Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer
Abstract
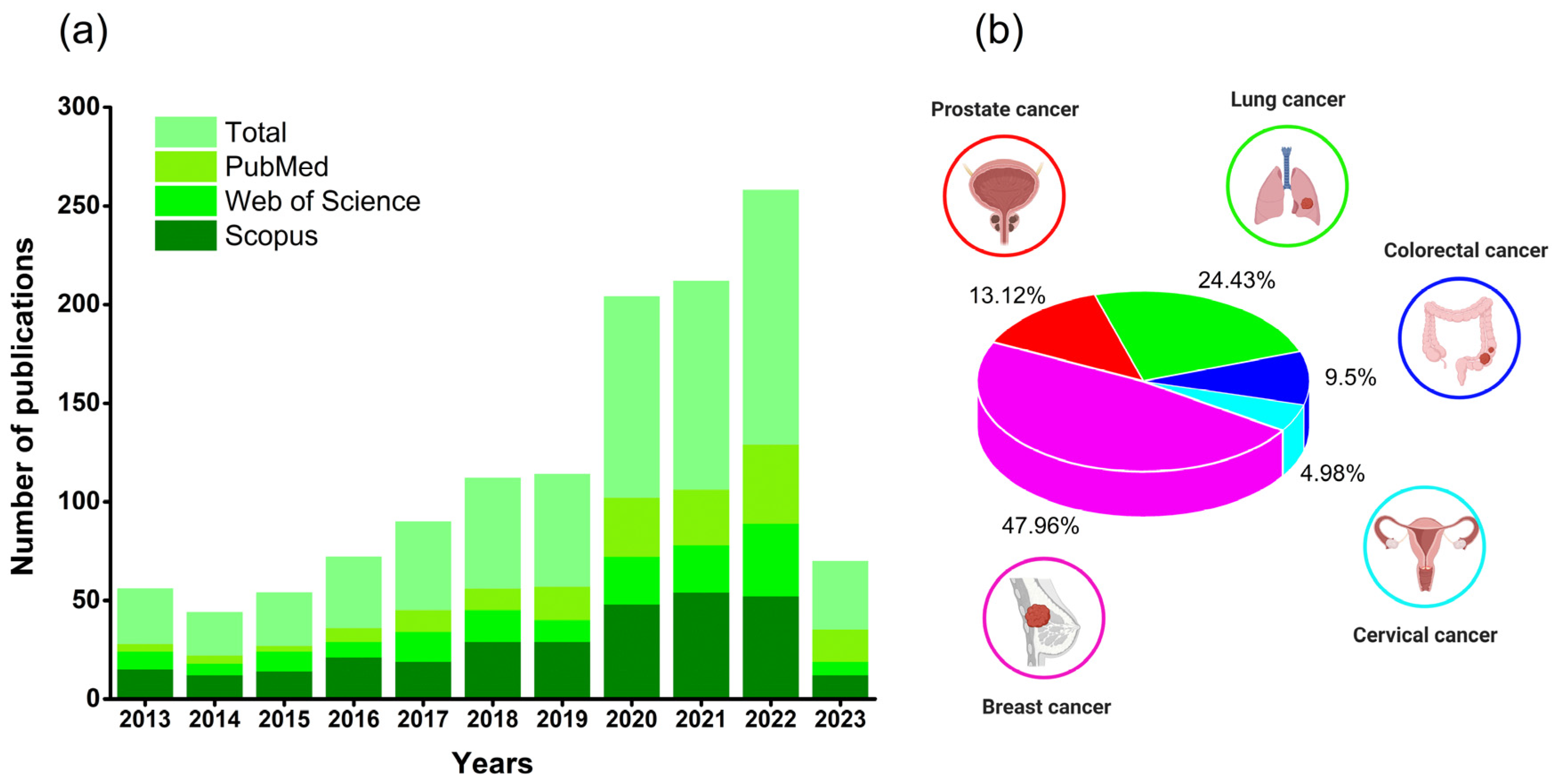
1. Introduction
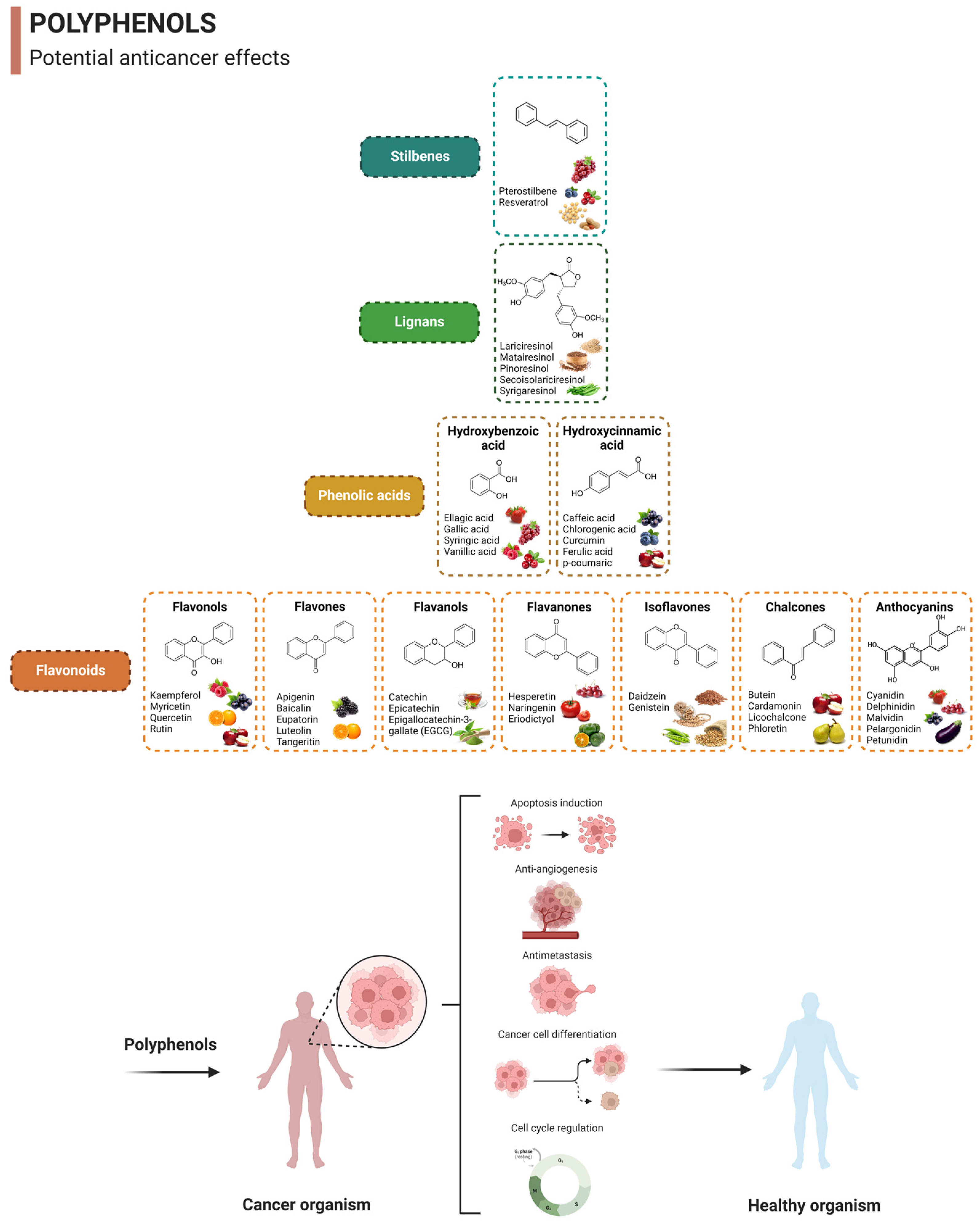
2. Overview of Polyphenols and Their Anticancer Properties
2.1. Quercetin
2.2. Curcumin
2.3. Epigallocatechin-3-Gallate (EGCG)
2.4. Resveratrol
3. Application of Nanotechnology Improving the Efficiency of Polyphenols for Cancer
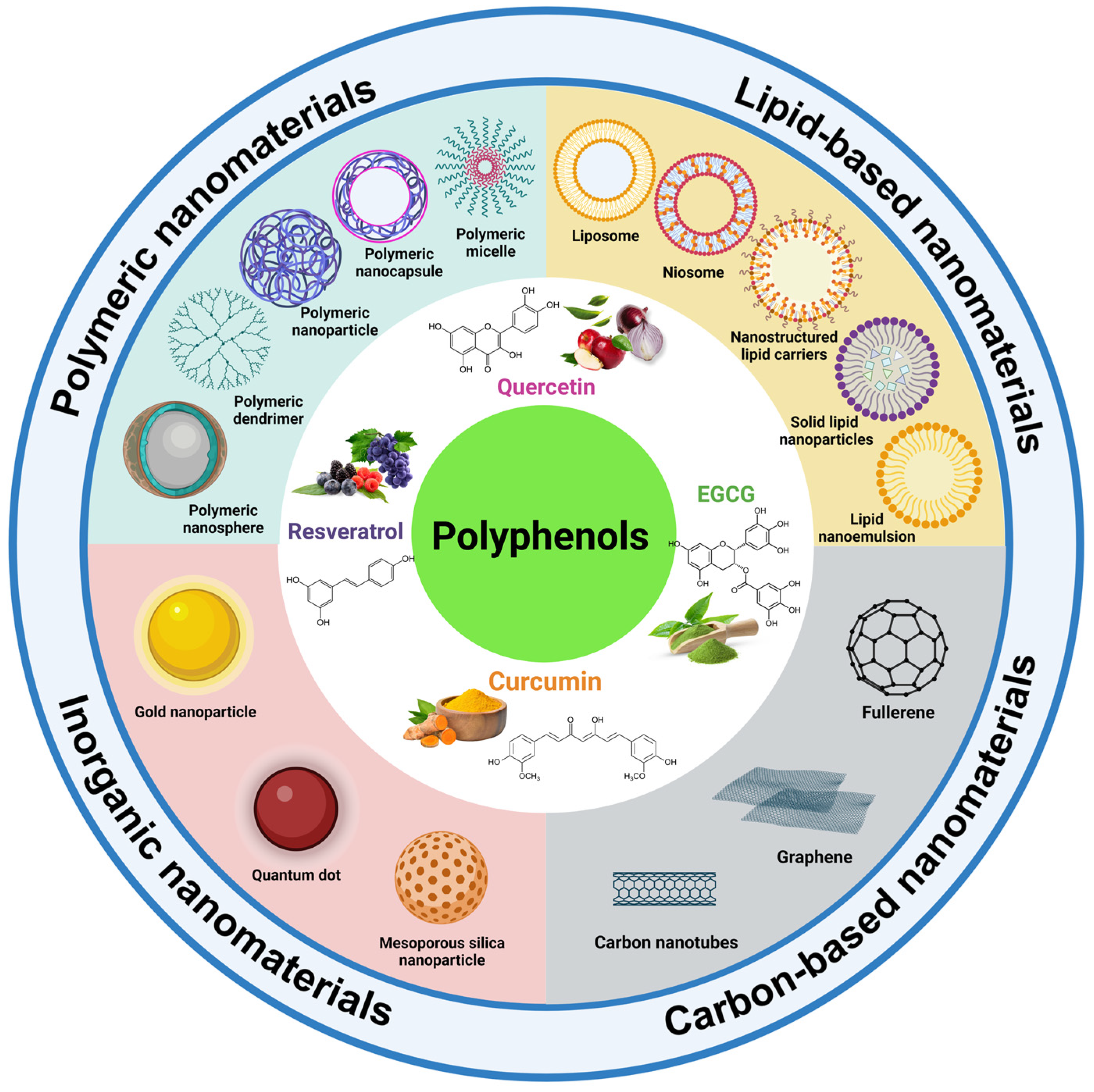
3.1. Polyphenol-Loaded Delivery Systems
3.1.1. Polymeric Nanomaterials
- Polymeric nanoparticles
| Polyphenol | Nanocarrier/ Nanoformulation | Particle Size | Cell Lines | Cell Viability Studies | Publication Year | References | |
|---|---|---|---|---|---|---|---|
| Free Compound | Nanocarrier | ||||||
| Quercetin | Chitosan/clay/graphitic-carbon nitride nanocomposite hydrogel | 454.65 nm | MCF-7 human breast cancer cell line | Cell viability = 100% (to 100 µM) | Cell viability = 60% (to 100 µM) | 2023 | [19] |
| Chitosan/SBE-β-CD nanoparticles | 272.07 nm | HeLa cervical cancer cells | IC50 = 59.84 µM Cell viability = 36.24% (to 150 µM) | IC50 = 66.68 at 43.55 µM Cell viability = 20.12 at 6.94% (to 150 µM) | 2023 | [28] | |
| Copper nanocluster-doped hydroxyapatite nanoparticles | 36.2 nm | HeLa cervical cancer cells | IC50 = 300 µM Cell viability = 70% (to 500 µM) | IC50 = 200 µM Cell viability = 30% (to 500 µM) | 2021 | [29] | |
| Gelatin/PVP/GO nanocarrier | 468 nm | MCF-7 human breast cancer cell line | Cell viability = 51.15% | Cell viability = 46.85% | 2023 | [20] | |
| GO nanocomposite film | - | BT474 breast cancer cells | IC50 = ND Cell viability = ~100% | IC50 = 99.29 µg/mL Cell viability = ~15% | 2022 | [53] | |
| Nanocochleates | 502 nm | 5000 KB human mouth cancer cells | IC50 = 5 µg/mL Cell viability = 20% (to 40 µg/mL) | IC50 = 10 µg/mL Cell viability = 40% (to 40 µg/mL) | 2022 | [30] | |
| PVP/PVA/TiO2 nanocomposite hydrogel | 330 nm | U87 human glioblastoma cell line | Cell viability = 61% | Cell viability = 74% | 2023 | [54] | |
| Vitamin-E TPGS nanoemulsion | 200 nm | HCT-116 colon cancer cell line | Cell viability = ~0 38% (to 100 µM) | Cell viability = ~0 25% (to 100 µM) | 2022 | [55] | |
| HT-29 colon cancer cell line | Cell viability = ~50% (to 100 µM) | Cell viability = ~0 35% (to 100 µM) | |||||
| Curcumin | Black-seed-oil-based nanoemulsion | 28.53 nm | MCF-7 human breast cancer cell line | IC50 = 6.67 µg/mL Cell viability = ~50% (to 6 µg/mL) | IC50 = 4.76 µg/mL Cell viability = ~35% (to 6 µg/mL) | 2020 | [56] |
| Chitosan-based microspheres | 5 nm | MDA-MB 231 model breast cancer cells | Cell viability = ~45% (to 96 µM) | Cell viability = ~50% (to 96 µM) | 2020 | [57] | |
| Curcumin–lactoferrin conjugated nanostructures | 166 nm | HCT116 human colon cancer cells | IC50 = 3.3 µg/mL Cell viability = 15% (to 5.2 µg/mL) | IC50 = 0.5 µg/mL Cell viability = 10% (to 5.2 µg/mL) | 2018 | [58] | |
| Emulsome nanoparticles | 184.21 nm | LNCaP prostate cancer cell line | IC50 = ND Cell viability = ~75% (to 30 µM) | IC50 = 17.1 µM Cell viability = 66% (to 30 µM) | 2023 | [59] | |
| Fe3O4/chitosan/agarose nanoemulsion | 279 nm | MCF-7 human breast cancer cell line | IC50 = ND Cell viability = ~60% (to 100 µM) | IC50 = 17.1 µM Cell viability = 48% (to 100 µM) | 2023 | [60] | |
| Fe3O4/PEG/folic acid nanoparticles | 650.1 nm | MCF-7 human breast cancer cell line | IC50 = 49.91 µM Cell viability = <10% (to 100 µM) | IC50 = ND Cell viability = >35% (to 100 µM) | 2022 | [61] | |
| A549 lung cancer cell | IC50 = 50.75 µM Cell viability = <10% (to 100 µM) | IC50 = ND Cell viability = ~40% (to 100 µM) | |||||
| PLGA/levan nano-micelles | 154.16 nm | MCF-7 human breast cancer cell line | IC50 = 0.01323 mg/mL Cell viability = ~75% (to 0.006 mg/mL) | IC50 = 0.01120 mg/mL Cell viability = ~65% (to 0.006 mg/mL) | 2021 | [62] | |
| PGS-based nanoparticles | 121 nm | HeLa cervical cancer cell | IC50 = 21.27 µM Cell viability = <25% (to 0.02 mg/mL) | IC50 = 15.95 µM Cell viability = <25% (to 0.02 mg/mL) | 2022 | [63] | |
| Pyromellitic dianhydride crosslinked cyclodextrin nanosponges | 70 nm | MCF-7 human breast cancer cell line | Cell viability = ~50% (to 130 mg/mL) | Cell viability = ~80% (to 200 mg/mL) | 2019 | [64] | |
| EGCG | Gold nanoparticles | 90.3 nm | A375SM human melanoma cell line | IC50 = ND Cell viability = ~60% (to 31.8 μM) | IC50 = 67.6 µg/mL Cell viability = ~20% (to 200 μM) | 2019 | [65] |
| MDA-MB-231 human breast cancer cell line | IC50 = ND Cell viability = ~60% (to 31.8 μM) | IC50 = 54.7 µg/mL Cell viability = 0% (to 200 μM) | |||||
