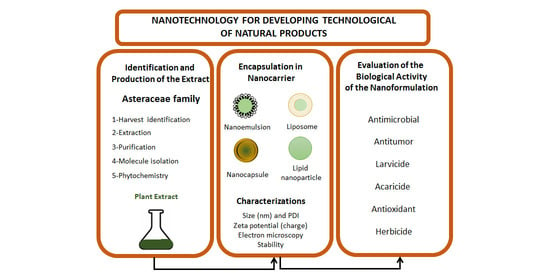
Nanotechnology Promoting the Development of Products from the Biodiversity of the Asteraceae Family
Abstract
1. Introduction
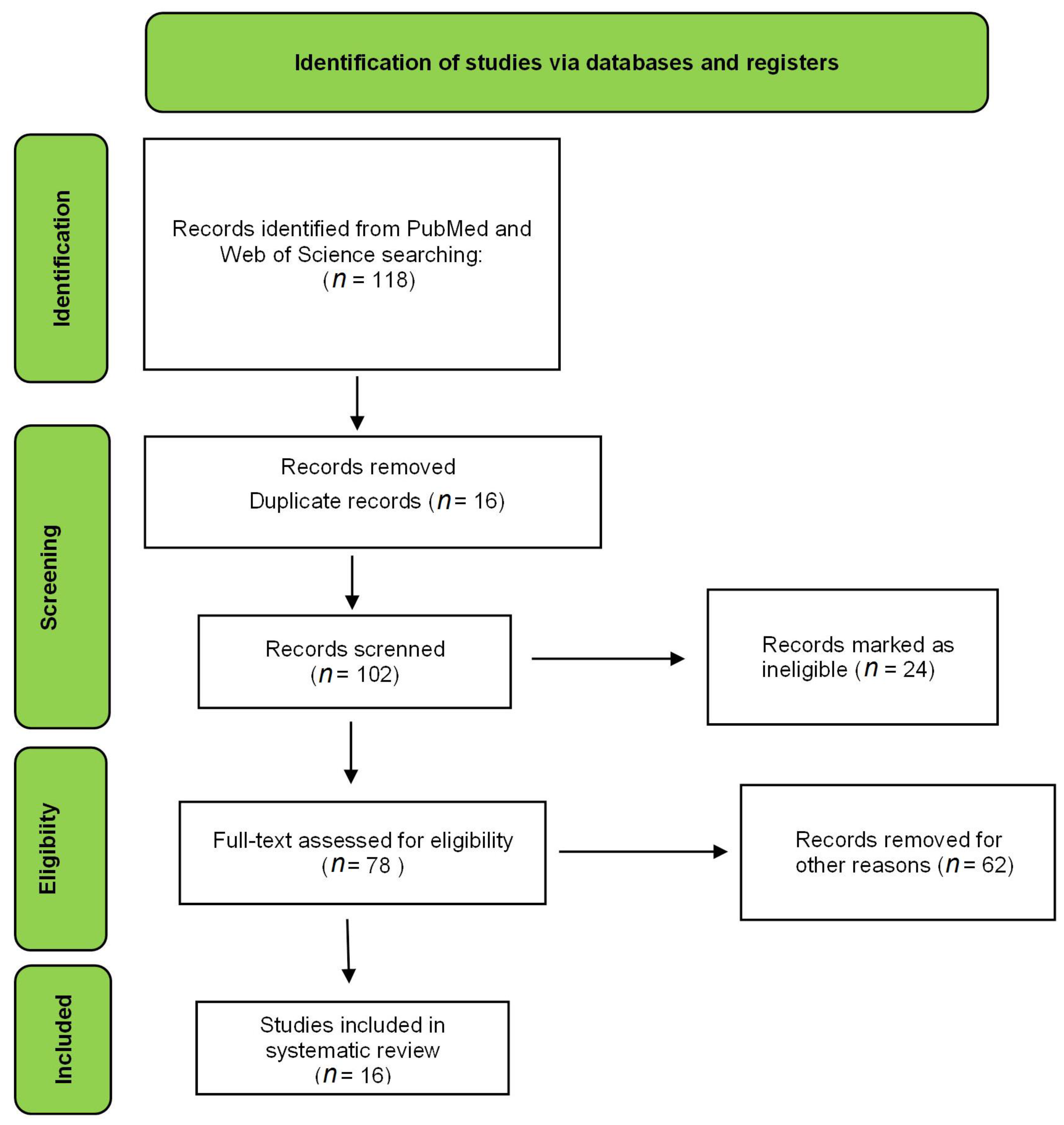
2. Literature Search
| References | [12] | [16] | [17] | [9] | [18] | [19] | [20] | [21] | [22] | [23] | [13] | [14] | [15] | [24] | [25] | [10] | [26] | [27] | [28] | [29] | [30] | [31] | [32] | [33] | [11] | [34] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Abstract | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Introduction | ||||||||||||||||||||||||||
| Background information | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Objectives | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Method | ||||||||||||||||||||||||||
| Phytochemistry | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Voucher specimen | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Formulation characterization | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Stability study | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Morphological study | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| In vitro studies | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Cytotoxicity assay | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| In vivo studies | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Therapeutic application | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Statistical analysis | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Results and discussion | ||||||||||||||||||||||||||
| Interpretation | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Conclusion | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Total punctuation | 11 | 13 | 11 | 14 | 12 | 10 | 13 | 12 | 12 | 11 | 11 | 12 | 11 | 11 | 10 | 14 | 10 | 11 | 10 | 11 | 10 | 10 | 11 | 10 | 14 | 11 |
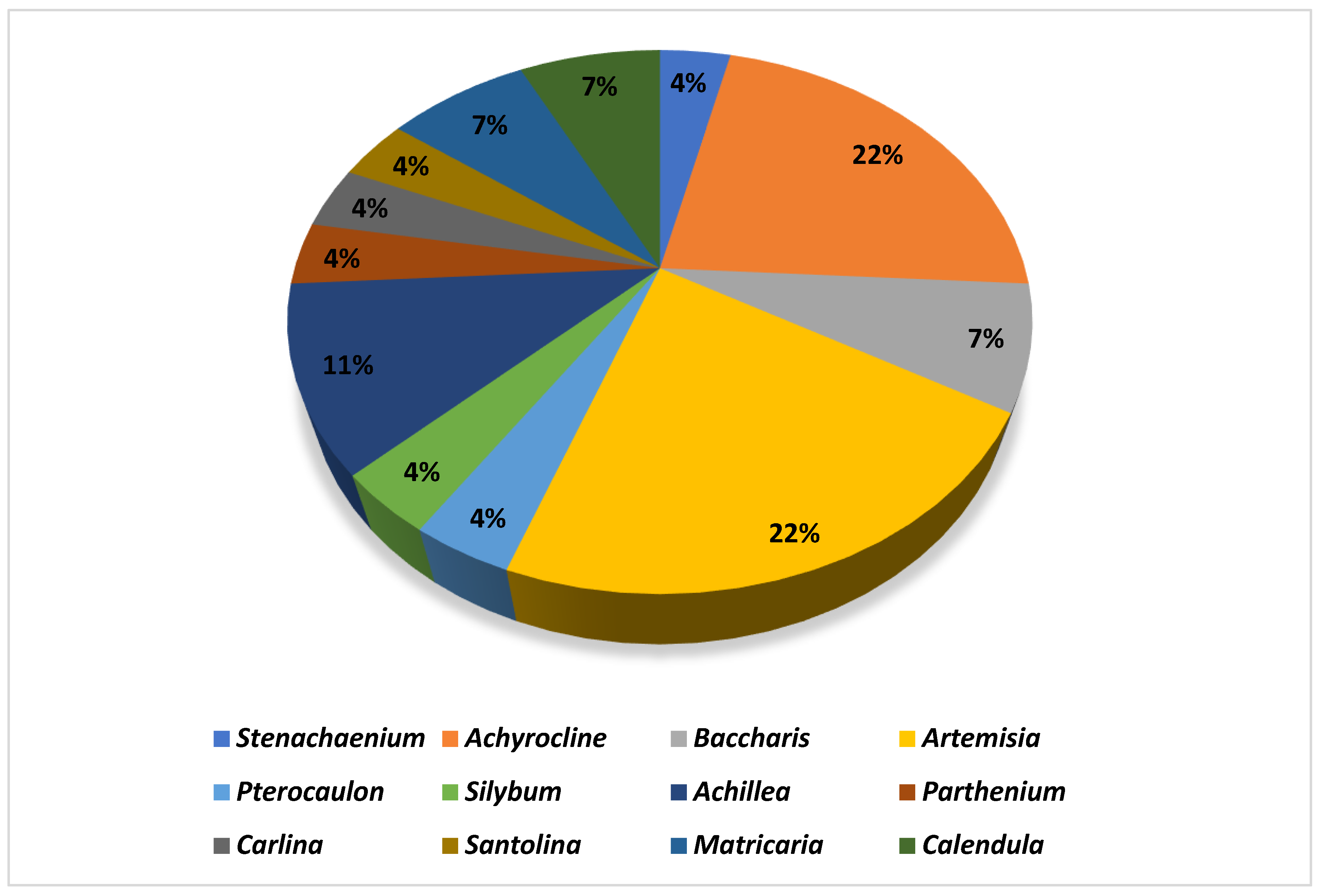
2.1. Phytochemistry Information
2.1.1. Artemisia spp.
2.1.2. Achyrocline spp.
2.1.3. Achillea spp.
2.1.4. Baccharis spp.
2.1.5. Calendula spp.
2.1.6. Matricaria spp.
2.1.7. Other Species
2.2. Biological Activities
2.3. Nanoformulation Information
2.4. Patents Targeting Products with Asteraceae Plants
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, F.A. Herbs—Useful Plants. Their Role in History and Today. Eur. J. Gastroenterol. Hepatol. 1996, 8, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, J.E.R.; Saldanha, H.C.; Freitas, G.R.O.E.; Morais, E.R. A Review of Medicinal Plants Used in the Brazilian Cerrado for the Treatment of Fungal and Bacterial Infections. J. Herb. Med. 2022, 31, 100523. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Asteraceae Species with Most Prominent Bioactivity and Their Potential Applications: A Review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Funk, V.A.; Bayer, R.J.; Keeley, S.; Chan, R.; Watson, L.; Gemeinholzer, B.; Schilling, E.E.; Panero, J.L.; Baldwin, B.G.; Garcia-Jacas, N.; et al. Everywhere but Antarctica: Using a Supertree to Understand the Diversity and Distribution of the Compositae. Biol. Skr. 2005, 55, 343–373. [Google Scholar]
