Serum and Urinary Soluble α-Klotho as Markers of Kidney and Vascular Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice Model with Mild CKD
2.1.1. Experimental Design
2.1.2. Biochemical Markers
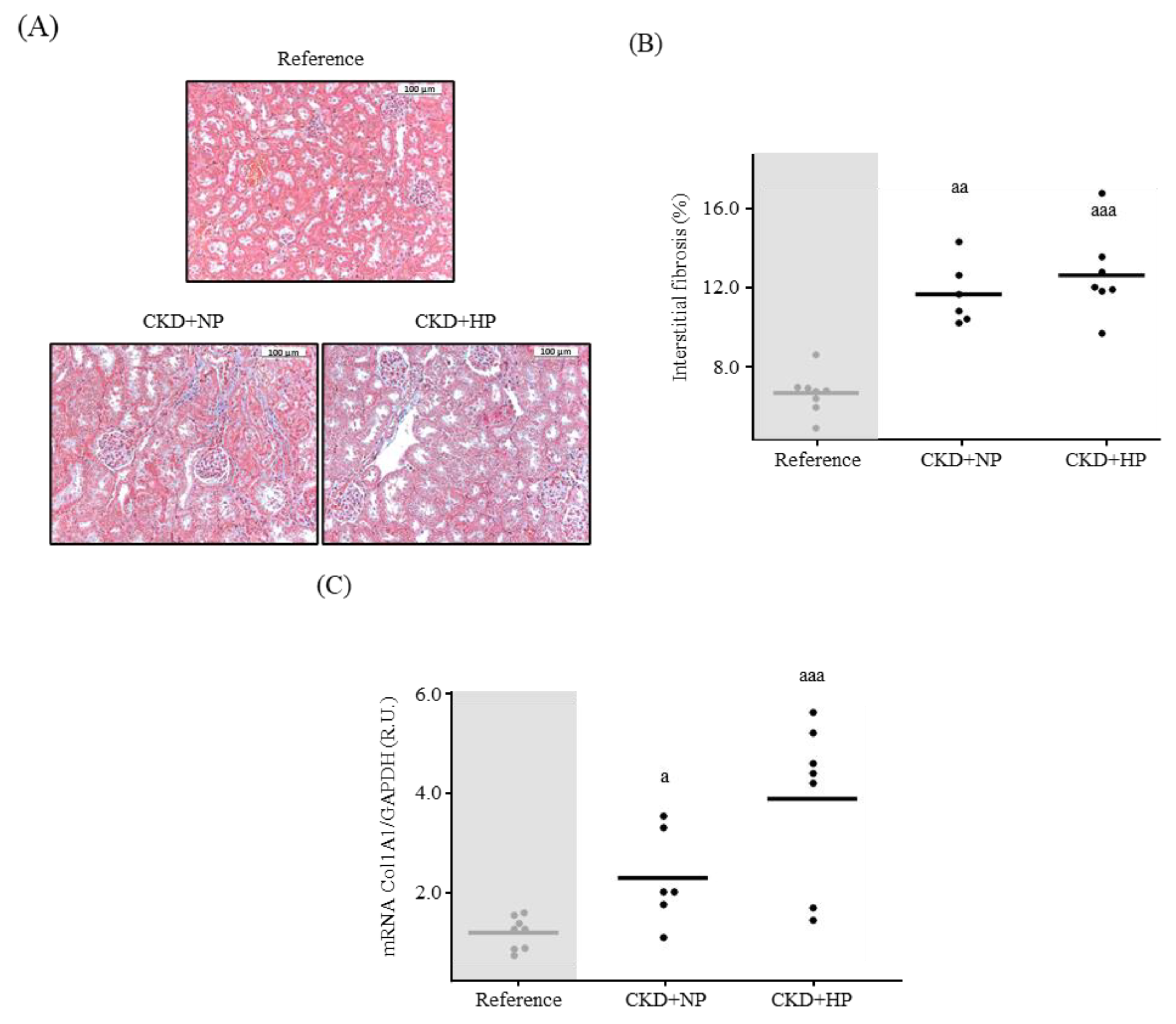
2.1.3. Kidney Interstitial Fibrosis and Immunohistochemistry for Kidney α-Klotho and Wnt/β-Catenin Signaling
2.1.4. Soft Tissue Quantification of Ca Deposition
2.1.5. Aortic Immunofluorescence for Autophagy Analysis
2.2. Patients CKD Stages 2–5 and Controls
2.3. In Vitro Studies
2.3.1. Experimental Design
2.3.2. Effect of sKlotho on VSMC Phenotypic Changes towards Osteogenic Differentiation
2.3.3. sKlotho and VSMCs Phenotypic Signaling towards Vascular Calcification and Autophagy
2.3.4. Quantification of Ca Deposition in the VSMCs
2.3.5. Evaluation of Autophagy Using LC3B-II Puncta VSMCs Immunofluorescence
2.4. Common Analytical and Technical Procedures Used in the Studies
2.4.1. RNA Extraction, cDNA Synthesis and Quantitative PCR
2.4.2. Western Blot Analysis
2.5. Statistical Analysis
3. Results
3.1. Mice Model with Mild CKD
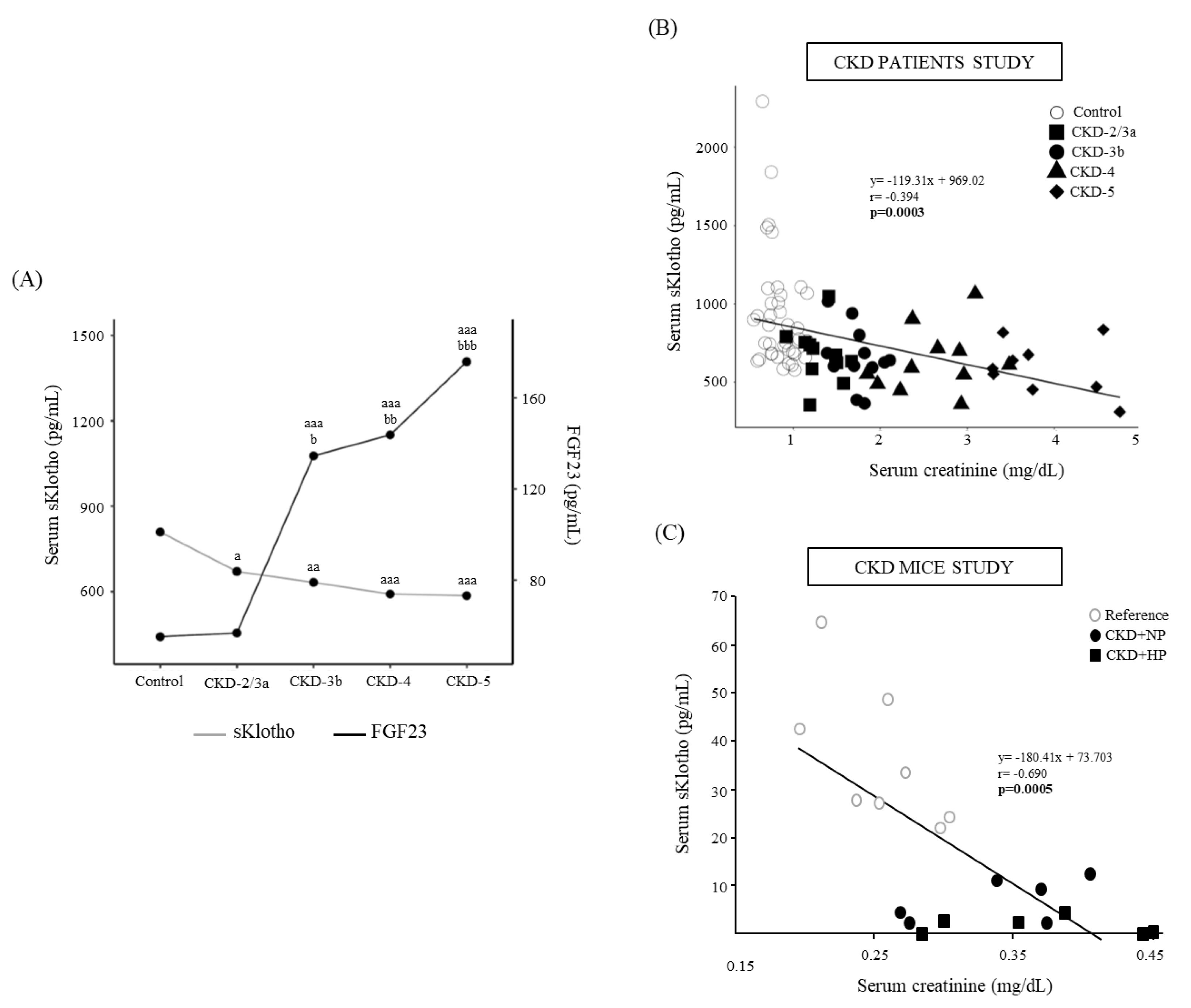
3.1.1. Biochemical Parameters and Kidney Function
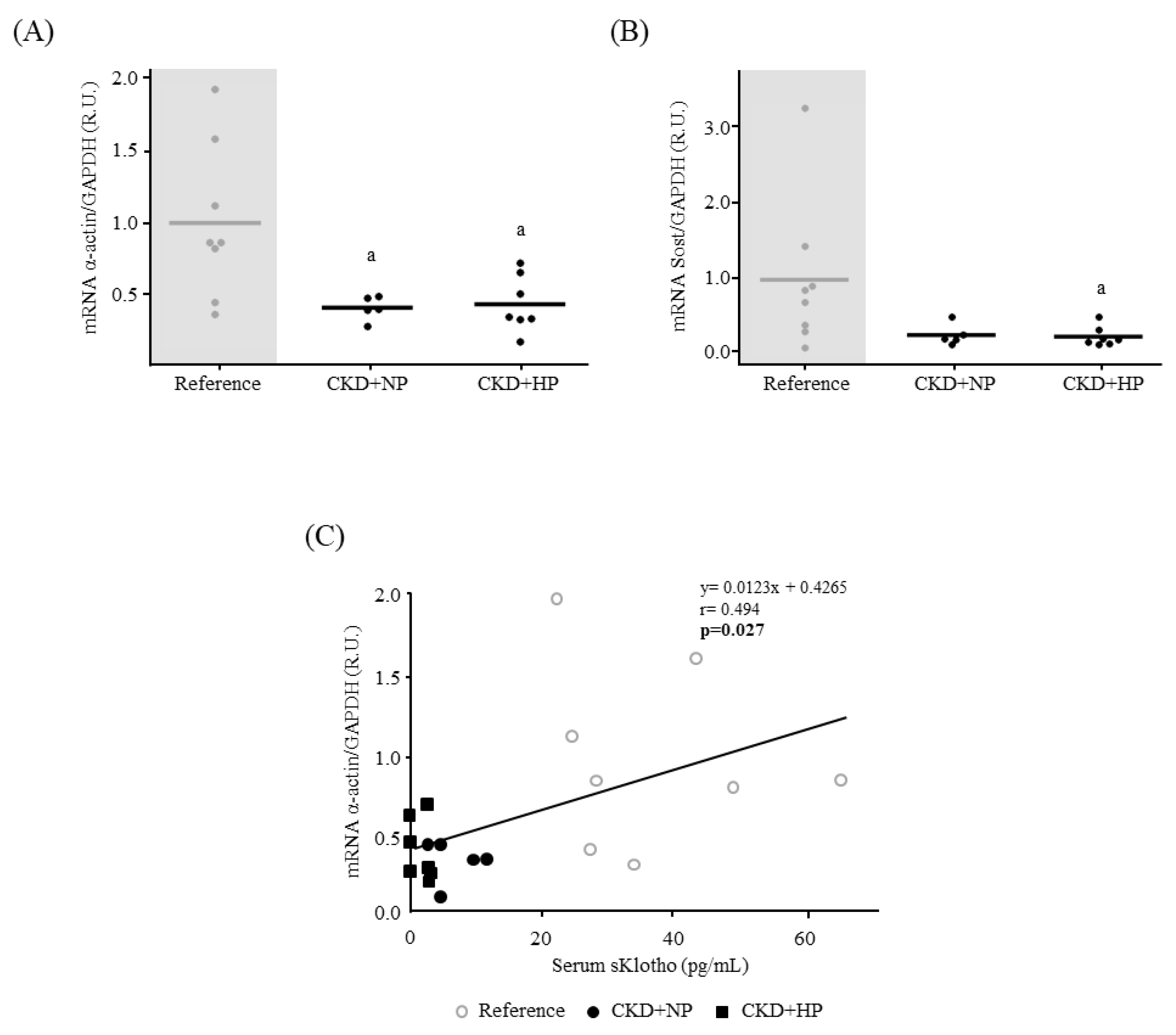
3.1.2. Kidney Fibrosis, α-Klotho Expression and Wnt/β-Catenin Signaling
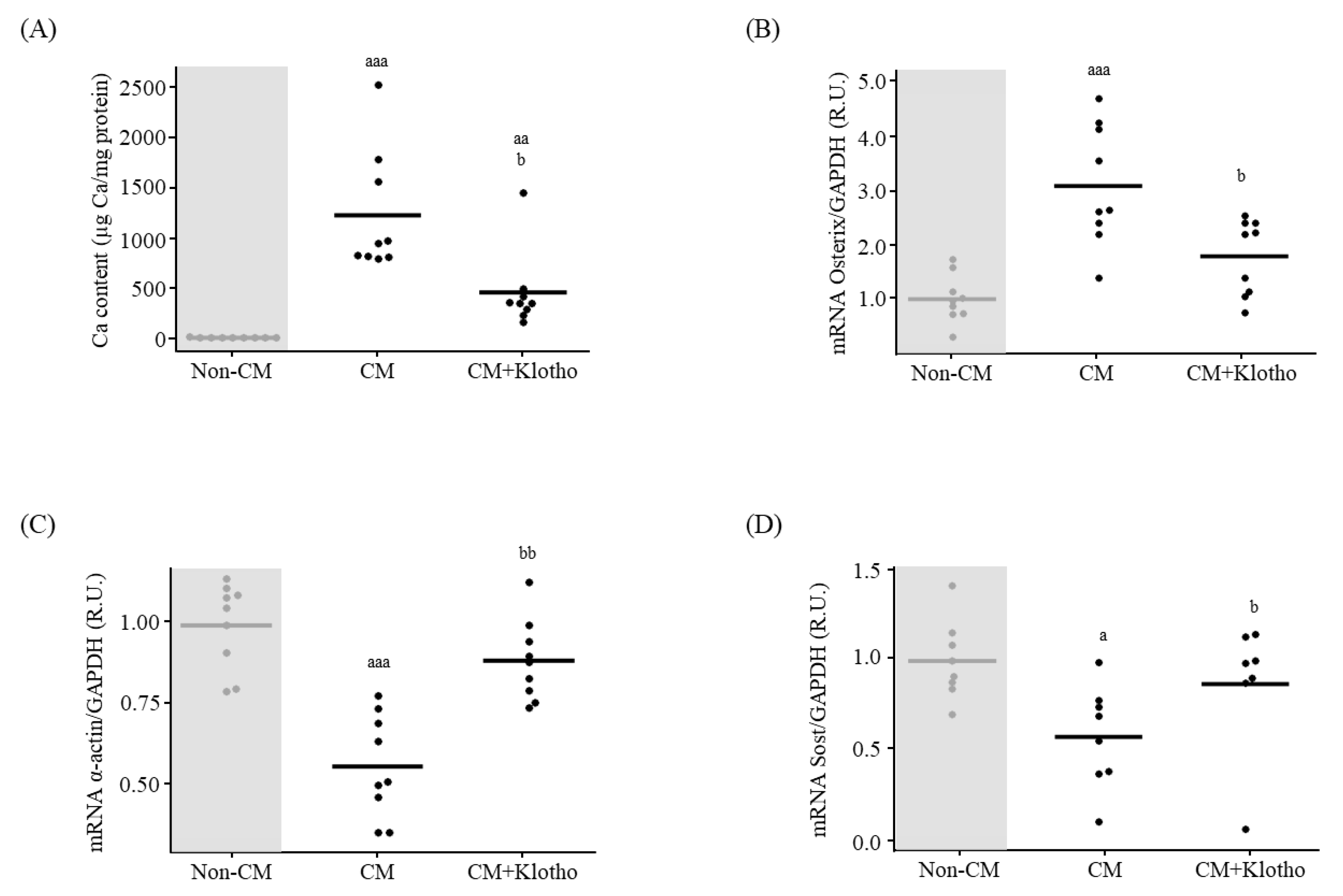
3.1.3. Osteogenic Differentiation and Ca Deposition
3.2. Patients CKD Stages 2 to 5 and Controls
3.3. In Vitro Effect of sKlotho on VSMCs Signaling towards Osteogenic Differentiation
3.4. Autophagy
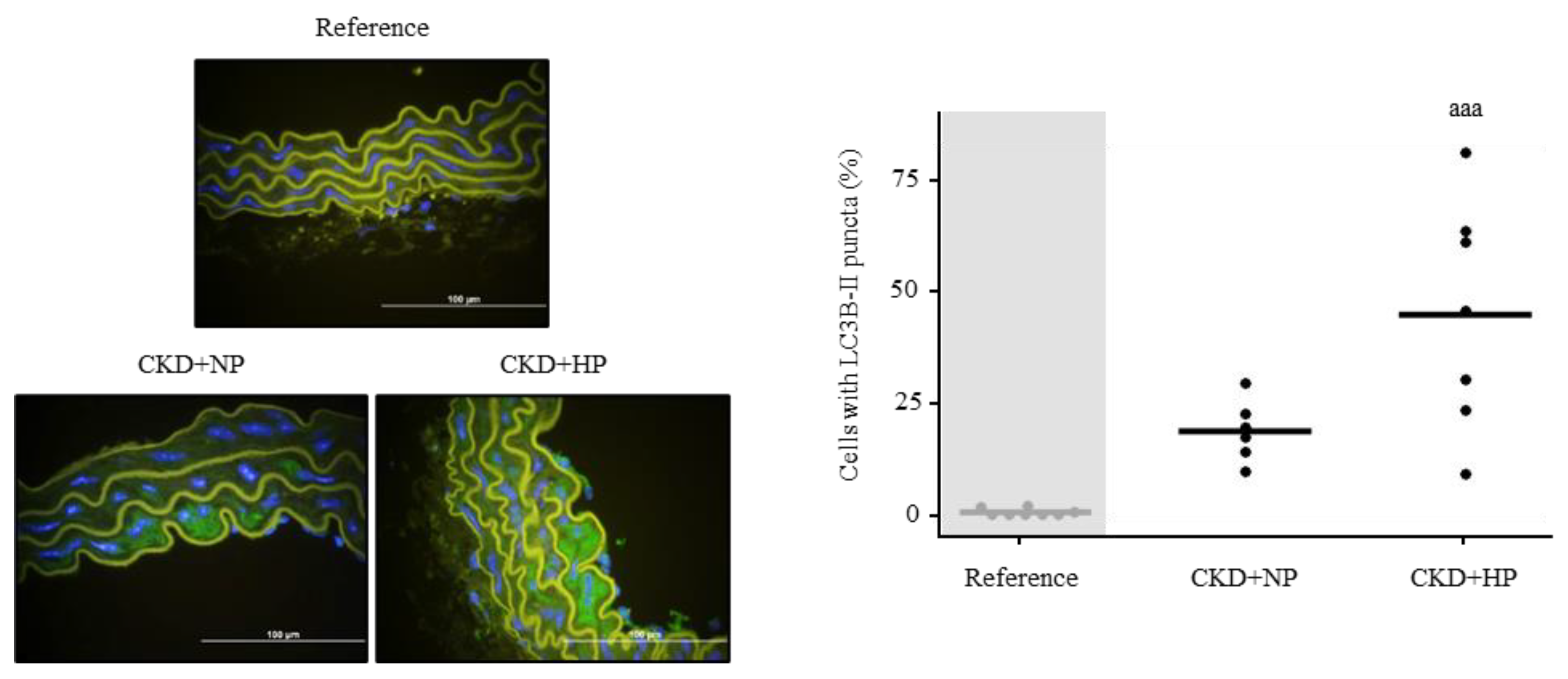
3.4.1. In Vivo: Autophagy Study in Aortas
3.4.2. In Vitro: Autophagic Effect of sKlotho in VSMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Coen, G.; Ballanti, P.; Bonucci, E.; Calabria, S.; Costantini, S.; Ferrannini, M.; Giustini, M.; Giordano, R.; Nicolai, G.; Manni, M.; et al. Renal osteodystrophy in predialysis and hemodialysis patients: Comparison of histologic patterns and diagnostic predictivity of intact PTH. Nephron 2002, 91, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef]
- Lu, X.; Hu, M.C. Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease. Kidney Dis. 2017, 3, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [PubMed]
- Bloch, L.; Sineshchekova, O.; Reichenbach, D.; Reiss, K.; Saftig, P.; Kuro-o, M.; Kaether, C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009, 583, 3221–3224. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef]