| MIA PaCa-2 human pancreatic cancer cell line | IC50 = ND Cell viability = ~60% (to 31.8 μM) | IC50 = 17.0 µg/mL Cell viability = ~10% (to 200 μM) | |||||
| PC3 human prostate cancer cell line | IC50 = ND Cell viability = ~40% (to 31.8 μM) | IC50 = 24.9 µg/mL Cell viability = ~5% (to 200 μM) | |||||
| Lecithin and non-ionic surfactant nanoemulsion | 10 nm | H1299 human lung cancer cell line | IC50 = 36.03 μM Cell viability = ~70% (to 40 μM) | IC50 = 4.71 μM Cell viability = ~50% (to 40 μM) | 2020 | [42] | |
| A549 human lung cancer cell line | IC50 = ND Cell viability = ~70% (to 40 μM) | IC50 = 16.05 μM Cell viability = ~65% (to 40 μM) | |||||
| PLGA nanoparticles | 175.8 nm | A549 lung cancer cell line | IC50 = 72.63 μM Cell viability = 85% (to 25 μM) | IC50 = 19.57 μM Cell viability = 35% (to 25 μM) | 2020 | [66] | |
| H1299 lung cancer cell line | IC50 = 68.73 μM Cell viability = 85% (to 25 μM) | IC50 = 16.98 μM Cell viability = 20% (to 25 μM) | |||||
| Self-assembled PEG and chlorin e6 (Ce6) nanoparticles | 190–132 nm | 4T1 mouse breast carcinoma cell line | Cell viability = >20% (to 100 μg/mL) | Cell viability = <10% (to 100 μg/mL) | 2020 | [67] | |
| A549 human non-small-cell lung cancer cell line | Cell viability = >15% (to 100 μg/mL) | Cell viability = <10% (to 100 μg/mL) | |||||
| HCT116 human colorectal cancer cell line | Cell viability = >15% (to 100 μg/mL) | Cell viability = <10% (to 100 μg/mL) | |||||
| Surface-active maghemite nanoparticles (SAMNs) | 208.4 nm | HeLa human cervical cancer cells | Cell viability = ~125% (to 50 μg/mL) | Cell viability = ~40% (to 50 μg/mL) | 2021 | [68] | |
| Resveratrol | Folic acid/PNIPAM hydrogels | 243.59 nm | MCF-7 human breast cancer cell line | IC50 = 26.27 μg/mL Cell viability = ~40% (to 100 μg/mL) | IC50 = 3.55 μg/mL Cell viability = ~30% (to 100 μg/mL) | 2023 | [69] |
| Gold nanoparticles crosslinked with PVP | 41 nm | PANC-1 human pancreatic cancer cell line | Cell viability = ~30% (to 40 μM) | Cell viability = ~20% (to 15 μM) | 2022 | [26] | |
| Mesoporous silica nanoparticles | 60 nm | MNT-1 human melanoma cell line | IC50 = 37.9 μM Cell viability = ~40% (to 50 μM) | IC50 = 25.5 μg/mL Cell viability = ~20% (to 100 μg/mL) | 2021 | [70] | |
| A375 human melanoma cell line | IC50 = 0.0026 μM Cell viability = ~10% (to 50 μM) | IC50 = 29.5 μg/mL Cell viability = ~5% (to 100 μg/mL) | |||||
| PLGA/chitosan nanoparticles | 341.56 nm | H1299 human non-small cell lung carcinoma cell line | IC50 = 57.31 μg/mL Cell viability = ~30% (to 100 μg/mL) | IC50 = 34.99 μg/mL Cell viability = ~15% (to 100 μg/mL) | 2020 | [71] | |
| Pluronic F127/vitamin-E TPGS micelles | 318 nm | MCF-7 human breast cancer cell line | ND | IC50 = 0.93 μg/mL Cell viability = ~20.7 (to 2.5 μg/mL) | 2021 | [72] | |
| MDA-MB-231 human breast cancer cell line | ND | IC50 = 0.76 μg/mL Cell viability = 7.1% (to 2.5 μg/mL) | |||||
| SBE-β-CD nanoparticles | 264.2 nm | A549 lung cancer cell line | IC50 = 50.79 μM Cell viability = >50% (to 50 μM) | IC50 = 3.31 μM Cell viability = <10% (to 50 μM) | 2020 | [73] | |
| H358 lung cancer cell line | IC50 = 49.96 μM Cell viability = >50% (to 50 μM) | IC50 = 0.97 μM Cell viability = <10% (to 50 μM) | |||||
| H460 lung cancer cell line | IC50 = 32.67 μM Cell viability = >30% (to 50 μM) | IC50 = 4.04 μM Cell viability = <10% (to 50 μM) | |||||
| H4006 lung cancer cell line | IC50 = 133.43 μM Cell viability = <70% (to 50 μM) | IC50 = 3.10 μM Cell viability = <10% (to 50 μM) | |||||
| H157 lung cancer cell line | IC50 = 30.81 μM Cell viability = >30% (to 50 μM) | IC50 = 5.42 μM Cell viability = <10% (to 50 μM) | |||||
| Starch and chitosan films | - | AGS human gastric epithelial cell lines | IC50 = 31.1 μg/mL | IC50 = 91.1 μg/mL | 2022 | [74] | |
- Polymeric micelles
- Polymeric dendrimers
3.1.2. Lipid-Based Nanomaterials
- Liposomes
- Niosomes
- Nanostructured lipid carriers (NLCs)
- Solid lipid nanoparticles (SLNs)
- Lipid nanoemulsions
3.1.3. Inorganic Nanomaterials
- Gold nanoparticles (AuNPs)
- Quantum dots (QDs)
- Mesoporous silica nanoparticles (MSNs)
3.1.4. Carbon-Based Nanomaterials
- Carbon nanotubes (CNTs)
- Graphene
3.2. Polyphenol Co-Delivery Systems
3.3. Polyphenol–Drug Delivery Systems
4. Cancer Models in Preclinical Studies
4.1. Breast Cancer
4.2. Prostate Cancer
4.3. Lung Cancer
4.4. Colorectal Cancer
4.5. Cervical Cancer
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 6 June 2023).
