Dietary Intake Levels of Iron, Copper, Zinc, and Manganese in Relation to Cognitive Function: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements of Dietary Intake of Iron, Zinc, Manganese, and Copper
2.3. Cognitive Function
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Participants Characteristics
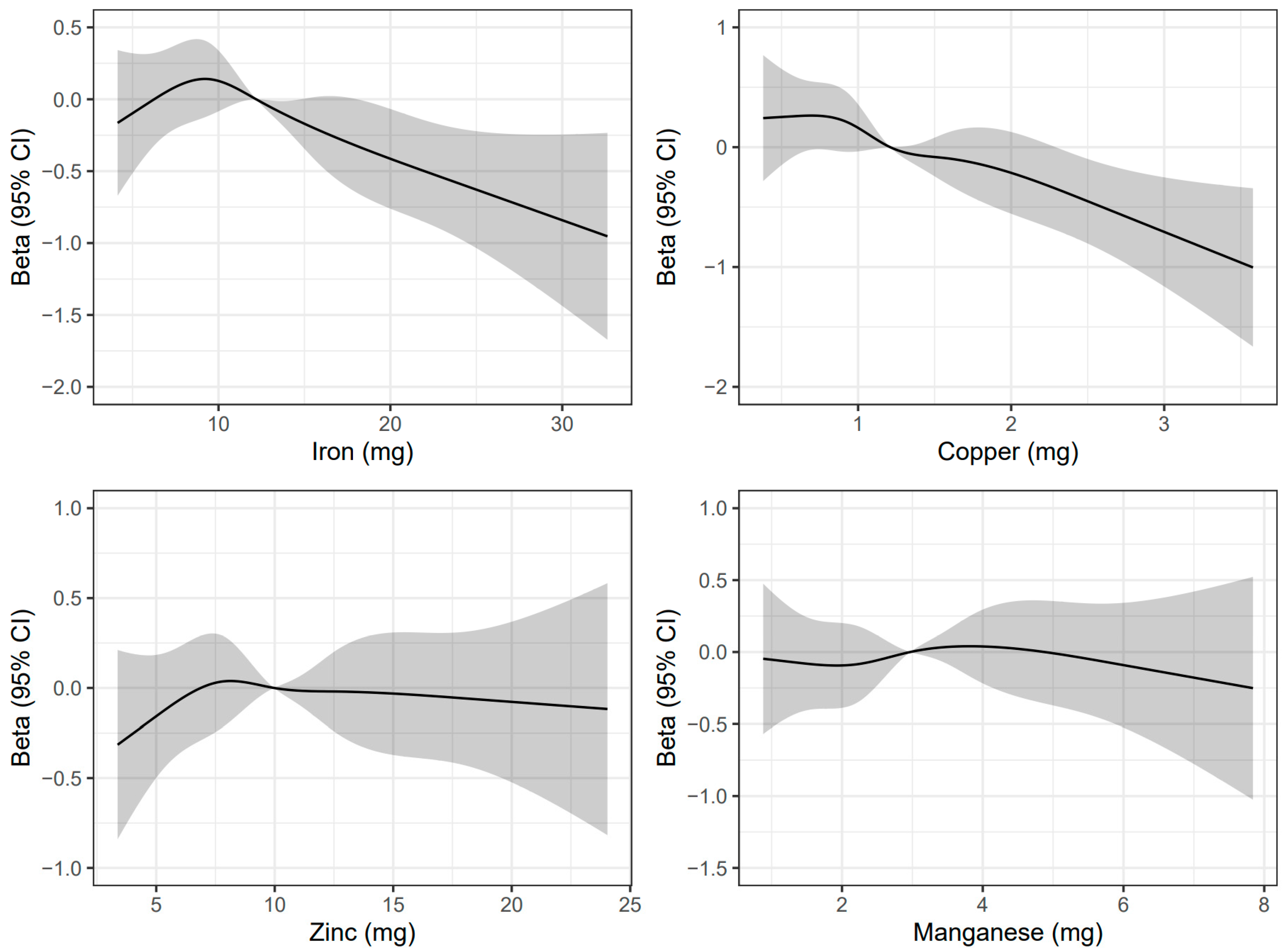
3.2. Associations of Mineral Intake Levels with Cognitive Function and Cognitive Impairment
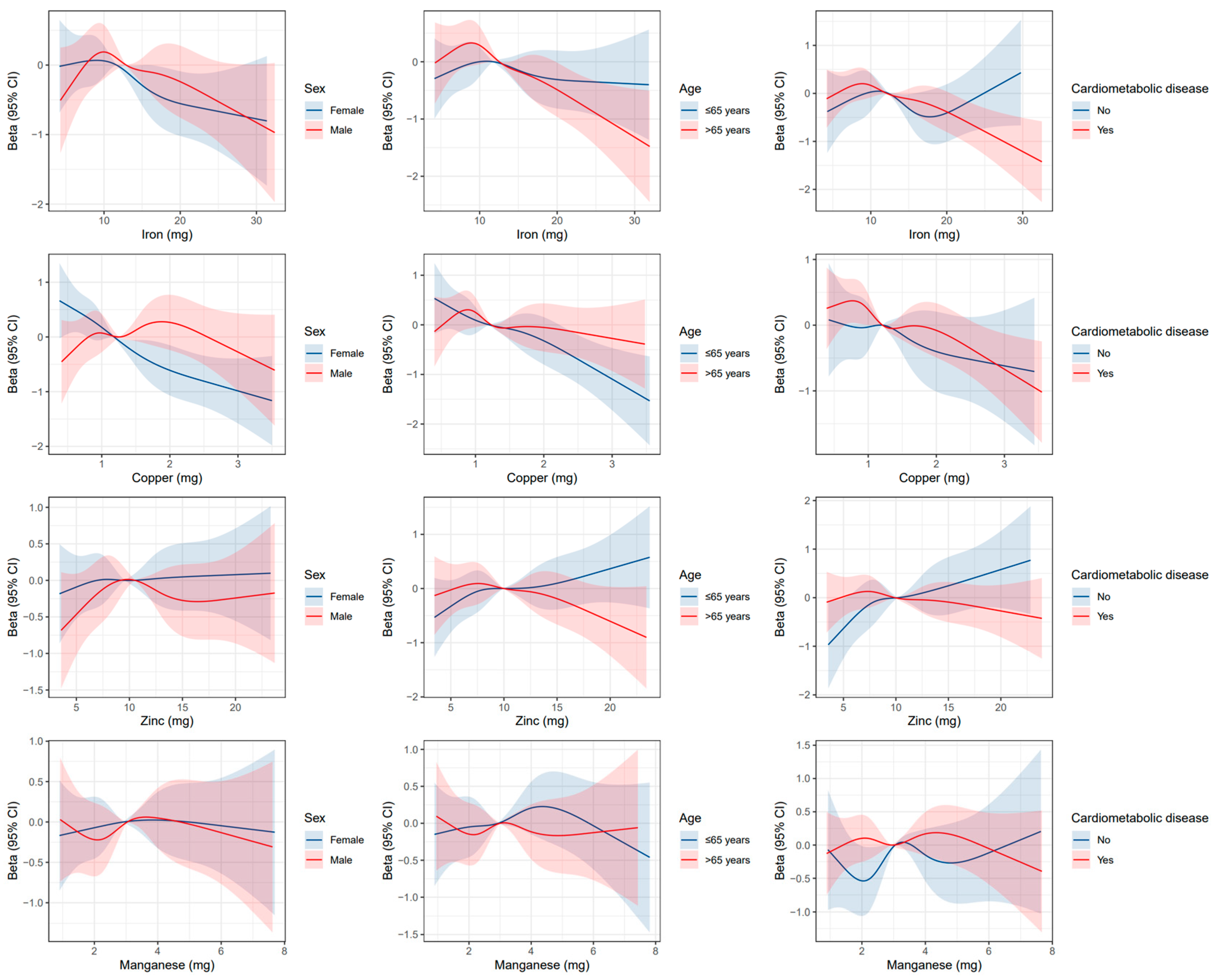
3.3. Subgroup Analyses
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kirkwood, T.B. Global aging and the brain. Nutr. Rev. 2010, 68, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc. 2018, 77, 152–163. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Consultation on the Development of the Global Dementia Observatory; Meeting Report; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kułak-Bejda, A.; Bejda, G.; Waszkiewicz, N. Mental Disorders, Cognitive Impairment and the Risk of Suicide in Older Adults. Front. Psychiatry 2021, 12, 695286. [Google Scholar] [CrossRef] [PubMed]
- Mayeda, E.R.; Whitmer, R.A.; Yaffe, K. Diabetes and cognition. Clin. Geriatr. Med. 2015, 31, 101–115. [Google Scholar] [CrossRef] [PubMed]
- McMaster, M.; Kim, S.; Clare, L.; Torres, S.J.; D’Este, C.; Anstey, K.J. Body, Brain, Life for Cognitive Decline (BBL-CD): Protocol for a multidomain dementia risk reduction randomized controlled trial for subjective cognitive decline and mild cognitive impairment. Clin. Interv. Aging 2018, 13, 2397–2406. [Google Scholar] [CrossRef]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef]
- Davies, R. Cognitive decline: Can diet be a preventive or treatment option? Nurs. Older People 2019, 31, 26–30. [Google Scholar]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Otaegui-Arrazola, A.; Amiano, P.; Elbusto, A.; Urdaneta, E.; Martınez-Lage, P. Diet, cognition, and Alzheimer’s disease: Food for thought. Eur. J. Nutr. 2014, 53, 24. [Google Scholar] [CrossRef]
- Roberts, B.R.; Ryan, T.M.; Bush, A.I.; Masters, C.L.; Duce, J.A. The role of metallobiology and amyloid-β peptides in Alzheimer’s disease. J. Neurochem. 2012, 120, 149–166. [Google Scholar] [CrossRef]
