Trajectories of Meat Intake and Risk of Type 2 Diabetes: Findings from the China Health and Nutrition Survey (1997–2018)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
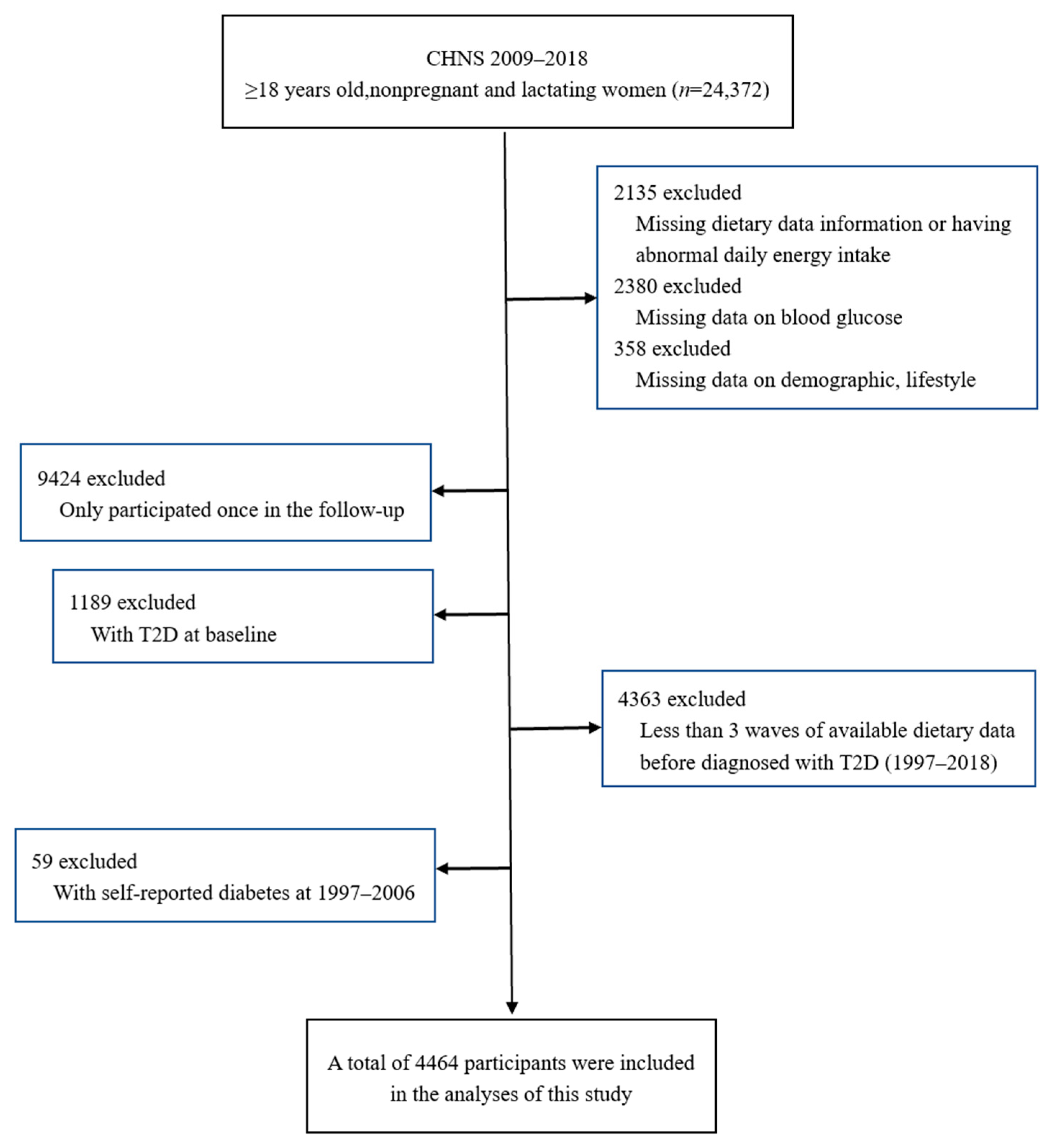
2.2. Study Participants
2.3. Measurement of Meat Intake
2.4. Diagnostic Criteria of T2D
2.5. Assessment of Covariates
2.6. Statistical Analysis
3. Results
3.1. Trajectory Groups of Meat Intake
3.2. Baseline Characteristics by Trajectory Groups
3.3. Trajectory Groups of Meat Intake and T2D
3.4. Cumulative Averages of Meat Intake and T2D
3.5. Dose–Response Relationship between Meat Intake and T2D
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109–119. [Google Scholar] [CrossRef]
- Hu, F.B.; Satija, A.; Manson, J.E. Curbing the Diabetes Pandemic: The Need for Global Policy Solutions. JAMA 2015, 313, 2319–2320. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Missbach, B.; König, J.; Hoffmann, G. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Steinbrecher, A.; Erber, E.; Grandinetti, A.; Kolonel, L.; Maskarinec, G. Meat consumption and risk of type 2 diabetes: The Multiethnic Cohort. Public Health Nutr. 2011, 14, 568–574. [Google Scholar] [CrossRef]
- Kouvari, M.; Notara, V.; Kalogeropoulos, N.; Panagiotakos, D.B. Diabetes mellitus associated with processed and unprocessed red meat: An overview. Int. J. Food Sci. Nutr. 2016, 67, 735–743. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, C.; Zhang, Z.; Liu, M.; Zhang, Y.; Li, H.; He, P.; Li, Q.; Qin, X. Variety and quantity of dietary protein intake from different sources and risk of new-onset diabetes: A Nationwide Cohort Study in China. BMC Med. 2022, 20, 6. [Google Scholar] [CrossRef]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.D.; Ley, S.H.; Vasanti, M.; Howard, A.G.; He, Y.; Hu, F.B. Time Trends of Dietary and Lifestyle Factors and Their Potential Impact on Diabetes Burden in China. Diabetes Care 2017, 40, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.J.; A, Y.X.; Xiao, Y. Investigation of the status of dietary intake in Uygur patients with type 2 diabetes mellitus. Chin. J. Nurs. 2007, 42, 976–979. [Google Scholar]