- Akimoto, T.; Yoshizawa, H.; Watanabe, Y.; Numata, A.; Yamazaki, T.; Takeshima, E.; Iwazu, K.; Komada, T.; Otani, N.; Morishita, Y.; et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012, 13, 155. [Google Scholar] [CrossRef]
- Drüeke, T.B.; Massy, Z.A. Circulating Klotho levels: Clinical relevance and relationship with tissue Klotho expression. Kidney Int. 2013, 83, 13–15. [Google Scholar] [CrossRef]
- Rotondi, S.; Pasquali, M.; Tartaglione, L.; Muci, M.L.; Mandanici, G.; Leonangeli, C.; Sales, S.; Farcomeni, A.; Mazzaferro, S. Soluble alpha-Klotho Serum Levels in Chronic Kidney Disease. Int. J. Endocrinol. 2015, 2015, 872193. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Moe, O.W.; Pastor, J.; Gianella, F.; Sidhu, S.S.; Sarnak, M.J.; Ix, J.H.; Drew, D.A. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin. Kidney J. 2020, 13, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal Production, Uptake, and Handling of Circulating alphaKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Adema, A.Y.; Vervloet, M.G.; Blankenstein, M.A.; Heijboer, A.C.; NIGRAM Consortium. α-Klotho is unstable in human urine. Kidney Int. 2015, 88, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Falk, E. Limitations of the SCORE-guided European guidelines on cardiovascular disease prevention. Eur. Heart J. 2017, 38, 2259–2263. [Google Scholar] [CrossRef]
- Marinelli, A.; Pistolesi, V.; Pasquale, L.; Di Lullo, L.; Ferrazzano, M.; Baudena, G.; Della Grotta, F.; Di Napoli, A. Diagnosis of Arterial Media Calcification in Chronic Kidney Disease. Cardiorenal. Med. 2013, 3, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Hu, M.C. Potential application of klotho in human chronic kidney disease. Bone 2017, 100, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhou, D.; Tan, R.J.; Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 2013, 24, 771–785. [Google Scholar] [CrossRef]
- Fernández, Á.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.C.; et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018, 558, 136–140. [Google Scholar] [CrossRef]