- Tõugu, V.; Tiiman, A.; Palumaa, P. Interactions of Zn(ii) and Cu(ii) ions with Alzheimer’s amyloid-beta peptide. Metal ion binding, contribution to fibrillization and toxicity. Metallomics 2011, 3, 250. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 1: Micronutrients. J. Nutr. Health Aging 2006, 10, 377–385. [Google Scholar] [PubMed]
- Choi, S.; Hong, D.K.; Choi, B.Y.; Suh, S.W. Zinc in the Brain: Friend or Foe? Int. J. Mol. Sci. 2020, 21, 8941. [Google Scholar] [CrossRef] [PubMed]
- Krall, R.F.; Tzounopoulos, T.; Aizenman, E. The Function and Regulation of Zinc in the Brain. Neuroscience 2021, 457, 235–258. [Google Scholar] [CrossRef]
- White, A.R.; Huang, X.; Jobling, M.F.; Barrow, C.J.; Beyreuther, K.; Masters, C.L.; Bush, A.I.; Cappai, R. Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: Possible risk factors in the Alzheimer’s-type neurodegenerative pathways: Homocysteine potentiates copper neurotoxicity. J. Neurochem. 2001, 76, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front. Aging Neurosci. 2014, 6, 92. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. NeuroToxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Ventriglia, M.; Brewer, G.J.; Simonelli, I.; Mariani, S.; Siotto, M.; Bucossi, S.; Squitti, R. Zinc in Alzheimer’s Disease: A Meta-Analysis of Serum, Plasma, and Cerebrospinal Fluid Studies. J. Alzheimer’s Dis. 2015, 46, 75–87. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.; Pan, Y.; Zhong, X.; Wei, M. Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 231. [Google Scholar] [CrossRef]
- Gao, S.; Jin, Y.; Unverzagt, F.W.; Ma, F.; Hall, K.S.; Murrell, J.R.; Cheng, Y.; Shen, J.; Ying, B.; Ji, R.; et al. Trace Element Levels and Cognitive Function in Rural Elderly Chinese. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 635–641. [Google Scholar] [CrossRef]
- Mueller, C.; Schrag, M.; Crofton, A.; Stolte, J.; Muckenthaler, M.U.; Magaki, S.; Kirsch, W. Altered Serum Iron and Copper Homeostasis Predicts Cognitive Decline in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2012, 29, 341–350. [Google Scholar] [CrossRef]