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2014, 56, 1097–1107. [Google Scholar] [CrossRef]
- Norat, T.; Scoccianti, C.; Boutron-Ruault, M.C.; Anderson, A.; Berrino, F.; Cecchini, M.; Espina, C.; Key, T.; Leitzmann, M.; Powers, H.; et al. European Code against Cancer 4th Edition: Diet and Cancer. Cancer Epidemiol. 2015, 39, S56–S66. [Google Scholar] [CrossRef]
- Williams, M.T.; Hord, N.G. The Role of Dietary Factors in Cancer Prevention: Beyond Fruits and Vegetables. Nutr. Clin. Pract. 2005, 20, 451–459. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef]
- Cara, K.C.; Goldman, D.M.; Kollman, B.K.; Amato, S.S.; Tull, M.D.; Karlsen, M.C. Commonalities among Dietary Recommendations from 2010 to 2021 Clinical Practice Guidelines: A Meta-Epidemiological Study from the American College of Lifestyle Medicine. Adv. Nutr. 2023, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-Delivery Systems for Food Bioactive Compounds in Cancer: Prevention, Therapy, and Clinical Applications. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary Polyphenols in Chemoprevention and Synergistic Effect in Cancer: Clinical Evidences and Molecular Mechanisms of Action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Shohag, S.; Akhter, S.; Islam, S.; Sarker, T.; Sifat, M.K.; Rahman, M.M.; Islam, M.R.; Sharma, R. Perspectives on the Molecular Mediators of Oxidative Stress and Antioxidant Strategies in the Context of Neuroprotection and Neurolongevity: An Extensive Review. Oxid. Med. Cell. Longev. 2022, 2022, 7743705. [Google Scholar] [CrossRef]
- Rivas, F.; Poblete-Aro, C.; Pando, M.E.; Allel, M.J.; Fernandez, V.; Soto, A.; Nova, P.; Garcia-Diaz, D. Effects of Polyphenols in Aging and Neurodegeneration Associated with Oxidative Stress. Curr. Med. Chem. 2021, 29, 1045–1060. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Saravanan, T.S.; Monteclaro, C.C.; Presser, N.; Ye, X.; Selvan, S.R.; Brosman, S. Epicatechins Purified from Green Tea (Camellia sinensis) Differentially Suppress Growth of Gender-Dependent Human Cancer Cell Lines. Evid.-Based Complement. Altern. Med. 2006, 3, 237–247. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-Inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin Inhibits NF-KB and Wnt/β-Catenin Pathways in Cervical Cancer Cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yi, K.; Zhou, X.; Li, X.; Zhong, C.; Cao, H.; Xie, C.; Zhu, J. Destruction of the Cellular Antioxidant Pool Contributes to Resveratrol-Induced Senescence and Apoptosis in Lung Cancer. Phytother. Res. 2023, 37, 2995–3008. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Quercetin- and Rutin-Based Nano-Formulations for Cancer Treatment: A Systematic Review of Improved Efficacy and Molecular Mechanisms. Phytomedicine 2022, 97, 153909. [Google Scholar] [CrossRef] [PubMed]
- Sabzini, M.; Pourmadadi, M.; Yazdian, F.; Khadiv-Parsi, P.; Rashedi, H. Development of Chitosan/Halloysite/Graphitic-carbon Nitride Nanovehicle for Targeted Delivery of Quercetin to Enhance Its Limitation in Cancer Therapy: An In Vitro Cytotoxicity against MCF-7 Cells. Int. J. Biol. Macromol. 2023, 226, 159–171. [Google Scholar] [CrossRef]
- Najafabadi, A.P.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Rahdar, A.; Díez-Pascual, A.M. PH-Sensitive Ameliorated Quercetin Delivery Using Graphene Oxide Nanocarriers Coated with Potential Anticancer Gelatin-Polyvinylpyrrolidone Nanoemulsion with Bitter Almond Oil. J. Drug Deliv. Sci. Technol. 2023, 82, 104339. [Google Scholar] [CrossRef]
- Saraswat, A.L.; Maher, T.J. Development and Optimization of Stealth Liposomal System for Enhanced in Vitro Cytotoxic Effect of Quercetin. J. Drug Deliv. Sci. Technol. 2020, 55, 101477. [Google Scholar] [CrossRef]
- Tabrez, S.; Jabir, N.R.; Adhami, V.M.; Khan, M.I.; Moulay, M.; Kamal, M.A.; Mukhtar, H. Nanoencapsulated Dietary Polyphenols for Cancer Prevention and Treatment: Successes and Challenges. Nanomedicine 2020, 15, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Kalam, N.; Shaikh, M.F.; Hasnain, M.S.; Hafiz, A.K.; Ansari, M.T. Nanoencapsulation of Polyphenols as Drugs and Supplements for Enhancing Therapeutic Profile—A Review. Curr. Mol. Pharmacol. 2021, 15, 77–107. [Google Scholar] [CrossRef]
- Mahdian, M.; Akbari Asrari, S.; Ahmadi, M.; Madrakian, T.; Rezvani Jalal, N.; Afkhami, A.; Moradi, M.; Gholami, L. Dual Stimuli-Responsive Gelatin-Based Hydrogel for PH and Temperature-Sensitive Delivery of Curcumin Anticancer Drug. J. Drug Deliv. Sci. Technol. 2023, 84, 104537. [Google Scholar] [CrossRef]
- Hasan, M.; Elkhoury, K.; Belhaj, N.; Kahn, C.; Tamayol, A.; Barberi-Heyob, M.; Arab-Tehrany, E.; Linder, M. Growth-Inhibitory Effect of Chitosan-Coated Liposomes Encapsulating Curcumin on MCF-7 Breast Cancer Cells. Mar. Drugs 2020, 18, 217. [Google Scholar] [CrossRef]
- Lee, D.G.; Lee, M.; Go, E.B.; Chung, N. Resveratrol-Loaded Gold Nanoparticles Enhance Caspase-Mediated Apoptosis in PANC-1 Pancreatic Cells via Mitochondrial Intrinsic Apoptotic Pathway. Cancer Nanotechnol. 2022, 13, 34. [Google Scholar] [CrossRef]
- Alizadeh, M.H.; Pooresmaeil, M.; Namazi, H. Carboxymethyl Cellulose@multi Wall Carbon Nanotubes Functionalized with Ugi Reaction as a New Curcumin Carrier. Int. J. Biol. Macromol. 2023, 234, 123778. [Google Scholar] [CrossRef]
- Ferreira, M.; Gomes, D.; Neto, M.; Passarinha, L.A.; Costa, D.; Sousa, Â. Development and Characterization of Quercetin-Loaded Delivery Systems for Increasing Its Bioavailability in Cervical Cancer Cells. Pharmaceutics 2023, 15, 936. [Google Scholar] [CrossRef]
- Simon, A.T.; Dutta, D.; Chattopadhyay, A.; Ghosh, S.S. Quercetin-Loaded Luminescent Hydroxyapatite Nanoparticles for Theranostic Application in Monolayer and Spheroid Cultures of Cervical Cancer Cell Line in Vitro. ACS Appl. Bio Mater. 2021, 4, 4495–4506. [Google Scholar] [CrossRef]
- Munot, N.; Kandekar, U.; Giram, P.S.; Khot, K.; Patil, A.; Cavalu, S. A Comparative Study of Quercetin-Loaded Nanocochleates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential. Pharmaceutics 2022, 14, 1601. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Q.; Peng, Q.; Kang, Z.; Xiao, S.; Zheng, P.; Li, J.; Chen, Y. Comparative Analysis of the Molecular Mechanism of Inhibiting Proliferation and Migration in Cervical Cancer HeLa Cell by Curcumin and Resveratrol. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Behroozaghdam, M.; Dehghani, M.; Zabolian, A.; Kamali, D.; Javanshir, S.; Hasani Sadi, F.; Hashemi, M.; Tabari, T.; Rashidi, M.; Mirzaei, S.; et al. Resveratrol in Breast Cancer Treatment: From Cellular Effects to Molecular Mechanisms of Action. Cell. Mol. Life Sci. 2022, 79, 539. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS Analysis and Characterization of Polyphenols in Food. TrAC-Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Li, Y.; Jiang, D.; Zhao, J.; Ge, J.F. Anticancer Effect and Apoptosis Induction by Quercetin in the Human Lung Cancer Cell Line A-549. Mol. Med. Rep. 2012, 5, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, P.; Zhang, H.; Su, X.; Guo, L.; Zhou, X.; Wang, J.; Huang, P.; Zhang, Q.; Sun, R. EGFR and ERK Activation Resists Flavonoid Quercetin-Induced Anticancer Activities in Human Cervical Cancer Cells In Vitro. Oncol. Lett. 2021, 22, 754. [Google Scholar] [CrossRef]
- Kazemi-Lomedasht, F.; Rami, A.; Zarghami, N. Comparison of Inhibitory Effect of Curcumin Nanoparticles and Free Curcumin in Human Telomerase Reverse Transcriptase Gene Expression in Breast Cancer. Adv. Pharm. Bull. 2013, 3, 127–130. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-Curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Hao, J.; Dai, X.; Gao, J.; Li, Y.; Hou, Z.; Chang, Z.; Wang, Y. Curcumin Suppresses Colorectal Tumorigenesis via the Wnt/β-Catenin Signaling Pathway by Downregulating Axin2. Oncol. Lett. 2021, 21, 186. [Google Scholar] [CrossRef]
- Sheng, J.; Shi, W.; Guo, H.; Long, W.; Wang, Y.; Qi, J.; Liu, J.; Xu, Y. The Inhibitory Effect of (-)-Epigallocatechin-3 -Gallate on Breast Cancer Progression via Reducing SCUBE2 Methylation and DNMT Activity. Molecules 2019, 24, 2899. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, C.; Chen, R.; Liu, C.; Chen, M. Lipophilized Epigallocatechin Gallate Derivative Exerts Anti-Proliferation Efficacy through Induction of Cell Cycle Arrest and Apoptosis on DU145 Human Prostate Cancer Cells. Nutrients 2019, 12, 92. [Google Scholar] [CrossRef]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer Effects of Epigallocatechin-3-Gallate Nanoemulsion on Lung Cancer Cells through the Activation of AMP-Activated Protein Kinase Signaling Pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The Potential Anti-Cancer Effects of Quercetin on Blood, Prostate and Lung Cancers: An Update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; da Silva, A.A.; da Silva, B.D.; Neto, L.T.; Tessaro, L.; Furtado, C.R.G.; de Sousa, A.M.F.; Carvalho, N.M.F.; Conte-Junior, C.A. Eco-Friendly Synthesis of ZnO Nanomaterial from Green Tea Extract: Photocatalytic, Antibacterial and Antioxidant Potential. Biomass Convers. Biorefinery 2023, 1, 1–15. [Google Scholar] [CrossRef]
