Enhancing the Antioxidant, Antibacterial, and Wound Healing Effects of Melaleuca alternifolia Oil by Microencapsulating It in Chitosan-Sodium Alginate Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TTO-Loaded CS NEMs
2.3. Preparation of SA-CS-TTO Microsphere
2.4. Characterization of Nanoemulsion and Microsphere
2.5. Water Adsorption and In Vitro Degradation Properties
2.6. TTO Loading, Encapsulation, and In Vitro Release
2.7. Biological Application of SA-CS-TTO Microsphere
2.7.1. Antibacterial Assay
2.7.2. Antioxidant Assays
2.7.3. Cell Viability Assay
2.7.4. Wound Healing Assay
2.8. Statistical Analysis
3. Results
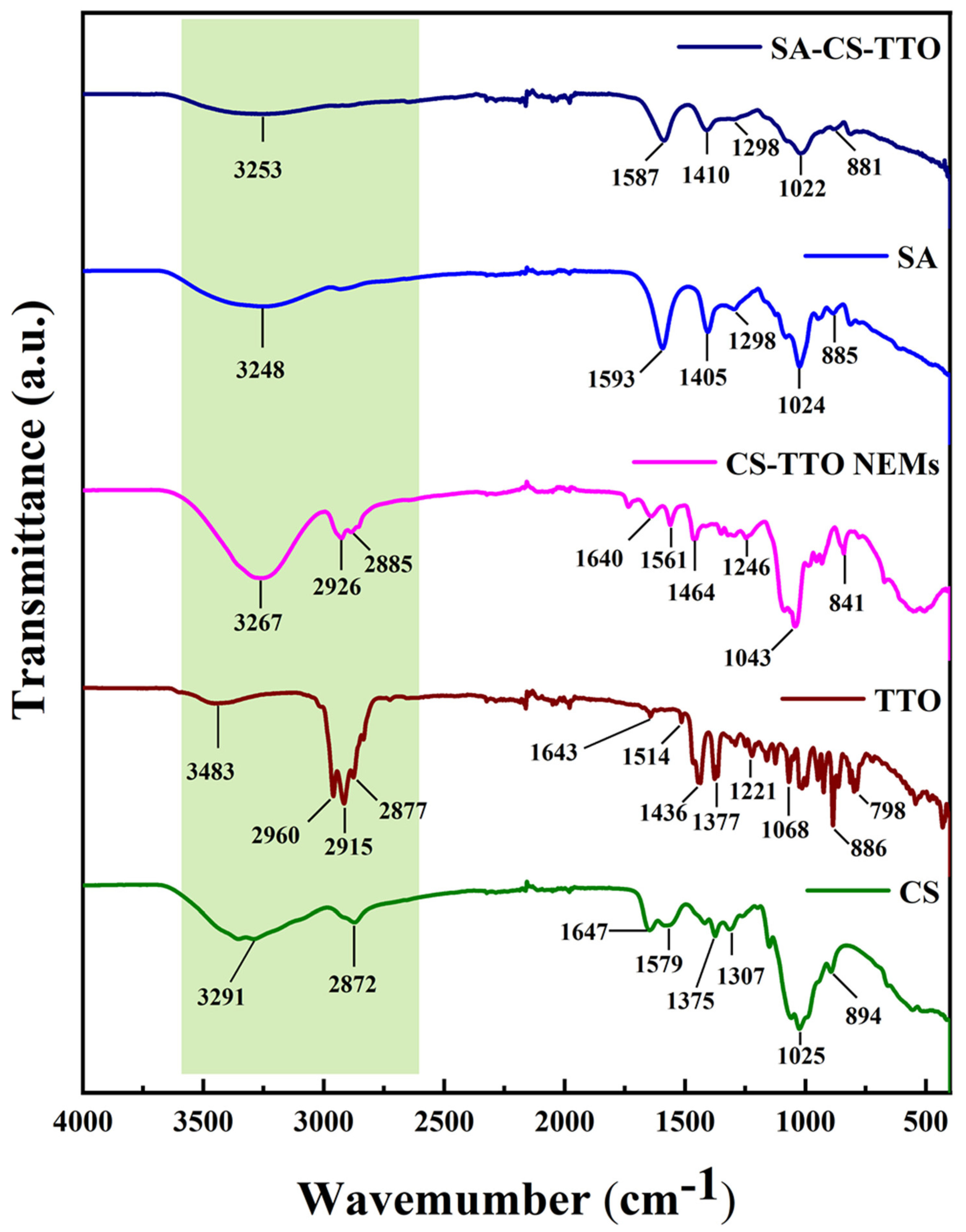
3.1. Functional Properties of CS-TTO Nanoemulsion and SA-CS-TTO Microsphere
3.2. Crystalline Properties CS-TTO and SA-CS-TTO Microsphere
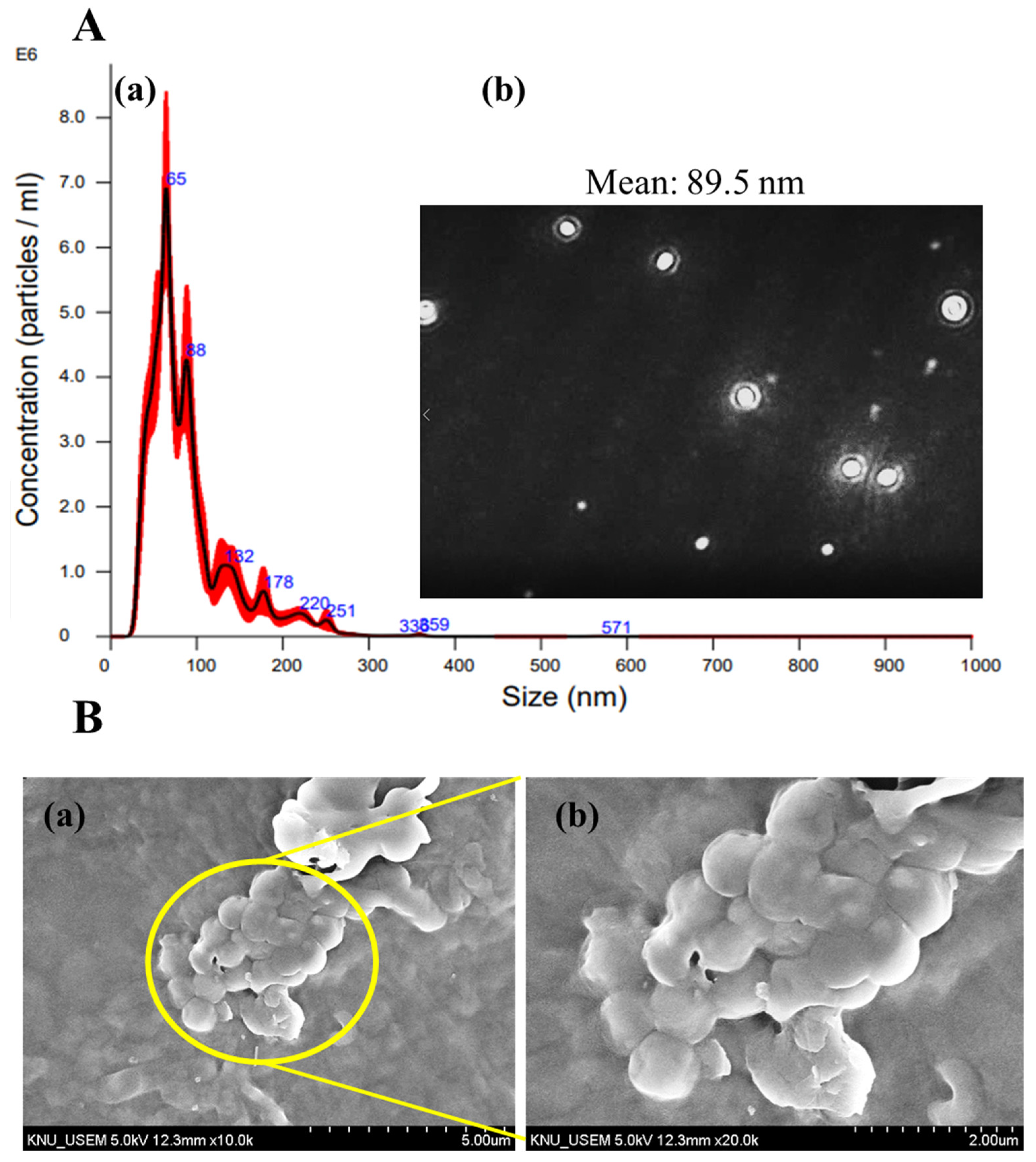
3.3. CS-TTO NEMs Size Distribution by NTA
3.4. SEM Analysis of CS-TT-SA Microsphere
3.5. Water Absorption, In Vitro Degradation, and Thermal Stability
3.6. TTO Encapsulation, Loading, and In Vitro Release
3.7. Antibacterial Activity
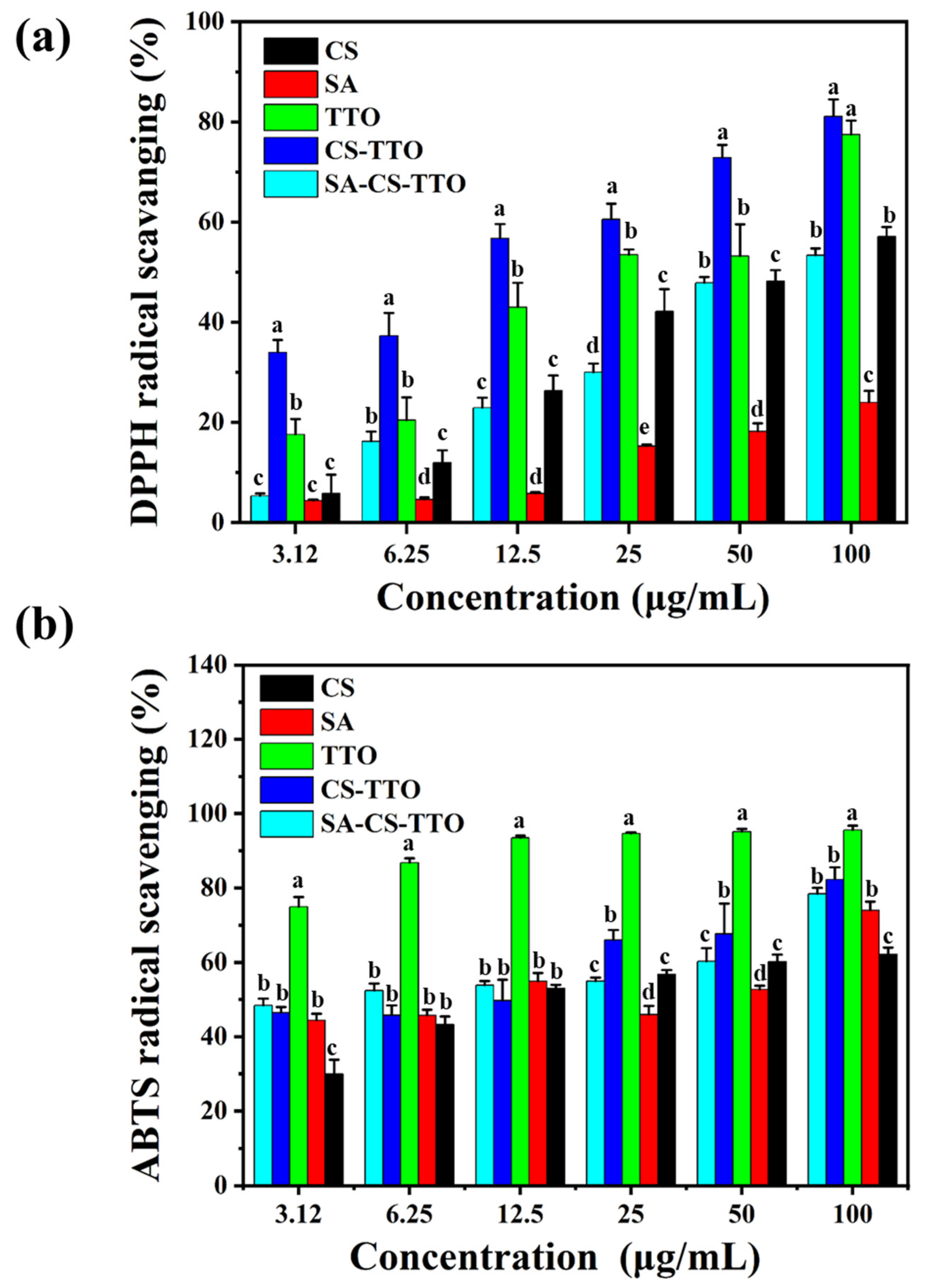
3.8. Antioxidant Activity
3.9. Cell Viability
3.10. In Vitro Wound Healing Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Wang, F. Core-Shell Chitosan Microsphere with Antimicrobial and Vascularized Functions for Promoting Skin Wound Healing. Mater. Des. 2021, 204, 109683. [Google Scholar] [CrossRef]
- Chuong, C.M.; Nickoloff, B.J.; Elias, P.M.; Goldsmith, L.A.; Macher, E.; Maderson, P.A.; Sundberg, J.P.; Tagami, H.; Plonka, P.M.; Thestrup-Pedersen, K.; et al. What Is the ‘True’ Function of Skin? Exp. Dermatol. 2002, 11, 159. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan Based-Asymmetric Membranes for Wound Healing: A Review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, M.C.; Evans, R. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Healy, B.; Freedman, A. ABC of Wound Healing: Infections. BMJ 2006, 332, 838. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Milagres de Almeida, J.; Crippa, B.L.; Martins Alencar de Souza, V.V.; Perez Alonso, V.P.; da Motta Santos Júnior, E.; Siqueira Franco Picone, C.; Prata, A.S.; Cirone Silva, N.C. Antimicrobial Action of Oregano, Thyme, Clove, Cinnamon and Black Pepper Essential Oils Free and Encapsulated against Foodborne Pathogens. Food Control 2023, 144, 109356. