Effects of a Losartan-Antioxidant Hybrid (GGN1231) on Vascular and Cardiac Health in an Experimental Model of Chronic Renal Failure
Abstract
1. Introduction
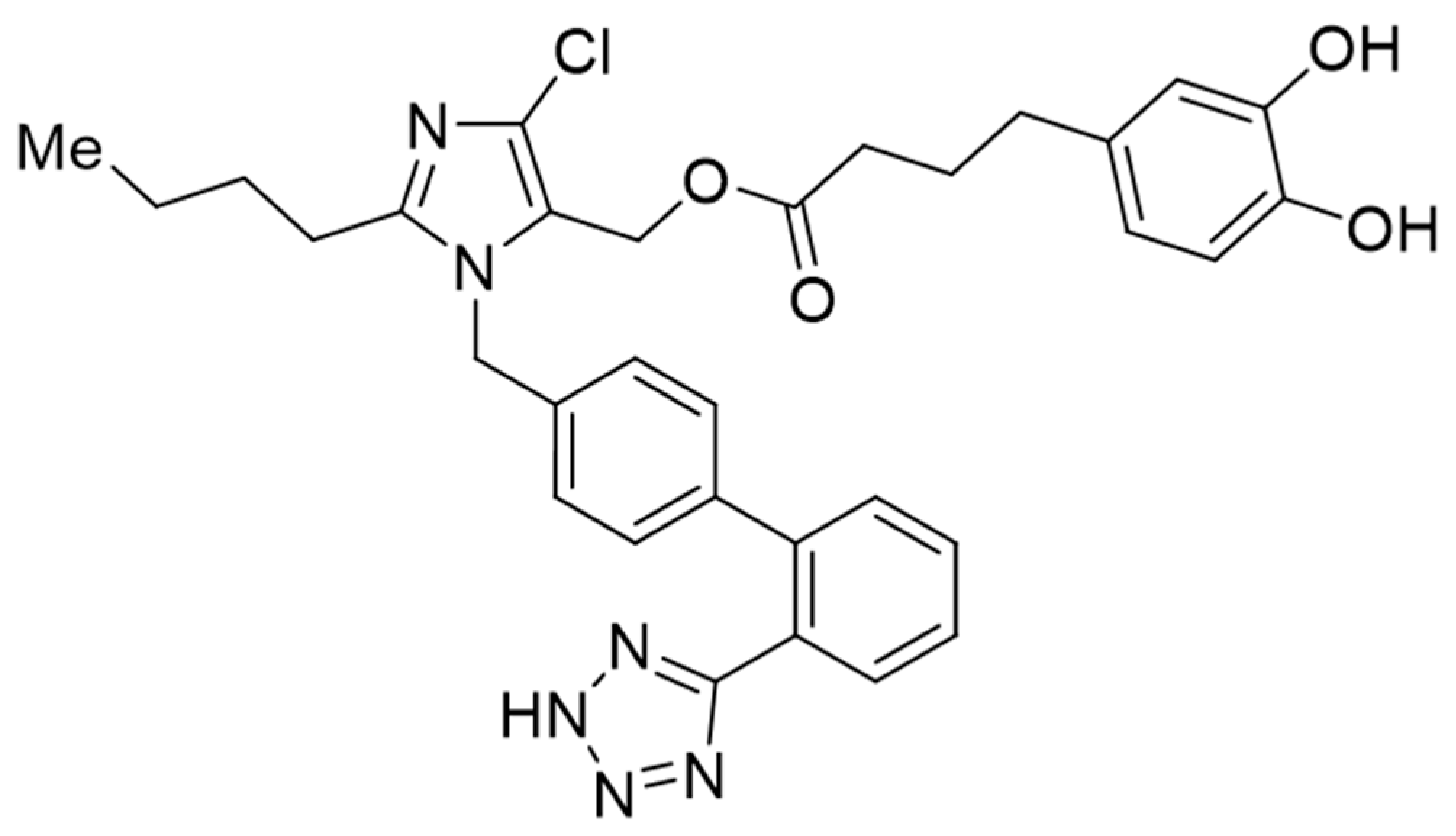
2. Materials and Methods
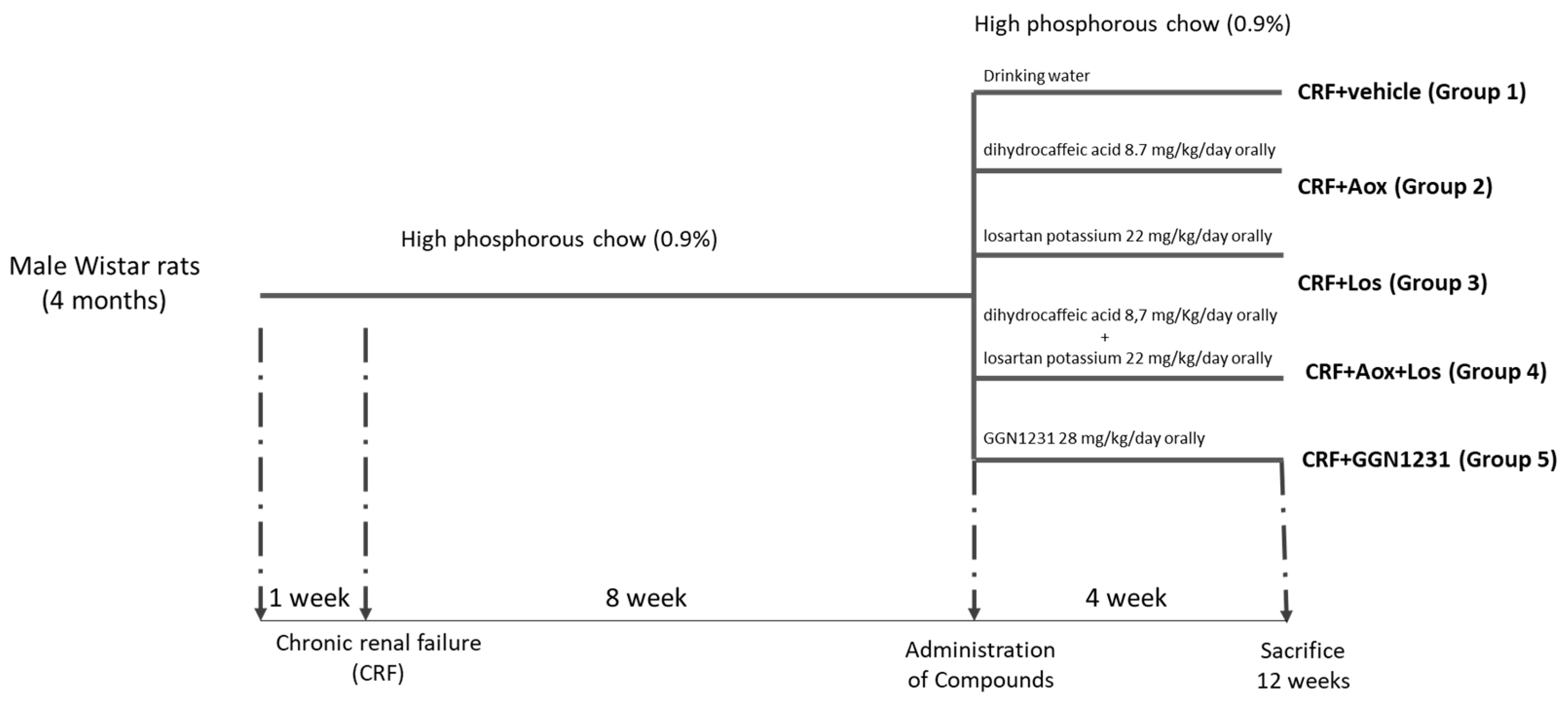
2.1. Experimental Studies
2.2. Analytical and Technical Procedures
2.2.1. Biochemical Markers
2.2.2. Aortic Calcium Content
2.2.3. Blood Pressure Measurement
2.2.4. Cardiac Morphological and Histological Changes
2.2.5. Immunohistochemistry
2.2.6. RNA Extraction, cDNA Synthesis, and Quantitative RT-PCR
2.3. Statistical Analysis
3. Results
3.1. Effect of Dihydrocaffeic Acid, Losartan, and Dihydrocaffeic Acid plus Losartan and GGN1231 on Weight, Biochemical, and Urinary Markers, and Vascular and Inflammation Parameters
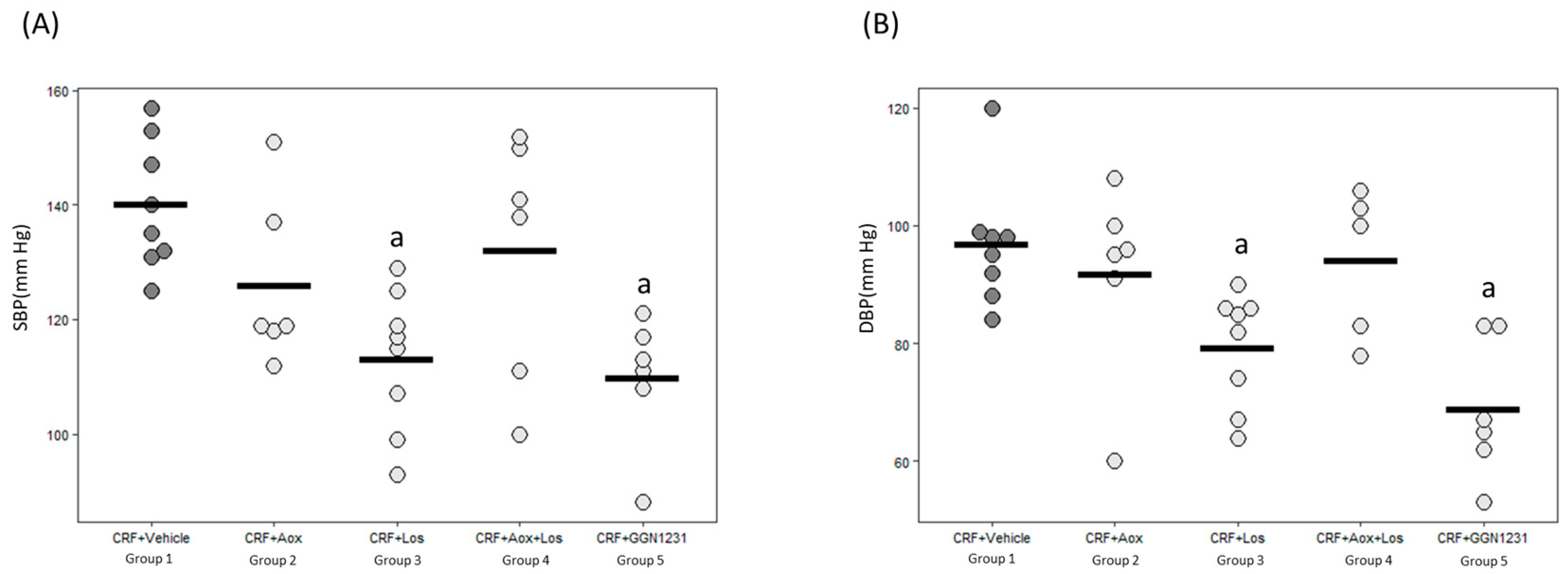
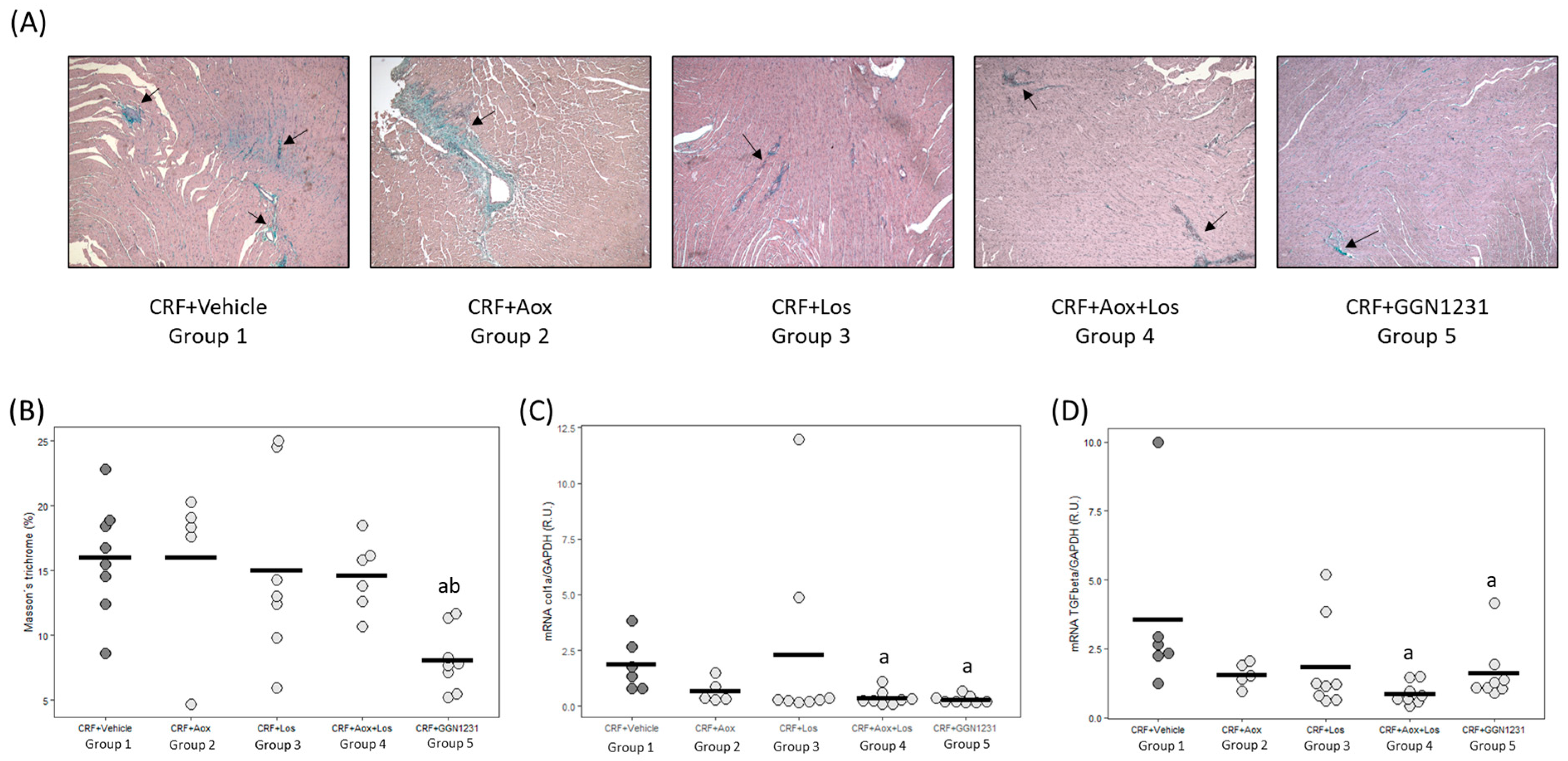
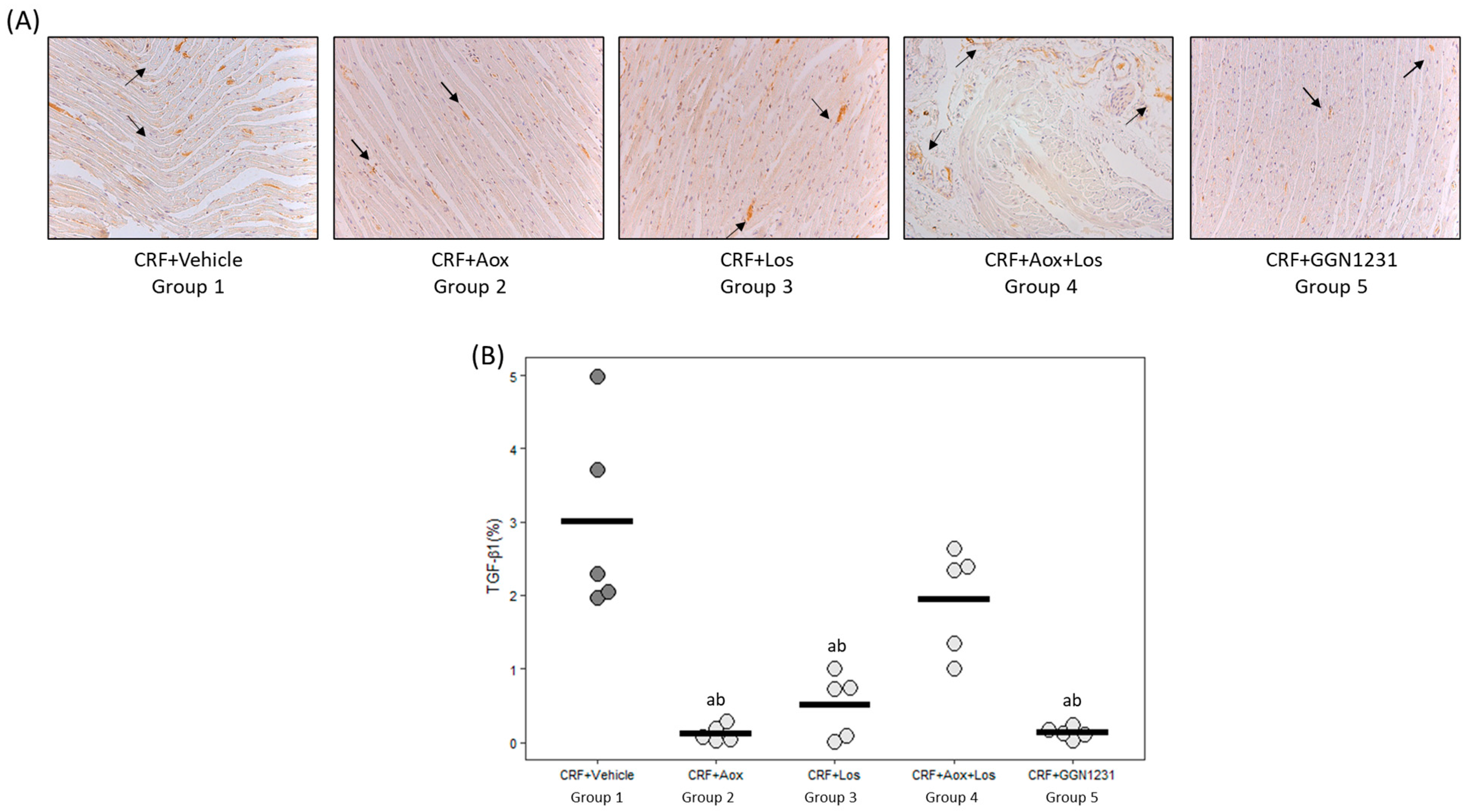
3.2. Effect of Dihydrocaffeic Acid, Losartan, Dihydrocaffeic Acid plus Losartan and GGN1231 on Blood Pressure, Left Ventricular Hypertrophy, Cardiac Inflammation, Cardiac Fibrosis, and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daiber, A.; Steven, S.; Euler, G.; Schulz, R. Vascular and Cardiac Oxidative Stress and Inflammation as Targets for Cardioprotection. Curr. Pharm. Des. 2021, 27, 2112–2130. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Rodríguez-Puyol, M.; Alajarín, R.; Serrano, I.; Sánchez-Alonso, P.; Griera, M.; Vaquero, J.J.; Rodríguez-Puyol, D.; Álvarez-Builla, J.; Díez-Marqués, M.L. Losartan-antioxidant hybrids: Novel molecules for the prevention of hypertension-induced cardiovascular damage. J. Med. Chem. 2009, 52, 7220–7227. [Google Scholar] [CrossRef]