- Kou, L.X.; Le, L.Z.; Qiu, L.Q.; Wu, Y.L. Investigation and analysis of dietary habits of type 2 diabetes patients in Guangzhou. Guangdong Med. J. 2008, 29, 1027–1028. [Google Scholar]
- He, Y.N.; Zhao, W.H.; Bai, G.Y.; Fang, Y.H.; Zhang, J.; Yang, X.G.; Ding, G.G. Relationship between meat consumption and metabolic syndrome in adults in China. Chin. J. Epidemiol. 2018, 39, 892–897. [Google Scholar]
- Du, H.; on behalf of the China Kadoorie Biobank Collaborative Group; Guo, Y.; Bennett, D.A.; Bragg, F.; Bian, Z.; Chadni, M.; Yu, C.; Chen, Y.; Tan, Y.; et al. Red meat, poultry and fish consumption and risk of diabetes: A 9 year prospective cohort study of the China Kadoorie Biobank. Diabetologia 2020, 63, 767–779. [Google Scholar] [CrossRef]
- Nagin, D.S. Group-Based Trajectory Modeling: An Overview. Ann. Nutr. Metab. 2014, 65, 205–210. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15 (Suppl. S1), 2–7. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. Chinese Food Composition Table; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes Mellit. 2021, 13, 315–409. [Google Scholar]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- van der Nest, G.; Lima Passos, V.; Candel, M.; van Breukelen, G.J.P. An overview of mixture modelling for latent evolutions in longitudinal data: Modelling approaches, fit statistics and software. Adv. Life Course Res. 2020, 43, 100323. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Dietary Guidelines for Chinese; People’s Medical Publishing House: Beijing, China, 2022. [Google Scholar]
- Mikkelsen, B.; Williams, J.; Rakovac, I.; Wickramasinghe, K.; Hennis, A.; Shin, H.-R.; Farmer, M.; Weber, M.; Berdzuli, N.; Borges, C.; et al. Life course approach to prevention and control of non-communicable diseases. BMJ 2019, 364, l257. [Google Scholar] [CrossRef]