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia-Llano, C.H.; Zapata, M.E.V.; Restrepo, Y.J.; Hernandez, J.H.M.; Navia-Porras, D.P.; Valencia, Y.; Valencia, C.; Grande-Tovar, C.D. Chitosan/Polyvinyl Alcohol/Tea Tree Essential Oil Composite Films for Biomedical Applications. Polymers 2021, 13, 3753. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A Review of Applications of Tea Tree Oil in Dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Ramachandran, C.; Hu, X.; Oh, D.H.; Wang, M.H. Chitosan-Tea Tree Oil Nanoemulsion and Calcium Chloride Tailored Edible Coating Increase the Shelf Life of Fresh Cut Red Bell Pepper. Prog. Org. Coat. 2020, 151, 106010. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A Review of the Toxicity of Melaleuca alternifolia (Tea Tree) Oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in Edible Nanoemulsions: Digestion, Bioavailability, and Potential Toxicity. Prog. Lipid. Res. 2021, 81, 101081. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.X. Nanoemulsions for Drug Delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Sugumar, S.; Mukherjee, A.; Chandrasekaran, N. Eucalyptus Oil Nanoemulsion-Impregnated Chitosan Film: Antibacterial Effects against a Clinical Pathogen, Staphylococcus aureus, in Vitro. Int. J. Nanomed. 2015, 10, 67–75. [Google Scholar] [CrossRef]
- Lin, X.; Sheng, Y.; Zhang, X.; Li, Z.; Yang, Y.; Wu, J.; Su, Z.; Ma, G.; Zhang, S. Oil-in-Ionic Liquid Nanoemulsion-Based Intranasal Delivery System for Influenza Split-Virus Vaccine. J. Control. Release 2022, 346, 380–391. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, V.; Sharma, V.; Sharma, R.; Kumar, S. Chitosan Nanoemulsion: Gleam into the Futuristic Approach for Preserving the Quality of Muscle Foods. Int. J. Biol. Macromol. 2022, 199, 121–137. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Ciacciarelli, A.; Quassinti, L.; Bramucci, M.; Miano, A.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Properties and Stability of Nanoemulsions: How Relevant Is the Type of Surfactant? J. Drug. Deliv. Sci. Technol. 2020, 58, 101772. [Google Scholar] [CrossRef]