- Chen, M.; Xu, E.; Zeng, C.; Zhu, W.; Zheng, J.; Chen, H. High Dietary Iron Has a Greater Impact on Brain Iron Homeostasis and Cognitive Function in Old Compared with Young C57BL/6J Male Mice. J. Nutr. 2021, 151, 2835–2842. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.; Liu, G.; Zeng, C.; Xu, E.; Zhu, W.; Anderson, G.J.; Chen, H. High Dietary Iron Disrupts Iron Homeostasis and Induces Amyloid-β and Phospho-τ Expression in the Hippocampus of Adult Wild-Type and APP/PS1 Transgenic Mice. J. Nutr. 2019, 149, 2247–2254. [Google Scholar] [CrossRef]
- Corona, C.; Masciopinto, F.; Silvestri, E.; Viscovo, A.D.; Lattanzio, R.; Sorda, R.L.; Ciavardelli, D.; Goglia, F.; Piantelli, M.; Canzoniero, L.M.T.; et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 2010, 1, e91. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-Reported Dietary Intake of Potassium, Calcium, and Magnesium and Risk of Dementia in the Japanese: The Hisayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Kumar, R.; Sachdev, P.S.; Anstey, K.J. Dietary Mineral Intake and Risk of Mild Cognitive Impairment: The PATH through Life Project. Front. Aging Neurosci. 2014, 6, 4. [Google Scholar] [CrossRef]
- Li, S.; Sun, W.; Zhang, D. Association of Zinc, Iron, Copper, and Selenium Intakes with Low Cognitive Performance in Older Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). J. Alzheimer’s Dis. 2019, 72, 1145–1157. [Google Scholar] [CrossRef]
- Shi, Z.; Li, M.; Wang, Y.; Liu, J.; El-Obeid, T. High iron intake is associated with poor cognition among Chinese old adults and varied by weight status—A 15-y longitudinal study in 4852 adults. Am. J. Clin. Nutr. 2019, 109, 109–116. [Google Scholar] [CrossRef]
- Antony, H.; Macreadie, I.G. Dietary copper and the brain. In Handbook of Behavior, Food and Nutrition; Preedy, V.R., Watson, R.R., Martin, C.R., Eds.; Springer: New York, NY, USA, 2011; pp. 2375–2392. [Google Scholar]