- Sami, W.; Ansari, T.; Butt, N.S.; Ab Hamid, M.R. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Richi, E.B.; Baumer, B.; Conrad, B.; Darioli, R.; Schmid, A.; Keller, U. Health Risks Associated with Meat Consumption: A Review of Epidemiological Studies. Int. J. Vitam. Nutr. Res. 2015, 85, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: Three cohorts of US men and women. JAMA Intern Med. 2013, 173, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, B.; Palli, D.; Masala, G. The InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: The EPIC-InterAct study. Diabetologia 2013, 56, 47–59. [Google Scholar]
- Yang, X.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Fan, M.; Cui, S.; Li, L. Meat and fish intake and type 2 diabetes: Dose–response meta-analysis of prospective cohort studies. Diabetes Metab. 2020, 46, 345–352. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Yang, X.; Hemler, E.C.; Fang, Y.; Zhao, L.; Zhang, J.; Yang, Z.; Wang, Z.; He, L.; et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982–2012: A cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019, 7, 540–548. [Google Scholar] [CrossRef]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: A systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Zhai, F.Y.; Du, S.F.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Popkin, B.M. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes. Rev. 2014, 15 (Suppl. S1), 16–26. [Google Scholar] [CrossRef] [PubMed]
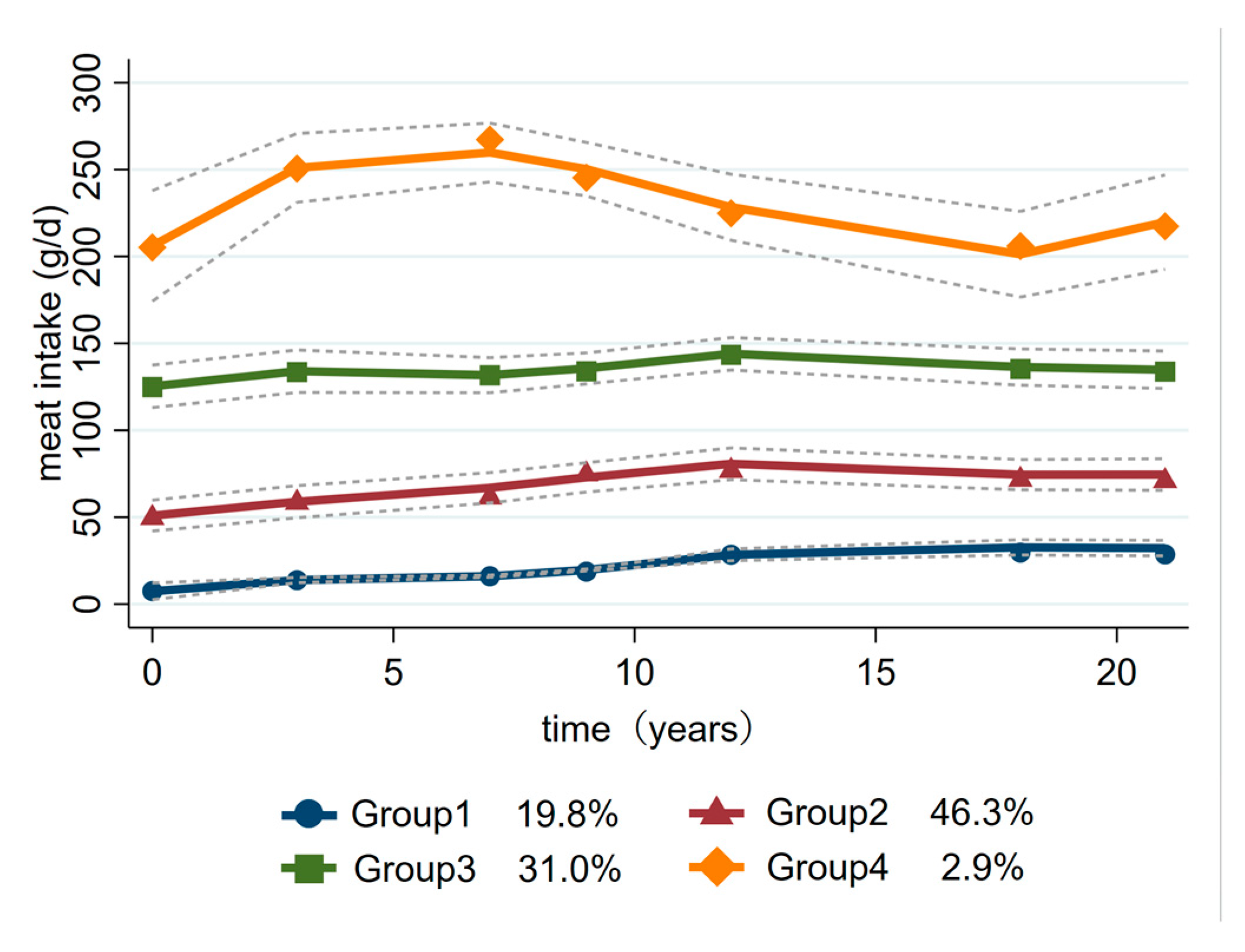

| Baseline Characteristics | Group 1 | Group 2 | Group 3 | Group 4 | p Value |
|---|---|---|---|---|---|
| (n = 883) | (n = 2066) | (n = 1384) | (n = 131) | ||
| Diabetes incidence rate (%) | 10.19 | 9.39 | 8.45 | 16.03 | 0.032 |
| Gender (%) | |||||
| Male | 40.20 | 40.71 | 54.48 | 71.76 | <0.001 |
| Female | 59.80 | 59.29 | 45.52 | 28.24 | |
| Age groups (%) | |||||