- de Vargas, M.R.W.; Raffin, F.N.; de Lima e Moura, T.F.A. Strategies Used for to Improve Aqueous Solubility of Simvastatin: A Systematic Review. Rev. Ciênc. Farm. Básica Apl. 2012, 33, 497–507. [Google Scholar]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation Design for Poorly Water-Soluble Drugs Based on Biopharmaceutics Classification System: Basic Approaches and Practical Applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.E.B.; Ricci-Júnior, E.; Mansur, C.R.E. Nanoemulsions as Delivery Systems for Lipophilic Drugs. J. Nanosci. Nanotechnol. 2012, 12, 2881–2890. [Google Scholar] [CrossRef]
- Balestrin, L.A.; Bidone, J.; Bortolin, R.C.; Moresco, K.; Moreira, J.C.; Teixeira, H.F. Protective Effect of a Hydrogel Containing Achyrocline satureioides Extract-Loaded Nanoemulsion against UV-Induced Skin Damage. J. Photochem. Photobiol. B Biol. 2016, 163, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Maxia, A.; Murgia, S.; Pons, R.; Demurtas, D.; Pando, D.; Falconieri, D.; Peris, J.E.; et al. Faceted Phospholipid Vesicles Tailored for the Delivery of Santolina insularis Essential Oil to the Skin. Colloids Surf. B Biointerfaces 2015, 132, 185–193. [Google Scholar] [CrossRef]
- Mughees, M.; Wajid, S.; Samim, M. Cytotoxic Potential of Artemisia Absinthium Extract Loaded Polymeric Nanoparticles against Breast Cancer Cells: Insight into the Protein Targets. Int. J. Pharm. 2020, 586, 119583. [Google Scholar] [CrossRef] [PubMed]
- Danielli, L.J.; dos Reis, M.; Bianchini, M.; Camargo, G.S.; Bordignon, S.A.L.; Guerreiro, I.K.; Fuentefria, A.; Apel, M.A. Antidermatophytic Activity of Volatile Oil and Nanoemulsion of Stenachaenium megapotamicum (Spreng.) Baker. Ind. Crops Prod. 2013, 50, 23–28. [Google Scholar] [CrossRef]
- Azizkhani, M.; Jafari Kiasari, F.; Tooryan, F.; Shahavi, M.H.; Partovi, R. Preparation and Evaluation of Food-Grade Nanoemulsion of Tarragon (Artemisia dracunculus L.) Essential Oil: Antioxidant and Antibacterial Properties. J. Food Sci. Technol. 2021, 58, 1341–1348. [Google Scholar] [CrossRef]
- Benelli, G.; Pavoni, L.; Zeni, V.; Ricciardi, R.; Cosci, F.; Cacopardo, G.; Gendusa, S.; Spinozzi, E.; Petrelli, R.; Cappellacci, L.; et al. Developing a Highly Stable Carlina acaulis Essential Oil Nanoemulsion for Managing Lobesia botrana. Nanomaterials 2020, 10, 1867. [Google Scholar] [CrossRef]
- Das, S.; Vörös-Horváth, B.; Bencsik, T.; Micalizzi, G.; Mondello, L.; Horváth, G.; Kószegi, T.; Széchenyi, A. Antimicrobial Activity of Different Artemisia Essential Oil. Molecules 2020, 25, 2390. [Google Scholar] [CrossRef]
- Bidone, J.; Argenta, D.F.; Kratz, J.; Pettenuzzo, L.F.; Horn, A.P.; Koester, L.S.; Bassani, V.L.; Simões, C.M.O.; Teixeira, H.F. Antiherpes Activity and Skin/Mucosa Distribution of Flavonoids from Achyrocline satureioides Extract Incorporated into Topical Nanoemulsions. Biomed Res. Int. 2015, 2015, 238010. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Caregnato, F.; Moreira, J.C.F.; Teixeira, H.F.; Carvalho, E.L.S. Antioxidant Effect of Nanoemulsions Containing Extract of Achyrocline satureioides (Lam) D.C.—Asteraceae. AAPS PharmSciTech 2015, 17, 844–850. [Google Scholar] [CrossRef]