- García, G.; Serrano, I.; Sánchez-Alonso, P.; Rodríguez-Puyol, M.; Alajarín, R.; Griera, M.; Vaquero, J.J.; Rodríguez-Puyol, D.; Álvarez-Builla, J.; Díez-Marqués, M.L. New losartan-hydrocaffeic acid hybrids as antihypertensive-antioxidant dual drugs: Ester, amide and amine linkers. Eur. J. Med. Chem. 2012, 50, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Kobo, O.; Abramov, D.; Davies, S.; Ahmed, S.B.; Sun, L.Y.; Mieres, J.H.; Parwani, P.; Siudak, Z.; Van Spall, H.G.; Mamas, M.A. CKD-Associated Cardiovascular Mortality in the United States: Temporal Trends From 1999 to 2020. Kidney Med. 2023, 5, 100597. [Google Scholar] [CrossRef] [PubMed]
- Amann, K.; Kronenberg, G.; Gehlen, F.; Wessels, S.; Orth, S.; Münter, K.; Ehmke, H.; Mall, G.; Ritz, E. Cardiac remodelling in experimental renal failure—An immunohistochemical study. Nephrol. Dial. Transplant. 1998, 13, 1958–1966. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Panizo, S.; Barrio-Vázquez, S.; Naves-Díaz, M.; Carrillo-López, N.; Rodríguez, I.; Fernández-Vázquez, A.; Valdivielso, J.M.; Thadhani, R.; Cannata-Andía, J.B. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol. Dial. Transplant. 2013, 28, 2735–2744. [Google Scholar] [CrossRef]
- Martínez-Arias, L.; Panizo-García, S.; Martín-Vírgala, J.; Martín-Carro, B.; Fernández-Villabrille, S.; Avello-Llano, N.; Miguel-Fernández, D.; Torres, M.P.R.; Cannata-Andía, J.B.; Carrillo-López, N.; et al. Contribution of phosphorus and PTH to the development of cardiac hypertrophy and fibrosis in an experimental model of chronic renal failure. Nefrologia (Engl. Ed.) 2021, 41, 640–651. [Google Scholar] [CrossRef]
- Fernández-Villabrille, S.; Martín-Carro, B.; Martín-Vírgala, J.; Alonso-Montes, C.; Palomo-Antequera, C.; García-Castro, R.; López-Ongil, S.; Dusso, A.S.; Fernández-Martín, J.L.; Naves-Díaz, M.; et al. MicroRNA-145 and microRNA-486 are potential serum biomarkers for vascular calcification. Nephrol. Dial. Transplant. 2023. [Google Scholar] [CrossRef] [PubMed]