- Frauscher, B.; Kirsch, A.H.; Schabhüttl, C.; Schweighofer, K.; Kétszeri, M.; Pollheimer, M.; Dragun, D.; Schröder, K.; Rosenkranz, A.R.; Eller, K.; et al. Autophagy Protects From Uremic Vascular Media Calcification. Front. Immunol. 2018, 9, 1866. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Zhao, M.M.; Cai, Y.; Guan, Q.C.; Zhao, Y.; Guan, Y.; Kong, W.; Zhu, W.G.; Xu, M.J.; Wang, X. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013, 83, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Alonso, C.; Menendez-Rodriguez, P.; Virgos-Soriano, M.J.; Fernandez-Martin, J.L.; Fernandez-Coto, M.T.; Cannata-Andia, J.B. Aluminum-induced osteogenesis in osteopenic rats with normal renal function. Calcif. Tissue Int. 1999, 64, 534–541. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 239, 77–84. [Google Scholar] [CrossRef]
- KDIGO. 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Panizo, S.; Naves-Díaz, M.; Carrillo-López, N.; Martínez-Arias, L.; Fernández-Martín, J.L.; Ruiz-Torres, M.P.; Cannata-Andía, J.B.; Rodríguez, I. MicroRNAs 29b, 133b, and 211 Regulate Vascular Smooth Muscle Calcification Mediated by High Phosphorus. J. Am. Soc. Nephrol. 2016, 27, 824–834. [Google Scholar] [CrossRef]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Martínez-Arias, L.; Avello, N.; Sosa, P.; Dusso, A.S.; Cannata-Andía, J.B.; Naves-Díaz, M. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol. Dial. Transpl. 2019, 34, 934–941. [Google Scholar] [CrossRef]
- Carrillo-López, N.; Martínez-Arias, L.; Alonso-Montes, C.; Martín-Carro, B.; Martín-Vírgala, J.; Ruiz-Ortega, M.; Fernández-Martín, J.L.; Dusso, A.S.; Rodriguez-García, M.; Naves-Díaz, M.; et al. The receptor activator of nuclear factor κΒ ligand receptor leucine-rich repeat-containing G-protein-coupled receptor 4 contributes to parathyroid hormone-induced vascular calcification. Nephrol. Dial. Transpl. 2021, 36, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, M.V.; Carrillo-López, N.; Panizo, S.; Castro-Grattoni, A.L.; Valcheva, P.; Ulloa, C.; Rodríguez-Carrio, J.; Cardús, A.; Quirós-Caso, C.; Martínez-Arias, L.; et al. Barley-ss-glucans reduce systemic inflammation, renal injury and aortic calcification through ADAM17 and neutral-sphingomyelinase2 inhibition. Sci. Rep. 2019, 9, 17810. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Román-García, P.; Rodríguez, I.; Martínez-Salgado, C.; Dusso, A.S.; Naves, M.; Cannata-Andía, J.B. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016, 90, 77–89. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, W.; Chen, J.; Liu, C.; Zhang, S.; Yu, K.; Wang, X.; Chen, M.; Wang, S.; Ran, X.; et al. Phosphate Metabolic Inhibition Contributes to Irradiation-Induced Myelosuppression through Dampening Hematopoietic Stem Cell Survival. Nutrients 2022, 14, 3395. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Cuervo, A.M.; Ravikumar, B.; Sarkar, S.; Korolchuk, V.I.; Kaushik, S.; Klionsky, D.J. In search of an “autophagomometer”. Autophagy 2009, 5, 585–589. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lim, K.; Groen, A.; Molostvov, G.; Lu, T.; Lilley, K.S.; Snead, D.; James, S.; Wilkinson, I.B.; Ting, S.; Hsiao, L.L.; et al. α-Klotho Expression in Human Tissues. J. Clin. Endocrinol. Metab 2015, 100, E1308–E1318. [Google Scholar] [CrossRef]
- Pavik, I.; Jaeger, P.; Ebner, L.; Wagner, C.A.; Petzold, K.; Spichtig, D.; Poster, D.; Wüthrich, R.P.; Russmann, S.; Serra, A.L. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol. Dial. Transpl. 2013, 28, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; LI, S.S.; Sha, M.Y.; Kong, J.W.; Ye, J.M.; Liu, Q.F. The controversy of klotho as a potential biomarker in chronic kidney disease. Front. Pharmacol. 2022, 13, 931746. [Google Scholar] [CrossRef]
- Devaraj, S.; Syed, B.; Chien, A.; Jialal, I. Validation of an immunoassay for soluble Klotho protein: Decreased levels in diabetes and increased levels in chronic kidney disease. Am. J. Clin. Pathol. 2012, 137, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bob, F.; Schiller, A.; Timar, R.; Lighezan, D.; Schiller, O.; Timar, B.; Bujor, C.G.; Munteanu, M.; Gadalean, F.; Mihaescu, A.; et al. Rapid decline of kidney function in diabetic kidney disease is associated with high soluble Klotho levels. Nefrologia 2019, 39, 250–257. [Google Scholar] [CrossRef]
- Nadkarni, G.N.; Uribarri, J. Phosphorus and the kidney: What is known and what is needed. Adv. Nutr. 2014, 5, 98–103. [Google Scholar] [CrossRef]
- Román-García, P.; Carrillo-López, N.; Fernández-Martín, J.L.; Naves-Díaz, M.; Ruiz-Torres, M.P.; Cannata-Andía, J.B. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone 2010, 46, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kong, J.; Yu, L.; Liu, Q. Abnormally decreased renal Klotho is linked to endoplasmic reticulum-associated degradation in mice. Int. J. Med. Sci. 2022, 19, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sakan, H.; Nakatani, K.; Asai, O.; Imura, A.; Tanaka, T.; Yoshimoto, S.; Iwamoto, N.; Kurumatani, N.; Iwano, M.; Nabeshima, Y.I.; et al. Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS ONE 2014, 9, e86301. [Google Scholar] [CrossRef] [PubMed]