| 18–44 years | 57.19 | 57.99 | 66.55 | 77.1 | <0.001 |
| 45–64 years | 39.75 | 38.38 | 31.14 | 20.61 | |
| ≥65 years | 3.06 | 3.63 | 2.31 | 2.29 | |
| Geographic region (%) | |||||
| Urban | 9.51 | 24.78 | 37.86 | 45.8 | <0.001 |
| Rural | 90.49 | 75.22 | 62.14 | 54.2 | |
| Education level (%) | |||||
| Primary school | 65.23 | 47.10 | 35.40 | 28.24 | <0.001 |
| Middle school | 26.50 | 34.27 | 37.21 | 41.98 | |
| High school and above | 8.27 | 18.64 | 27.38 | 29.77 | |
| Household income per capita (%) | |||||
| Low | 55.72 | 32.33 | 21.75 | 20.61 | <0.001 |
| Median | 29.22 | 35.04 | 33.60 | 31.30 | |
| High | 15.06 | 32.62 | 44.65 | 48.09 | |
| Smoking (%) | |||||
| Nonsmoker | 69.31 | 72.85 | 66.18 | 48.09 | <0.001 |
| Current smoker | 30.69 | 27.15 | 33.82 | 51.91 | |
| Alcohol drinking (%) | |||||
| Nondrinker | 67.16 | 66.26 | 58.67 | 45.80 | <0.001 |
| Current drinker | 32.84 | 33.74 | 41.33 | 54.20 | |
| Physical activity (MET h/week) | 423.17 ± 237.52 | 316.82 ± 254.31 | 266.13 ± 229.99 | 189.89 ± 162.84 | <0.001 |
| Urbanicity score (mean [SD]) | 46.59 ± 10.83 | 56.72 ± 12.57 | 60.80 ± 11.62 | 63.33 ± 11.86 | <0.001 |
| Total energy intake (kcal, mean [SD]) | 2462.88 ± 721.58 | 2377.75 ± 707.98 | 2537.40 ± 687.79 | 2771.13 ± 688.70 | <0.001 |
| BMI (mg/kg, mean [SD]) | 22.54 ± 3.05 | 22.7 ± 3.09 | 22.42 ± 2.93 | 22.64 ± 3.15 | 0.136 |
| WC (cm, mean [SD]) | 78.74 ± 8.82 | 78.59 ± 9.75 | 77.62 ± 8.89 | 79.83 ± 10.06 | 0.004 |
| SBP (mmHg, mean [SD]) | 119.35 ± 16.84 | 117.93 ± 16.36 | 116.28 ± 14.62 | 116.18 ± 13.40 | <0.001 |
| DBP (mmHg, mean [SD]) | 77.58 ± 11.03 | 77.08 ± 10.97 | 75.90 ± 9.76 | 77.41 ± 10.00 | <0.001 |
| Baseline Characteristics | n | Cumulative Number of Cases/Person-Year | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||
| Group 2 | 2066 | 194/15,619 | 1 | 1 | 1 | 1 |
| Group 1 | 833 | 90/6249 | 1.10 (0.86, 1.42) | 1.14 (0.88, 1.48) | 1.16 (0.89, 1.50) | 1.02 (0.78, 1.33) |
