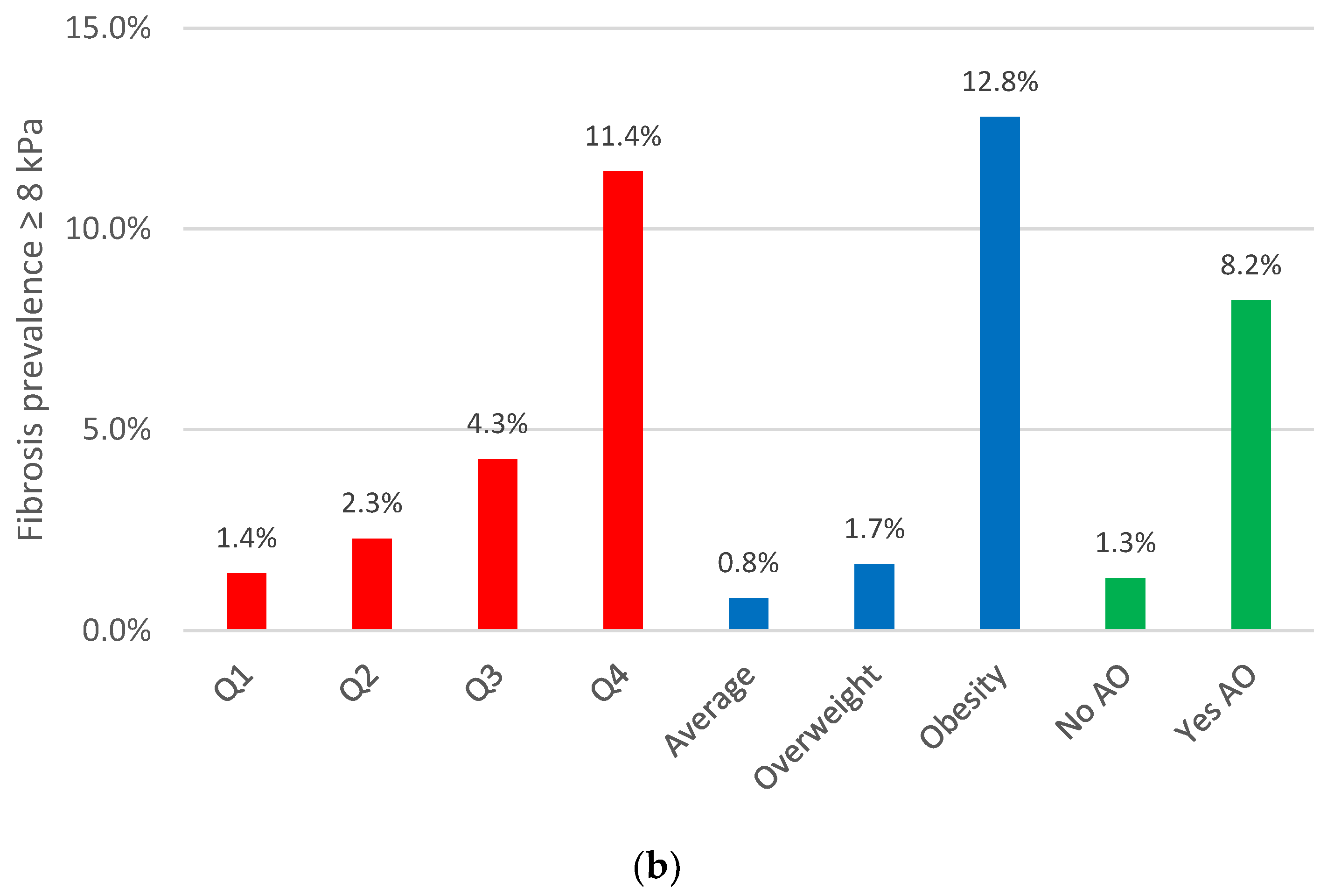
3.2. Abdominal Obesity Indices: Their Relationship with MASLD and Liver Fibrosis Prevalence
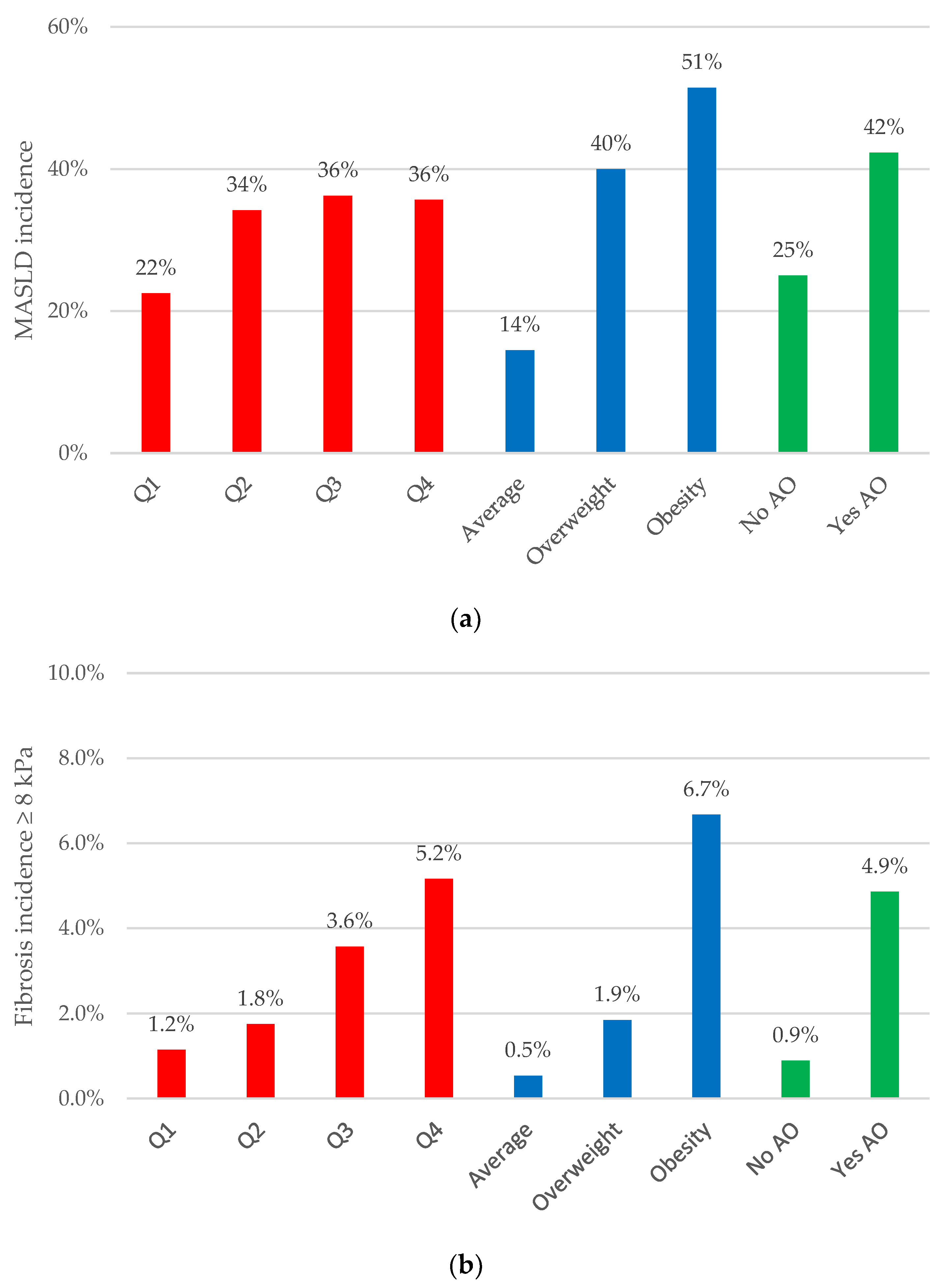
3.3. Abdominal Obesity Indices: Their Relationship with MASLD Incidence and Liver Fibrosis Progression
3.4. Abdominal Obesity Indices in Dysglycemic Subjects: Their Relationship with MASLD and Liver Fibrosis Prevalence
3.5. Abdominal Obesity Indices in Dysglycemic Subjects: Their Relationship with MASLD Incidence and Liver Fibrosis Progression
4. Discussion
- (1)
- We did not compare our results with histology data to evaluate fibrosis progression, which is reasonable given the challenges associated with such testing, particularly in a study involving the general population. Instead, we utilized TE, an invasive yet straightforward and reproducible test, to assess the presence and progression of fibrosis.