- van Loon, E.P.; Pulskens, W.P.; van der Hagen, E.A.; Lavrijsen, M.; Vervloet, M.G.; van Goor, H.; Bindels, R.J.; Hoenderop, J.G. Shedding of klotho by ADAMs in the kidney. Am. J. Physiol. Renal. Physiol. 2015, 309, F359–F368. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Nagasu, H.; Morita, Y.; Yamaguchi, T.P.; Kanwar, Y.S.; Kashihara, N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am. J. Physiol. Renal. Physiol. 2012, 302, F1641–F1651. [Google Scholar] [CrossRef]
- Miao, J.; Liu, J.; Niu, J.; Zhang, Y.; Shen, W.; Luo, C.; Liu, Y.; Li, C.; Li, H.; Yang, P.; et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 2019, 18, e13004. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Pérez-Delgado, N.; Górriz, J.L.; Martínez-Castelao, A.; Ortiz, A.; Mora-Fernández, C. Effects of Pentoxifylline on Soluble Klotho Concentrations and Renal Tubular Cell Expression in Diabetic Kidney Disease. Diabetes Care 2018, 41, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Wen, M.; Roth, H.J.; Fehrenz, M.; Flügge, F.; Herath, E.; Weihrauch, A.; Fliser, D.; Heine, G.H. Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int. 2013, 83, 121–128. [Google Scholar] [CrossRef]
- Lan, Q.; Du, C.; Xiong, J.; Wu, Y.; Liao, W.; Liu, C.; Chen, J.; Ran, L.; Wang, Y.; Wang, Y.; et al. Renal Klotho safeguards platelet lifespan in advanced chronic kidney disease through restraining Bcl-xL ubiquitination and degradation. J. Thromb. Haemost. 2022, 20, 2972–2987. [Google Scholar] [CrossRef]
- Kim, H.R.; Nam, B.Y.; Kim, D.W.; Kang, M.W.; Han, J.H.; Lee, M.J.; Shin, D.H.; Doh, F.M.; Koo, H.M.; Ko, K.I.; et al. Circulating alpha-klotho levels in CKD and relationship to progression. Am. J. Kidney Dis. 2013, 61, 899–909. [Google Scholar] [CrossRef]
- Pillebout, E.; Burtin, M.; Yuan, H.T.; Briand, P.; Woolf, A.S.; Friedlander, G.; Terzi, F. Proliferation and remodeling of the peritubular microcirculation after nephron reduction: Association with the progression of renal lesions. Am. J. Pathol. 2001, 159, 547–560. [Google Scholar] [CrossRef]