- Naves-Diaz, M.; Carrillo-López, N.; Rodríguez-Rodríguez, A.; Braga, S.; Fernández-Coto, T.; Lopez-Novoa, J.M.; López-Hernández, F.; Cannata-Andía, J.B. Differential effects of 17β-estradiol and raloxifene on bone and lipid metabolism in rats with chronic kidney disease and estrogen insufficiency. Menopause 2010, 17, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Martínez-Arias, L.; Avello, N.; Sosa, P.; Dusso, A.S.; Cannata-Andía, J.B.; Naves-Díaz, M. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Connerty, H.V.; Briggs, A.R. Determination of serum calcium by means of orthocresolphthalein complexone. Am. J. Clin. Pathol. 1966, 45, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Cho, S.; Kim, S.R.; Jang, H.R.; Lee, J.E.; Huh, W.; Kim, D.J.; Oh, H.Y.; Kim, Y.-G. Effect of losartan on proteinuria and urinary angiotensinogen excretion in non-diabetic patients with chronic kidney disease. Postgrad. Med. J. 2011, 87, 664–669. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Wang, Y.; Yang, M.; Shi, G.; Yin, D.; Xuan, Z.; Xu, F. Activation of TRPC6 by AngII Induces Podocyte Injury and Participates in Proteinuria of Nephrotic Syndrome. Front. Pharmacol. 2022, 13, 915153. [Google Scholar] [CrossRef]
- Ohashi, H.; Oda, H.; Ohno, M.; Watanabe, S.; Itou, H.; Araki, H.; Yokoyama, H.; Sakata, S. Losartan reduces proteinuria and preserves renal function in hypertensive patients with IgA nephropathy. Clin. Exp. Nephrol. 2002, 6, 224–228. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; de Zeeuw, D. The antihypertensive and renal effects of angiotensin II receptor antagonists: Remaining questions. Curr. Opin. Nephrol. Hypertens 2000, 9, 57–61. [Google Scholar] [CrossRef]
- Perna, A.; Remuzzi, G. Abnormal permeability to proteins and glomerular lesions: A meta-analysis of experimental and human studies. Am. J. Kidney Dis. 1996, 27, 34–41. [Google Scholar] [CrossRef]