- Niederhofer, A.; Müller, B.W. A Method for Direct Preparation of Chitosan with Low Molecular Weight from Fungi. Eur. J. Pharm. Biopharm. 2004, 57, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Shajahan, A.; Kalaichelvan, P.T.; Kaviyarasan, V. Fungal Chitosan Based Nanocomposites Sponges—An Alternative Medicine for Wound Dressing. Int. J. Biol. Macromol. 2017, 104, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-Chitosan Nanoemulsions by Ultrasound-Mediated Emulsification: Formulation, Characterization and Antimicrobial Activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. PH-Controlled Nucleolin Targeted Release of Dual Drug from Chitosan-Gold Based Aptamer Functionalized Nano Drug Delivery System for Improved Glioblastoma Treatment. Carbohydr. Polym. 2021, 262, 117907. [Google Scholar] [CrossRef]
- Lv, C.; Zhou, X.; Wang, P.; Wu, Z.; Jiao, Z.; Guo, M.; Wang, Z.; Wang, Y.; Wang, L.; Zhang, P. Antibacterial Microspheres with a Bionic Red-Blood-Cell like Hollow Structure and Superior Swelling Recovery Capacity for Efficient Traumatic Hemostasis. Appl. Mater. Today 2022, 29, 101559. [Google Scholar] [CrossRef]
- Chen, M.; Hu, Y.; Zhou, J.; Xie, Y.; Wu, H.; Yuan, T.; Yang, Z. Facile Fabrication of Tea Tree Oil-Loaded Antibacterial Microcapsules by Complex Coacervation of Sodium Alginate/Quaternary Ammonium Salt of Chitosan. RSC Adv. 2016, 6, 13032–13039. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Naveen, K.V.; Han, K.S.; Zhang, X.; Jeong, M.S.; Wang, M.H. Combination of Paraconiothyrium Brasiliense Fabricated Titanium Dioxide Nanoparticle and Antibiotics Enhanced Antibacterial and Antibiofilm Properties: A Toxicity Evaluation. Environ. Res. 2022, 212, 113237. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Park, S.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Evaluation of Phytochemicals, Antioxidants, and Antidiabetic Efficacy of Various Solvent Fractions of Gynura procumbens (Lour.) Merr. Process Biochem. 2021, 111, 51–62. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Antimicrobial and Wound Healing Properties of FeO Fabricated Chitosan/PVA Nanocomposite Sponge. Antibiotics 2021, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.H. Bimetallic Silver-Platinum (AgPt) Nanoparticles and Chitosan Fabricated Cotton Gauze for Enhanced Antimicrobial and Wound Healing Applications. Int. J. Biol. Macromol. 