- Wei, J.; Gianattasio, K.Z.; Bennett, E.E.; Stewart, J.D.; Xu, X.; Park, E.S.; Smith, R.L.; Ying, Q.; Whitsel, E.A.; Power, M.C. The Associations of Dietary Copper With Cognitive Outcomes. Am. J. Epidemiol. 2022, 191, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Surette, C.; Cormier, P.; Foucher, D. Low level exposure to manganese from drinking water and cognition in school-age children. NeuroToxicology 2018, 64, 110–117. [Google Scholar] [CrossRef]
- Bryan, J.; Osendarp, S.; Hughes, D.; Calvaresi, E.; Baghurst, K.; Klinken, J.-W. Nutrients for Cognitive Development in School-aged Children. Nutr. Rev. 2004, 62, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Zhang, F.; Cui, Y.; Zhang, D.; Shen, X. Threshold effects and interactive effects of total zinc and selenium intake on cognitive function in older adults. Clin. Nutr. ESPEN 2022, 47, 383–390. [Google Scholar] [CrossRef]
- Welcome to the Health and Retirement Study [EB/OL]. Available online: https://hrs.isr.umich.edu/ (accessed on 5 April 2022).
- Sonnega, A.; Faul, J.D.; Ofstedal, M.B.; Langa, K.M.; Phillips, J.W.; Weir, D.R. Cohort Profile: The Health and Retirement Study (HRS). Int. J. Epidemiol. 2014, 43, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed]
- Harvard T.H. Chan School of Public Health [EB/OL]. Nutrition Questionnaire Service Center. Available online: https://www.hsph.harvard.edu/nutrition-questionnaire-service-center/general-documentation/ (accessed on 2 May 2022).
- McEvoy, C.T.; Guyer, H.; Langa, K.M.; Yaffe, K. Neuroprotective Diets Are Associated with Better Cognitive Function: The Health and Retirement Study. J. Am. Geriatr. Soc. 2017, 65, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Papassotiriou, I.; Shariful Islam, S.M. Adherence to Mediterranean Diet Is Associated With Lung Function in Older Adults: Data From the Health and Retirement Study. J. Am. Coll. Nutr. 2021, 40, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Harvard T.H. Chan School of Public Health [EB/OL]. Nutrient Tables. Nutrition Questionnaire Service Center. Available online: https://www.hsph.harvard.edu/nutrition-questionnaire-service-center/nutrient-tables/ (accessed on 2 May 2022).
