Abstract
The aim of this study was to assess the relationship between Nutrition Risk Screening 2002 (NRS-2002) and the prevalence of concomitant chronic diseases among hospitalized older adults. This study included 2122 consecutively hospitalized older participants with an average age of 82 years. The criteria to participate were the ability to communicate and give consent. In multivariate design, the prevalence of nutritional risk with at least 3 points in the NRS-2002 score was associated with the presence of stroke, atrial fibrillation, dementia and pressure ulcers. Patients with arterial hypertension, lipid disorders, osteoarthritis and urine incontinence had a significantly lower (better) NRS-2002 score. The explanation of the inverse relationship between some disorders and nutritional risk may be their occurrence in relatively earlier age and the relationship with body mass index. In conclusion, the study revealed which medical conditions coexist with the increased nutritional risk in a “real-world” hospitalized geriatric population. The hospital admission of an older subject with stroke, atrial fibrillation, dementia or pressure ulcers should primarily draw attention to the nutritional risk of the patient.
1. Introduction
The term malnutrition is often used to describe a deficiency in nutrition that causes adverse effects on the body and its normal functions []. Malnutrition in hospitalized patients represents a heavy healthcare burden worldwide [,]. An increase in malnutrition-related diseases in people with multiple comorbidities is a growing health concern, and it is strictly related to both the aging of the general population and the improvement in healthcare []. Malnutrition adversely affects physical well-being, interferes with treatment, and increases the duration of hospital stay []. Improvements in nutrition are known to bring tangible benefits to older people and many age-related diseases and conditions can be prevented, modulated or ameliorated by good nutrition []. In order to develop an appropriate nutritional plan and intervene immediately, the screening of patients’ nutritional status is normally performed upon hospital admission [].
The determinants of malnutrition are especially important in the most vulnerable older population, with the risk of naturally developing general poor health or chronic diseases []. Some of these factors could be potentially influenced by the environment of the geriatric population, which has been shown to have a significant impact on nutrition []. If an older person is residing in a hospital or long-term care facility, it has been shown that they are more likely to have a poor nutrition status compared to a community-dwelling older person []. Indeed, some investigators have shown that up to 60–80% of European geriatric hospital patients are malnourished [,]. Several factors contribute to the worsening of nutritional status during hospitalization: illness-related loss of appetite, fasting for diagnostic procedures, drug-related side effects, diseases that compromise the regular functioning of the digestive system and the poor patient management [].
For this purpose, numerous screening tools have been developed and validated [,]. An effective nutritional screening tool must be practical, i.e., those who are going to use the tool must find it rapid and simple, and such a tool must also have high validity and reliability []. The Nutritional Risk Screening (NRS-2002) is a validated tool for medical services to identify malnourished patients who would benefit from nutritional intervention [,]. It may predict the probability of a better or worse outcome due to nutritional factors, but also whether nutritional treatment is likely to influence the clinical prognosis []. Unlike other nutritional assessment tools that mainly focus on laboratory indicators such as the albumin or lymphocyte count, NRS-2002 takes the effect of the changes in food intake and disease severity into consideration [,]. Nowadays, NRS-2002 stands out as an effective, flexible, and comprehensive nutritional assessment tool and has been extensively used in the clinical nutrition practice to provide nutritional information [,].
The severity of concomitant diseases is the basic determinant of overall NRS-2002 score []. While the severity of acute disease or surgery may be quite reliably assessed, the contribution of concomitant chronic diseases to overall NRS-2002 is less clear. In clinical practice, the influence of the most common accompanying diseases should be appropriately weighed. In the currently available literature, there is a relatively wide range of papers comparing NRS-2002 with specific individual disorders [,,,,] or with different nutritional tools [,]. However, there is an insufficiency of research relating to nutritional risk and the variety of age-related diseases compared concomitantly. Hence, the aim of this study was to assess the relationship between NRS and the prevalence of concomitant chronic diseases among hospitalized older adults.
2. Materials and Methods
2.1. Design of the Study and Participants
The study population that was selected consisted of older adults, aged 60 years old and above, who were hospitalized in the Geriatric Department, Central Veterans Hospital located in Lodz, Poland. Patients were recruited from January 2012 to December 2019. During the period 2020–2022, the department served partially as a COVID-19 ward. The total number of individuals was selected with the following inclusion criteria: admission to the department, aged 60 years and above, ability to communicate efficiently, complete data, and giving informed consent. After screening, 2122 patients (631 men and 1491 women) who met the criteria were enrolled into the analysis.
2.2. Nutritional Questionnaire
NRS-2002 was designed as a tool to identify patients at nutritional risk. It assesses risk through two criteria: impaired nutritional status and disease severity []. Nutritional status was determined by variables which comprised age, body mass index (BMI), reduced dietary intake and recent body mass loss. BMI was calculated by dividing the weight (in kilograms) by height squared (in meters). The NRS-2002 score consisted of the total nutritional score (impaired nutritional status), the severity of disease score (increase in nutritional requirement) and the age adjustment score (<70 or ≥70 years). The total number of points scales from 0 to 7. Patients with a score of 3 or more are suggested to be nutritionally at risk.
2.3. Concomitant Diseases
The prevalence of diseases such as arterial hypertension, diabetes, lipid disorders, current or previous stroke, coronary artery disease, current or previous myocardial infarction, atrial fibrillation, heart failure, obstructive lung diseases (chronic obstructive pulmonary disease and asthma), osteoarthritis, osteoporosis, current or previous fracture, gastrointestinal diseases, neoplastic diseases, depression, dementia, pressure ulcers and urine incontinence was scrutinized from the patients’ database. The vast majority of subjects presented with multiple comorbidities; however, the presented diseases were analyzed separately.