- Da Botas, G.S.; Cruz, R.A.S.; De Almeida, F.B.; Duarte, J.L.; Araújo, R.S.; Souto, R.N.P.; Ferreira, R.; Carvalho, J.C.T.; Santos, M.G.; Rocha, L.; et al. Baccharis reticularia DC. and Limonene Nanoemulsions: Promising Larvicidal Agents for Aedes aegypti (Diptera: Culicidae) Control. Molecules 2017, 22, 1990. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Amani, A.; Sereshti, H.; Abai, M.R.; Esmaeili, F.; Sedaghat, M.M. Preparation and Optimization Nanoemulsion of Tarragon (Artemisia dracunculus) Essential Oil as Effective Herbal Larvicide against Anopheles stephensi. Ind. Crops Prod. 2017, 109, 214–219. [Google Scholar] [CrossRef]
- Panatieri, L.F.; Brazil, N.T.; Faber, K.; Medeiros-Neves, B.; von Poser, G.L.; Rott, M.B.; Zorzi, G.K.; Teixeira, H.F. Nanoemulsions Containing a Coumarin-Rich Extract from Pterocaulon balansae (Asteraceae) for the Treatment of Ocular Acanthamoeba Keratitis. AAPS PharmSciTech 2017, 18, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Rosseti, C.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Prediction of Permeation and Cellular Transport of Silybum marianum Extract Formulated in a Nanoemulsion by Using PAMPA and Caco-2 Cell Models. Planta Med. 2017, 83, 1184–1193. [Google Scholar] [CrossRef]
- Al-Assiuty, B.A.; Nenaah, G.E.; Ageba, M.E. Chemical Profile, Characterization and Acaricidal Activity of Essential Oils of Three Plant Species and Their Nanoemulsions against Tyrophagus putrescentiae, a Stored-Food Mite. Exp. Appl. Acarol. 2019, 79, 359–376. [Google Scholar] [CrossRef]
- Zainuddin, N.J.; Ashari, S.E.; Salim, N.; Asib, N.; Omar, D.; Lian, G.E.C. Optimization and Characterization of Palm Oil-Based Nanoemulsion Loaded with Parthenium hysterophorus Crude Extract for Natural Herbicide Formulation. J. Oleo Sci. 2019, 68, 747–757. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Toit, L.C.; Parry, A.; Pillay, V.; Choonara, Y.E. Encapsulation of Essential Oils within a Polymeric Liposomal Formulation for Enhancement of Antimicrobial Efficacy. Nat. Prod. Commun. 2010, 5, 1401–1408. [Google Scholar] [CrossRef]
- Arsic, I.; Tadic, V.; Vlaovic, D.; Homšek, I.; Vesic, S.; Wiley, J. Preparation of Novel Apigenin-Enriched, Liposomal and Non-Liposomal, Antiinflammatory Topical Formulations as Substitutes for Corticosteroid Therapy. Phyther. Res. 2011, 233, 228–233. [Google Scholar] [CrossRef]
- Hassanzadeh-kiabi, F.; Negahdari, B. Antinociceptive Synergistic Interaction between Achillea millefolium and Origanum vulgare L. Extract Encapsulated in Liposome in Rat. Artif. Cells Nanomed. Biotechnol. 2018, 46, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo-Rinhel, A.S.G.; De Andrade, M.F.; Landi-librandi, A.P.; Elisa, A.; Seixas, C.; Mariko, L.; Bastos, J.K.; Lucisano-valim, Y.M.; Landi-librandi, A.P.; Elisa, A.; et al. Incorporation of Baccharis dracunculifolia DC (Asteraceae) Leaf Extract into Phosphatidylcholine-Cholesterol Liposomes Improves Its Anti-Inflammatory Effect in Vivo. Nat. Prod. Res. 2018, 6419, 2521–2525. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Kok, S.H.L.; Bian, Z.X.; Lam, K.H.; Tang, J.C.O.; Lee, K.K.H.; Gambari, R.; Chui, C.H. D-Glucose as a Modifying Agent in Gelatin/Collagen Matrix and Reservoir Nanoparticles for Calendula Officinalis Delivery. Colloids Surf. B Biointerfaces 2014, 117, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.; Salado, C.; Vega, S.; Aizpurua-Olaizola, O.; de la Arada, I.; Suarez, T.; Usobiaga, A.; Arrondo, J.L.R.; Alonso, A.; Goñi, F.M.; et al. Solid Lipid Nanoparticles for Delivery of Calendula Officinalis Extract. Colloids Surf. B Biointerfaces 2015, 135, 18–26. [Google Scholar] [CrossRef]
- Do Carmo, G.M.; Baldissera, M.D.; Vaucher, R.A.; Rech, V.C.; Oliveira, C.B.; Sagrillo, M.R.; Boligon, A.A.; Athayde, M.L.; Alves, M.P.; França, R.T.; et al. Effect of the Treatment with Achyrocline satureioides (Free and Nanocapsules Essential Oil) and Diminazene Aceturate on Hematological and Biochemical Parameters in Rats Infected by Trypanosoma evansi. Exp. Parasitol. 2015, 149, 39–46. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Cossetin, L.F.; Dalla Lana, D.F.; Monteiro, S.G. Achyrocline satureioides Essential Oil Loaded in Nanocapsules Ameliorate the Antioxidant/Oxidant Status in Heart of Rats Infected with Trypanosoma evansi. Microb. Pathog. 2017, 105, 30–36. [Google Scholar] [CrossRef]
- Ritter, C.S.; Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Sagrillo, M.R.; da Silva, A.P.T.; Moresco, R.N.; Guarda, N.S.; da Silva, A.S.; Stefani, L.M.; et al. Achyrocline satureioides Essential Oil-Loaded in Nanocapsules Reduces Cytotoxic Damage in Liver of Rats Infected by Trypanosoma evansi. Microb. Pathog. 2017, 103, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Moradkhani, M.R.; Karimi, A. Effect of Artemisia AucheriL and Bupivacaine Encapsulated Nanoparticles on Nociceptive Pain. Drug Res. 2019, 69, 401–405. [Google Scholar] [CrossRef]
- Karam, T.K.; Ortega, S.; Ueda Nakamura, T.; Auzély-Velty, R.; Nakamura, C.V. Development of Chitosan Nanocapsules Containing Essential Oil of Matricaria chamomilla L. for the Treatment of Cutaneous Leishmaniasis. Int. J. Biol. Macromol. 2020, 162, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, A.S.; Walter, B.M. Artemisia in Flora Do Brasil 2020. Jardim Botânico Do Rio de Janeiro. Available online: http://reflora.jbrj.gov.br/reflora/floradobrasil/FB15959 (accessed on 5 January 2022).
- Fuzimoto, A.D. An Overview of the Anti-SARS-CoV-2 Properties of Artemisia Annua, Its Antiviral Action, Protein-Associated Mechanisms, and Repurposing for COVID-19 Treatment. J. Integr. Med. 2021, 19, 375–388. [Google Scholar] [CrossRef]
- Deble, L.P. Achyrocline in Flora Do Brasil 2020. Jardim Botânico Do Rio de Janeiro. Available online: http://reflora.jbrj.gov.br/reflora/floradobrasil/FB102953 (accessed on 7 January 2022).
- Fernandes, F.; Heiden, G. Achillea in Flora Do Brasil 2020. Jardim Botânico Do Rio de Janeiro. Available online: http://reflora.jbrj.gov.br/reflora/floradobrasil/FB613446 (accessed on 5 January 2022).