- Borutinskaitė, V.; Virkšaitė, A.; Gudelytė, G.; Navakauskienė, R. Green Tea Polyphenol EGCG Causes Anti-Cancerous Epigenetic Modulations in Acute Promyelocytic Leukemia Cells. Leuk. Lymphoma 2018, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Chakraborty, P.; Bhattacharya, H.; Singh, S.K.; Dua, K.; Dey, A.; Jha, N.K. Recent Advances in Flavonoid-Based Nanocarriers as an Emerging Drug Delivery Approach for Cancer Chemotherapy. Drug Discov. Today 2023, 28, 103409. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y.; et al. Nanoparticle-Based Medicines in Clinical Cancer Therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gahtori, R.; Govarthanan, K.; Sharma, V.; Pappuru, S.; Pandit, S.; Mathuriya, A.S.; Dholpuria, S.; Bishi, D.K. Recent Trends in Biodegradable Polyester Nanomaterials for Cancer Therapy. Mater. Sci. Eng. C 2021, 127, 112198. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol. Symp. 2018, 380, 1800114. [Google Scholar] [CrossRef]
- Rosendahl, J.; Solberg, A.; Ståhlberg, A.; Håkansson, J.; Chinga-Carrasco, G.; Deng, J.; Demitri, C.; Pasquier, E.; Rosendahl, J.; Solberg, A.; et al. Polysaccharides and Structural Proteins as Components in Three-Dimensional Scaffolds for Breast Cancer Tissue Models: A Review. Bioengineering 2023, 10, 682. [Google Scholar] [CrossRef]
- Pranav; Laskar, P.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Biomolecule-Functionalized Nanoformulations for Prostate Cancer Theranostics. J. Adv. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.M.; Morosan, A.; Tihauan, B.; Oprea, O.; Motelica, L.; Trusca, R.; Nicoara, A.I.; Popescu, R.C.; Savu, D.; Mihaiescu, D.E.; et al. Novel Graphene Oxide/Quercetin and Graphene Oxide/Juglone Nanostructured Platforms as Effective Drug Delivery Systems with Biomedical Applications. Nanomaterials 2022, 12, 1943. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, M.M.; Pourmadadi, M.; Rahdar, A.; Díez-Pascual, A.M. Improving Quercetin Anticancer Activity through a Novel Polyvinylpyrrolidone/Polyvinyl Alcohol/TiO2 Nanocomposite. J. Drug Deliv. Sci. Technol. 2023, 81, 104304. [Google Scholar] [CrossRef]
- Enin, H.A.A.; Alquthami, A.F.; Alwagdani, A.M.; Yousef, L.M.; Albuqami, M.S.; Alharthi, M.A.; Alsaab, H.O. Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition. Colloids Interfaces 2022, 6, 49. [Google Scholar] [CrossRef]
- Kazi, M.; Nasr, F.A.; Noman, O.; Alharbi, A.; Alqahtani, M.S.; Alanazi, F.K. Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Pharmaceutics 2020, 12, 1107. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Facile Preparation of PH-Sensitive Chitosan Microspheres for Delivery of Curcumin; Characterization, Drug Release Kinetics and Evaluation of Anticancer Activity. Int. J. Biol. Macromol. 2020, 162, 501–511. [Google Scholar] [CrossRef]
- Chaharband, F.; Kamalinia, G.; Atyabi, F.; Mortazavi, S.; Mirzaie, Z.H.; Dinarvand, R. Formulation and In Vitro Evaluation of Curcumin-Lactoferrin Conjugated Nanostructures for Cancerous Cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 626–636. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Sahin, F.; Ucisik, M.H. Delivery of Curcumin within Emulsome Nanoparticles Enhances the Anti-Cancer Activity in Androgen-Dependent Prostate Cancer Cell. Mol. Biol. Rep. 2023, 50, 2531–2543. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ahmadi, M.; Yazdian, F. Synthesis of a Novel PH-Responsive Fe3O4/Chitosan/Agarose Double Nanoemulsion as a Promising Nanocarrier with Sustained Release of Curcumin to Treat MCF-7 Cell Line. Int. J. Biol. Macromol. 2023, 235, 123786. [Google Scholar] [CrossRef]
- Movileanu, C.; Anghelache, M.; Turtoi, M.; Voicu, G.; Neacsu, I.A.; Ficai, D.; Trusca, R.; Oprea, O.; Ficai, A.; Andronescu, E.; et al. Folic Acid-Decorated PEGylated Magnetite Nanoparticles as Efficient Drug Carriers to Tumor Cells Overexpressing Folic Acid Receptor. Int. J. Pharm. 2022, 625, 122064. [Google Scholar] [CrossRef]
- Eskandari, Z.; Bahadori, F.; Yenigun, V.B.; Demiray, M.; Eroğlu, M.S.; Kocyigit, A.; Oner, E.T. Levan Enhanced the NF-ΚB Suppression Activity of an Oral Nano PLGA-Curcumin Formulation in Breast Cancer Treatment. Int. J. Biol. Macromol. 2021, 189, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Massironi, A.; Marzorati, S.; Marinelli, A.; Toccaceli, M.; Gazzotti, S.; Ortenzi, M.A.; Maggioni, D.; Petroni, K.; Verotta, L. Synthesis and Characterization of Curcumin-Loaded Nanoparticles of Poly(Glycerol Sebacate): A Novel Highly Stable Anticancer System. Molecules 2022, 27, 6997. [Google Scholar] [CrossRef]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic Dianhydride Crosslinked Cyclodextrin Nanosponges for Curcumin Controlled Release; Formulation, Physicochemical Characterization and Cytotoxicity Investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Tu, G.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Enhanced Chemotherapeutic Efficacy of Plga-Encapsulated Epigallocatechin Gallate (EGCG) against Human Lung Cancer. Int. J. Nanomed. 2020, 15, 4417–4429. [Google Scholar]
- Chen, X.; Yi, Z.; Chen, G.; Ma, X.; Su, W.; Deng, Z.; Ma, L.; Tong, Q.; Ran, Y.; Li, X. Carrier-Enhanced Photodynamic Cancer Therapy of Self-Assembled Green Tea Polyphenol-Based Nanoformulations. ACS Sustain. Chem. Eng. 2020, 8, 16372–16384. [Google Scholar] [CrossRef]
- Fasolato, L.; Magro, M.; Cozza, G.; Sbarra, F.; Molinari, S.; Novelli, E.; Vianello, F.; Venerando, A. An Iron Shield to Protect Epigallocatehin-3-Gallate from Degradation: Multifunctional Self-Assembled Iron Oxide Nanocarrier Enhances Protein Kinase CK2 Intracellular Targeting and Inhibition. Pharmaceutics 2021, 13, 1266. [Google Scholar] [CrossRef] [PubMed]
- Metawea, O.R.M.; Teleb, M.; Haiba, N.S.; Elzoghby, A.O.; Khafaga, A.F.; Noreldin, A.E.; Khattab, S.N.; Khalil, H.H. Folic Acid-Poly(N-Isopropylacrylamide-Maltodextrin) Nanohydrogels as Novel Thermo-/PH-Responsive Polymer for Resveratrol Breast Cancer Targeted Therapy. Eur. Polym. J. 2023, 182, 111721. [Google Scholar] [CrossRef]
- Marinheiro, D.; Ferreira, B.J.M.L.; Oskoei, P.; Oliveira, H.; Daniel-da-silva, A.L. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Alhakamy, N.A.; Padder, R.; Husain, M.; Md, S. Preparation and Characterization of Chitosan Coated Plga Nanoparticles of Resveratrol: Improved Stability, Antioxidant and Apoptotic Activities in H1299 Lung Cancer Cells. Coatings 2020, 10, 439. [Google Scholar] [CrossRef]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol Loaded Polymeric Micelles for Theranostic Targeting of Breast Cancer Cells. Nanotheranostics 2021, 5, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Muth, A.; Kunda, N.K.; Gupta, V. Inhalable Resveratrol-Cyclodextrin Complex Loaded Biodegradable Nanoparticles for Enhanced Efficacy against Non-Small Cell Lung Cancer. Int. J. Biol. Macromol. 2020, 164, 638–650. [Google Scholar] [CrossRef]
- Boontawee, R.; Issarachot, O.; Kaewkroek, K.; Wiwattanapatapee, R. Foldable/Expandable Gastro-Retentive Films Based on Starch and Chitosan as a Carrier for Prolonged Release of Resveratrol. Curr. Pharm. Biotechnol. 2022, 23, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, D.; Xue, G.; Yu, S.; Yuan, C.; Huang, M.; Jiang, L. Improved Therapeutic Efficacy of Quercetin-Loaded Polymeric Nanoparticles on Triple-Negative Breast Cancer by Inhibiting UPA. RSC Adv. 2020, 10, 34517–34526. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ghosh, S.; Kumar, P.; Basu, B.; Nagpal, K. Ellagic Acid-Loaded, Tween 80-Coated, Chitosan Nanoparticles as a Promising Therapeutic Approach against Breast Cancer: In-Vitro and In-Vivo Study. Life Sci. 2021, 284, 119927. [Google Scholar] [CrossRef] [PubMed]
- García, M.C. Stimuli-Responsive Self-Assembled Nanocarriers Based on Amphiphilic Block Copolymers for Cancer Therapy. Appl. Multifunct. Nanomater. 2023, 365–409. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric Micelles and Cancer Therapy: An Ingenious Multimodal Tumor-Targeted Drug Delivery System. Drug Deliv. Transl. Res. 2022, 13, 135–163. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent Advances in Developing Polymeric Micelles for Treating Cancer: Breakthroughs and Bottlenecks in Their Clinical Translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef]
- Karabasz, A.; Lachowicz, D.; Karewicz, A.; Mezyk-Kopec, R.; Stalińska, K.; Werner, E.; Cierniak, A.; Dyduch, G.; Bereta, J.; Bzowska, M. Analysis of Toxicity and Anticancer Activity of Micelles of Sodium Alginate-Curcumin. Int. J. Nanomed. 2019, 14, 7249–7262. [Google Scholar] [CrossRef]
- Xuan, M.; Liang, J.; Li, J.; Wu, W. Multi-Functional Lipopeptide Micelles as a Vehicle for Curcumin Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126208. [Google Scholar] [CrossRef]
- Gligor, G.; Loghin, F.; Juncan, M.; Frum, A.; Dobrea, C.M.; Zenze, M.; Daniels, A.; Singh, M. Dendrimers as Modifiers of Inorganic Nanoparticles for Therapeutic Delivery in Cancer. Pharmaceutics 2023, 15, 398. [Google Scholar] [CrossRef]
- Marcelo, G.; Vega, M.A.; Nieto, C.; Rai, D.B.; Medicherla, K.; Kulhari, H. Dendrimer-Mediated Delivery of Anticancer Drugs for Colon Cancer Treatment. Pharmaceutics 2023, 15, 801. [Google Scholar] [CrossRef]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (Propylene Imine) Dendrimer as an Emerging Polymeric Nanocarrier for Anticancer Drug and Gene Delivery. Eur. Polym. J. 2021, 158, 110683. [Google Scholar] [CrossRef]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as Efficient Nanocarriers for the Protection and Delivery of Bioactive Phytochemicals. Adv. Colloid Interface Sci. 2020, 278, 102125. [Google Scholar] [CrossRef]