2.4. Statistical Analysis
The normality of distribution was analyzed using a Shapiro–Wilk test. As several variables were not normally distributed, data were expressed both as the mean ± standard deviation (SD) and median (25–75% quartiles). The quantitative variables were compared using a Mann–Whitney U-test and qualitative variables using a chi-square test. Spearman correlation coefficients were used to calculate the relationship between the two quantitative variables. The results of NRS-2002 were presented in two different ways: for patients’ characteristics as a raw variable and for logistic regression NRS-2002 was dichotomized as <3 and ≥3 points. Multivariate ordinal logistic regression analysis was further used to investigate the relationship between concomitant diseases and NRS-2002 (≥3 points against <3 points). Statistical significance was set at p ≤ 0.05. Statistical analysis was performed using Statistica 13.1.
2.5. Ethical Certification
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Lodz with the approval number RNN/300/17/KE. Patients signed informed consent for all the diagnostic and therapeutic procedures during hospitalization. All of the gathered data were confidential.
3. Results
The mean age for the whole study populations was 82.18 ± 7.90 years. Table 1 shows the characteristics of 2122 patients according to gender. Men had a higher body mass compared to the women. The prevalence of diabetes, myocardial infarction, atrial fibrillation and neoplastic disease was higher in men than in women. The prevalence of arterial hypertension, lipid disorders, osteoarthritis, osteoporosis, fractures, depression, dementia and urine incontinence was higher in women than in men.

Table 1.
Characteristics of the patients according to gender.
Table 2 shows the comparison of NRS-2002 scores according to the prevalence of concomitant diseases. The presence of arterial hypertension (in men), lipid disorders, osteoarthritis, osteoporosis (in women) and urine incontinence was associated with significantly lower (better) NRS-2002 scores in bivariate analyses. The presence of stroke, atrial fibrillation, heart failure, neoplastic diseases (in men), dementia and pressure ulcers was associated with significantly higher (worse) NRS-2002.

Table 2.
Comparison of NRS-2002 according to the prevalence of diseases.
Additional bivariate analyses were performed between the presence of diseases and age (Table 3). The patients presenting with diseases such as arterial hypertension (women), coronary artery disease, myocardial infarction, atrial fibrillation, heart failure, osteoporosis (men), fractures (women), neoplastic diseases (men), dementia and pressure ulcers were significantly older, whereas the patients with lipid disorders and gastrointestinal diseases (women) were significantly younger in comparison to those without disease.

Table 3.
Comparison of age according to the prevalence of diseases.
In order to simultaneously assess an association of accompanying diseases to NRS-2002, logistic regression was performed. For the whole study group, the analysis is shown in Figure 1. Diseases which expressed significance in bivariable analysis were employed in multivariable analysis. Diseases such as stroke, atrial fibrillation, dementia, and pressure ulcers were independently associated with increased values of NRS-2002 (increased odds ratio for nutritional risk). Arterial hypertension, lipid disorders, osteoarthritis and urine incontinence were associated with lower values of NRS-2002.
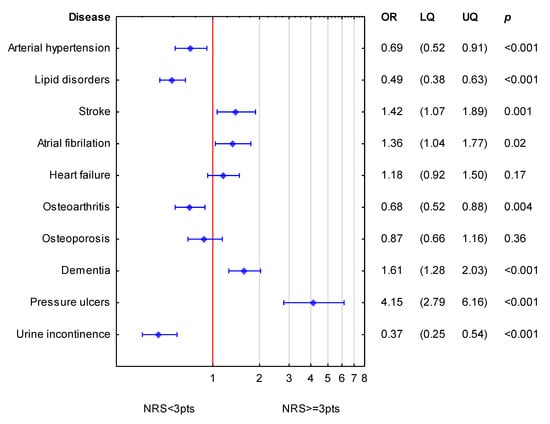
Figure 1.
Simultaneous association of diseases accompanying NRS-2002 for the whole group of patients. (OR—odds ratio; LQ—lower quartile; UQ—upper quartile; p—p value). Reference value (OR = 1; red line) corresponds to the absence of particular disease. Blue symbols correspond to odds ratios and 25–75% confidence intervals for the presence of particular disease.
Figure 2 shows the simultaneous statistical impact of concomitant diseases on NRS-2002 scores in women. Stroke, atrial fibrillation, dementia and pressure ulcers were related to higher values of NRS-2002 (increased odds ratio for nutritional risk). Arterial hypertension, lipid disorders, and urine incontinence were related to lower values of NRS-2002.

Figure 2.
Simultaneous association of accompanying diseases to NRS-2002 in women. (OR—odds ratio; LQ—lower quartile; UQ—upper quartile; p—p value). Reference value (OR = 1; red line) corresponds to the absence of particular disease. Blue symbols correspond to odds ratios and 25–75% confidence intervals for the presence of particular disease.
Figure 3 shows the simultaneous statistical impact of concomitant diseases on NRS-2002 in men. Dementia and pressure ulcers were related to higher values of NRS-2002 (increased odds ratio for nutritional risk). Lipid disorders, osteoarthritis and urine incontinence were related to lower values of NRS-2002.