- Xu, F.; Mao, C.; Hu, Y.; Rui, C.; Xu, Z.; Zhang, L. Cardiovascular effects of losartan and its relevant clinical application. Curr. Med. Chem. 2009, 16, 3841–3857. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Sadoshima, J.; Brosius, F.C.; Izumo, S. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes In vitro. Circ. Res. 1999, 85, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, Y.; Gallagher, P.E.; Averill, D.B.; Tallant, E.A.; Brosnihan, K.B.; Ferrario, C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004, 43, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: More than regulation of blood pressure? Hypertension 2007, 50, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.V. Antihypertensive effect of caffeic acid and its analogs through dual renin–angiotensin–aldosterone system inhibition. Eur. J. Pharmacol. 2014, 730, 125–132. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Zhou, J.J.; Oura, H.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Effects on Blood Pressure of Caffeic Acid Analogues Isolated from Salviae Miltiorrhizae Radix in Rats with Adenine-induced Renal Hypertension. Phytother. Res. 1995, 9, 105–109. [Google Scholar] [CrossRef]
- Dahlöf, B.; Keller, S.E.; Makris, L.; Goldberg, A.I.; Sweet, C.S.; Lim, N.Y. Efficacy and tolerability of losartan potassium and atenolol in patients with mild to moderate essential hypertension. Am. J. Hypertens. 1995, 8, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Kovács, M.G.; Kovács, Z.Z.A.; Varga, Z.; Szűcs, G.; Freiwan, M.; Farkas, K.; Kővári, B.; Cserni, G.; Kriston, A.; Kovács, F.; et al. Investigation of the Antihypertrophic and Antifibrotic Effects of Losartan in a Rat Model of Radiation-Induced Heart Disease. Int. J. Mol. Sci. 2021, 22, 12963. [Google Scholar] [CrossRef]