- Zeynalzadeh, S.; Dehghani, E.; Hassani, A.; Baradar Khoshfetrat, A.; Salami-Kalajahi, M. Effect of Curcumin-Loaded Poly(Amidoamine) Dendrimer on Cancer Cell Lines: A Comparison between Physical Loading and Chemical Conjugation of Drug. Polym. Bull. 2023, 1–14. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Jogdeo, C.M.; Panja, S.; Kanvinde, S.; Kapoor, E.; Siddhanta, K.; Oupický, D. Advances in Lipid-Based Codelivery Systems for Cancer and Inflammatory Diseases. Adv. Healthc. Mater. 2023, 12, 2202400. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Shayan, M.; Bourbour, M.; Moghtaderi, M.; Noorbazargan, H.; Eshrati Yeganeh, F.; Saffar, S.; Tahriri, M. Preparation, Optimization and In-Vitro Evaluation of Curcumin-Loaded Niosome@calcium Alginate Nanocarrier as a New Approach for Breast Cancer Treatment. Biology 2021, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Gadag, S.; Narayan, R.; Nayak, A.S.; Catalina Ardila, D.; Sant, S.; Nayak, Y.; Garg, S.; Nayak, U.Y. Development and Preclinical Evaluation of Microneedle-Assisted Resveratrol Loaded Nanostructured Lipid Carriers for Localized Delivery to Breast Cancer Therapy. Int. J. Pharm. 2021, 606, 120877. [Google Scholar] [CrossRef]
- Hatami, M.; Kouchak, M.; Kheirollah, A.; Khorsandi, L.; Rashidi, M. Effective Inhibition of Breast Cancer Stem Cell Properties by Quercetin-Loaded Solid Lipid Nanoparticles via Reduction of Smad2/Smad3 Phosphorylation and β-Catenin Signaling Pathway in Triple-Negative Breast Cancer. Biochem. Biophys. Res. Commun. 2023, 664, 69–76. [Google Scholar] [CrossRef]
- Mahmoud, K.; Swidan, S.; El-Nabarawi, M.; Teaima, M. Lipid Based Nanoparticles as a Novel Treatment Modality for Hepatocellular Carcinoma: A Comprehensive Review on Targeting and Recent Advances. J. Nanobiotechnol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Rethi, L.; Mutalik, C.; Anurogo, D.; Lu, L.S.; Chu, H.Y.; Yougbaré, S.; Kuo, T.R.; Cheng, T.M.; Chen, F.L. Lipid-Based Nanomaterials for Drug Delivery Systems in Breast Cancer Therapy. Nanomaterials 2022, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Baskararaj, S.; Panneerselvam, T.; Govindaraj, S.; Arunachalam, S.; Parasuraman, P.; Pandian, S.R.K.; Sankaranarayanan, M.; Mohan, U.P.; Palanisamy, P.; Ravishankar, V.; et al. Formulation and Characterization of Folate Receptor-Targeted PEGylated Liposome Encapsulating Bioactive Compounds from Kappaphycus Alvarezii for Cancer Therapy. 3 Biotech 2020, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Allahou, L.W.; Madani, S.Y.; Seifalian, A. Investigating the Application of Liposomes as Drug Delivery Systems for the Diagnosis and Treatment of Cancer. Int. J. Biomater. 2021, 2021, 3041969. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wang, X.; Yin, J.; Mou, Y.; Huang, H.; Ren, Z. Selective Delivery of Curcumin to Breast Cancer Cells by Self-Targeting Apoferritin Nanocages with PH-Responsive and Low Toxicity. Drug Deliv. 2022, 29, 986–996. [Google Scholar] [CrossRef]
- Esmaeili, M.; Can, A.; Ozaydin, G.; Yuce, M.; Zarrabi, A. Colloids and Surfaces A: Physicochemical and Engineering Aspects Optimization of Curcumin Loaded Niosomes for Drug Delivery Applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129921. [Google Scholar] [CrossRef]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef]
- Baranei, M.; Taheri, R.A.; Tirgar, M.; Saeidi, A.; Oroojalian, F.; Uzun, L.; Asefnejad, A.; Wurm, F.R.; Goodarzi, V. Anticancer Effect of Green Tea Extract (GTE)-Loaded PH-Responsive Niosome Coated with PEG against Different Cell Lines. Mater. Today Commun. 2021, 26, 101751. [Google Scholar] [CrossRef]
- Abtahi, N.A.; Naghib, S.M.; Ghalekohneh, S.J.; Mohammadpour, Z.; Nazari, H.; Mosavi, S.M.; Gheibihayat, S.M.; Haghiralsadat, F.; Reza, J.Z.; Doulabi, B.Z. Multifunctional Stimuli-Responsive Niosomal Nanoparticles for Co-Delivery and Co-Administration of Gene and Bioactive Compound: In Vitro and In Vivo Studies. Chem. Eng. J. 2022, 429, 132090. [Google Scholar] [CrossRef]
- Khakbaz, F.; Mirzaei, M.; Mahani, M. Lecithin Sensitized Thermo-Sensitive Niosome Using NIR-Carbon Dots for Breast Cancer Combined Chemo-Photothermal Therapy. J. Photochem. Photobiol. A Chem. 2023, 434, 114236. [Google Scholar] [CrossRef]
- Garg, J.; Pathania, K.; Sah, S.P.; Pawar, S.V. Nanostructured Lipid Carriers: A Promising Drug Carrier for Targeting Brain Tumours. Future J. Pharm. Sci. 2022, 8, 25. [Google Scholar] [CrossRef]
- Truong, T.H.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Muangnoi, C.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-Coated Nanostructured Lipid Carriers for Transdermal Delivery of Tetrahydrocurcumin for Breast Cancer Therapy. Carbohydr. Polym. 2022, 288, 119401. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, Y.; Zhao, D.; Fang, C.; He, D.; Yang, Q.; Yang, L.; Chen, R.; Tan, Q.; Zhang, J. Oral Administration of Natural Polyphenol-Loaded Natural Polysaccharide-Cloaked Lipidic Nanocarriers to Improve Efficacy against Small-Cell Lung Cancer. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102261. [Google Scholar] [CrossRef]
- Katopodi, A.; Detsi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Natural Products as Promising Systems for Their Bioactivity Enhancement: The Case of Essential Oils and Flavonoids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127529. [Google Scholar] [CrossRef]
- Shamsuddin, N.A.M.; Zulfakar, M.H. Nanostructured Lipid Carriers for the Delivery of Natural Bioactive Compounds. Curr. Drug Deliv. 2022, 20, 127–143. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin Nanoformulations: Beneficial Nanomedicine against Cancer. Phyther. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of Oleuropein in Nanostructured Lipid Carriers: Biocompatibility and Antioxidant Efficacy in Lung Epithelial Cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Sguizzato, M.; Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Singh, V.; Yusuf, M.; Akhtar, N.; Sulaiman, G.M.; Albukhaty, S.; Abdellatif, A.A.H.; Khan, M.; Mohammed, S.A.A.; et al. Solid Lipid Nanoparticles for Targeted Natural and Synthetic Drugs Delivery in High-Incidence Cancers, and Other Diseases: Roles of Preparation Methods, Lipid Composition, Transitional Stability, and Release Profiles in Nanocarriers’ Development. Nanotechnol. Rev. 2023, 12, 20220517. [Google Scholar] [CrossRef]
- Teaima, M.H.; Badawi, N.M.; Attia, D.A.; El-Nabarawi, M.A.; Elmazar, M.M.; Mousa, S.A. Efficacy of Pomegranate Extract Loaded Solid Lipid Nanoparticles Transdermal Emulgel against Ehrlich Ascites Carcinoma. Nanomed. Nanotechnol. Biol. Med. 2022, 39, 102466. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ali, A.; Rahamathulla, M.; Salam, S.; Hani, U.; Wahab, S.; Warsi, M.H.; Yusuf, M.; Ali, A.; Mittal, V.; et al. Fabrication of Sustained Release Curcumin-Loaded Solid Lipid Nanoparticles (Cur-SLNs) as a Potential Drug Delivery System for the Treatment of Lung Cancer: Optimization of Formulation and In Vitro Biological Evaluation. Polymers 2023, 15, 542. [Google Scholar] [CrossRef]
- Mohite, P.; Rajput, T.; Pandhare, R.; Sangale, A.; Singh, S.; Prajapati, B.G. Nanoemulsion in Management of Colorectal Cancer: Challenges and Future Prospects. Nanomanufacturing 2023, 3, 139–166. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion Delivery System of Tea Polyphenols Enhanced the Bioavailability of Catechins in Rats. Food Chem. 2018, 242, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Goyal, A.; Gürer, E.S.; Yapar, E.A.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Opazo, P.; Gotteland, M.; Oyarzun-Ampuero, F.A.; Garcia, L. Design, Development and Evaluation of Nanoemulsion Containing Avocado Peel Extract with Anticancer Potential: A Novel Biological Active Ingredient to Enrich Food. Food Hydrocoll. 2021, 111, 106370. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, H. The Recent Progress of Inorganic-Based Intelligent Responsive Nanoplatform for Tumor Theranostics. View 2022, 3, 20220009. [Google Scholar] [CrossRef]
- Amaldoss, M.J.N.; Yang, J.L.; Koshy, P.; Unnikrishnan, A.; Sorrell, C.C. Inorganic Nanoparticle-Based Advanced Cancer Therapies: Promising Combination Strategies. Drug Discov. Today 2022, 27, 103386. [Google Scholar] [CrossRef]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.-X.; Chen, Z.-S.; Hou, K.; Dong, P.; Li, J.; Wang, S.; et al. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Hsing, M.T.; Hsu, H.T.; Chang, C.H.; Chang, K.B.; Cheng, C.Y.; Lee, J.H.; Huang, C.L.; Yang, M.Y.; Yang, Y.C.; Liu, S.Y.; et al. Improved Delivery Performance of N-Butylidenephthalide-Polyethylene Glycol-Gold Nanoparticles Efficient for Enhanced Anti-Cancer Activity in Brain Tumor. Cells 2022, 11, 2172. [Google Scholar] [CrossRef] [PubMed]
- Thipe, V.C.; Amiri, K.P.; Bloebaum, P.; Karikachery, A.R.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of Resveratrol-Conjugated Gold Nanoparticles: Interrelationship of Increased Resveratrol Corona on Anti-Tumor Efficacy against Breast, Pancreatic and Prostate Cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, K.R.; Ghate, M.D.; Lalhlenmawia, H.; Kumar, D.; Singh, J. Bioinspired Quantum Dots for Cancer Therapy: A Mini-Review. Mater. Lett. 2022, 313, 131742. [Google Scholar] [CrossRef]
- Rakovich, A.; Rakovich, T. Semiconductor versus Graphene Quantum Dots as Fluorescent Probes for Cancer Diagnosis and Therapy Applications. J. Mater. Chem. B 2018, 6, 2690–2712. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene Quantum Dots for Cancer Targeted Drug Delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef]
- Murali, G.; Kwon, B.; Kang, H.; Modigunta, J.K.R.; Park, S.; Lee, S.; Lee, H.; Park, Y.H.; Kim, J.; Park, S.Y.; et al. Hematoporphyrin Photosensitizer-Linked Carbon Quantum Dots for Photodynamic Therapy of Cancer Cells. ACS Appl. Nano Mater. 2022, 5, 4376–4385. [Google Scholar] [CrossRef]