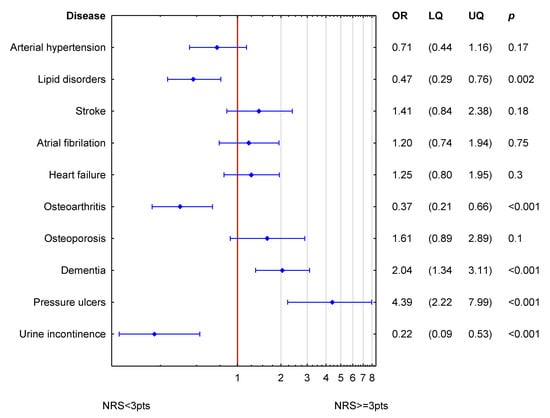
Figure 3.
Simultaneous association of accompanying diseases to NRS-2002 in men. (OR odds ratio; LQ—lower quartile; UQ—upper quartile; p—p value). Reference value (OR = 1; red line) corresponds to the absence of particular disease. Blue symbols correspond to odds ratios and 25–75% confidence intervals for the presence of particular disease.
Although NRS-2002 is connected with BMI (as contains BMI in its definition), some further adjustments for the four different levels of BMI were performed. For BMI cut-off points of 23, 25, 27 and 30 kg/m2, the positive relationship between these two variables was extremely strong (chi-square p < 0.001 for all BMI cut-offs against dichotomized NRS-2002).
When BMI was entered into the regression models, it was such a strong predictor of NRS-2002 that only some diseases were still present in the model. Pressure ulcers, atrial fibrillation and dementia (borderline significance) were related to a higher nutritional risk, while lipid disorders, urine incontinence and osteoarthritis (only in one model) were related to lower nutritional risk.
4. Discussion
Presented research focuses on the association between NRS-2002 and a variety of concomitant diseases among older people. To the best of our knowledge this is the first study concurrently assessing the relationship between nutritional risk and the most common chronic diseases in a large hospitalized geriatric population. Our data indicate that this relationship is complex and inhomogeneous—not all concomitant disorders are associated with worse nutritional status. The results indicate that there is a bidirectional association between nutritional risk and disorders typical for hospitalized geriatric patients. The presence of stroke, atrial fibrillation, dementia and pressure ulcers are associated with increased risk of malnutrition. On the other hand, lipid disorders, arterial hypertension, osteoarthritis or urine incontinence are statistically bounded with less nutritional risk.
The screening and assessment of malnutrition, also with NRS-2002, have proven their predictive values in different populations of patients [,,,,,,,]. In several studies, either targeting specific patients or performing in more general setting, malnutrition has been linked to diverse clinical conditions: age, gender, low BMI, infections, cancer, diabetes mellitus, grade of renal function, acute kidney injury, pulmonary diseases, gastrointestinal disorders, depression, cognitive/functional geriatric tests, or generally high comorbidity and polypharmacy [,,,,,,].
In the present study, we investigated the relationship between NRS-2002 at admission and the presence of chronic diseases among hospitalized older adults. The prevalence of nutritional risk with at least three points in the NRS-2002 score was associated with the presence of stroke, atrial fibrillation, heart failure (in bivariate analyses), dementia, and pressure ulcers. All these conditions are closely related to advancing age. Malnutrition is frequently observed in patients with stroke []. Patients with ischemic stroke at risk of malnutrition are more likely to develop infection complications than those with normal nutrition []. The nutritional status can affect the occurrence of new-onset atrial fibrillation in acute myocardial infarction patients []. Atrial fibrillation is related to obesity with long-term increased incidence independently of other risk factors [,]. Our findings suggest that malnutrition may be also related to the higher prevalence of atrial fibrillation and this fraction of patients seems to be significantly older. The incidence of complications and the median length of the hospital stay are significantly higher in heart failure patients at nutritional risk []. In a retrospective study including 2830 heart failure patients, a high NRS-2002 score was strongly and independently associated with the incidence of 1-year re-hospitalization and the length of initial hospital stay []. Our results reveal a significantly higher NRS-2002 among patients with heart failure. As for atrial fibrillation, these patients are older as compared to those without the disease.
In dementia, many contributing factors must be considered, including nutrition []. Having a cognitive impairment determines malnutrition in successfully ageing populations whilst dementia is reported to be associated with malnutrition within usual and accelerated ageing populations []. The prevalence of malnutrition was strongly correlated with the severity of dementia in hospitalized patients []. In 4095 geriatric hospital patients, subjects with cognitive dysfunction had a higher NRS-2002 score compared to cognitively intact subjects []. Our results comply with previous reports with dementia contributing to a worse NRS-2002 in the whole study population and separately in women and men.
Pressure ulcers are one of the most common occurrences in bedridden subjects []. There is significantly higher risk for pressure injury in patients who are at risk for undernutrition compared with those who are not at risk []. Prolonged immobilization, sensory deficit, circulatory disturbances and poor nutrition have been identified as important risk factors in the development of pressure ulcers formation []. Litchford’s et al. study has reported associations between the declining nutrition status and risk for pressure ulcers []. The significant association between the NRS-2002 and pressure ulcers in a mixed hospital population was described []. Likewise in our study, pressure ulcers, more evident in the oldest hospitalized subjects, were the most powerful condition related to a worse nutritional status.
Interestingly, we found that patients with arterial hypertension, lipid disorders, osteoarthritis, and urine incontinence have a significantly lower NRS-2002. These data contrast with a few previous studies. An elevated Geriatric Nutritional Risk Index was associated with an earlier age of hypertension onset in the older Chinese population []. In a cross-sectional study conducted in Sri Lanka with 999 participants aged on average 70.8 years, hypertension, alcohol consumption and increased age were positively associated with malnutrition []. In a cross-sectional study involving 330 Thai community-dwelling older adults, factors significantly associated with an increased nutritional risk were: age ≥80 years, low income, living alone, moderate-to-severe pain, dyslipidemia, osteoarthritis, poor physical performance and ≥1 fall in the previous year [].