- Crimmins, E.M.; Kim, J.K.; Langa, K.M.; Weir, D.R. Assessment of Cognition Using Surveys and Neuropsychological Assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2011, 66B, i162–i171. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Lu, Y.; Sugawara, Y.; Zhang, S.; Tomata, Y.; Tsuji, I. Smoking cessation and incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Eur. J. Epidemiol. 2020, 35, 851–860. [Google Scholar] [CrossRef]
- Mareschi, J.P.; Cousin, F.; De La Villeon, B.; Brubacher, G.B. Valeur calorique de I’alimentation et couverture des apports nutritionnels conseill’s en vitamines de I’homme adulte. Principaux vecteurs alimentaires de vitamines. Ann. Nutr. Metab. 1984, 28, 11–23. [Google Scholar] [CrossRef]
- Shi, Z.; El-Obeid, T.; Li, M.; Xu, X.; Liu, J. Iron-related dietary pattern increases the risk of poor cognition. Nutr. J. 2019, 18, 48. [Google Scholar] [CrossRef]
- Shi, Z.; El-Obeid, T.; Li, M.; Xu, X.; Liu, J. Plant-based Iron-related Dietary Pattern Increases the Risk of Poor Cognition (P18-043-19). Curr. Dev. Nutr. 2019, 3, nzz039.P18-043-19. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Copper and iron in Alzheimer’s disease: A systematic review and its dietary implications. Br. J. Nutr. 2012, 107, 7–19. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Sheng, L.-T.; Pan, X.-F.; Feng, L.; Yuan, J.-M.; Pan, A.; Koh, W.-P. Meat consumption in midlife and risk of cognitive impairment in old age: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1729–1738. [Google Scholar] [CrossRef]
- Institute Of Medicine (US) Panel On Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Li, K.; Reichmann, H. Role of iron in neurodegenerative diseases. J. Neural Transm. 2016, 123, 389–399. [Google Scholar] [CrossRef]
- Erikson, K.M.; Pinero, D.J.; Connor, J.R.; Beard, J.L. Regional Brain Iron, Ferritin and Transferrin Concentrations during Iron Deficiency and Iron Repletion in Developing Rats. J. Nutr. 1997, 127, 2030–2038. [Google Scholar] [CrossRef]
- Li, W.; Sun, L.; Yue, L.; Li, G.; Xiao, S. The Association Between Eating Green Vegetables Every Day And Mild Cognitive Impairment: A Community-Based Cross-Sectional Study In Shanghai. Neuropsychiatr. Dis. Treat. 2019, 15, 3213–3218. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xing, Y.; Wang, W.; Li, S.; Zhang, D.; Zheng, W.; Shen, X. Threshold Effects of Total Copper Intake on Cognitive Function in US Older Adults and the Moderating Effect of Fat and Saturated Fatty Acid Intake. J. Acad. Nutr. Diet. 2021, 121, 2429–2442. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Schneider, J.A.; Wilson, R.S.; Scherr, P.A. Dietary Copper and High Saturated and trans Fat Intakes Associated With Cognitive Decline. Arch. Neurol. 2006, 63, 1085. [Google Scholar] [CrossRef] [PubMed]
- Hureau, C.; Faller, P. Aβ-mediated ROS production by Cu ions: Structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie 2009, 91, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Bush, A.I.; La Fontaine, S. Links between copper and cholesterol in Alzheimer’s disease. Front. Physiol. 2013, 4, 111. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, D.-W.; Park, K.S. Elevated serum copper and ceruloplasmin levels in Alzheimer’s disease: Serum copper and Alzheimer’s disease. Asia-Pac. Psychiatry 2014, 6, 38–45. [Google Scholar] [CrossRef]