2022, 220, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Preparation of Chitosan-Rosmarinic Acid Derivatives with Enhanced Antioxidant and Anti-Inflammatory Activities. Carbohydr. Polym. 2022, 296, 119943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Kong, L. Chitosan-Modified PLGA Nanoparticles with Versatile Surface for Improved Drug Delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.H. Cerium Oxide Decorated 5-Fluorouracil Loaded Chitosan Nanoparticles for Treatment of Hepatocellular Carcinoma. Int. J. Biol. Macromol. 2022, 216, 52–64. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A New Look towards the Thermal Decomposition of Chitins and Chitosans with Different Degrees of Deacetylation by Coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- Bajas, D.; Vlase, G.; Mateescu, M.; Grad, O.A.; Bunoiu, M.; Vlase, T.; Avram, C. Formulation and Characterization of Alginate-Based Membranes for the Potential Transdermal Delivery of Methotrexate. Polymers 2021, 13, 161. [Google Scholar] [CrossRef]
- Muñoz-Nuñez, C.; Cuervo-Rodríguez, R.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A. Synthesis and Characterization of Thiazolium Chitosan Derivative with Enhanced Antimicrobial Properties and Its Use as Component of Chitosan Based Films. Carbohydr. Polym. 2023, 302, 120438. [Google Scholar] [CrossRef]
- Han, X.; Zheng, Z.; Yu, C.; Deng, Y.; Ye, Q.; Niu, F.; Chen, Q.; Pan, W.; Wang, Y. Preparation, Characterization and Antibacterial Activity of New Ionized Chitosan. Carbohydr. Polym. 2022, 290, 119490. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Zhang, X. Determination of the Degree of Deacetylation of Chitin and Chitosan by X-Ray Powder Diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Suo, L.; Wu, H.; Wang, P.; Xue, Z.; Gao, J.; Shen, J. The Improvement of Periodontal Tissue Regeneration Using a 3D-Printed Carbon Nanotube/Chitosan/Sodium Alginate Composite Scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 73–84. [Google Scholar] [CrossRef]
- Alnawmasi, J.S. Construction of Amino-Thiol Functionalized Ion-Imprinted Chitosan for Lead (II) Ion Removal. Carbohydr. Polym. 2023, 308, 120596. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Huang, L. Feasibility of Chitosan-Alginate (Chi-Alg) Hydrogel Used as Scaffold for Neural Tissue Engineering: A Pilot Study in Vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef]