The explanation of an inverse relationship of some disorders to nutritional risk found in our study may be their occurrence in relatively earlier age and in relationships with overweight/obesity. In the present study, BMI decreased (rho = −0.23) while the NRS-2002 score increased (rho = 0.20) with age of the patients. NRS-2002 was inversely related to BMI (rho = −0.27) and BMI was a strong and independent determinant of a lower (better) NRS-2002 score in multivariate analyses. Obesity is not a key problem in older hospitalized patients. The prevalence of obesity among adults aged 65–74 years is higher than in those aged 75 and over []. Age is an independent risk factor associated with an elevation in lipid levels in middle-age, and is associated with declining lipid levels in older (>55–60) years []. The incidence rates of symptomatic osteoarthritis of either hand, or knee or hip rapidly increase around the age of 50 and then level off after age 70 [,]. The prevalence of symptomatic knee osteoarthritis was reported to non-linearly increase with age to show the highest annual incidence between the 55th and 64th year of life [].
In the literature, there are papers indicating a positive correlation between the occurrence of osteoarthritis and obesity []. According to Australian research, the prevalence of osteoarthritis is seven-fold higher among obese subjects []. Likewise, overweight and obesity are important risk factors for urinary incontinence []. The association of hypertension and lipid disorders with obesity is well known [,]. Therefore, this relationship to age and BMI may explain our results of better (lower) NRS-2002 scores in patients with those four disorders, while taking into account other comorbidities. All four selected medical conditions are also characterized by their relatively non-debilitating course—when appropriately treated. Lower NRS-2002 scores in all four of these medical conditions do not necessarily mean that these groups of patients are at less nutritional risk. It only shows that, in a “real-world” mixed geriatric hospitalized population, the nutritional impact of these conditions is age- and BMI-dependent.
Several medical conditions highlighted in previous reports were not selected as independent statistical determinants of malnutrition in our analysis. COPD is one of the risk factors for malnutrition among older subjects [] and, based on some research, the prevalence of malnutrition according to NRS-2002 in COPD patients is as much as 57% []. NRS-2002 has been indicated as a tool for predicting 1 year mortality prognosis in patients with COPD []. In contrast to arthritis, osteoporosis is strictly linked with undernutrition. It is particularly connected with protein [] and calcium/vitamin D3 deficiency []. A low BMI is an independent risk factor of that condition in the Frax algorithm []. Fractures are predominantly linked with older age and malnutrition [,]. Additionally, subjects who are malnourished often have negative outcomes after hip fracture []. Malnutrition is connected with worse functional recovery, and an increase in mortality after hip fracture [,]. Hip fracture patients who are both overweight or obese, and malnourished, have significantly and substantially worse clinical outcomes than their well-nourished—albeit overweight or obese—counterparts [].
Gastrointestinal diseases are associated with malnutrition as the variety of symptoms and disorders hinders proper nutrition [,]. The validity of NRS-2002 in the nutritional assessment was confirmed in disorders such as acute diverticulitis [] and inflammatory bowel disease []. Malnutrition predicts poorer clinical outcomes for people with cancer [,]. Malnutrition negatively impacts the quality of life and treatment, and it has been estimated that up to 10–20% of cancer patients die due to consequences of malnutrition rather than for the tumor itself []. The prevalence of malnutrition was related to the presence and severity of depression in different groups of patients [,]. In the present study, of these medical conditions, heart failure (in both women and men) and neoplastic diseases (in men) were related to a higher NRS-2002, while osteoporosis was linked with a significantly lower NRS-2002 in women, but only in bivariable analyses. These results indicate that in a multimorbid geriatric inpatient population, the relative burden of these conditions may be less pronounced compared to other diseases.
The main strengths of our study are the number of subjects and the assessment of a wide variety of geriatric disorders. Nevertheless, there are several limitations in this study that could be addressed in future research. This study focused on older inpatients in central Poland. The patients had multiple medical problems but not all the concomitant disorders were scrutinized. Larger multicenter studies in different populations would enable one to draw more conclusions. The relationship between nutritional status and concomitant diseases may also be different during long-term hospitalization or in an institutional environment []. We only used one short nutritional screening test—NRS-2002. Other nutritional assessment tools might have performed differently. Finally, it is a cross-sectional analysis, monitoring the nutritional status and its predictive value during and after the hospitalization would probably bring new information.
5. Conclusions
This study revealed which medical conditions coexist with the increased nutritional risk in a “real-world” hospitalized geriatric population. The hospital admission of older subjects with stroke, atrial fibrillation, heart failure, dementia or pressure ulcers should primarily draw attention to the nutritional risk of the patient.
Author Contributions
Conceptualization, T.K. and S.S.S.; Methodology, S.S.S.; Software, G.K. and S.S.S.; Validation, B.K.S. and T.K.; Formal Analysis, S.S.S.; Investigation, S.S.S. and A.G.; Resources, A.C.-S.; Data Curation, S.S.S.; Writing—Original Draft Preparation, S.S.S. and B.K.S.; Writing—Review and Editing, T.K.; Visualization, B.K.S.; Supervision, T.K. and B.K.S.; Project Administration, A.W.; Funding Acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors were supported by the grant from the Medical University of Lodz, Poland (503/6-077-01/503-61-001).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board or Ethics Committee of Medical University of Łódź, Poland (protocol code RNN/300/17/KE).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The statistical data used to support the presented findings may be obtained upon request to corresponding author.
Acknowledgments
Special thanks to Łukasz Kroc, Edyta Piechocka-Wochniak and Elizaveta Fife for their support in collecting research material.