- Evenepoel, P.; Daenen, K.; Bammens, B.; Claes, K.; Meijers, B.; Naesens, M.; Sprangers, B.; Kuypers, D.; Lerut, E. Microscopic nephrocalcinosis in chronic kidney disease patients. Nephrol. Dial. Transpl. 2015, 30, 843–848. [Google Scholar] [CrossRef]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. A J. Vasc. Biol. 1994, 14, 133–140. [Google Scholar] [CrossRef]
- Lau, W.L.; Linnes, M.; Chu, E.Y.; Foster, B.L.; Bartley, B.A.; Somerman, M.J.; Giachelli, C.M. High phosphate feeding promotes mineral and bone abnormalities in mice with chronic kidney disease. Nephrol. Dial. Transpl. 2013, 28, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, L.; Yin, X.; Ye, J.; Li, S. Correlation Between Soluble Klotho and Vascular Calcification in Chronic Kidney Disease: A Meta-Analysis and Systematic Review. Front. Physiol. 2021, 12, 711904. [Google Scholar] [CrossRef]
- Yamada, S. and C.M. Giachelli, Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 2017, 100, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Zhang, S.Y.; Guan, S.M.; Zhou, S.Q.; Fang, X. Role of Wnt/β-Catenin Pathway in the Arterial Medial Calcification and Its Effect on the OPG/RANKL System. Curr. Med. Sci. 2019, 39, 28–36. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Yang, T.T.; Zhou, H.J.; Zhao, Y.; Kuang, X.; Duan, W.; Du, J.R. Lentiviral vector-mediated overexpression of Klotho in the brain improves Alzheimer’s disease-like pathology and cognitive deficits in mice. Neurobiol. Aging 2019, 78, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.A.; Andres, A.M.; Sin, J.; Taylor, D.P. Untangling autophagy measurements: All fluxed up. Circ. Res. 2015, 116, 504–514. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Kuro-o, M.; Moe, O.W. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010, 78, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, M.; Rakugi, H.; Ishikawa, K.; Maekawa, Y.; Yamamoto, K.; Ohta, J.; Chihara, Y.; Kida, I.; Ogihara, T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006, 339, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Hum, J.M.; O’Bryan, L.M.; Tatiparthi, A.K.; Cass, T.A.; Clinkenbeard, E.L.; Cramer, M.S.; Bhaskaran, M.; Johnson, R.L.; Wilson, J.M.; Smith, R.C.; et al. Chronic Hyperphosphatemia and Vascular Calcification Are Reduced by Stable Delivery of Soluble Klotho. J. Am. Soc. Nephrol. 2017, 28, 1162–1174. [Google Scholar] [CrossRef] [PubMed]