- Schultz, J.E.J.; Witt, S.A.; Glascock, B.J.; Nieman, M.L.; Reiser, P.J.; Nix, S.L.; Reiser, P.J.; Nix, S.L.; Kimball, T.R.; Doetschman, T. TGF-β1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J. Clin. Investig. 2002, 109, 787–796. [Google Scholar] [CrossRef]
- Duangrat, R.; Parichatikanond, W.; Morales, N.P.; Pinthong, D.; Mangmool, S. Sustained AT(1)R stimulation induces upregulation of growth factors in human cardiac fibroblasts via G(αq)/TGF-β/ERK signaling that influences myocyte hypertrophy. Eur. J. Pharmacol. 2022, 937, 175384. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A.; Carver, W.; Giles, W.; Borg, T.K. Cardiac fibroblasts: Friend or foe? Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1015–H1026. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Gamaro, G.D.; Suyenaga, E.; Borsoi, M.; Lermen, J.; Pereira, P.; Ardenghi, P. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. ISRN Pharmacol. 2011, 2011, 451682. [Google Scholar] [CrossRef] [PubMed]
- Bıçakçı, N.; Karaboğa, I.; Dökmeci, A.H.; Güzel, S.; Erboğa, Z.F. Cardioprotective effect of caffeic acid phenethyl ester on cardiac contusion following blunt chest trauma in rats. Biotech. Histochem. 2019, 94, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef]
- Niu, Z.; Qiang, T.; Lin, W.; Li, Y.; Wang, K.; Wang, D.; Wang, X. Evaluation of Potential Herb-Drug Interactions Between Shengmai Injection and Losartan Potassium in Rat and In Vitro. Front. Pharmacol. 2022, 13, 878526. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Zhang, X.; Wang, J.; Wang, S.; Xiao, X.; Wang, R.; Li, P.; Wang, Y. Angiotensin II induces connective tissue growth factor expression in human hepatic stellate cells by a transforming growth factor β-independent mechanism. Sci. Rep. 2017, 7, 7841. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chou, Y.-H.; Liao, F.-L.; Lin, C.-C.; Chang, F.-C.; Liu, C.-H.; Huang, T.-M.; Lai, C.-F.; Lin, Y.-F.; Wu, V.-C.; et al. Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci. Rep. 2016, 6, 34265. [Google Scholar] [CrossRef]