Conflicts of Interest
There is no conflict of interest to be declared.
References
- Morley, J.E. Editorial: Defining Undernutrition (Malnutrition) in Older Persons. J. Nutr. Health Aging 2018, 22, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Perman, M.I.; Waitzberg, D.L. Hospital malnutrition in Latin America: A systematic review. Clin. Nutr. 2017, 36, 958–967. [Google Scholar] [CrossRef]
- Fan, Y.; Yao, Q.; Liu, Y.; Jia, T.; Zhang, J.; Jiang, E. Underlying Causes and Co-existence of Malnutrition and Infections: An Exceedingly Common Death Risk in Cancer. Front. Nutr. 2022, 9, 814095. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Pellegrino, G.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Vendemiale, G. Comparison of Three Nutritional Screening Tools with the New Glim Criteria for Malnutrition and Association with Sarcopenia in Hospitalized Older Patients. J. Clin. Med. 2020, 9, 1898. [Google Scholar] [CrossRef] [PubMed]
- Budzyński, J.; Tojek, K.; Czerniak, B.; Banaszkiewicz, Z. Scores of nutritional risk and parameters of nutritional status assessment as predictors of in-hospital mortality and readmissions in the general hospital population. Clin. Nutr. 2016, 35, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.E.; Williams, E.A. Optimizing nutrition in older people. Maturitas 2018, 112, 34–38. [Google Scholar] [CrossRef]
- Trollebø, M.A.; Skeie, E.; Revheim, I.; Stangeland, H.; Erstein, M.-A.H.; Grønning, M.K.; Tangvik, R.J.; Morken, M.H.; Nygård, O.; Eagan, T.M.L.; et al. Comparison of nutritional risk screening with NRS2002 and the GLIM diagnostic criteria for malnutrition in hospitalized patients. Sci. Rep. 2022, 12, 19743. [Google Scholar] [CrossRef]
- Kroc, Ł.; Fife, E.; Piechocka-Wochniak, E.; Sołtysik, B.; Kostka, T. Comparison of Nutrition Risk Screening 2002 and Subjective Global Assessment Form as Short Nutrition Assessment Tools in Older Hospitalized Adults. Nutrients 2021, 13, 225. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kostka, T.; Guligowska, A. Do Determinants of Quality of Life Differ in Older People Living in the Community and Nursing Homes? Int. J. Environ. Res. Public Health 2023, 20, 916. [Google Scholar] [CrossRef]
- Fávaro-Moreira, N.C.; Krausch-Hofmann, S.; Matthys, C.; Vereecken, C.; Vanhauwaert, E.; Declercq, A.; Bekkering, G.E.; Duyck, J. Risk Factors for Malnutrition in Older Adults: A Systematic Review of the Literature Based on Longitudinal Data. Adv. Nutr. 2016, 7, 507–522. [Google Scholar] [CrossRef]
- van Bokhorst-de van der Schueren, M.A.; Lonterman-Monasch, S.; de Vries, O.J.; Danner, S.A.; Kramer, M.H.; Muller, M. Prevalence and determinants for malnutrition in geriatric outpatients. Clin. Nutr. 2013, 32, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef]
- Neelemaat, F.; Meijers, J.; Kruizenga, H.; van Ballegooijen, H.; van Bokhorst-de van der Schueren, M. Comparison of five malnutrition screening tools in one hospital inpatient sample. J. Clin. Nurs. 2011, 20, 2144–2152. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 3, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Dahl, H.; Warz, S.I.; Welland, N.L.; Arnesen, I.; Marti, H.P.; Dierkes, J. Factors associated with nutritional risk in patients receiving haemodialysis assessed by Nutritional Risk Screening 2002 (NRS2002). J. Ren. Care 2022, 48, 112–118. [Google Scholar] [CrossRef]
- Kyle, U.G.; Kossovsky, M.P.; Karsegard, V.L.; Pichard, C. Comparison of tools for nutritional assessment and screening at hospital admission: A population study. Clin. Nutr. 2006, 25, 409–417. [Google Scholar] [CrossRef]
- Sahli, L.; Hagenbuch, N.; Ballmer, P.E.; Rühlin, M.; Imoberdorf, R. NRS-2002 components, nutritional score and severity of disease score, and their association with hospital length of stay and mortality. Swiss Med. Wkly. 2021, 151, w20517. [Google Scholar] [CrossRef]
- Rabito, E.I.; Marcadenti, A.; da Silva Fink, J.; Figueira, L.; Silva, F.M. Nutritional Risk Screening 2002, Short Nutritional Assessment Questionnaire, Malnutrition Screening Tool, and Malnutrition Universal Screening Tool Are Good Predictors of Nutrition Risk in an Emergency Service. Nutr. Clin. Pract. 2017, 32, 526–532. [Google Scholar] [CrossRef]
- Borek, P.; Chmielewski, M.; Małgorzewicz, S.; Dębska Ślizień, A. Analysis of Outcomes of the NRS 2002 in Patients Hospitalized in Nephrology Wards. Nutrients 2017, 9, 287. [Google Scholar] [CrossRef]
- Czapla, M.; Karniej, P.; Juárez-Vela, R.; Łokieć, K. The Association between Nutritional Status and In-Hospital Mortality among Patients with Acute Coronary Syndrome-A Result of the Retrospective Nutritional Status Heart Study (NSHS). Nutrients 2020, 12, 3091. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Fu, H.; Du, J. Application value of NRS2002 and PG-SGA in nutritional assessment for patients with cervical cancer surgery. Am. J. Transl. Res. 2021, 13, 7186–7192. [Google Scholar] [PubMed]