- Hole, P.; Sillence, K.; Hannell, C.; Maguire, C.M.; Roesslein, M.; Suarez, G.; Capracotta, S.; Magdolenova, Z.; Horev-Azaria, L.; Dybowska, A.; et al. Interlaboratory Comparison of Size Measurements on Nanoparticles Using Nanoparticle Tracking Analysis (NTA). J. Nanoparticle Res. 2013, 15, 2101. [Google Scholar] [CrossRef]
- Chaves Moreira dos Santos, D.; Leite Silvério de Souza, M.; Morais Teixeira, E.; Leidicy Alves, L.; Mário Carneiro Vilela, J.; Andrade, M.; das Graças Carvalho, M.; Paula Fernandes, A.; Antônio Miranda Ferreira, L.; Marques Gontijo Aguiar, M.; et al. A New Nanoemulsion Formulation Improves Antileishmanial Activity and Reduces Toxicity of Amphotericin B. J. Drug Target. 2017, 26, 357–364. [Google Scholar] [CrossRef]
- Brandão Seibert, J.; Vasconcelos Rodrigues, I.; Pinto Carneiro, S.; Roquete Amparo, T.; Sousa Lanza, J.; Jean Frézard, F.G.; Henrique Bianco de Souza, G.; David Henrique dos Santos, O. Seasonality Study of Essential Oil from Leaves of Cymbopogon Densiflorus and Nanoemulsion Development with Antioxidant Activity. Flavour Fragr. J. 2018, 34, 5–14. [Google Scholar] [CrossRef]
- Dhorm Pimentel de Moraes, A.R.; Tavares, G.D.; Soares Rocha, F.J.; de Paula, E.; Giorgio, S. Effects of Nanoemulsions Prepared with Essential Oils of Copaiba- and Andiroba against Leishmania infantum and Leishmania amazonensis Infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fang, Q.; Ding, M.; Wu, J.; Ye, F.; Lv, Z.; Jin, J. Microspheres of Carboxymethyl Chitosan, Sodium Alginate and Collagen for a Novel Hemostatic in Vitro Study. J. Biomater. Appl. 2015, 30, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and Self-Healing Chitosan-Alginate Hydrogel Encapsulated Gelatin Microspheres via Covalent Cross-Linking for Drug Delivery. Mater. Sci. Eng. C 2019, 101, 619–629. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M. Enhanced PH-Responsive Carrier System Based on Alginate and Chemically Modified Carboxymethyl Chitosan for Oral Delivery of Protein Drugs: Preparation and in-Vitro Assessment. Carbohydr. Polym. 