- Shivaji, K.; Mani, S.; Ponmurugan, P.; De Castro, C.S.; Lloyd Davies, M.; Balasubramanian, M.G.; Pitchaimuthu, S. Green-Synthesis-Derived CdS Quantum Dots Using Tea Leaf Extract: Antimicrobial, Bioimaging, and Therapeutic Applications in Lung Cancer Cells. ACS Appl. Nano Mater. 2018, 1, 1683–1693. [Google Scholar] [CrossRef]
- Shivaji, K.; Balasubramanian, M.G.; Devadoss, A.; Asokan, V.; De Castro, C.S.; Davies, M.L.; Ponmurugan, P.; Pitchaimuthu, S. Utilization of Waste Tea Leaves as Bio-Surfactant in CdS Quantum Dots Synthesis and Their Cytotoxicity Effect in Breast Cancer Cells. Appl. Surf. Sci. 2019, 487, 159–170. [Google Scholar] [CrossRef]
- Seyyedi Zadeh, E.; Ghanbari, N.; Salehi, Z.; Derakhti, S.; Amoabediny, G.; Akbari, M.; Asadi Tokmedash, M. Smart PH-Responsive Magnetic Graphene Quantum Dots Nanocarriers for Anticancer Drug Delivery of Curcumin. Mater. Chem. Phys. 2023, 297, 127336. [Google Scholar] [CrossRef]
- Li, T.; Shi, S.; Goel, S.; Shen, X.; Xie, X.; Chen, Z.; Zhang, H.; Li, S.; Qin, X.; Yang, H.; et al. Recent Advancements in Mesoporous Silica Nanoparticles towards Therapeutic Applications for Cancer. Acta Biomater. 2019, 89, 1–13. [Google Scholar] [CrossRef]
- Rani, R.; Malik, P.; Dhania, S.; Mukherjee, T.K. Recent Advances in Mesoporous Silica Nanoparticle-Mediated Drug Delivery for Breast Cancer Treatment. Pharmaceutics 2023, 15, 227. [Google Scholar] [CrossRef]
- Li, D.; Song, C.; Zhang, J.; Zhao, X. Targeted Delivery and Apoptosis Induction Activity of Peptide-Transferrin Targeted Mesoporous Silica Encapsulated Resveratrol in MCF-7 Cells. J. Pharm. Pharmacol. 2023, 75, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ionita, S.; Lincu, D.; Mitran, R.A.; Ziko, L.; Sedky, N.K.; Deaconu, M.; Brezoiu, A.M.; Matei, C.; Berger, D. Resveratrol Encapsulation and Release from Pristine and Functionalized Mesoporous Silica Carriers. Pharmaceutics 2022, 14, 203. [Google Scholar] [CrossRef]
- Mohebian, Z.; Babazadeh, M.; Zarghami, N. In Vitro Efficacy of Curcumin-Loaded Amine-Functionalized Mesoporous Silica Nanoparticles against MCF-7 Breast Cancer Cells. Adv. Pharm. Bull. 2023, 13, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Carbon-Based Nanomaterials for Targeted Cancer Nanotherapy: Recent Trends and Future Prospects. J. Drug Target. 2021, 29, 716–741. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Baby, A.; Raman, V.; Balakrishnan, S.P. Carbon-Based Nanomaterials for Cancer Treatment and Diagnosis: A Review. ChemistrySelect 2022, 7, e202202455. [Google Scholar] [CrossRef]
- Jiang, B.P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. A Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef]
- Chary, P.S.; Bhawale, R.; Vasave, R.; Rajana, N.; Singh, P.K.; Mehra, N.K. A Review on Emerging Role of Multifunctional Carbon Nanotubes as an Armament in Cancer Therapy, Imaging and Biosensing. J. Drug Deliv. Sci. Technol. 2023, 85, 104588. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Pan, T.; Yin, Y.; Mei, Y.; Xiao, Q.; Wang, R.; Yan, Z.; Wang, W. Versatile Carbon Nanoplatforms for Cancer Treatment and Diagnosis: Strategies, Applications and Future Perspectives. Theranostics 2022, 12, 2290–2321. [Google Scholar] [CrossRef]
- Thakur, C.K.; Neupane, R.; Karthikeyan, C.; Ashby, C.R.; Babu, R.J.; Boddu, S.H.S.; Tiwari, A.K.; Moorthy, N.S.H.N. Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells. Molecules 2022, 27, 7461. [Google Scholar] [CrossRef]
- Farokh, A.; Pourmadadi, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M. Assessment of Synthesized Chitosan/Halloysite Nanocarrier Modified by Carbon Nanotube for PH-Sensitive Delivery of Curcumin to Cancerous Media. Int. J. Biol. Macromol. 2023, 237, 123937. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wu, J.; Lin, J.; Liu, W.; Chen, P.; Yu, M.; Zhou, D.; Yao, G. Graphene-Based Nanomaterials for Breast Cancer Treatment: Promising Therapeutic Strategies. J. Nanobiotechnol. 2021, 19, 211. [Google Scholar] [CrossRef]
- Patel, S.C.; Lee, S.; Lalwani, G.; Suhrland, C.; Chowdhury, S.M.; Sitharaman, B. Graphene-Based Platforms for Cancer Therapeutics. Ther. Deliv. 2016, 7, 101. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Costa, L.D.F.D.O.; Miranda, G.D.S.; Nardecchia, S.; Monteiro, M.S.D.S.D.B.; Ricci-Júnior, E.; Delpech, M.C. Waterborne Poly(Urethane-Urea)s Nanocomposites Reinforced with Clay, Reduced Graphene Oxide and Respective Hybrids: Synthesis, Stability and Structural Characterization. J. Polym. Environ. 2020, 28, 74–90. [Google Scholar] [CrossRef]
- Al-Ani, L.A.; Kadir, F.A.; Hashim, N.M.; Julkapli, N.M.; Seyfoddin, A.; Lu, J.; AlSaadi, M.A.; Yehye, W.A. The Impact of Curcumin-Graphene Based Nanoformulation on Cellular Interaction and Redox-Activated Apoptosis: An In Vitro Colon Cancer Study. Heliyon 2020, 6, e05360. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.L.; Lima-Sousa, R.; Alves, C.G.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Chitosan-Based Injectable in Situ Forming Hydrogels Containing Dopamine-Reduced Graphene Oxide and Resveratrol for Breast Cancer Chemo-Photothermal Therapy. Biochem. Eng. J. 2022, 185, 108529. [Google Scholar] [CrossRef]
- Moasses Ghafary, S.; Rahimjazi, E.; Hamzehil, H.; Modarres Mousavi, S.M.; Nikkhah, M.; Hosseinkhani, S. Design and Preparation of a Theranostic Peptideticle for Targeted Cancer Therapy: Peptide-Based Codelivery of Doxorubicin/Curcumin and Graphene Quantum Dots. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102544. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Emamy, H. Nontoxic Concentrations of PEGylated Graphene Nanoribbons for Selective Cancer Cell Imaging and Photothermal Therapy. J. Mater. Chem. 2012, 22, 20626–20633. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Chen, Y.; Fang, Z. In-Vitro Photothermal Therapy Using Plant Extract Polyphenols Functionalized Graphene Sheets for Treatment of Lung Cancer. J. Photochem. Photobiol. B Biol. 2020, 204, 111587. [Google Scholar] [CrossRef]
- Saqezi, A.S.; Kermanian, M.; Ramazani, A.; Sadighian, S. Synthesis of Graphene Oxide/Iron Oxide/Au Nanocomposite for Quercetin Delivery. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1541–1550. [Google Scholar] [CrossRef]
- Sheik, A.; Huh, Y.S. Nano-Formulation for Curcumin and Resveratrol in Colorectal Cancer Therapy. Onco Ther. 2022, 9, 83–91. [Google Scholar] [CrossRef]
- Ghobadi, N.; Asoodeh, A. Co-Administration of Curcumin with Other Phytochemicals Improves Anticancer Activity by Regulating Multiple Molecular Targets. Phyther. Res. 2023, 37, 1688–1702. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zichri, S.; Meltzer, M.; Lacham-Hartman, S.; Kolusheva, S.; Hadad, U.; Papo, N.; Jelinek, R. Synergistic Activity of Anticancer Polyphenols Embedded in Amphiphilic Dendrimer Nanoparticles. ACS Appl. Polym. Mater. 2022, 4, 8913–8925. [Google Scholar] [CrossRef]
- Palliyage, G.H.; Hussein, N.; Mimlitz, M.; Weeder, C.; Alnasser, M.H.A.; Singh, S.; Ekpenyong, A.; Tiwari, A.K.; Chauhan, H. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm. Res. 2021, 38, 851–871. [Google Scholar] [CrossRef]
- Rasouli, S.; Montazeri, M.; Mashayekhi, S.; Sadeghi-Soureh, S.; Dadashpour, M.; Mousazadeh, H.; Nobakht, A.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Synergistic Anticancer Effects of Electrospun Nanofiber-Mediated Codelivery of Curcumin and Chrysin: Possible Application in Prevention of Breast Cancer Local Recurrence. J. Drug Deliv. Sci. Technol. 2020, 55, 101402. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Kucinska, M.; Tomczak, S.; Mlynarczyk, D.T.; Piskorz, J.; Goslinski, T.; Murias, M.; Jelinska, A. Liposomal Nanoformulation as a Carrier for Curcumin and PEGCG—Study on Stability and Anticancer Potential. Nanomaterials 2022, 12, 1274. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, X.; Tian, X.; Zhang, P.; Chen, Z.; Hu, X.; Mei, X. GSH and Enzyme Responsive Nanospheres Based on Self-Assembly of Green Tea Polyphenols and BSA Used for Target Cancer Chemotherapy. Colloids Surf. B Biointerfaces 2019, 173, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.P.; Ratnacaram, C.K.; Kumar, L.; Jose, J. Combinatorial Approaches of Nanotherapeutics for Inflammatory Pathway Targeted Therapy of Prostate Cancer. Drug Resist. Updat. 2022, 64, 100865. [Google Scholar] [CrossRef] [PubMed]
- Fatemizadeh, M.; Tafvizi, F.; Shamsi, F.; Amiri, S. Apoptosis Induction, Cell Cycle Arrest and Anti-Cancer Potential of Tamoxifen-Curcumin Loaded Niosomes Against MCF-7 Cancer Cells. Iran. J. Pathol. 2022, 17, 183–190. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, T.; Gao, C.; Abdulhadi El-Ali, H.A.; Gao, N.; Yang, C.; Zhang, R.; Jing, J.; Zhang, X. A Multi-Crosslinking Nanocapsule-Based Serial-Stimuli-Responsive Leakage-Free Drug-Delivery System In Vitro. Chem. A Eur. J. 2019, 25, 13017–13024. [Google Scholar] [CrossRef]
- Firouzi Amandi, A.; Jokar, E.; Eslami, M.; Dadashpour, M.; Rezaie, M.; Yazdani, Y.; Nejati, B. Enhanced Anti-Cancer Effect of Artemisinin- and Curcumin-Loaded Niosomal Nanoparticles against Human Colon Cancer Cells. Med. Oncol. 2023, 40, 170. [Google Scholar] [CrossRef]