- Illa, P.; Tomiskova, M.; Skrickova, J. Nutritional Risk Screening Predicts Tumor Response in Lung Cancer Patients. J. Am. Coll. Nutr. 2015, 34, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Long, J.; Fang, S.; Mai, H.; Lu, W.; Liu, Y.; Wei, J.; Yan, F. Nutritional Risk Screening in patients with chronic kidney disease. Asia Pac. J. Clin. Nutr. 2016, 25, 249–256. [Google Scholar] [CrossRef]
- Silva, D.F.O.; Lima, S.; Sena-Evangelista, K.C.M.; Marchioni, D.M.; Cobucci, R.N.; Andrade, F.B. Nutritional Risk Screening Tools for Older Adults with COVID-19: A Systematic Review. Nutrients 2020, 12, 2956. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, Z.; Zhu, Y.X.; Tao, J.; Zhang, Y.; Wang, Y.Y.; Ke, Y.Y.; Ren, C.X.; Xu, J.; Zhang, X.Y. Comparison of the efficacy of Nutritional Risk Screening 2002 and Mini Nutritional Assessment Short Form in recognizing sarcopenia and predicting its mortality. Eur. J. Clin. Nutr. 2020, 74, 1029–1037. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Zhang, Q.; Rao, S.; Wu, X.; Zhang, J.; Li, J. Comparison of the Suitability Between NRS2002 and MUST as the First-Step Screening Tool for GLIM Criteria in Hospitalized Patients with GIST. Front. Nutr. 2022, 9, 864024. [Google Scholar] [CrossRef]
- Hersberger, L.; Bargetzi, L.; Bargetzi, A.; Tribolet, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: Secondary analysis of a prospective randomised trial. Clin. Nutr. 2020, 39, 2720–2729. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Yu, J.; Jia, Y.; Jiang, Y.; Chen, X.; Gao, Y.; Ye, L.; Wan, Z.; Cao, Y.; et al. Prognostic Value of the Nutritional Risk Screening 2002 Scale in Patients With Acute Myocardial Infarction: Insights From the Retrospective Multicenter Study for Early Evaluation of Acute Chest Pain. J. Cardiovasc. Nurs. 2021, 36, 546–555. [Google Scholar] [CrossRef]
- Sanson, G.; Sadiraj, M.; Barbin, I.; Confezione, C.; De Matteis, D.; Boscutti, G.; Zaccari, M.; Zanetti, M. Prediction of early- and long-term mortality in adult patients acutely admitted to internal medicine: NRS-2002 and beyond. Clin. Nutr. 2020, 39, 1092–1100. [Google Scholar] [CrossRef]
- Boban, M.; Laviano, A.; Persic, V.; Rotim, A.; Jovanovic, Z.; Vcev, A. Characteristics of NRS-2002 Nutritional Risk Screening in patients hospitalized for secondary cardiovascular prevention and rehabilitation. J. Am. Coll. Nutr. 2014, 33, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, L.; Guan, C.; Zhao, L.; Luo, C.; Zhou, B.; Zhang, X.; Wang, J.; Zhao, J.; Huang, J.; et al. Malnutrition screening and acute kidney injury in hospitalised patients: A retrospective study over a 5-year period from China. Br. J. Nutr. 2020, 123, 337–346. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; Gutiérrez-Lora, C.; Izaola-Jauregui, O.; Primo-Martín, D.; Gómez-Hoyos, E.; Jiménez-Sahagún, R.; De Luis-Román, D.A. Real World Practice Study of the Effect of a Specific Oral Nutritional Supplement for Diabetes Mellitus on the Morphofunctional Assessment and Protein Energy Requirements. Nutrients 2022, 14, 4802. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, D. Relationship between the depression levels and nutritional statuses of advanced stage cancer patients. Palliat. Support. Care 2022, 20, 654–661. [Google Scholar] [CrossRef]
- Abdulan, I.M.; Onofriescu, M.; Stefaniu, R.; Mastaleru, A.; Mocanu, V.; Alexa, I.D.; Covic, A. The predictive value of malnutrition for functional and cognitive status in elderly hemodialysis patients. Int. Urol. Nephrol. 2019, 51, 155–162. [Google Scholar] [CrossRef]
- Zugasti Murillo, A.; Petrina-Jáuregui, M.E.; Ripa-Ciáurriz, C.; Sánchez Sánchez, R.; Villazón-González, F.; González-Díaz Faes, Á.; Fernández-López, C.; Calles-Romero, L.; Martín Palmero, M.; Riestra-Fernández, M.; et al. SeDREno study—Prevalence of hospital malnutrition according to GLIM criteria, ten years after the PREDyCES study. Nutr. Hosp. 2021, 38, 1016–1025. [Google Scholar] [CrossRef]
- Tangvik, R.J.; Tell, G.S.; Guttormsen, A.B.; Eisman, J.A.; Henriksen, A.; Nilsen, R.M.; Ranhoff, A.H. Nutritional risk profile in a university hospital population. Clin. Nutr. 2015, 34, 705–711. [Google Scholar] [CrossRef]
- Çoban, E. Malnutrition Rate in Stroke Patients on Admission. Şişli Etfal Hastan. Bülteni 2019, 53, 272–275. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Fan, G.; Gao, F.; Wang, J.; Gu, S. Correlation between nutritional status screening by MNA-SF and acute stroke-associated infections in older adults. Aging Clin. Exp. Res. 2023, 35, 717–721. [Google Scholar] [CrossRef]