- Tai, C.; Gan, T.; Zou, L.; Sun, Y.; Zhang, Y.; Chen, W.; Li, J.; Zhang, J.; Xu, Y.; Lu, H.; et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on cardiovascular events in patients with heart failure: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2017, 17, 257. [Google Scholar] [CrossRef]

| CRF+Vehicle Group 1 (n = 8) | CRF+Aox Group 2 (n = 8) | CRF+Los Group 3 (n = 8) | CRF+Aox+Los Group 4 (n = 8) | CRF+GGN1231 Group 5 (n = 8) | |
|---|---|---|---|---|---|
| Weight (g) | 388 [375–407] | 387 [371–401] | 373 [352–375] | 388 [382–396] | 374 [365–398] |
| Creatinine (mg/dL) | 2.1 [1.7–2.9] | 1.9 [1.6–2.7] | 1.7 [1.3–3.7] | 2.2 [1.6–4.0] | 2.1 [1.4–2.6] |
| Calcium (mg/dL) | 10.1 [9.3–10.4] | 9.3 [8.4–9.3] | 9.5 [9.2–10.2] | 9.6 [8.1–9.7] | 8.8 [8.1–9.1] |
| Phosphorus (mg/dL) | 12.2 [9.6–14.4] | 12.3 [11.1–14.1] | 10.1 [6.9–19.7] | 13.5 [9.7–22.2] | 14.0 [11.7–15.5] |
| PTH (pg/mL) | 6745 [4975–7168] | 6706 [6538–7331] | 6169 [4395–6480] | 6389 [5057–7072] | 6608 [6403–6961] |
| Creatinine clearance (mL/min) | 0.5 [0.4–0.8] | 0.5 [0.4–0.6] | 0.7 [0.5–0.9] | 0.5 [0.2–0.6] | 0.4 [0.3–0.6] |
| Creatinine clearance (mL/min/kg) | 1.4 [0.9–1.7] | 1.2 [0.8–1.8] | 1.8 [0.6–2.3] | 1.4 [0.9–1.7] | 1.1 [0.9–1.8] |
| Proteinuria (mg/24 h) | 74 [41–823] | 92 [67–101] | 19 [12–30] a,b,c | 66 [37–92] | 22 [19–29] a,b,c |
| Urinary calcium (mg/dL) | 5.3 [3.5–5.9] | 4.9 [3.2–7.3] | 5.3 [3.2–5.9] | 4.1 [2.9–6.6] | 4.1 [3.4–6.2] |
| Urinary phosphorus (mg/dL) | 109 [104–198] | 109 [106–180] | 174 [117–192] | 157 [140–198] | 138 [85–176] |
| CRF+Vehicle Group 1 (n = 8) | CRF+Aox Group 2 (n = 8) | CRF+Los Group 3 (n = 8) | CRF+Aox+Los Group 4 (n = 8) | CRF+GGN1231 Group 5 (n = 8) | |