2010, 80, 1125–1136. [Google Scholar] [CrossRef]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently Polysaccharide-Based Alginate/Chitosan Hydrogel Embedded Alginate Microspheres for BSA Encapsulation and Soft Tissue Engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile Synthesis of PH-Responsive Sodium Alginate/Carboxymethyl Chitosan Hydrogel Beads Promoted by Hydrogen Bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, Y.; Chen, K.; Zhang, Y.; Zhang, H. Enhanced Physical and Antimicrobial Properties of Alginate/Chitosan Composite Aerogels Based on Electrostatic Interactions and Noncovalent Crosslinking. Carbohydr. Polym. 2021, 266, 118102. [Google Scholar] [CrossRef]
- Lin, G.; Chen, H.; Zhou, H.; Zhou, X.; Xu, H. Preparation of Tea Tree Oil/Poly(Styrene-Butyl Methacrylate) Microspheres with Sustained Release and Anti-Bacterial Properties. Materials 2018, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of Chitosan Nanofibers Containing Tea Tree Oil Liposomes against Salmonella Spp. in Chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Mcdermott, A.M.; Zasloff, M. Antimicrobial Peptides and Wound Healing: Biological and Therapeutic Considerations. Exp. Dermatol. 2016, 25, 167. [Google Scholar] [CrossRef]
- Altoé, L.S.; Alves, R.S.; Sarandy, M.M.; Morais-Santos, M.; Novaes, R.D.; Gonçalves, R.V. Does Antibiotic Use Accelerate or Retard Cutaneous Repair? A Systematic Review in Animal Models. PLoS ONE 2019, 14, e0223511. [Google Scholar] [CrossRef]
- Majhi, A.; Kundu, K.; Adhikary, R.; Banerjee, M.; Mahanti, S.; Basu, A.; Bishayi, B. Combination Therapy with Ampicillin and Azithromycin in an Experimental Pneumococcal Pneumonia Is Bactericidal and Effective in down Regulating Inflammation in Mice. J. Inflamm. 2014, 11, 5. [Google Scholar] [CrossRef]
- Ko, K.; Lee, W.K.; Oh, C.Y.; Lee, S.H.; Cho, S.T.; Bang, W.J.; Shin, T.Y.; Choo, M.S.; Cho, J.S.; Lee, Y.G.; et al. Is A Combination of Antibiotics and Non-Steroidal Anti-Inflammatory Drugs More Beneficial Than Antibiotic Monotherapy For The Treatment of Female Acute Uncomplicated Cystitis? A Randomized Controlled Pilot Study. Urol. J. 2018, 15, 365–369. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds With Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 2405. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jia, E.; Zhang, Q.; Zheng, W.; Sun, R.; Qian, M.; Tan, Y.; Hu, W. Injectable Carboxymethyl Chitosan-Genipin Hydrogels Encapsulating Tea Tree Oil for Wound Healing. Carbohydr. Polym. 