- Wu, L.; Wang, W.; Gui, Y.; Yan, Q.; Peng, G.; Zhang, X.; Ye, L.; Wang, L. Nutritional Status as a Risk Factor for New-Onset Atrial Fibrillation in Acute Myocardial Infarction. Clin. Interv. Aging 2023, 18, 29–40. [Google Scholar] [CrossRef]
- Tedrow, U.B.; Conen, D.; Ridker, P.M.; Cook, N.R.; Koplan, B.A.; Manson, J.E.; Buring, J.E.; Albert, C.M. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J. Am. Coll. Cardiol. 2010, 55, 2319–2327. [Google Scholar] [CrossRef]
- Goudis, C.A.; Korantzopoulos, P.; Ntalas, I.V.; Kallergis, E.M.; Ketikoglou, D.G. Obesity and atrial fibrillation: A comprehensive review of the pathophysiological mechanisms and links. J. Cardiol. 2015, 66, 361–369. [Google Scholar] [CrossRef]
- Tevik, K.; Thürmer, H.; Husby, M.I.; de Soysa, A.K.; Helvik, A.S. Nutritional risk screening in hospitalized patients with heart failure. Clin. Nutr. 2015, 34, 257–264. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, H.; He, W.; Li, D.; Lin, M.; Wang, M.; Shang, M.; Zhang, W. The Association of Nutritional Risk Screening 2002 With 1-Year Re-hospitalization and the Length of Initial Hospital Stay in Patients With Heart Failure. Front. Nutr. 2022, 9, 849034. [Google Scholar] [CrossRef]
- Cohen, D. Dementia, depression, and nutritional status. Prim. Care 1994, 21, 107–119. [Google Scholar] [CrossRef]
- Bardon, L.A.; Corish, C.A.; Lane, M.; Bizzaro, M.G.; Loayza Villarroel, K.; Clarke, M.; Power, L.C.; Gibney, E.R.; Dominguez Castro, P. Ageing rate of older adults affects the factors associated with, and the determinants of malnutrition in the community: A systematic review and narrative synthesis. BMC Geriatr. 2021, 21, 676. [Google Scholar] [CrossRef]
- Konturek, P.C.; Herrmann, H.J.; Schink, K.; Neurath, M.F.; Zopf, Y. Malnutrition in hospitals: It was, is now, and must not remain a problem! Med. Sci. Monit. 2015, 21, 2969–2975. [Google Scholar] [CrossRef]
- Wirth, R.; Smoliner, C.; Sieber, C.C.; Volkert, D. Cognitive function is associated with body composition and nutritional risk of geriatric patients. J. Nutr. Health Aging 2011, 15, 706–710. [Google Scholar] [CrossRef]
- Nancy, G.A.; Kalpana, R.; Nandhini, S. A Study on Pressure Ulcer: Influencing Factors and Diagnostic Techniques. Int. J. Low. Extrem. Wounds 2022, 21, 254–263. [Google Scholar] [CrossRef]
- Serpa, L.F.; Oliveira, A.S.; Nogueira, P.C.; de Gouveia Santos, V.L.C. Risk for undernutrition and development of pressure injury in hospitalised patients in Brazil: Multicentre prospective cohort study. Int. Wound J. 2020, 17, 916–924. [Google Scholar] [CrossRef]
- Edlich, R.F.; Winters, K.L.; Woodard, C.R.; Buschbacher, R.M.; Long, W.B.; Gebhart, J.H.; Ma, E.K. Pressure ulcer prevention. J. Long-Term Eff. Med. Implant. 2004, 14, 285–304. [Google Scholar] [CrossRef]
- Litchford, M.D.; Dorner, B.; Posthauer, M.E. Malnutrition as a Precursor of Pressure Ulcers. Adv. Wound Care 2014, 3, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Alhaug, J.; Gay, C.L.; Henriksen, C.; Lerdal, A. Pressure ulcer is associated with malnutrition as assessed by Nutritional Risk Screening (NRS 2002) in a mixed hospital population. Food Nutr. Res. 2017, 61, 1324230. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zhang, X.; Zheng, N.; Xiao, Y.; Liu, X.; Yang, Y.; Deng, L.; Chen, Y.; Li, B. Association of the Geriatric Nutritional Risk Index with incident hypertension in the older Chinese population: A 6-year cohort study. J. Int. Med. Res. 2021, 49, 3000605211010051. [Google Scholar] [CrossRef]
- Damayanthi, H.; Moy, F.M.; Abdullah, K.L.; Dharmaratne, S.D. Prevalence of malnutrition and associated factors among community-dwelling older persons in Sri Lanka: A cross-sectional study. BMC Geriatr. 2018, 18, 199. [Google Scholar] [CrossRef]
- Nawai, A.; Phongphanngam, S.; Khumrungsee, M.; Leveille, S.G. Factors associated with nutrition risk among community-dwelling older adults in Thailand. Geriatr. Nurs. 2021, 42, 1048–1055. [Google Scholar] [CrossRef]
- Fakhouri, T.H.; Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Obesity among Older Adults in the United States, 2007–2010; NCHS Data Brief; NCHS: Hyattsville, ML, USA, 2012; pp. 1–8. [Google Scholar]
- Feng, L.; Nian, S.; Tong, Z.; Zhu, Y.; Li, Y.; Zhang, C.; Bai, X.; Luo, X.; Wu, M.; Yan, Z. Age-related trends in lipid levels: A large-scale cross-sectional study of the general Chinese population. BMJ Open 2020, 10, e034226. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Oliveria, S.A.; Felson, D.T.; Reed, J.I.; Cirillo, P.A.; Walker, A.M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995, 38, 1134–1141. [Google Scholar] [CrossRef]
- Arthritis Foundation. Arthritis by the Numbers; Arthritis Foundation: Atlanta, GA, USA, 2019; Available online: https://www.arthritis.org/getmedia/e1256607-fa87-4593-aa8a-8db4f291072a/2019-abtn-final-march-2019.pdf (accessed on 19 March 2023).