|---|---|---|---|---|---|
| Calcium (µg de Ca/mg protein) | 25.2 [6.1–73] | 8.73 [6.7–8.9] | 11.1 [6.2–44] | 11.2 [7.7–15.3] | 6.8 [4.0–8.7] |
| mRNA RUNX2/GAPDH (R.U.) | 2.8 [1.6–7.0] | 6.5 [3.0–9.2] | 2.7 [0.7–9.3] | 3.0 [2.2–5.2] | 3.2 [1.2–4.4] |
| mRNA α-ACTIN/GAPDH (R.U.) | 0.2 [0.1–0.3] | 0.5 [0.3–1.5] | 0.1 [0.1–0.2] b,c | 0.3 [0.2–0.5] | 0.1 [0.1–0.6] |
| mRNA TNF-α/GAPDH (R.U.) | 6.5 [1.6–8.8] | 4.6 [4.6–8.0] | 3.3 [1.66.45] | 5.9 [2.5–7.3] | 0.6 [0.5–2.1] a,b,c,d |
| CRF+Vehicle Group 1 (n = 8) | CRF+Aox Group 2 (n = 8) | CRF+Los Group 3 (n = 8) | CRF+Aox+Los Group 4 (n = 8) | CRF+GGN1231 Group 5 (n = 8) | |
|---|---|---|---|---|---|
| Heart/body weight (mg/g) | 4.8 [3.8–5.2] | 4.9 [4.2–7.0] | 3.4 [2.7–4.8] | 4.2 [3.7–4.7] | 3.6 [3.3–4.0] |
| LV Wall (μm) | 2539 [2443–2786] | 2364 [2199–2406] | 2477 [2285–2665] | 2396 [2330–2455] | 2107 [2027–2215] a |
| Septum (μm) | 2125 [2041–2207] | 2025 [1987–2601] | 1861 [1767–2179] | 1995 [1906–2141] | 2012 [1892–2183] |
| Cardiomyocytes diameter (μm) | 11.9 [11.0–12.4] | 9.3 [8.9–10.6] a | 10.1 [8.7–10.5] a | 8.9 [8.7–9.6] a | 9.0 [8.6–9.4] a |
| Heart/body weight (mg/g) | 4.8 [3.8–5.2] | 4.9 [4.2–7.0] | 3.4 [2.7–4.8] | 4.2 [3.7–4.7] | 3.6 [3.3–4.0] |
| CRF+Vehicle Group 1 (n = 8) | CRF+Aox Group 2 (n = 8) | CRF+Los Group 3 (n = 8) | CRF+Aox+Los Group 4 (n = 8) | CRF+GGN1231 Group 5 (n = 8) | |
|---|---|---|---|---|---|
| mRNA ATR1/GAPDH (R.U.) | 2.5 [2.1–2.7] | 0.7 [0.4–0.8] a | 0.5 [0.4–0.8] a | 0.5 [0.4–0.6] a | 0.3 [0.3–0.5] a |
| mRNA ATR2/GAPDH (R.U.) | 5.6 [2.6–15.2] | 12.7 [6.0–20.8] | 4.7 [4.1–39.6] | 14.8 [5.5–19.9] | 8.7 [4.9–24.7] |
| mRNA MAS/GAPDH (R.U.) | 0.9 [0.7–1.2] | 1.2 [1.0–1.4] | 1.1 [0.5–1.6] | 1.2 [0.7–2.2] | 1.1 [0.9–2.1] |