- Squitti, R.; Simonelli, I.; Ventriglia, M.; Siotto, M.; Pasqualetti, P.; Rembach, A.; Doecke, J.; Bush, A.I. Meta-Analysis of Serum Non-Ceruloplasmin Copper in Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 38, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Requejo, A.M.; Andrés, P.; López-Sobaler, A.M.; Quintas, M.E.; Redondo, M.R.; Navia, B.; Rivas, T. Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 1997, 66, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, X.; Ma, H.; Qu, X.; Wang, H. Iron, Zinc and Copper from Cereal Food Sources and Cognitive Performance in Older Adults in China. Iran. J. Public Health 2021, 50, 2546–2554. [Google Scholar] [CrossRef]
- Miyagawa, N. Dietary Intake of Manganese in the Japanese Diet and its Association with Cardiometabolic and Cardiovascular Diseases. J. Atheroscler. Thromb. 2022, 29, 1421–1422. [Google Scholar] [CrossRef]
- Shiraishi, K. Dietary intakes of trace elements. Jap. J. Clin. Nutr. 1994, 84, 381–389. [Google Scholar]
- Cook, J.D.; Dassenko, S.A.; Whittaker, P. Calcium supplementation: Effect on iron absorption. Am. J. Clin. Nutr. 1991, 53, 106–111. [Google Scholar] [CrossRef]
| Characteristics | Overall (n = 6863) | Gender | |
|---|---|---|---|
| Male (n = 2794) | Female (n = 4069) | ||
| Age, years, mean (SD) | 66.7 (10.5) | 66.8 (10.2) | 66.7 (10.7) |
| Race, n (%) | |||
| White | 4783 (69.7) | 1993 (71.3) | 2790 (68.6) |
| Black | 1098 (16.0) | 394 (14.1) | 704 (17.3) |
| Hispanic | 765 (11.1) | 320 (11.5) | 445 (10.9) |
| Others | 217 (3.2) | 87 (3.1) | 130 (3.2) |
| Married or partnered, n (%) | 4417 (64.4) | 2191 (78.4) | 2226 (54.7) |
| Education, years, mean (SD) | 13.0 (2.9) | 13.2 (3.1) | 12.9 (2.8) |
| Total energy intake, kcal, mean (SD) | 1755.2 (682.3) | 1876.0 (730.9) | 1672.2 (633.8) |
| Frequency of vigorous activity, n (%) | |||
| <1/month | 3674 (53.5) | 1250 (44.7) | 2424 (59.6) |
| 1–4/month | 1448 (21.1) | 701 (25.1) | 747 (18.4) |
| >1/week | 1741 (25.4) | 843 (30.2) | 898 (22.1) |
| Household income, n (%) | |||
| 0–<20,000 | 1508 (22.0) | 439 (15.7) | 1069 (26.3) |
| 20,000–<40,000 | 1595 (23.2) | 622 (22.3) | 973 (23.9) |
| 40,000–<80,000 | 1893 (27.6) | 830 (29.7) | 1063 (26.1) |
| ≥80,000 | 1867 (27.2) | 903 (32.3) | 964 (23.7) |
| Smoking status, n (%) | |||
| Never | 3133 (45.7) | 972 (34.8) | 2161 (53.1) |
| Former | 2989 (43.6) | 1493 (53.4) | 1496 (36.8) |
| Current | 741 (10.8) | 329 (11.8) | 412 (10.1) |
| Drinking status, n (%) | |||
| Never | 3104 (45.2) | 1043 (37.3) | 2061 (50.7) |
| Former | 1073 (15.6) | 404 (14.5) | 669 (16.4) |
| Current | 2686 (39.1) | 1347 (48.2) | 1339 (32.9) |
| Body weight status, n (%) | |||
| Underweight | 63 (0.9) | 13 (0.5) | 50 (1.2) |
| Normal weight | 1407 (20.5) | 467 (16.7) | 940 (23.1) |
| Overweight | 2316 (33.7) | 1084 (38.8) | 1232 (30.3) |
| Obesity | 3077 (44.8) | 1230 (44.0) | 1847 (45.4) |
| Hypertension, n (%) | 4178 (60.9) | 1739 (62.2) | 2439 (59.9) |
| Diabetes mellitus, n (%) | 1630 (23.8) | 708 (25.3) | 922 (22.7) |
| Heart diseases, n (%) | 1724 (25.1) | 843 (30.2) | 881 (21.7) |
| Depression, n (%) | 936 (13.6) | 314 (11.2) | 622 (15.3) |
| Global cognitive score, mean (SD) | 15.3 (4.4) | 15.0 (4.2) | 15.5 (4.5) |