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Osborne, R.H. Obesity and increased burden of hip and knee joint disease in Australia: Results from a national survey. BMC Musculoskelet. Disord. 2012, 13, 254. [Google Scholar] [CrossRef]
- Subak, L.L.; Richter, H.E.; Hunskaar, S. Obesity and urinary incontinence: Epidemiology and clinical research update. J. Urol. 2009, 182, S2–S7. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Nicholas, S.B. Lipid disorders in obesity. Curr. Hypertens. Rep. 1999, 1, 131–136. [Google Scholar] [CrossRef]
- Baldemir, R.; Cirik, M. Practical parameters that can be used for nutritional assessment in patients hospitalized in the intensive care unit with the diagnosis of chronic obstructive pulmonary disease: Prognostic nutritional index, neutrophil-to-lymphocyte, platelet-to-lymphocyte, and lymphocyte-to-monocyte ratio. Medicine 2022, 101, e29433. [Google Scholar] [CrossRef]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Wieczorowska-Tobis, K.; Deskur-Śmielecka, E. Optimal Assessment of Nutritional Status in Older Subjects with the Chronic Obstructive Pulmonary Disease-A Comparison of Three Screening Tools Used in the GLIM Diagnostic Algorithm. Int. J. Environ. Res. Public Health 2022, 19, 1025. [Google Scholar] [CrossRef]
- Chen, R.; Xing, L.; You, C.; Ou, X. Prediction of prognosis in chronic obstructive pulmonary disease patients with respiratory failure: A comparison of three nutritional assessment methods. Eur. J. Intern. Med. 2018, 57, 70–75. [Google Scholar] [CrossRef]
- Rizzoli, R.; Bonjour, J.P. Malnutrition and osteoporosis. Z. Gerontol. Geriatr. 1999, 32 (Suppl. S1), I31–I37. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Anbar, R.; Gross Nevo, R.F.; Schlesinger, A.; Frishman, S.; Salai, M.; Beloosesky, Y. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin. Nutr. 2016, 35, 1053–1058. [Google Scholar] [CrossRef]
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Pre-fracture nutritional status is predictive of functional status at discharge during the acute phase with hip fracture patients: A multicenter prospective cohort study. Clin. Nutr. 2017, 36, 1320–1325. [Google Scholar] [CrossRef]
- Nuotio, M.; Tuominen, P.; Luukkaala, T. Association of nutritional status as measured by the Mini-Nutritional Assessment Short Form with changes in mobility, institutionalization and death after hip fracture. Eur. J. Clin. Nutr. 2016, 70, 393–398. [Google Scholar] [CrossRef]
- Malafarina, V.; Reginster, J.Y.; Cabrerizo, S.; Bruyère, O.; Kanis, J.A.; Martinez, J.A.; Zulet, M.A. Nutritional Status and Nutritional Treatment Are Related to Outcomes and Mortality in Older Adults with Hip Fracture. Nutrients 2018, 10, 555. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Tsubaki, A. Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients 2020, 12, 3743. [Google Scholar] [CrossRef]
- Bell, J.J.; Pulle, R.C.; Lee, H.B.; Ferrier, R.; Crouch, A.; Whitehouse, S.L. Diagnosis of overweight or obese malnutrition spells DOOM for hip fracture patients: A prospective audit. Clin. Nutr. 2021, 40, 1905–1910. [Google Scholar] [CrossRef]
- Wang, F.; Chen, W.; Bruening, K.S.; Raj, S.; Larsen, D.A. Nutrition Screening Tools and the Prediction of Clinical Outcomes among Chinese Hospitalized Gastrointestinal Disease Patients. PLoS ONE 2016, 11, e0159436. [Google Scholar] [CrossRef]
- Chávez-Tostado, M.; Cervantes-Guevara, G.; López-Alvarado, S.E.; Cervantes-Pérez, G.; Barbosa-Camacho, F.J.; Fuentes-Orozco, C.; Hernández-Corona, D.M.; González-Heredia, T.; Cervantes-Cardona, G.A.; González-Ojeda, A. Comparison of nutritional screening tools to assess nutritional risk and predict clinical outcomes in Mexican patients with digestive diseases. BMC Gastroenterol. 2020, 20, 79. [Google Scholar] [CrossRef]
- Giorgetti, G.; Fabiocchi, F.; Brandimarte, G.; Tursi, A. Acute Diverticulitis Is at Significant Risk of Malnutrition: An Analysis of Hospitalized Patients in a Medicine Department. J. Gastrointest. Liver Dis. 2019, 28, 53–56. [Google Scholar] [CrossRef]
- Fiorindi, C.; Dragoni, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery. Nutrients 2021, 13, 3899. [Google Scholar] [CrossRef]
- Bullock, A.F.; Greenley, S.L.; McKenzie, G.A.G.; Paton, L.W.; Johnson, M.J. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: Systematic review, narrative synthesis and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1519–1535. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Mariani, L.; Lo Vullo, S.; Amerio, M.L.; Biffi, R.; Caccialanza, G.; Capuano, G.; Correja, I.; Cozzaglio, L.; Di Leo, A.; et al. The nutritional risk in oncology: A study of 1,453 cancer outpatients. Support. Care Cancer 2012, 20, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Piglowska, M.; Guligowska, A.; Kostka, T. Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents. Nutrients 2020, 12, 2042. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).