| Group 3 | 1384 | 117/10,477 | 0.89 (0.71, 1.12) | 0.88 (0.70, 1.11) | 0.88 (0.70, 1.12) | 1.01 (0.78, 1.32) |
| Group 4 | 131 | 21/942 | 1.87 (1.19, 2.96) ** | 1.81 (1.14, 2.88) ** | 1.85 (1.16, 2.94) ** | 2.37 (1.41, 3.98) ** |
| Quintile of Meat Intake (g/day) | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| n = 892 | n = 893 | n = 893 | n = 893 | n = 893 | |
| Median (g/day) | 13.97 | 45.28 | 75.45 | 112.78 | 166.25 |
| Model 1 | 1.47 (1.09, 2.00) * | 1.25 (0.91, 1.71) | 1.00 (ref) | 0.96 (0.69, 1.34) | 1.29 (0.95, 1.76) |
| Model 2 | 1.54 (1.12, 2.10) * | 1.29 (0.94, 1.77) | 1.00 (ref) | 0.95 (0.68, 1.32) | 1.27 (0.93, 1.74) |
| Model 3 | 1.57 (1.15, 2.15) * | 1.31 (0.96, 1.80) | 1.00 (ref) | 0.95 (0.68, 1.32) | 1.29 (0.95, 1.77) |
| Model 4 | 1.46 (1.07, 2.01) * | 1.35 (0.98, 1.85) | 1.00 (ref) | 1.00 (0.72, 1.40) | 1.41 (1.03, 1.94) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, H.; Du, S.; Jiao, Y.; Wang, Q.; Su, C.; Zhang, B.; Ding, G. Trajectories of Meat Intake and Risk of Type 2 Diabetes: Findings from the China Health and Nutrition Survey (1997–2018). Nutrients 2023, 15, 3277. https://doi.org/10.3390/nu15143277
Liu M, Wang H, Du S, Jiao Y, Wang Q, Su C, Zhang B, Ding G. Trajectories of Meat Intake and Risk of Type 2 Diabetes: Findings from the China Health and Nutrition Survey (1997–2018). Nutrients. 2023; 15(14):3277. https://doi.org/10.3390/nu15143277
Chicago/Turabian StyleLiu, Mengran, Huijun Wang, Shufa Du, Yingying Jiao, Qi Wang, Chang Su, Bing Zhang, and Gangqiang Ding. 2023. "Trajectories of Meat Intake and Risk of Type 2 Diabetes: Findings from the China Health and Nutrition Survey (1997–2018)" Nutrients 15, no. 14: 3277. https://doi.org/10.3390/nu15143277
APA StyleLiu, M., Wang, H., Du, S., Jiao, Y., Wang, Q., Su, C., Zhang, B., & Ding, G. (2023). Trajectories of Meat Intake and Risk of Type 2 Diabetes: Findings from the China Health and Nutrition Survey (1997–2018). Nutrients, 15(14), 3277. https://doi.org/10.3390/nu15143277