| mRNA TNF-α/GAPDH (R.U.) | 14.5 [14.2–18.0] | 1.2 [1.0–1.7] a | 1.4 [1.1–2.4] a | 1.3 [1.1–1.4] a | 1.4 [1.3–1.7] a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Arias, L.; Fernández-Villabrille, S.; Alonso-Montes, C.; García-Navazo, G.; Ruíz-Torres, M.P.; Alajarín, R.; Alvarez-Builla, J.; Gutiérrez-Calabres, E.; Vaquero-López, J.J.; Carrillo-López, N.; et al. Effects of a Losartan-Antioxidant Hybrid (GGN1231) on Vascular and Cardiac Health in an Experimental Model of Chronic Renal Failure. Nutrients 2023, 15, 1820. https://doi.org/10.3390/nu15081820
Martínez-Arias L, Fernández-Villabrille S, Alonso-Montes C, García-Navazo G, Ruíz-Torres MP, Alajarín R, Alvarez-Builla J, Gutiérrez-Calabres E, Vaquero-López JJ, Carrillo-López N, et al. Effects of a Losartan-Antioxidant Hybrid (GGN1231) on Vascular and Cardiac Health in an Experimental Model of Chronic Renal Failure. Nutrients. 2023; 15(8):1820. https://doi.org/10.3390/nu15081820
Chicago/Turabian StyleMartínez-Arias, Laura, Sara Fernández-Villabrille, Cristina Alonso-Montes, Gonzalo García-Navazo, María P. Ruíz-Torres, Ramón Alajarín, Julio Alvarez-Builla, Elena Gutiérrez-Calabres, Juan José Vaquero-López, Natalia Carrillo-López, and et al. 2023. "Effects of a Losartan-Antioxidant Hybrid (GGN1231) on Vascular and Cardiac Health in an Experimental Model of Chronic Renal Failure" Nutrients 15, no. 8: 1820. https://doi.org/10.3390/nu15081820
APA StyleMartínez-Arias, L., Fernández-Villabrille, S., Alonso-Montes, C., García-Navazo, G., Ruíz-Torres, M. P., Alajarín, R., Alvarez-Builla, J., Gutiérrez-Calabres, E., Vaquero-López, J. J., Carrillo-López, N., Rodríguez-Puyol, D., Cannata-Andía, J. B., Panizo, S., & Naves-Díaz, M. (2023). Effects of a Losartan-Antioxidant Hybrid (GGN1231) on Vascular and Cardiac Health in an Experimental Model of Chronic Renal Failure. Nutrients, 15(8), 1820. https://doi.org/10.3390/nu15081820