| Reference (n = 8) | CKD+NP (n = 6) | CKD+HP (n = 7) | |
|---|---|---|---|
| Final body weight (g) | 32.21 ± 5.73 | 27.23 ± 1.89 | 25.54 ± 2.86 aa |
| Creatinine (mg/dL) | 0.25 ± 0.04 | 0.34 ± 0.06 a | 0.37 ± 0.06 aaa |
| BUN (mg/dL) | 23.19 ± 4.46 | 41.67 ± 7.14 aaa | 36.45 ± 8.86 aaa |
| Calcium (mg/dL) | 9.13 ± 0.56 | 9.56 ± 0.73 | 9.67 ± 0.46 |
| Phosphorus (mg/dL) | 5.29 ± 1.23 | 5.81 ± 0.79 | 7.21 ± 1.66 a |
| PTH (pg/mL) | 172.68 ± 58.35 | 353.29 ± 121.95 a | 442.04 ± 219.37 aaa |
| FGF23 (pg/mL) | 196.75 ± 132.13 | 186.00 ± 75.42 | 1643.86 ± 892.96 aaa,bbb |
| 1,25(OH)2D3 (pg/mL) | 88.00 ± 29.25 | 104.71 ± 15.91 | 109.76 ± 20.30 |
| sKlotho (pg/mL) | 36.78 ± 14.81 | 7.33 ± 4.56 aa | 1.85 ± 1.85 aaa |
| Calciuria (mg/24 h) | 23.50 ± 15.41 | 26.50 ± 13.00 | 9.90 ± 6.82 aaa,bb |
| Phosphaturia (mg/24 h) | 7.20 ± 7.54 | 2.90 ± 6.40 | 788.20 ± 483.95 aa,bbb |
| Urine albumin (µg/24 h) | 32.14 [14.35–49.75] | 12.96 [8.37–29.19] | 100.85 [37.62–118.32] b |
| Urine Klotho (pg/24 h) | 2269 [1801–13,052] | 1592 [1158–24,249] | 537 [373–857] a,b |
| Urine Klotho (pg Klotho/mg urine creatinine) | 6384 [4988–64,903] | 5876 [4100–13,483] | 2365 [377–2482] a,b |
| Control (n = 38) | CKD-2/3a (n = 11) | CKD-3b (n = 12) | CKD+NP (n = 6) | CKD+HP (n = 7) | |
|---|---|---|---|---|---|
| Age (years) | 66.50 ± 4.63 | 61.72 ± 10.11 | 67.33 ± 7.41 | 69.09 ± 8.94 | 68.78 ± 5.97 |
| BMI (kg/m2) | 27.19 ± 4.57 | 31.34 ± 4.70 | 26.79 ± 2.84 | 30.52 ± 6.60 | 26.74 ± 3.99 |
| Creatinine (mg/dL) | 0.83 (0.72–0.98) | 1.23 (1.19–1.50) aaa | 1.75 (1.63–1.84) aaa | 2.66 (2.30–2.95) aaa,b | 3.70 (3.41–4.48) aaa,bbb,c |
| eGFR (mL/min/1.73 m2) | 81.5 (75–87.8) | 49.0 (47.0–53.0) aaa | 39.0 (33.8–40.3) aaa | 23.0 (20.5–24.5) aaa,b | 12.0 (12.0–13.0) aaa,bbb,c |
| Total protein (mg/dL) | 70.70 ± 3.49 | 71.20 ± 3.08 | 69.45 ± 2.88 | 69.05 ± 3.56 | 68.50 ± 5.96 |
| Calcium (mg/dL) | 9.53 ± 0.27 | 9.53 ± 0.32 | 9.55 ± 0.37 | 9.66 ± 0.60 | 9.35 ± 0.45 |
| Phosphorus (mg/dL) | 3.64 (3.54–4.00) | 3.16 (3.54–3.53) a | 3.30 (2.69–3.47) aaa | 3.41 (3.10–3.88) | 4.09 (3.84–4.87) a,bbb,ccc,ddd |
| PTH (pg/mL) | 52.0 (41.5–60.5) | 56.0 (43.0–75.0) | 86.5 (71.8–117.5) aaa | 114.0 (71.5–198.5) aaa,bb | 176.0 (155.0–212.0) aaa,bbb,c |
| 1,25(OH)2D3 (pg/mL) | 45.56 ± 11.55 | 38.80 ± 11.50 | 29.13 ± 10.27 aaa | 38.57 ± 17.89 | 24.03 ± 12.14 aaa |