- Mogheri, F.; Jokar, E.; Afshin, R.; Akbari, A.A.; Dadashpour, M.; Firouzi-amandi, A.; Serati-Nouri, H.; Zarghami, N. Co-Delivery of Metformin and Silibinin in Dual-Drug Loaded Nanoparticles Synergistically Improves Chemotherapy in Human Non-Small Cell Lung Cancer A549 Cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102752. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Cui, H.; Zhang, F.; Zhao, L.; Liu, Y.; Meng, Q. Combined and Targeted Drugs Delivery System for Colorectal Cancer Treatment: Conatumumab Decorated, Reactive Oxygen Species Sensitive Irinotecan Prodrug and Quercetin Co-Loaded Nanostructured Lipid Carriers. Drug Deliv. 2022, 29, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, J.; Chen, J.; Yang, M.; Wang, H.; Qiao, H.; Chen, Z.; Hu, L.; Di, L.; Li, J. Enhanced Anti-Colon Cancer Efficacy of 5-Fluorouracil by Epigallocatechin-3- Gallate Co-Loaded in Wheat Germ Agglutinin-Conjugated Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; He, K.; Qiu, T.; Sun, J.; Liu, Q.; Zhang, X.; Zheng, H. Tumor-Targeted Delivery of Silibinin and IPI-549 Synergistically Inhibit Breast Cancer by Remodeling the Microenvironment. Int. J. Pharm. 2020, 581, 119239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, C.; Liu, M.; Zhao, Y.; Wu, Y.; Fan, Z.; Ding, Z.; Zhang, H.; Wang, Z.; Han, J. Drug-Binding Albumins Forming Stabilized Nanoparticles for Co-Delivery of Paclitaxel and Resveratrol: In Vitro/In Vivo Evaluation and Binding Properties Investigation. Int. J. Biol. Macromol. 2020, 153, 873–882. [Google Scholar] [CrossRef]
- Chen, S.; Fan, J.-X.; Zheng, D.-W.; Liu, F.; Zeng, X.; Yan, G.-P.; Zhang, X.-Z. A Multi-Functional Drug Delivery System Based on Polyphenols for Efficient Tumor Inhibition and Metastasis Prevention. Biomater. Sci. 2020, 8, 702–711. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Yao, P.; Dai, Q.; Xia, P.; Zhang, D.; et al. Folic Acid-Modified ROS-Responsive Nanoparticles Encapsulating Luteolin for Targeted Breast Cancer Treatment. Drug Deliv. 2021, 28, 1695–1708. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Yao, B.; Lu, X.; Zhang, X.; He, P.; Vasilatos, S.N.; Ren, X.; Bian, W.; Yao, C. Transferrin Receptor-Targeted Redox/PH-Sensitive Podophyllotoxin Prodrug Micelles for Multidrug-Resistant Breast Cancer Therapy. J. Mater. Chem. B 2019, 7, 5814–5824. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.A.; Almatar, H.M.A.; Al-Ramadan, A.S.; Buheazah, T.M.; AlHomoud, H.S.; Al-Nasif, H.A.; Alam, M.A. A Chitosan-PLGA Based Catechin Hydrate Nanoparticles Used in Targeting of Lungs and Cancer Treatment. Saudi J. Biol. Sci. 2020, 27, 2344–2357. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, H.Y.; Zhao, H.; Wang, T. RGD-Modified Nanoliposomes Containing Quercetin for Lung Cancer Targeted Treatment. Onco Targets Ther. 2018, 11, 5397–5405. [Google Scholar] [CrossRef]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Al-Debsi, T.; Al-Shalabi, E.; Hasan Ibrahim, L.; Faruqu, F.N.; Walters, A.; Palgrave, R.; Al-Jamal, K.T. Bioinspired Polymerization of Quercetin to Produce a Curcumin-Loaded Nanomedicine with Potent Cytotoxicity and Cancer-Targeting Potential in Vivo. ACS Biomater. Sci. Eng. 2019, 5, 6036–6045. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Liu, X.Y.; Cai, J.X.; Hu, X.B.; Wang, J.M.; Tang, T.T.; Xiang, D.X. 3,5,4′-Trimethoxy-Trans-Stilbene Loaded PEG-PE Micelles for the Treatment of Colon Cancer. Int. J. Nanomed. 2019, 14, 7489–7502. [Google Scholar] [CrossRef]
- Sudha, T.; El-Far, A.H.; Mousa, D.S.; Mousa, S.A. Resveratrol and Its Nanoformulation Attenuate Growth and the Angiogenesis of Xenograft and Orthotopic Colon Cancer Models. Molecules 2020, 25, 1412. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and Optimization of Poly (Lactic Acid) Nanoparticles Loaded with Fisetin to Improve Anti-Cancer Therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Kazi, J.; Sen, R.; Ganguly, S.; Jha, T.; Ganguly, S.; Chatterjee Debnath, M. Folate Decorated Epigallocatechin-3-Gallate (EGCG) Loaded PLGA Nanoparticles; in-Vitro and in-Vivo Targeting Efficacy against MDA-MB-231 Tumor Xenograft. Int. J. Pharm. 2020, 585, 119449. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, K.P.; Beak, S.; Park, J.S.; Kim, Y.J.; Kim, K.-N.; Kim, S.-R.; Yoon, M.S. Antibreast Cancer Activity of Aspirin-Conjugated Chalcone Polymeric Micelles. Macromol. Res. 2021, 29, 105–110. [Google Scholar] [CrossRef]
- Yilmaz, M.; Karanastasis, A.A.; Chatziathanasiadou, M.V.; Oguz, M.; Kougioumtzi, A.; Clemente, N.; Kellici, T.F.; Zafeiropoulos, N.E.; Avgeropoulos, A.; Mavromoustakos, T.; et al. Inclusion of Quercetin in Gold Nanoparticles Decorated with Supramolecular Hosts Amplifies Its Tumor Targeting Properties. ACS Appl. Bio Mater. 2019, 2, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Upadhyay, P.K.; Dewangan, H.K. Development, Evaluation, Pharmacokinetic and Biodistribution Estimation of Resveratrol-Loaded Solid Lipid Nanoparticles for Prostate Cancer Targeting. J. Microencapsul. 2022, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Lai, C.J.; Lin, Y.N.; Huang, C.M.; Lin, Y.H. Multifunctional Nanoparticles for Targeting the Tumor Microenvironment to Improve Synergistic Drug Combinations and Cancer Treatment Effects. J. Mater. Chem. B 2020, 8, 10416–10427. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, W.; Tao, W.; Zhou, Y.; Xue, B. Delivery of Curcumin Nanoliposomes Using Surface Modified with CD133 Aptamers for Prostate Cancer. Drug Des. Devel. Ther. 2019, 13, 4021–4033. [Google Scholar] [CrossRef]
- Shitole, A.A.; Sharma, N.; Giram, P.; Khandwekar, A.; Baruah, M.; Garnaik, B.; Koratkar, S. LHRH-Conjugated, PEGylated, Poly-Lactide-Co-Glycolide Nanocapsules for Targeted Delivery of Combinational Chemotherapeutic Drugs Docetaxel and Quercetin for Prostate Cancer. Mater. Sci. Eng. C 2020, 114, 111035. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Z.M.; Chen, Y.; Gao, D.F.; Wang, P.; Lin, Y.X.; Wang, Y.M.; Wang, F.; Han, Y.; Yuan, H.Q. Co-Delivery of Docetaxel and Resveratrol by Liposomes Synergistically Boosts Antitumor Efficiency against Prostate Cancer. Eur. J. Pharm. Sci. 2022, 174, 106199. [Google Scholar] [CrossRef]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Kumari, S.; Kashyap, V.K.; Tripathi, M.K.; Wagh, S.; Meibohm, B.; Chauhan, S.C.; Jaggi, M.; et al. Cross-Linked Polyphenol-Based Drug Nano-Self-Assemblies Engineered to Blockade Prostate Cancer Senescence. ACS Appl. Mater. Interfaces 2019, 11, 38537–38554. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deng, Y.; Zhu, C.; Xiang, C. Anti Prostate Cancer Therapy: Aptamer-Functionalized, Curcumin and Cabazitaxel Co-Delivered, Tumor Targeted Lipid-Polymer Hybrid Nanoparticles. Biomed. Pharmacother. 2020, 127, 110181. [Google Scholar] [CrossRef] [PubMed]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In Vitro and In Vivo Anticancer Efficacy Potential of Quercetin Loaded Polymeric Nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted Delivery of Quercetin by Nanoparticles Based on Chitosan Sensitizing Paclitaxel-Resistant Lung Cancer Cells to Paclitaxel. Mater. Sci. Eng. C 2021, 119, 111442. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, H.; Zhang, Y.; Cai, H.; Zhang, P.; Li, L.; Zhou, J.; Yin, T. Co-Delivery of Silybin and Paclitaxel by Dextran-Based Nanoparticles for Effective Anti-Tumor Treatment through Chemotherapy Sensitization and Microenvironment Modulation. J. Control. Release 2020, 321, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial Growth Factor Receptor-Targeted and Reactive Oxygen Species-Responsive Lung Cancer Therapy by Docetaxel and Resveratrol Encapsulated Lipid-Polymer Hybrid Nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef]
- Riaz, M.K.; Zhang, X.; Wong, K.H.; Chen, H.; Liu, Q.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Pulmonary Delivery of Transferrin Receptors Targeting Peptide Surface-Functionalized Liposomes Augments the Chemotherapeutic Effect of Quercetin in Lung Cancer Therapy. Int. J. Nanomed. 2019, 14, 2879. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic Acid (FA)-Conjugated Mesoporous Silica Nanoparticles Combined with MRP-1 SiRNA Improves the Suppressive Effects of Myricetin on Non-Small Cell Lung Cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Gastroenterol. Rev. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, N.; Georgieva, M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics 2022, 14, 1213. [Google Scholar] [CrossRef]
- Chaurasia, S.; Patel, R.R.; Vure, P.; Mishra, B. Potential of Cationic-Polymeric Nanoparticles for Oral Delivery of Naringenin: In Vitro and In Vivo Investigations. J. Pharm. Sci. 2018, 107, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, Y.; Hu, N.; Yu, Q.; Zhang, X.; Li, J.; Wu, F.; Xu, H.; Tang, Q.; Li, X. Ferroptosis-Induced Anticancer Effect of Resveratrol with a Biomimetic Nano-Delivery System in Colorectal Cancer Treatment. Asian J. Pharm. Sci. 2022, 17, 751–766. [Google Scholar] [CrossRef]
- Sen, K.; Banerjee, S.; Mandal, M. Dual Drug Loaded Liposome Bearing Apigenin and 5-Fluorouracil for Synergistic Therapeutic Efficacy in Colorectal Cancer. Colloids Surf. B Biointerfaces 2019, 180, 9–22. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Zhang, B.; Xu, Z.P. MnO2-Shelled Doxorubicin/Curcumin Nanoformulation for Enhanced Colorectal Cancer Chemo-Immunotherapy. J. Colloid Interface Sci. 2022, 617, 315–325. [Google Scholar] [CrossRef]
- Mishra, S.; Manna, K.; Kayal, U.; Saha, M.; Chatterjee, S.; Chandra, D.; Hara, M.; Datta, S.; Bhaumik, A.; Das Saha, K. Folic Acid-Conjugated Magnetic Mesoporous Silica Nanoparticles Loaded with Quercetin: A Theranostic Approach for Cancer Management. RSC Adv. 2020, 10, 23148–23164. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, S.; Guan, X.; Xie, Z. One-Step Synthesis of Nanoscale Zeolitic Imidazolate Frameworks with High Curcumin Loading for Treatment of Cervical Cancer. ACS Appl. Mater. Interfaces 2015, 7, 22181–22187. [Google Scholar] [CrossRef]