| Dietary Iron intake, mg, mean (SD) | 13.3 (6.3) | 14.1 (6.4) | 12.8 (6.2) |
| Dietary Copper intake, mg, mean (SD) | 1.4 (0.7) | 1.4 (0.8) | 1.3 (0.7) |
| Dietary Zinc intake, mg, mean (SD) | 10.7 (4.6) | 11.4 (4.9) | 10.3 (4.4) |
| Dietary Manganese intake, mg, mean (SD) | 3.3 (1.6) | 3.3 (1.6) | 3.2 (1.6) |
| Vitamin supplement use, n (%) | 4578 (66.7) | 1640 (58.7) | 2938 (72.2) |
| Iron supplement intake, n (%) | 1960 (28.6) | 752 (26.9) | 1208 (29.7) |
| Zinc supplement intake, n (%) | 756 (11.0) | 260 (9.3) | 496 (12.2) |
| Range | Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | |
|---|---|---|---|---|---|---|---|
| Iron | |||||||
| Quintile 1 | <8.1 | 0.00 [Reference] | 0.00 [Reference] | 0.00 [Reference] | |||
| Quintile 2 | 8.1–<10.8 | 0.72 [0.41, 1.04] | <0.001 | 0.37 [0.08, 0.66] | 0.012 | 0.30 [0.02, 0.59] | 0.038 |
| Quintile 3 | 10.8–<13.7 | 0.64 [0.30, 0.97] | <0.001 | 0.13 [−0.18, 0.44] | 0.411 | 0.01 [−0.30, 0.33] | 0.941 |
| Quintile 4 | 13.7–<17.7 | 0.78 [0.40, 1.15] | <0.001 | 0.20 [−0.15, 0.54] | 0.264 | 0.05 [−0.31, 0.40] | 0.794 |
| Quintile 5 | ≥17.7 | 0.31 [−0.14, 0.76] | 0.178 | −0.26 [−0.68, 0.16] | 0.221 | −0.50 [−0.94, −0.06] | 0.027 |
| P-trend | 0.847 | 0.116 | 0.007 | ||||
| Copper | |||||||
| Quintile 1 | <0.8 | 0.00 [Reference] | 0.00 [Reference] | 0.00 [Reference] | |||
| Quintile 2 | 0.8–<1.1 | 0.30 [−0.01, 0.61] | 0.061 | −0.05 [−0.33, 0.24] | 0.745 | −0.11 [−0.39, 0.18] | 0.461 |
| Quintile 3 | 1.1–<1.4 | 0.44 [0.12, 0.77] | 0.008 | −0.03 [−0.33, 0.27] | 0.832 | −0.17 [−0.47, 0.14] | 0.285 |
| Quintile 4 | 1.4–<1.8 | 0.46 [0.09, 0.83] | 0.014 | −0.06 [−0.40, 0.28] | 0.726 | −0.27 [−0.63, 0.08] | 0.124 |
| Quintile 5 | ≥1.8 | 0.34 [−0.09, 0.77] | 0.117 | −0.19 [−0.59, 0.20] | 0.344 | −0.52 [−0.94, −0.10] | 0.014 |
| P-trend | 0.176 | 0.064 | 0.002 | ||||
| Zinc | |||||||
| Quintile 1 | <6.8 | 0.00 [Reference] | 0.00 [Reference] | 0.00 [Reference] | |||
| Quintile 2 | 6.8–<9.0 | 0.50 [0.18, 0.81] | 0.002 | 0.15 [−0.14, 0.44] | 0.314 | 0.10 [−0.19, 0.39] | 0.494 |
| Quintile 3 | 9.0–<11.1 | 0.86 [0.52, 1.21] | <0.001 | 0.26 [−0.06, 0.57] | 0.110 | 0.18 [−0.14, 0.50] | 0.266 |
| Quintile 4 | 11.1–<14.2 | 0.99 [0.60, 1.37] | <0.001 | 0.28 [−0.08, 0.64] | 0.124 | 0.17 [−0.19, 0.53] | 0.348 |
| Quintile 5 | ≥14.2 | 1.05 [0.57, 1.53] | <0.001 | 0.21 [−0.23, 0.66] | 0.345 | 0.06 [−0.39, 0.51] | 0.804 |
| P-trend | 0.003 | 0.553 | 0.785 | ||||
| Manganese | |||||||
| Quintile 1 | <1.9 | 0.00 [Reference] | 0.00 [Reference] | 0.00 [Reference] | |||
| Quintile 2 | 1.9–<2.6 | 0.75 [0.44, 1.06] | <0.001 | 0.17 [−0.11, 0.46] | 0.240 | 0.10 [−0.19, 0.39] | 0.500 |
| Quintile 3 | 2.6–<3.4 | 1.04 [0.71, 1.36] | <0.001 | 0.20 [−0.11, 0.50] | 0.209 | 0.06 [−0.26, 0.38] | 0.717 |
| Quintile 4 | 3.4–<4.4 | 1.51 [1.16, 1.86] | <0.001 | 0.48 [0.15, 0.81] | 0.004 | 0.26 [−0.10, 0.62] | 0.161 |
| Quintile 5 | ≥4.4 | 1.68 [1.26, 2.09] | <0.001 | 0.36 [−0.03, 0.75] | 0.072 | −0.03 [−0.50, 0.44] | 0.912 |
| P-trend | <0.001 | 0.001 | 0.368 |
| Range | Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | |
|---|---|---|---|---|---|---|---|
| Iron | |||||||
| Quintile 1 | <8.1 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| Quintile 2 | 8.1–<10.8 | 0.69 [0.56, 0.86] | 0.001 | 0.79 [0.63, 0.99] | 0.043 | 0.82 [0.65, 1.02] | 0.079 |