| FGF23 (pg/mL) | 55.2 (46.3–64.7) | 56.8 (52.1–62.8) | 134.6 (80.7–166.2) aaa,b | 143.8 (101.1–240.8) aaa,bb | 175.9 (141.2–1,689.0) aaa,bbb |
| sKlotho (pg/mL) | 809.2 (680.3–1042.2) | 670.6 (604.2–746.0) a | 632.2 (599.8–713.0) aa | 591.4 (517.2- 706.8) aaa | 585.80 (469.40–674.20) aaa |
| Urine creatinine (mg/dL) | 105.65 (74.63–150.80) | 44.80 (35.25–100.75) a | 95.95 (57.58–114.38) | 82.75 (56.75–95.73) | 47.20 (46.60–71.30) aaa |
| Proteinuria (g/L) | 0.07 (0.05–0.09) | 0.05 [0.03–0.16] | 0.44 (0.15–0.77) aaa,bb | 0.32 (0.09–0.94) aaa,bb | 0.58 (0.26–1.06) aaa,bbb |
| Urine Klotho (pg Klotho/mg urine creatinine) | 433.4 (189.1–726.9) | 1143.1 (539.4–2220) aa | 1055.1 (910.8–2,103.5) aaa | 1003.3 (849.2–2819.2) aaa | 1099.7 (905.9–1503) aaa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Vírgala, J.; Fernández-Villabrille, S.; Martín-Carro, B.; Tamargo-Gómez, I.; Navarro-González, J.F.; Mora-Fernández, C.; Calleros, L.; Astudillo-Cortés, E.; Avello-Llano, N.; Mariño, G.; et al. Serum and Urinary Soluble α-Klotho as Markers of Kidney and Vascular Impairment. Nutrients 2023, 15, 1470. https://doi.org/10.3390/nu15061470
Martín-Vírgala J, Fernández-Villabrille S, Martín-Carro B, Tamargo-Gómez I, Navarro-González JF, Mora-Fernández C, Calleros L, Astudillo-Cortés E, Avello-Llano N, Mariño G, et al. Serum and Urinary Soluble α-Klotho as Markers of Kidney and Vascular Impairment. Nutrients. 2023; 15(6):1470. https://doi.org/10.3390/nu15061470
Chicago/Turabian StyleMartín-Vírgala, Julia, Sara Fernández-Villabrille, Beatriz Martín-Carro, Isaac Tamargo-Gómez, Juan F. Navarro-González, Carmen Mora-Fernández, Laura Calleros, Elena Astudillo-Cortés, Noelia Avello-Llano, Guillermo Mariño, and et al. 2023. "Serum and Urinary Soluble α-Klotho as Markers of Kidney and Vascular Impairment" Nutrients 15, no. 6: 1470. https://doi.org/10.3390/nu15061470
APA StyleMartín-Vírgala, J., Fernández-Villabrille, S., Martín-Carro, B., Tamargo-Gómez, I., Navarro-González, J. F., Mora-Fernández, C., Calleros, L., Astudillo-Cortés, E., Avello-Llano, N., Mariño, G., Dusso, A. S., Alonso-Montes, C., Panizo, S., Cannata-Andía, J. B., Naves-Díaz, M., & Carrillo-López, N. (2023). Serum and Urinary Soluble α-Klotho as Markers of Kidney and Vascular Impairment. Nutrients, 15(6), 1470. https://doi.org/10.3390/nu15061470