- Chen, X.; Tong, R.; Liu, B.; Liu, H.; Feng, X.; Ding, S.; Lei, Q.; Tang, G.; Wu, J.; Fang, W. Duo of (-)-Epigallocatechin-3-Gallate and Doxorubicin Loaded by Polydopamine Coating ZIF-8 in the Regulation of Autophagy for Chemo-Photothermal Synergistic Therapy. Biomater. Sci. 2020, 8, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Zeng, X.; Wu, Y.; Yang, C.; Mei, L.; Wang, Z.; Huang, L. Fabrication of Genistein-Loaded Biodegradable TPGS-b-PCL Nanoparticles for Improved Therapeutic Effects in Cervical Cancer Cells. Int. J. Nanomed. 2015, 10, 2461–2473. [Google Scholar] [CrossRef]
- Zaman, M.S.; Chauhan, N.; Yallapu, M.M.; Gara, R.K.; Maher, D.M.; Kumari, S.; Sikander, M.; Khan, S.; Zafar, N.; Jaggi, M.; et al. Curcumin Nanoformulation for Cervical Cancer Treatment. Sci. Rep. 2016, 6, 20051. [Google Scholar] [CrossRef] [PubMed]
- Thulasidasan, A.K.T.; Retnakumari, A.P.; Shankar, M.; Vijayakurup, V.; Anwar, S.; Thankachan, S.; Pillai, K.S.; Pillai, J.J.; Nandan, C.D.; Alex, V.V.; et al. Folic Acid Conjugation Improves the Bioavailability and Chemosensitizing Efficacy of Curcumin-Encapsulated PLGA-PEG Nanoparticles towards Paclitaxel Chemotherapy. Oncotarget 2017, 8, 107374–107389. [Google Scholar] [CrossRef]
- Luo, C.-L.; Liu, Y.-Q.; Wang, P.; Song, C.-H.; Wang, K.-J.; Dai, L.-P.; Zhang, J.-Y.; Ye, H. The Effect of Quercetin Nanoparticle on Cervical Cancer Progression by Inducing Apoptosis, Autophagy and Anti-Proliferation via JAK2 Suppression. Biomed. Pharmacother. 2016, 82, 595–605. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Ma, B.; Niu, R.; Zhang, H.; Kun, L. Antitumor Activity and Safety Evaluation of Nanaparticle-Based Delivery of Quercetin through Intravenous Administration in Mice. Mater. Sci. Eng. C 2017, 77, 803–810. [Google Scholar] [CrossRef]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a Phase I Pilot Clinical Trial Examining the Effect of Plant-Derived Resveratrol and Grape Powder on Wnt Pathway Target Gene Expression in Colonic Mucosa and Colon Cancer. Cancer Manag. Res. 2009, 1, 25. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M.; et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic Efficacy of Curcumin and Resveratrol against Cancer: Chemoprevention, Chemoprotection, Drug Synergism and Clinical Pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef] [PubMed]
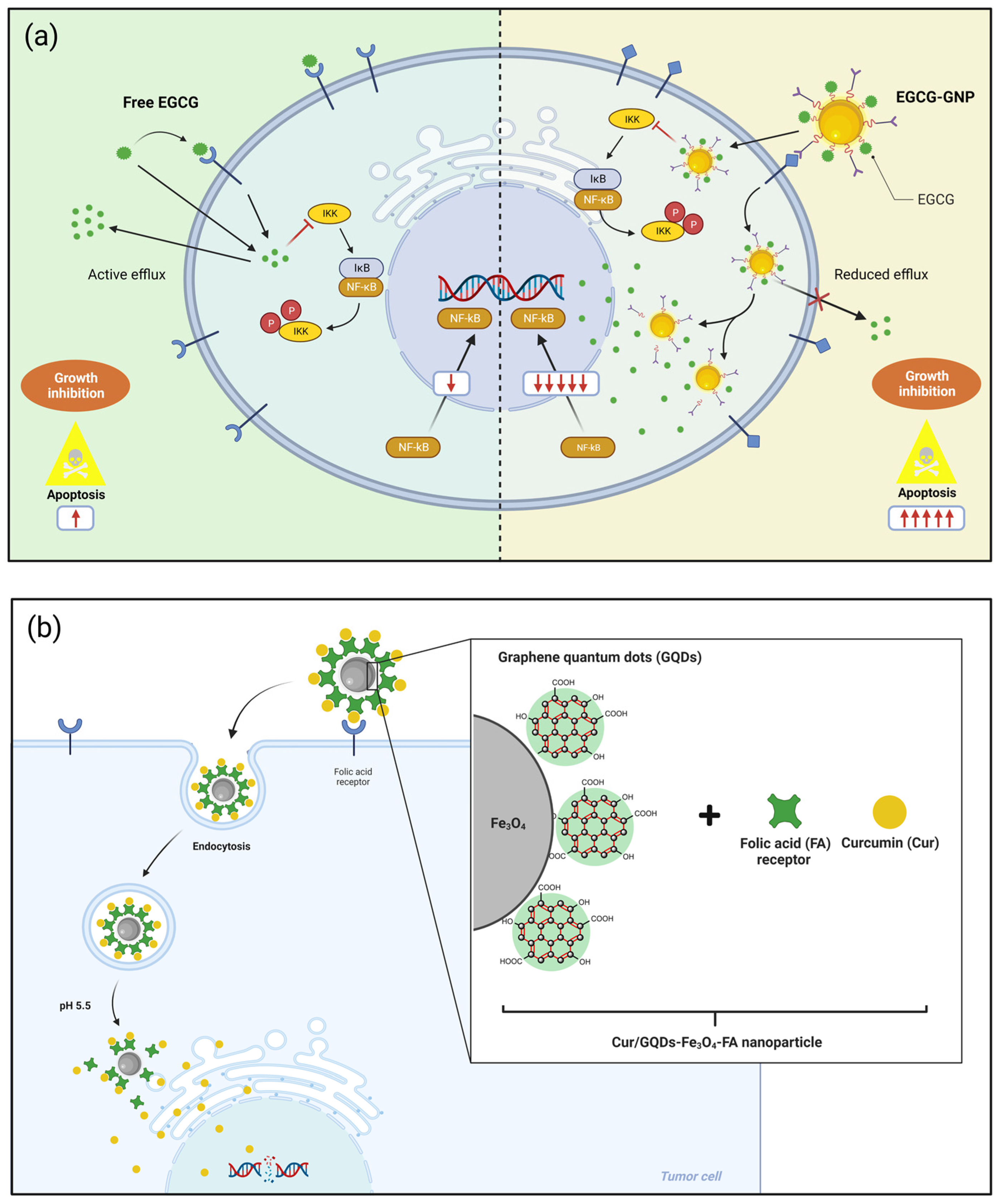
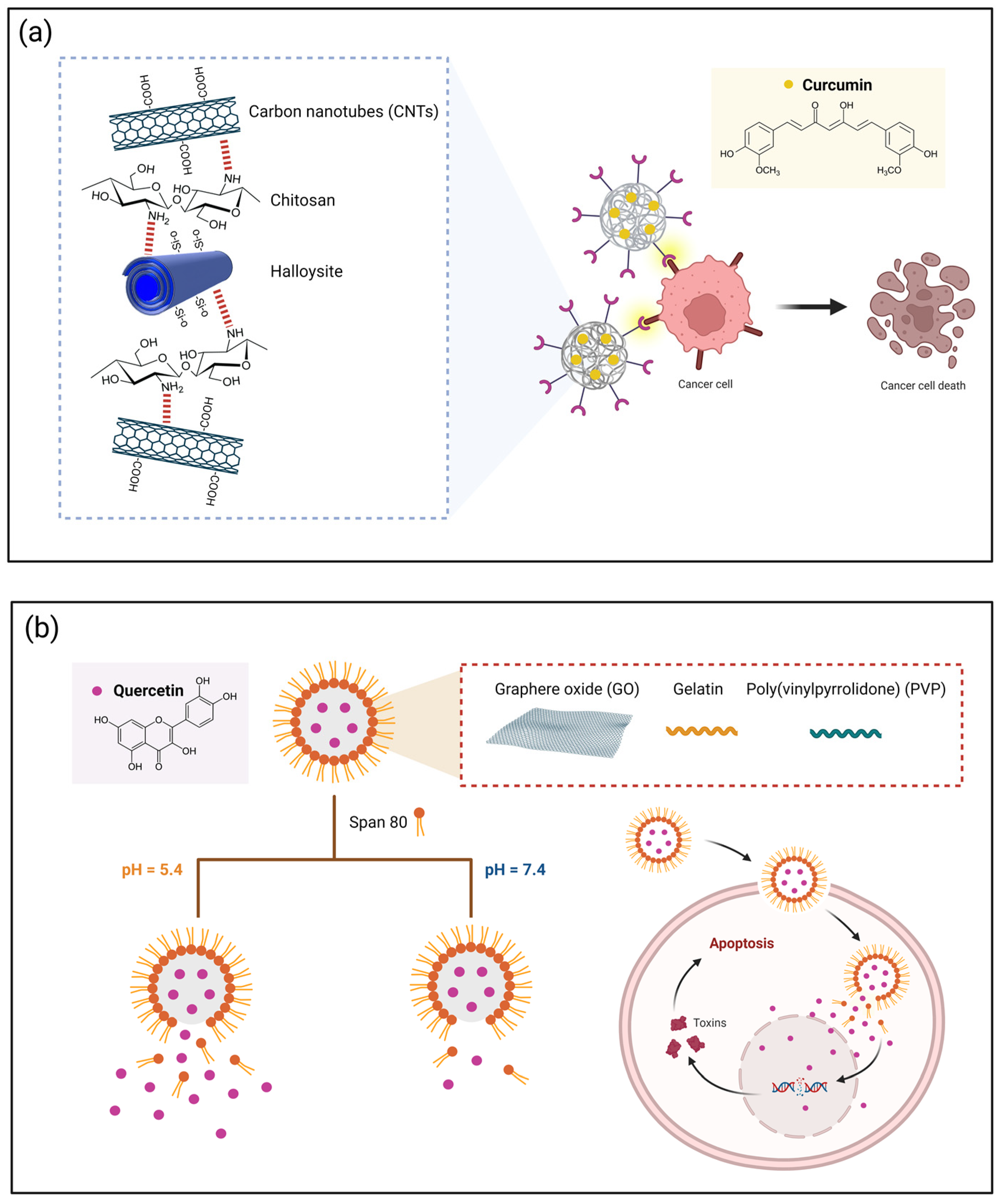


| Polyphenol | Main Class/ Sub-Class | Food Source | Type of Cancer | Therapeutic Effects on Cancer Cells | References |
|---|---|---|---|---|---|
| Quercetin | Flavonoids/Flavonols | Apples, raspberry, blackcurrant, blueberry, orange, cherry, grapes, raspberry, cranberry, strawberry, and green vegetables | Breast | - A significant difference in tumor sizes compared to the control group. ↑ The survival rate of tumor-carrying mice. | [15] |
| Prostate | ↑ Inhibition of cell growth inducing apoptosis. | [15] | |||
| Lung | - Inhibited the growth of A-549 cancer cells. ↑ Apoptosis of A-549 cells. | [35] | |||
| Cervical | ↓ Cell viability of cancer cells. - Paralyzed the cell cycle in the G2/M phase and cellular apoptosis. - Inhibited cell migration and invasion. | [36] | |||
| Curcumin | Non-flavonoid/Phenolic acids | Saffron | Breast | ↑ Inhibit the telomerase gene expression in T47D cell line. | [37] |
| Cervical | ↑ Inhibition of cell proliferation in Hella. | [16] | |||
| Prostate | ↑ Uptake of cancer cells DU145. ↑ Therapeutic effect. ↓ Cell viability. | [38] | |||
| Colorectal | - Suppressed tumorigenesis in AOM-DSS in mice. ↓ Decreased both the tumor number and tumor size compared with the AOM-DSS treatment group. ↓ Expression of IL-1β, IL-6, Cox-2, and β-catenin. | [39] | |||
| EGCG | Flavonoids/Flavonols | Green tea | Breast | ↓ Cellular viability of MCF-7 and MDA-MB-231 strains. ↓ Expression and DNA methyltransferase (DNMT) activity. | [40] |
| Prostate | ↓ Cell proliferation of DU145. - Induction of apoptosis. | [41] | |||
| Lung | ↑ Inhibition of cell proliferation of H1299. - No formation of H1299 colonies. | [42] | |||
| Resveratrol | Non-flavonoid/Stilbenes | Grapes, berries, and peanuts | Lung | ↓ Cellular viability. ↑ Senescent and apoptotic cells. - Inhibited cell proliferation of A549 and H1299. | [17] |
| Cervical | - Inhibited the proliferation and migration of HeLa cells. ↓ Expression of MAPK3. | [31] |
| Type of Cancer | Polyphenol | Nanocarrier/ Nanoformulation | Animal Model and Dose/Treatment | In Vivo Effects | Publication Year | References |
|---|---|---|---|---|---|---|
| Breast | EGCG | Self-assembled nanoparticles containing EGCG, Fe2+ ions, and DOX | Tumors induced by 4T1 cells in female BALB/c mice using subcutaneous route. | - Remarkable performance in diagnosing tumors using magnetic resonance imaging. - Inhibition of tumor cell metastasis. | 2020 | [168] |
| Lutein | Self-assembled nanoparticles conjugated with DSPE-PEG and folic acid | 4T1 cells inoculated into the right breast or tail vein of female BALB/c mice, and administration of luteolin-loaded nanoparticles (10 mg/kg) intravenously. | - Accumulation at tumor sites. - Efficient inhibition of tumor growth regarding free polyphenol. ↓ Systemic toxicity. - Cell death by apoptosis. | 2021 | [169] | |
| Podophyllotoxin | PEG polymer micelles modified with T7 peptide and mPEG | MCF-7 cells inoculated subcutaneously into the hind flank of female mice, and intravenous administration of polyphenol-loaded micelles (80 mg/kg). | ↑ Maximum tolerated dose of the polyphenolic compound. ↓ Weight loss of animals. - Inhibition of tumor growth. | 2019 | [170] | |
| Resveratrol | Folic-acid-linked polymer nanogels | Ehrlich ascites tumor (EAT) cells injected into the mammary gland of female BALB/c mice, and intravenous administration of resveratrol-loaded nanoparticles (2 mg/kg). | - Suppression of tumor growth. ↓ VEGF-1 and Ki-67 expression levels. - Upregulation of caspase-3 (apoptosis induction). - Necrosis in tumor tissues. | 2023 | [69] | |
| Lung | Catechin | Chitosan-PLGA-based polymer nanoparticles | Nanoparticles administered by different routes (intravenous, oral, intranasal) in Wistar rats. | ↑ Bioavailability. - No apparent tissue toxicity regardless of route of administration. | 2020 | [171] |
| EGCG | PLGA nanoparticles | Human lung tumor xenograft implanted in the flank of male NOD/SCID mice and BALB/c mice, and administration intraperitoneally of EGCG-loaded nanoparticles (5 mg/kg). | - No changes in body weight of animals. ↓ Tumor volume and weight. ↓ Expression of Ki-67 protein and negative regulation of phospho-NF-κB. ↑ Cell death by apoptosis. | 2020 | [66] | |
| Quercetin | Liposomes modified with RGD peptide | A549 cells injected into the right flank of C57BL/6 mice, and intravenous administration of polyphenol-loaded nanoparticles (5 and 10 mg/kg). | Tumor targeting ability. Considerable half-life and average rate of residence in plasma. ↓ Tumor volume. ↓ Organ toxicity of animals. | 2018 | [172] | |
| Lung | Resveratrol | Casein nanoparticles | Resveratrol-loaded nanoparticles (15 mg/kg) injected intravenously in Wistar rats. | ↑ Availability compared to free polyphenol after oral administration. ↑ Average stay rate and half-life. | 2018 | [173] |
| Colorectal | EGCG | Gelatin/chitosan nanoparticles | Mice-bearing orthotopic colon cancer was treated with EGCG-loaded nanoparticles (15 mg/kg) using oral injection. | ↑ Half-life improving pharmacokinetics. ↓ Tumor volume. ↑ Tumor inhibition rate. - The appearance of necrosis and apoptosis regions in treated tissue and no damage to non-target organs. | 2019 | [165] |
| Quercetin | PEG-functionalized quercetin nanoparticles | CT26 cells inoculated subcutaneously into the right flank of female BALB/c mice, and polyphenol-loaded nanoparticles (6 mg/kg) inoculated into the caudal vein. | ↑ Accumulation at tumor site compared to other organs after 24 h. | 2019 | [174] | |
| Resveratrol | PEG-PE polymer micelles | CT26 cells were inoculated subcutaneously in the armpit of female BALB/c mice, and the resveratrol nanoformulation (5 mg/kg) was injected into the animals’ caudal vein. | Longer plasma residence time compared to free polyphenol. ↓ Tumor growth. ↓ Systemic toxicity reflected little change in body weight and lower tumor weight. ↑ Survival over time. | 2019 | [175] | |
| Resveratrol | PLGA-PEG nanoparticles coated with chitosan | COLO205-luc cells injected subcutaneously in the axilla of female mice induced a colorectal tumor. An orthotopic model of cancer was treated using resveratrol-loaded nanoparticles (2 mg/kg) introduced into the animals’ cecum. | ↓ Tumor growth and angiogenesis. ↑ Bioavailability than polyphenol in free form. | 2020 | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, I.R.S.; Tessaro, L.; Lima, A.K.O.; Velloso, I.P.S.; Conte-Junior, C.A. Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients 2023, 15, 3136. https://doi.org/10.3390/nu15143136
Vieira IRS, Tessaro L, Lima AKO, Velloso IPS, Conte-Junior CA. Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients. 2023; 15(14):3136. https://doi.org/10.3390/nu15143136
Chicago/Turabian StyleVieira, Italo Rennan Sousa, Leticia Tessaro, Alan Kelbis Oliveira Lima, Isabela Portella Silva Velloso, and Carlos Adam Conte-Junior. 2023. "Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer" Nutrients 15, no. 14: 3136. https://doi.org/10.3390/nu15143136
APA StyleVieira, I. R. S., Tessaro, L., Lima, A. K. O., Velloso, I. P. S., & Conte-Junior, C. A. (2023). Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients, 15(14), 3136. https://doi.org/10.3390/nu15143136