- (2)
- The observed changes in LSM values during follow-up were relatively minor. This could be attributed to several factors. Firstly, our study does not target specific hospital, clinic, or specialized unit populations where individuals with known liver pathologies are specifically referred. Secondly, we excluded individuals with a history of liver disease, including those with viral hepatitis or hazardous alcohol consumption. Thirdly, a longer follow-up period may be necessary to detect significant changes in LSMs.
- (3)
- The XL probe was unavailable for use in our study. Although this probe might have potentially reduced the failure rate, particularly among the subjects with obesity, we believe it would not have significantly affected our main findings. This is because we excluded patients with obesity with invalid TE readings or those in whom the technique could not be performed, resulting in a low rate of unreliable liver stiffness measurements (only 1.5%).
- (4)
- Data on the medication used by the study participants, such as glucose and lipid-lowering drugs, was not available, which could influence the metabolic characteristics of certain patient subgroups, particularly those with dysglycemia.
- (5)
- The HOMA was not utilized in our cohort to assess insulin resistance, which could have provided a more precise evaluation of the relationship between the VAI and insulin resistance in MASLD. Similarly, we lack data on inflammatory parameters and adipokines, which could have helped establish a connection between the VAI and the development of liver fibrosis.
- (6)
- Although we had sufficient statistical power for the primary objectives of our study, the sample size might be inadequate for specific subgroup analyses, posing a potential limitation.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, L.E.; Langefeld, C.D.; Scherzinger, A.L.; Norris, J.M.; Haffner, S.M.; Saad, M.F.; Bergman, R.N. Insulin sensitivity, insulin secretion, and abdominal fat: The Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 2003, 52, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Kim, W.; Kim, D.; Yoon, J.H.; Lee, K.; Kim, J.H.; Cho, E.J.; Lee, J.H.; Kim, H.Y.; Kim, Y.J.; et al. Visceral Obesity Predicts Significant Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Medicine 2015, 94, e2159. [Google Scholar] [CrossRef] [PubMed]