| Quintile 3 | 10.8–<13.7 | 0.77 [0.62, 0.96] | 0.022 | 0.96 [0.76, 1.22] | 0.764 | 1.02 [0.80, 1.30] | 0.866 |
| Quintile 4 | 13.7–<17.7 | 0.80 [0.63, 1.03] | 0.085 | 1.03 [0.79, 1.34] | 0.825 | 1.11 [0.85, 1.45] | 0.462 |
| Quintile 5 | ≥17.7 | 0.97 [0.73, 1.31] | 0.861 | 1.26 [0.92, 1.71] | 0.151 | 1.41 [1.02, 1.95] | 0.040 |
| P-trend | 0.197 | 0.059 | 0.009 | ||||
| Copper | |||||||
| Quintile 1 | <0.8 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| Quintile 2 | 0.8–<1.1 | 0.96 [0.78, 1.18] | 0.707 | 1.12 [0.90, 1.39] | 0.320 | 1.14 [0.92, 1.43] | 0.234 |
| Quintile 3 | 1.1–<1.4 | 0.91 [0.73, 1.14] | 0.407 | 1.11 [0.88, 1.40] | 0.367 | 1.18 [0.93, 1.49] | 0.177 |
| Quintile 4 | 1.4–<1.8 | 0.88 [0.69, 1.13] | 0.331 | 1.11 [0.86, 1.44] | 0.425 | 1.21 [0.93, 1.58] | 0.157 |
| Quintile 5 | ≥1.8 | 1.08 [0.81, 1.43] | 0.606 | 1.35 [1.00, 1.81] | 0.048 | 1.54 [1.13, 2.10] | 0.006 |
| P-trend | 0.052 | 0.062 | 0.018 | ||||
| Zinc | |||||||
| Quintile 1 | <6.8 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| Quintile 2 | 6.8–<9.0 | 0.77 [0.62, 0.95] | 0.014 | 0.88 [0.70, 1.09] | 0.242 | 0.90 [0.72, 1.12] | 0.332 |
| Quintile 3 | 9.0–<11.1 | 0.75 [0.60, 0.93] | 0.011 | 0.99 [0.78, 1.26] | 0.962 | 1.03 [0.81, 1.31] | 0.806 |
| Quintile 4 | 11.1–<14.2 | 0.63 [0.49, 0.81] | <0.001 | 0.86 [0.66, 1.13] | 0.290 | 0.91 [0.69, 1.19] | 0.489 |
| Quintile 5 | ≥14.2 | 0.59 [0.43, 0.81] | 0.001 | 0.85 [0.60, 1.20] | 0.354 | 0.92 [0.65, 1.29] | 0.625 |
| P-trend | 0.057 | 0.887 | 0.668 | ||||
| Manganese | |||||||
| Quintile 1 | <1.9 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| Quintile 2 | 1.9–<2.6 | 0.62 [0.51, 0.76] | <0.001 | 0.79 [0.63, 0.98] | 0.033 | 0.82 [0.66, 1.02] | 0.078 |
| Quintile 3 | 2.6–<3.4 | 0.66 [0.53, 0.82] | <0.001 | 0.96 [0.76, 1.21] | 0.720 | 1.03 [0.81, 1.31] | 0.831 |
| Quintile 4 | 3.4–<4.4 | 0.50 [0.39, 0.63] | <0.001 | 0.79 [0.61, 1.02] | 0.071 | 0.88 [0.67, 1.16] | 0.379 |
| Quintile 5 | ≥4.4 | 0.49 [0.37, 0.64] | <0.001 | 0.87 [0.65, 1.18] | 0.379 | 1.06 [0.74, 1.53] | 0.743 |
| P-trend | <0.001 | 0.187 | 0.944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Huang, Y.; Wang, B.; Chen, H.; Pan, W.; Yang, M.; Xia, Z.; Zhang, R.; Yuan, C. Dietary Intake Levels of Iron, Copper, Zinc, and Manganese in Relation to Cognitive Function: A Cross-Sectional Study. Nutrients 2023, 15, 704. https://doi.org/10.3390/nu15030704
Zhao D, Huang Y, Wang B, Chen H, Pan W, Yang M, Xia Z, Zhang R, Yuan C. Dietary Intake Levels of Iron, Copper, Zinc, and Manganese in Relation to Cognitive Function: A Cross-Sectional Study. Nutrients. 2023; 15(3):704. https://doi.org/10.3390/nu15030704
Chicago/Turabian StyleZhao, Dong, Yilun Huang, Binghan Wang, Hui Chen, Wenfei Pan, Min Yang, Zhidan Xia, Ronghua Zhang, and Changzheng Yuan. 2023. "Dietary Intake Levels of Iron, Copper, Zinc, and Manganese in Relation to Cognitive Function: A Cross-Sectional Study" Nutrients 15, no. 3: 704. https://doi.org/10.3390/nu15030704
APA StyleZhao, D., Huang, Y., Wang, B., Chen, H., Pan, W., Yang, M., Xia, Z., Zhang, R., & Yuan, C. (2023). Dietary Intake Levels of Iron, Copper, Zinc, and Manganese in Relation to Cognitive Function: A Cross-Sectional Study. Nutrients, 15(3), 704. https://doi.org/10.3390/nu15030704