- Verdi, L.G.; Maria, I.; Brighente, C.; Pizzolatti, G.; De Química, D.; Federal, U.; Catarina, D.S. Gênero Baccharis (Asteraceae): Aspectos químicos, econômicos e biológicos. Quím. Nova. 2005, 28, 85–94. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A Systematic Review of Calendula Officinalis Extract for Wound Healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Singh, S.; Kumar, V.; Kumar, A.; Kumari, A.; Rathore, S.; Kumar, R.; Singh, S. A Comprehensive Review on Biology, Genetic Improvement, Agro and Process Technology of German Chamomile (Matricaria chamomilla L.). Plants 2022, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and Traditional Medical Applications of Carlina acaulis L.—A Critical Ethnopharmacological Review. J. Ethnopharmacol. 2019, 239, 111842. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Malhi, D.S.; Cooper, R.; Kaur, M.; Sohal, H.S.; Mutreja, V.; Sharma, A. Comprehensive Review on Ethnobotanical Uses, Phytochemistry, Biological Potential and Toxicology of Parthenium hysterophorus L.: A Journey from Noxious Weed to a Therapeutic Medicinal Plant. J. Ethnopharmacol. 2021, 281, 114525. [Google Scholar] [CrossRef]
- Medeiros-Neves, B.; Teixeira, H.F.; von Poser, G.L. The Genus Pterocaulon (Asteraceae)—A Review on Traditional Medicinal Uses, Chemical Constituents and Biological Properties. J. Ethnopharmacol. 2018, 224, 451–464. [Google Scholar] [CrossRef]
- Porwal, O.; Mohammed Ameen, M.S.; Anwer, E.T.; Uthirapathy, S.; Ahamad, J.; Tahsin, A. Silybum marianum (Milk Thistle): Review on Its Chemistry, Morphology, Ethno Medical Uses, Phytochemistry and Pharmacological Activities. J. Drug Deliv. Ther. 2019, 9, 199–206. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical Composition of the Essential Oils and Extracts of Achillea Species and Their Biological Activities: A Review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef]
- Kourniatis, L.R.; Spinelli, L.S.; Mansur, C.R.E.; González, G. Nanoemulsões Óleo de Laranja/Água Preparadas Em Homogeneizador de Alta Pressão. Quim. Nova 2010, 33, 295–300. [Google Scholar] [CrossRef]
- Apolinário, A.C.; Salata, G.C.; Bianco, A.F.R.; Fukumori, C.; Lopes, L.B. Opening the Pandora’s Box of Nanomedicine: There Is Needed Plenty of Room at the Bottom. Quim. Nova 2020, 43, 212–225. [Google Scholar] [CrossRef]
- Mahdi, E.S.; Noor, A.M.; Sakeena, M.H.; Abdullah, G.Z.; Abdulkarim, M.F.; Sattar, M.A. Formulation and in Vitro Release Evaluation of Newly Synthesized Palm Kernel Oil Esters-Based Nanoemulsion Delivery System for 30% Ethanolic Dried Extract Derived from Local Phyllanthus Urinaria for Skin Antiaging. Int. J. Nanomed. 2011, 6, 2499–2512. [Google Scholar] [CrossRef]
- Rossi-Bergman, B. A Nanotecnologia: Da Saúde Para Além Do Determinismo Tecnológico. Ciênc. Cult. 2008, 60, 54–57. [Google Scholar]
- Ferreira, A.P.; Sant’Anna, L.S. A Nanotecnologia e a Questão Da Sua Regulação No Brasil: Impactos à Saúde e Ao Ambiente. Rev. Uniandrade 2015, 16, 119–128. [Google Scholar] [CrossRef]
- Yang, M. New Nanoparticle Medicine “Yizhihao Shangshiqutong” Useful for Relaxing Pain Due to Injury and Dampness, Comprises Nanoparticle Powders of 6 Chinese Medicinal Materials Including Artemisia Repestris Fluid Extract, Menthol and Borneol. CN 102552414-A, 11 September 2002. [Google Scholar]
- Basulto Heras, L.G.; Pacheco, G.C.; Pulido, M.R.; Gonzalez Enriquez, G.V.; Villalvazo, R.M. Nanotechnological Ophthalmic Solution Used for Preventing and Combating Dry Eyes, and Maintaining Integrity of Corneal Epithelium with Mitigating Eye Discomfort, Comprises Extract of Matricaria Recutita and Calendula Officinalis in Liposome. MX 2011013407-A1, 28 June 2013. [Google Scholar]
- Si, H.; Zhou, X. Preparation of Antibacterial Resin Used for e.g. Environmentally-Friendly Paint, Involves Dissolving Stearyl Dimethylaminopropylamine in Polyvinyl Acetate, Stirring Artemisia Argyi Oil and Liposome Mixture and Emulsifying Mixture. CN 105017913-A, 4 November 2015. [Google Scholar]
- Ouyang, S.; Ouyang, W.; Zheng, X. Oil-in-Water Compound Juniper Berry Oil Nanoemulsion Composition for Treating e.g. Acne and Eczema Comprises Surfactant, Cosurfactant, Juniper Berry Oil, Matricaria chamomilla L. Oil, Eucalyptus Oil, Tea Tree Oil, and Distilled Water. CN 102552414-A, 11 July 2012. [Google Scholar]
| Species | Vegetal Part Used | Extraction Method | Extracted | Chemical Marker | Extract Characterization | References |
|---|---|---|---|---|---|---|
| Achyrocline satureioides | Inflorescences | Maceration | Ethanolic extract (80%) | 3-O-methylquercetin | HPLC-UV | [17] |
| Achillea fragantissimaand Achillea santolina | Aerial parts | Hydrodistillation | Essential oil | cis-thujone and 1,8 cineole | GC-MS | [22] |