- Julian, M.T.; Ballesta, S.; Pera, G.; Perez-Montes de Oca, A.; Soldevila, B.; Caballeria, L.; Morillas, R.; Exposito, C.; Martinez-Escude, A.; Puig-Domingo, M.; et al. Abdominal obesity and dsyglycemia are risk factors for liver fibrosis progression in NAFLD subjects: A population-based study. Front. Endocrinol. 2022, 13, 1051958. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Poirier, P.; Despres, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, M.C.; Despres, J.P.; Lemieux, S.; Moorjani, S.; Bouchard, C.; Tremblay, A.; Nadeau, A.; Lupien, P.J. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. 1994, 73, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study, G. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Coccia, F.; Testa, M.; Guarisco, G.; Bonci, E.; Di Cristofano, C.; Silecchia, G.; Leonetti, F.; Gastaldelli, A.; Capoccia, D. Noninvasive assessment of hepatic steatosis and fibrosis in patients with severe obesity. Endocrine 2020, 67, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Vural Keskinler, M.; Mutlu, H.H.; Sirin, A.; Erkalma Senates, B.; Colak, Y.; Tuncer, I.; Oguz, A. Visceral Adiposity Index as a Practical Tool in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Metab. Syndr. Relat. Disord. 2021, 19, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.T.; Lee, M.Y.; Wang, C.W.; Wu, D.W.; Chen, S.C. Gender Differences in the Relationships among Metabolic Syndrome and Various Obesity-Related Indices with Nonalcoholic Fatty Liver Disease in a Taiwanese Population. Int. J. Environ. Res. Public Health 2021, 18, 857. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Amato, M.C.; Di Marco, V.; Camma, C.; Pizzolanti, G.; Barcellona, M.R.; Cabibi, D.; Galluzzo, A.; Sinagra, D.; Giordano, C.; et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol. Ther. 2012, 35, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Vongsuvanh, R.; George, J.; McLeod, D.; van der Poorten, D. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. J. Hepatol. 2012, 57, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Caballeria, L.; Pera, G.; Arteaga, I.; Rodriguez, L.; Aluma, A.; Morillas, R.M.; de la Ossa, N.; Diaz, A.; Exposito, C.; Miranda, D.; et al. High Prevalence of Liver Fibrosis Among European Adults with Unknown Liver Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2018, 16, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Koehler, E.M.; Schouten, J.N.; Hansen, B.E.; Hofman, A.; Stricker, B.H.; Janssen, H.L. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin. Gastroenterol. Hepatol. 2013, 11, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Roulot, D.; Costes, J.L.; Buyck, J.F.; Warzocha, U.; Gambier, N.; Czernichow, S.; Le Clesiau, H.; Beaugrand, M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011, 60, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Koehler, E.M.; Plompen, E.P.; Schouten, J.N.; Hansen, B.E.; Darwish Murad, S.; Taimr, P.; Leebeek, F.W.; Hofman, A.; Stricker, B.H.; Castera, L.; et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology 2016, 63, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.; Lai, C.L.; Wong, D.K.; Seto, W.K.; Hung, I.; Yuen, M.F. Significant changes in liver stiffness measurements in patients with chronic hepatitis B: 3-year follow-up study. J. Viral. Hepat. 