2023, 301, 120348. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Physical and Antimicrobial Properties of Chitosan-Tea Tree Essential Oil Composite Films. J. Food Eng. 2010, 98, 443–452. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of Antioxidant Activity of Australian Tea Tree (Melaleuca alternifolia) Oil and Its Components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, Physicochemical and Antioxidant Properties of Sodium Alginate Isolated from a Tunisian Brown Seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. Biocompatible Fungal Chitosan Encapsulated Phytogenic Silver Nanoparticles Enhanced Antidiabetic, Antioxidant and Antibacterial Activity. Int. J. Biol. Macromol. 2020, 153, 63–71. [Google Scholar] [CrossRef]






| Sample | B. cereus | S. aureus | E. coli | S. enterica |
|---|---|---|---|---|
| MIC (µg/mL) | ||||
| CS | 250 b | 250 c | >250 c | 125 b |
| SA | 250 b | >250 c | >250 c | >250 c |
| TTO | 62.5 a | 125 b | 125 b | 125 c |
| CS-TTO | 62.5 a | 62.5 a | 62.5 a | 31.25–62.5 a |
| SA-CS-TTO | 62.5 a | 125 b | 125 b | 125 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathiyaseelan, A.; Zhang, X.; Wang, M.-H. Enhancing the Antioxidant, Antibacterial, and Wound Healing Effects of Melaleuca alternifolia Oil by Microencapsulating It in Chitosan-Sodium Alginate Microspheres. Nutrients 2023, 15, 1319. https://doi.org/10.3390/nu15061319
Sathiyaseelan A, Zhang X, Wang M-H. Enhancing the Antioxidant, Antibacterial, and Wound Healing Effects of Melaleuca alternifolia Oil by Microencapsulating It in Chitosan-Sodium Alginate Microspheres. Nutrients. 2023; 15(6):1319. https://doi.org/10.3390/nu15061319
Chicago/Turabian StyleSathiyaseelan, Anbazhagan, Xin Zhang, and Myeong-Hyeon Wang. 2023. "Enhancing the Antioxidant, Antibacterial, and Wound Healing Effects of Melaleuca alternifolia Oil by Microencapsulating It in Chitosan-Sodium Alginate Microspheres" Nutrients 15, no. 6: 1319. https://doi.org/10.3390/nu15061319
APA StyleSathiyaseelan, A., Zhang, X., & Wang, M.-H. (2023). Enhancing the Antioxidant, Antibacterial, and Wound Healing Effects of Melaleuca alternifolia Oil by Microencapsulating It in Chitosan-Sodium Alginate Microspheres. Nutrients, 15(6), 1319. https://doi.org/10.3390/nu15061319