| Achillea millefolium | Leave | Refluxed | Aquous extract | - | - | [26] |
| Achyrocline satureioides | Aerial parts | Maceration | Ethanolic extract (80%) | 3-O-methylquercetin | LC-UV | [16] |
| Achyrocline satureioides | - | - | Essential oil (purchased) | α-Pinene | CG-FID | [30] |
| Achyrocline satureioides | - | - | Essential oil (purchased) | α-Pinene | CG-FID | [31] |
| Achyrocline satureioides | - | - | Essential oil (purchased) | α-Pinene | CG-FID | [32] |
| Achyrocline satureioides | Inflorescences | Maceration | Ethanolic extract (80%) | 3-O-methylquercetin | HPLC-UV | [9] |
| Artemisia absinthium | Whole plant | Maceration | Ethanolic extract | - | GC-MS | [11] |
| Artemisia afra | - | Hydrodistillation | Essential oil | - | - | [24] |
| Artemisia annua | Whole plant | Steam distillation | Essential oil | β-selinene | GC-FID and GC/MS | [15] |
| Artemisia aucheri | Aerial parts | Maceration | Methanolic extract | - | - | [33] |
| Artemisia dracunculus | - | - | Essential oil (purchased) | P-Allylanisole | GC-MS | [19] |
| Artemisia dracunculus | - | - | Essential oil (purchased) | Estragole | GC-FID and GC/MS | [13] |
| Baccharis dracunculifolia | Aerial parts | Maceration | Ethanolic extract | - | - | [27] |
| Baccharis reticularia | Leaves | Hydrodistillation | Essential oil | D-limonene | GC-FID and GC/MS | [18] |
| Calendula offinalis | - | - | Powder and oil (purchased) | - | - | [28] |
| Calendula offinalis | - | Supercritical CO2 extract | - | - | - | [29] |
| Carlina acaulis | Root | Hydrodistillation | Essential oil | Carlina oxide | GC-MS | [14] |
| Matricaria chamomilla | Flower | Percolation | Aqueous extract | Apigenin | HPLC | [25] |
| Matricaria chamomilla | - | - | Essential oil (purchased) | - | - | [34] |
| Parthenium hysterophorus | Whole plant | Maceration | Methanolic extract | - | - | [23] |
| Pterocaulon balansae | Aerial parts | Maceration | Hexanic extract | 5-methoxy-6,7-methylenedioxycoumarin | HPLC-PDA/UPLC-MS | [20] |
| Santolina insularis | Aerial parts | Steam distillation | Essential oil | β-phellandrene | GC-FID and GC/MS | [10] |
| Silybum marianum | - | - | Commercial extract | Silybin | HPLC-PDA/UPLC-MS | [21] |
| Stenachaenium megapotamicum | Leaves and flowers | Hydrodistillation | Essential oil | Fokienol | GC-MS | [12] |
| Chemical Marker | Species | Identification | References |
|---|---|---|---|
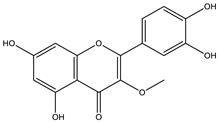 | Achyrocline satureioides | HPLC-UV | [17] |
 | Achillea fragantissima | GC-MS | [22] |
 | Achillea santolina | GC-MS | [22] |
 | Achyrocline satureioides | LC-UV | [16] |
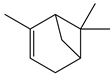 | Achyrocline satureioides | CG-FID | [30] |
 | Achyrocline satureioides | CG-FID | [31] |
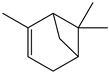 | Achyrocline satureioides | CG-FID | [32] |
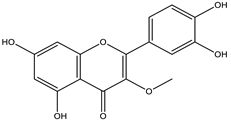 | Achyrocline satureioides | HPLC-UV | [9] |
 | Artemisia annua | GC-FID and GC/MS | [15] |
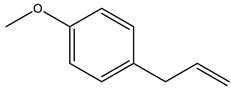 | Artemisia dracunculus | GC-MS | [19] |
 | Artemisia dracunculus | GC-FID and GC/MS | [13] |
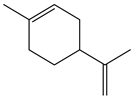 | Baccharis reticularia | GC-FID and GC/MS | [18] |
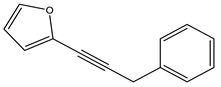 | Carlina acaulis | GC-MS | [14] |
 | Chamomile | HPLC | [25] |
 | Pterocaulon balansae | HPLC-PDA/UPLC-MS | [20] |
 | Santolina insularis | GC-FID and GC/MS | [10] |
 | Silybum marianum | HPLC-PDA/UPLC-MS | [21] |
 | Stenachaenium megapotamicum | GC-MS | [12] |
| Nanoemulsion | |||||
|---|---|---|---|---|---|
| Species | Biologic Activity | Assay | Vector/Microorganism | Result | References |
| Achillea fragantissima | Acaricidal | Fumigant acaricidal activity | Tyrophagus putrescentiae | LC50 = 4.7 μL/L (3.2 ± 6.8) | [22] |
| Achillea santolina | LC50 = 9.6 μL/L (7.1 ± 13.3) | ||||
| Achyrocline satureioides | Antiviral | Antiherpes activity | Herpes Simplex Virus type 1 (HSV-1/KOS strain | IC50 = 1.40 ± 0.88 μg/mL | [16] |
| Achyrocline satureioides | Antioxidant | TBA-RS | - | 77.6% inhibition of lipoperoxidation | [17] |
| Achyrocline satureioides | Antioxidant | TRAP | - | Six-fold higher reduction in the chemiluminescence | [9] |
| Artemisia annua | Antibacterial and antifungal | MIC | Escherichia coli | 1.68 ± 0.72 µg/mL | [15] |
| Staphylococcus aureus | 1.62 ± 0.37 µg/mL | ||||
| P. aeruginosa | 1.46 ± 0.22 µg/mL | ||||
| S. pyogenes | 3.15 ± 0.16 µg/mL | ||||
| S. pombe | 2.01 ± 0.46 µg/mL | ||||
| C. albicans | 3.62 ± 0.65 µg/mL | ||||
| C. tropicalis | 4.29 ± 0.82 µg/mL | ||||
| C. dubliniensis | 3.63 ± 0.57 µg/mL | ||||
| C. krusei | 3.79 ± 0.57 µg/mL | ||||
| Artemisia dracunculus | Larvicidal | Larvicidal activity | Anopheles stephensi (3rd and 4th instar larvae) | 82% of mortality | [19] |