2011, 18, e200–e205. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Lukenda Zanko, V.; Jakopcic, I.; Domislovic, V.; Mijic, A.; Stevanovic, T.; Delija, B.; Bokun, T.; Dinjar Kujundzic, P.; Ostojic, A.; et al. Prospective evaluation of non-alcoholic fatty liver disease by elastographic methods of liver steatosis and fibrosis; controlled attenuation parameter and liver stiffness measurements. J. Diabetes Complicat. 2020, 34, 107512. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Sabir, N.; Sermez, Y.; Kazil, S.; Zencir, M. Correlation of abdominal fat accumulation and liver steatosis: Importance of ultrasonographic and anthropometric measurements. Eur. J. Ultrasound 2001, 14, 121–128. [Google Scholar] [CrossRef]
- Park, B.J.; Kim, Y.J.; Kim, D.H.; Kim, W.; Jung, Y.J.; Yoon, J.H.; Kim, C.Y.; Cho, Y.M.; Kim, S.H.; Lee, K.B.; et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J. Gastroenterol. Hepatol. 2008, 23, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Son, J.Y.; Kim, J.M.; Hwang, S.S.; Han, J.S.; Heo, N.J. Body fat distribution is more predictive of all-cause mortality than overall adiposity. Diabetes Obes. Metab. 2018, 20, 141–147. [Google Scholar] [CrossRef] [PubMed]
- van der Poorten, D.; Milner, K.L.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, L.; Wu, J.; Wang, J.; Wang, Y.; Zeng, X. Role of age, gender and ethnicity in the association between visceral adiposity index and non-alcoholic fatty liver disease among US adults (NHANES 2003–2018): Cross-sectional study. BMJ Open 2022, 12, e058517. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, F.; Xiao, J.; Huang, F.; Cheng, F.; Wang, L.; Ju, Y.; Zhou, Y.; Jia, H. Associations Between Abdominal Obesity Indices and Nonalcoholic Fatty Liver Disease: Chinese Visceral Adiposity Index. Front. Endocrinol. 2022, 13, 831960. [Google Scholar] [CrossRef] [PubMed]
- Ercin, C.N.; Dogru, T.; Genc, H.; Celebi, G.; Aslan, F.; Gurel, H.; Kara, M.; Sertoglu, E.; Tapan, S.; Bagci, S.; et al. Insulin Resistance but Not Visceral Adiposity Index Is Associated with Liver Fibrosis in Nondiabetic Subjects with Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord 2015, 13, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Nobarani, S.; Alaei-Shahmiri, F.; Aghili, R.; Malek, M.; Poustchi, H.; Lahouti, M.; Khamseh, M.E. Visceral Adipose Tissue and Non-alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes. Dig. Dis. Sci. 2022, 67, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Amato, M.; Cabibi, D.; Camma, C.; Di Marco, V.; Giordano, C.; Galluzzo, A.; Craxi, A. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology 2010, 52, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Ali-Eldin, Z.A.; Ali-Eldin, F.A.; Mohamed, I.E. Visceral Adiposity Index and the Degree of Hepatic Fibrosis and Inflammation in Egyptian Patients with Chronic Hepatitis C. J. Clin. Diagn. Res. 2017, 11, OC11–OC14. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.N.; Du, S.S.; Wang, C.; Li, Y.C.; Liu, L.Y.; Guo, F.C.; Sun, C.H. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J. Gastroenterol. 2014, 20, 17932–17940. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Bugianesi, E. NASH in Lean Individuals. Semin. Liver Dis. 2019, 39, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.T.; Gidener, T.; Mara, K.C.; Larson, J.J.; Therneau, T.M.; Allen, A.M. Natural History of Nonalcoholic Fatty Liver Disease with Normal Body Mass Index: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1374–1381.e1376. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [PubMed]

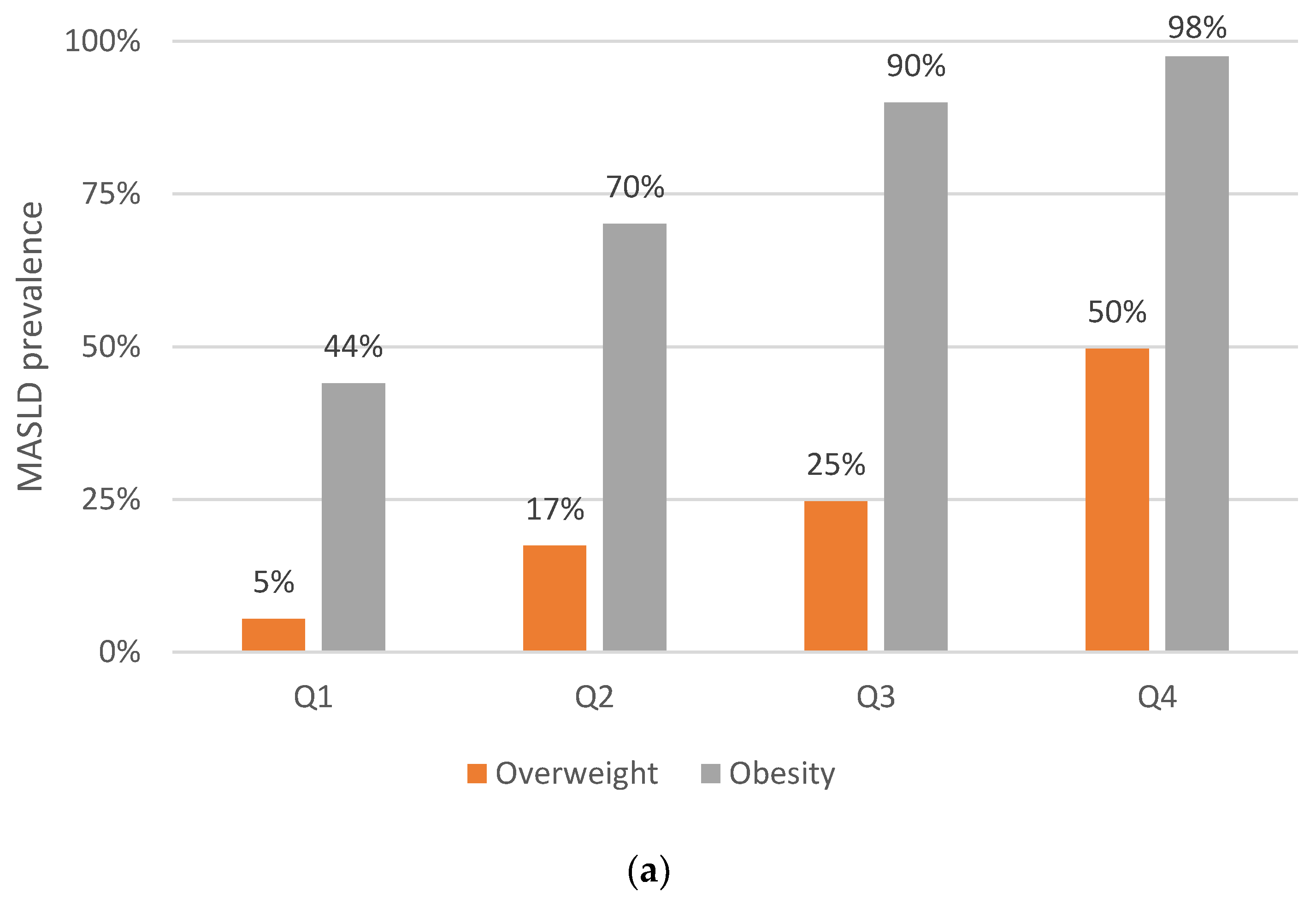

| Total (n = 1403) | ||||
|---|---|---|---|---|
| Baseline | Follow-Up | |||
| n/Mean | sd/% | n/Mean | sd/% | |
| Age, years | 56 | ±11 | 60 | ±11 |
| Female, n (%) | 873 | (62%) | 873 | (62%) |
| VAI | 1.7 | 1.4 | 1.7 | 1.1 |
| Body mass index, kg/m2 | 28 | ±5 | 28 | ±5 |
| Abdominal obesity, n (%) | 718 | (51%) | 767 | (55%) |
| T2D, n (%) | 170 | (12%) | 231 | (16%) |
| Prediabetes, n (%) | 187 | (13%) | 149 | (11%) |
| Dysglycemia *, n (%) | 357 | (25%) | 380 | (27%) |
| Glucose, mg/dL | 99 | ±24 | 101 | ±23 |
| Glycated hemoglobin (%) | 5.7 | 0.7 | 5.7 | 0.7 |
| Triglyceride, mg/dL | 120 | ±72 | 115 | ±57 |
| Total cholesterol, mg/dL | 214 | ±38 | 208 | ±39 |
| LDL-cholesterol, mg/dL | 135 | ±33 | 130 | ±34 |
| HDL-cholesterol, mg/dL | 56 | ±13 | 55 | ±13 |
| Cholesterol remnants †, mg/dL | 23 | ±13 | 23 | ±14 |
| Atherogenic dyslipemia §, n (%) | 132 | (9%) | 136 | (10%) |
| ALT and/or AST > 40 U/L, n (%) | 108 | (8%) | 100 | (7%) |
| FLI ¶ | 47 | ±28 | 49 | ±28 |
| FLI ≥ 60, n (%) | 490 | (35%) | 482 | (37%) |
| MASLD, n (%) | 511 | (36%) | 677 | (51%) |
| Mean liver fibrosis by LSM (kPa) | 4.9 | 2.2 | 4.9 | 2.1 |
| Liver fibrosis by Fibroscan ≥ 8.0 kPa, n (%) | 68 | (5%) | 62 | (4%) |
| Liver fibrosis by Fibroscan ≥ 9.2 kPa, n (%) | 35 | (2%) | 40 | (3%) |
| FIB-4 > 2.67, n (%) | 24 | (2%) | 53 | (4%) |
| High NFS, n (%) | 16 | (1%) | 73 | (6%) |
| Cross-Sectional | Longitudinal | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Dysglycemia | Overall | Dysglycemia | |||||
| MASLD | Fibrosis | MASLD | Fibrosis | MASLD | Fibrosis | MASLD | Fibrosis | |
| BMI | 33.9 (24–48) p < 0.001 | 8.8 (4.7–16) p < 0.001 | 32 (16–64) p < 0.001 | 7.3 (3.1–17) p < 0.001 | 2.8 (1.7–4.6) p < 0.001 | 4.7 (2.3–9.4) p < 0.001 | 6.7 (1.7–26) p < 0.006 | 6.3 (2.0–20) p < 0.002 |