| Artemisia dracunculus | Antibacterial | MIC and MBC | Escherichia coli | 5.75/6.25 µg/mL | [13] |
| Listeria monocytogenes | 3.25/3.75 µg/mL | ||||
| Salmonella enteritidis | 4.75/5.75 µg/mL | ||||
| Shigella dysenteriae | 3.80/4.45 µg/mL | ||||
| Staphylococcus aureus | 2.5/3.25 µg/mL | ||||
| Antioxidant | DPPH | - | IC50 = 0.052 mg/mL | ||
| FRAP | 70.15 ± 0.63 µmol/mL of antioxidant capacity in the concentration of 10µg/mL | ||||
| Baccharis reticularia | Larvicidal | Larvicidal activity | Aedes aegypti | LC50 = 221.273 µL/mL (24 h); LC50 = 144.685 µL/mL (48 h) | [18] |
| Carlina acaulis | Larvicidal | Larvicidal activity | Lobesia botrana (1st instar larvae) | LC50 = 9.04 µL/mL; LC90 = 17.70 µL/mL | [14] |
| Parthenium hysterophorus | Herbicide | Seed germination bioassay | Diodia ocimifolia | 100% of germination inhibition in the concentration of 5 g L−1 | [23] |
| Stenachaenium megapotamicum | Antifungal | MIC | Epidermophyton floccosum | 5.18 μg/mL | [12] |
| Trichophyton rubrum | 41.5 μg/mL | ||||
| Polymeric Liposome | |||||
| Achillea millefolium | Antinociceptive | Formalin test | Male Wistar rat (220–280 g) Approved from Ethical Committee | 66% of pain inhibition | [26] |
| Artemisia afra | Antimicrobial | MIC | E. coli | 270 μg/mL | [24] |
| P. aeruginosa | >270 μg/mL | ||||
| S. aureus | >270 μg/mL | ||||
| C. albicans | 17 μg/mL | ||||
| Baccharis dracunculifolia | Anti-inflammatory | Zymosan-induced joint inflammation | Male Wistar rat (180–200 g) Approved from Ethical Committee | Decreased joint swelling and inflammatory interleukins | [27] |
| Chamomile | Anti-inflammatory | Clinical trial | Human being (24–65 years old) Approved from Ethical Committee | Reduction in erythema, edema, vesicular, and excoriation | [25] |
| Santolina insularis | Skin permeation | - | - | Improvement of the active permeation delivering in the skin | [10] |
| Polymeric Nanoparticles | |||||
| Achyrocline satureioides | Antiprotozoan (Trypanosoma evansi) | Hematological analysis in vivo | Female Wistar rat (203 g average) Approved from Ethical Committee | The treatment controls the infection but does not eliminate it. | [30] |
| Achyrocline satureioides | Antioxidant (Trypanosoma evans infection) | TBARS | Female Wistar rat (200 g average) Approved from Ethical Committee | The treatment with avoided the increase in ROS and TBARS levels of infected rats. | [31] |
| Achyrocline satureioides | Hepatic protection (Trypanosoma evans infection) | MTT assay | Female Wistar rat (200 g average) Approved from Ethical Committee | Increase the hepatic protection against the infection and reduces cytotoxic damage in liver | [32] |
| Artemisia aucheri | Antinociceptive | Formalin test | Male Sprague-Dawley rats (260–300 g) | Bupivacaine in combination with A. aucheri gave a synergic effect in antinociceptive activity | [33] |
| Artemisia absinthium | Anticancer | MTT assay | MCF-7; MDA MB-231 | IC50 = 176.83/181.39 µg/mL | [11] |
| Calendula officinalis | Anticancer | Cytotoxicity assay | Human breast adenocarcinoma MCF7 | Improvement of anticancer effects | [28] |
| Calendula officinalis | Wound healing | Delivery study | - | Improve epithelium repair in ocular surface and works as good delivery system | [29] |
| Matricaria chamomilla | Antileshmaniasis | Antiproliferative assay | Leshmaniasis amazonensis | IC50 = 3.33 µL/mL | [34] |
| Formulation | Species | Obtaining Method | Physicochemical Characterization | Method | Result | References |
|---|---|---|---|---|---|---|
| Nanoemulsion | Stenachaenium. megapotamicum | Spontaneous emulsification | Particle size | PCS (photon correlation spectroscopy) | 210 nm | [12] |
| PDI | 0.369 | |||||
| Zeta potential | - | |||||
| Nanoemulsion | Achyrocline satureioides | Spontaneous emulsification | Particle size | PCS | 237.35 ± 12.71nm | [16] |
| PDI | 0.09 ± 0.04 | |||||
| Zeta potential | −42.45 ± 1.96 mV | |||||
| Nanoemulsion | A. satureioides | Spontaneous emulsification | Particle size | DLS | 295.6 ± 9.0nm | [17] |
| PDI | 0.211 ± 0.021 | |||||
| Zeta potential | −43.6 ± 2.1 mV | |||||
| Nanoemulsion incorporated into hydrogel | A. satureioides | Spontaneous emulsification | Particle size | PCS | 246.8 ± 3.3 nm | [9] |
| PDI | 0.22 ± 0.1 nm | |||||
| Zeta potential | −42.5 ± 2.2 mV | |||||
| Nanoemulsion | Baccharis reticularia | Non-heating and low energy method | Particle size | PCS | 94.5 ± 1.9 nm | [18] |
| PDI | 0.382 ± 0.048 | |||||
| Zeta potential | −21.5 ± 1.4 mV | |||||
| Nanoemulsion | Artemisa draucunlus | Spontaneous method | Particle size | DLS | 11.20 ± 1.10 nm | [19] |
| PDI | 2.1 ± 0.08 | |||||
| Nanoemulsion | Pterocaulon balansae | Spontaneous emulsification | Particle size | DLS | 276 ± 54 nm | [20] |