| VAI Q4 | 5.1 (3.9–6.8) p < 0.001 | 3.5 (2.1–5.9) p < 0.001 | 4.0 (2.5–6.4) p < 0.001 | 7.1 (3.2–16) p < 0.001 | 1.2 (0.8–1.8) p = 0.454 | 2.0 (1.0–4.1) p = 0.041 | 1.0 (0.5–2.3) p = 0.905 | 1.8 (0.7–45) p = 0.231 |
| Abdominal obesity | 37.5 (23–60) p < 0.001 | 7.6 (3.6–16) p < 0.001 | 42 (17–109) p < 0.001 | 8.4 (2.8–25) p < 0.001 | 3.1 (2.2–4.4) p < 0.001 | 7.9 (3.2–20) p < 0.001 | 3.2 (1.3–7.9) p = 0.012 | 10 (2.3–46) p = 0.003 |
| Index | n | ∆ LSM (kPa) | F < 8 to F ≥ 8 (%) | MASLD Incidence (%) |
|---|---|---|---|---|
| VAI | ||||
| Q1–Q3 | 1053 | 0.14 | 2.2 | 29.9 |
| Q4 | 350 | −0.13 | 5.2 | 35.7 |
| BMI | ||||
| Non-obese | 973 | 0.13 | 1.4 | 28.8 |
| Obese | 430 | −0.04 | 6.7 | 51.4 |
| Abdominal obesity | ||||
| No | 685 | 0.10 | 0.9 | 25.0 |
| Yes | 718 | 0.05 | 4.9 | 42.3 |
| VAI and BMI | ||||
| Q1–Q3 non-obese | 786 | 0.17 | 1.2 | 27.7 |
| Q1–Q3 obese | 267 | 0.08 | 5.3 | 51.5 |
| Q4 non-obese | 187 | −0.04 | 2.2 | 35.1 |
| Q4 obese | 163 | −0.23 | 9.3 | 50.0 |
| VAI and BMI and AO | ||||
| Non-factors | 561 | 0.12 | 0.9 | 24.2 |
| Only obese | 22 | −0.48 | 0.0 | 50.0 |
| Only VAI Q4 | 94 | 0.16 | 1.1 | 27.7 |
| Only AO | 225 | 0.28 | 1.8 | 38.2 |
| Obese and VAI Q4 | 8 | −0.30 | 0.0 | 100 |
| Obese and AO | 245 | 0.13 | 5.8 | 51.7 |
| AO and VAI Q4 | 93 | −0.4 | 3.4 | 45.7 |
| All 3 factors | 155 | −0.23 | 9.8 | 33.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julián, M.T.; Arteaga, I.; Torán-Monserrat, P.; Pera, G.; Pérez-Montes de Oca, A.; Ruiz-Rojano, I.; Casademunt-Gras, E.; Chacón, C.; Alonso, N. The Link between Abdominal Obesity Indices and the Progression of Liver Fibrosis: Insights from a Population-Based Study. Nutrients 2024, 16, 1586. https://doi.org/10.3390/nu16111586
Julián MT, Arteaga I, Torán-Monserrat P, Pera G, Pérez-Montes de Oca A, Ruiz-Rojano I, Casademunt-Gras E, Chacón C, Alonso N. The Link between Abdominal Obesity Indices and the Progression of Liver Fibrosis: Insights from a Population-Based Study. Nutrients. 2024; 16(11):1586. https://doi.org/10.3390/nu16111586
Chicago/Turabian StyleJulián, María Teresa, Ingrid Arteaga, Pere Torán-Monserrat, Guillem Pera, Alejandra Pérez-Montes de Oca, Irene Ruiz-Rojano, Elena Casademunt-Gras, Carla Chacón, and Nuria Alonso. 2024. "The Link between Abdominal Obesity Indices and the Progression of Liver Fibrosis: Insights from a Population-Based Study" Nutrients 16, no. 11: 1586. https://doi.org/10.3390/nu16111586
APA StyleJulián, M. T., Arteaga, I., Torán-Monserrat, P., Pera, G., Pérez-Montes de Oca, A., Ruiz-Rojano, I., Casademunt-Gras, E., Chacón, C., & Alonso, N. (2024). The Link between Abdominal Obesity Indices and the Progression of Liver Fibrosis: Insights from a Population-Based Study. Nutrients, 16(11), 1586. https://doi.org/10.3390/nu16111586