| PDI | 0.215 ± 0.09 | |||||
| Zeta potential | −21.5 ± 5.9 mV | |||||
| Nanoemulsion | Silybum marianum | Stirred for 24 h at 25 °C | Particle size | DLS | 20.09 ± 0.04 nm | [21] |
| PDI | 0.059 ± 0.014 | |||||
| Zeta potential | −6.63 ± 1.73 mV | |||||
| Nanoemulsion | Achillea fragantissima and A. santolina | High energy (ultrasonication) | Particle size | DLS | 91.3 ± 9.6 nm/104.6 ± 14.1 | [22] |
| PDI | 0.20 ± 0.02/0.26 ± 0.01 | |||||
| Zeta potential | - | |||||
| Nanoemulsion | Parthenium hysterophorus | High energy emulsification | Particle size | DLS | 218 nm | [23] |
| PDI | 0.08 | |||||
| Zeta potential | −26.80 mV | |||||
| Nanoemulsion | Artemisia draucunlus | Ultrasound at ambient temperature | Particle size | DLS | 50 nm | [13] |
| PDI | - | - | ||||
| Zeta potential | Electrophoretic mobility | −30mV | ||||
| Nanoemulsion | Carlina acaulis | High-pressure homogenizer | Particle size | DLS | 143.9 nm | [14] |
| PDI | 0.28 ± 0.005 | |||||
| Zeta potential | 153.93 ± 1.58 | |||||
| Nanoemulsion | Artemisia annua | Sonication | Particle size | DLS | 160 ± 2.2 | [15] |
| PDI | 0.041 | |||||
| Zeta potential | - | |||||
| Liposome | Artemisia afra | Sonication | Particle size | DLS | 8.269 ± 0.796 μm | [24] |
| PDI | 0.429 ± 0.053 | |||||
| Zeta potential | 9.0 ± 2.40 mV | |||||
| Liposome | Matricaria chamomilla | Stirring in ultrasound bath | Particle size | DLS | 304 nm | [25] |
| PDI | 0.14 | |||||
| Zeta potential | - | |||||
| Liposome | Santolina insularis | Ultrasonication | Particle size | DLS | 111 ± 7 nm | [10] |
| PDI | 0.15 | |||||
| Zeta potential | −17 ± 2 mV | |||||
| Liposome nanoparticle | Achillea millefolium | Ultrasonication | Particle size | DLS | 160 nm | [26] |
| PDI | 0.25 | |||||
| Zeta potential | −30 to −60 mV | |||||
| Liposome | Baccharis dracuncufolia | - | Particle size | Laser light scattering | 182.5 ± 9.2 | [27] |
| PDI | 0.13 ± 0.05 | |||||
| Zeta potential | −0.08 ± 0.26 mV | |||||
| Nanoparticle | Calendula officinalis | Stirring | Particle size | Scanning electron microscope | 541.68 ± 85.14 nm | [28] |
| PDI | - | |||||
| Zeta potential | - | |||||
| Solid lipid nanoparticle | Calendula officinalis | Warm microemulsion technique | Particle size | PCS | 80 nm | [29] |
| PDI | - | |||||
| Zeta potential | −35 to 48 mV | |||||
| Nanocapsule | Achyrocline satureioides | Deposition of preformed polymer | Particle size | DLS | 256.6 ± 1.27 nm | [30] |
| PDI | 0.097 ± 0.007 | |||||
| Zeta potential | −30 ± 0.18 mV | |||||
| Nanocapsule | Achyrocline satureioides | Deposition of preformed polymer | Particle size | DLS | 235.9 nm | [31] |
| PDI | 0.112 | |||||
| Zeta potential | −29.3 mV | |||||
| Nanocapsule | Achyrocline satureioides | Deposition of preformed polymer | Particle size | DLS | 235.9 nm | [32] |
| PDI | 0.112 | |||||
| Zeta potential | −29.3 mV |
| Patent Number | Species | Nanoformulation | Indication | References |
|---|---|---|---|---|
| CN1368306-A | Artemisia sp. | Nanoparticle | Antinociceptive formulation | [52] |
| MX2011013407-A1 | Matricaria recutita and Calendula officinalis | Liposome | Phthalmic solution used for preventing and combating dry eyes | [53] |
| CN105017913-A | Artemisia argyi | Liposome | Antibacterial resin used for environmentally friendly paint | [54] |
| CN102552414-A | Matricaria chamomilla | Nanoemulsion | Acne, eczema, psoriasis, skin inflammation, pustule, and wound infection. | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yien, R.M.K.; Matos, A.P.d.S.; Gomes, A.C.C.; Garófalo, D.d.A.; Santos-Oliveira, R.; Simas, N.K.; Ricci-Júnior, E. Nanotechnology Promoting the Development of Products from the Biodiversity of the Asteraceae Family. Nutrients 2023, 15, 1610. https://doi.org/10.3390/nu15071610
Yien RMK, Matos APdS, Gomes ACC, Garófalo DdA, Santos-Oliveira R, Simas NK, Ricci-Júnior E. Nanotechnology Promoting the Development of Products from the Biodiversity of the Asteraceae Family. Nutrients. 2023; 15(7):1610. https://doi.org/10.3390/nu15071610
Chicago/Turabian StyleYien, Raíssa Mara Kao, Ana Paula dos Santos Matos, Anne Caroline Candido Gomes, Denise de Abreu Garófalo, Ralph Santos-Oliveira, Naomi Kato Simas, and Eduardo Ricci-Júnior. 2023. "Nanotechnology Promoting the Development of Products from the Biodiversity of the Asteraceae Family" Nutrients 15, no. 7: 1610. https://doi.org/10.3390/nu15071610
APA StyleYien, R. M. K., Matos, A. P. d. S., Gomes, A. C. C., Garófalo, D. d. A., Santos-Oliveira, R., Simas, N. K., & Ricci-Júnior, E. (2023). Nanotechnology Promoting the Development of Products from the Biodiversity of the Asteraceae Family. Nutrients, 15(7), 1610. https://doi.org/10.3390/nu15071610