Intermittent Fasting on Neurologic Diseases: Potential Role of Gut Microbiota
Abstract
1. Introduction
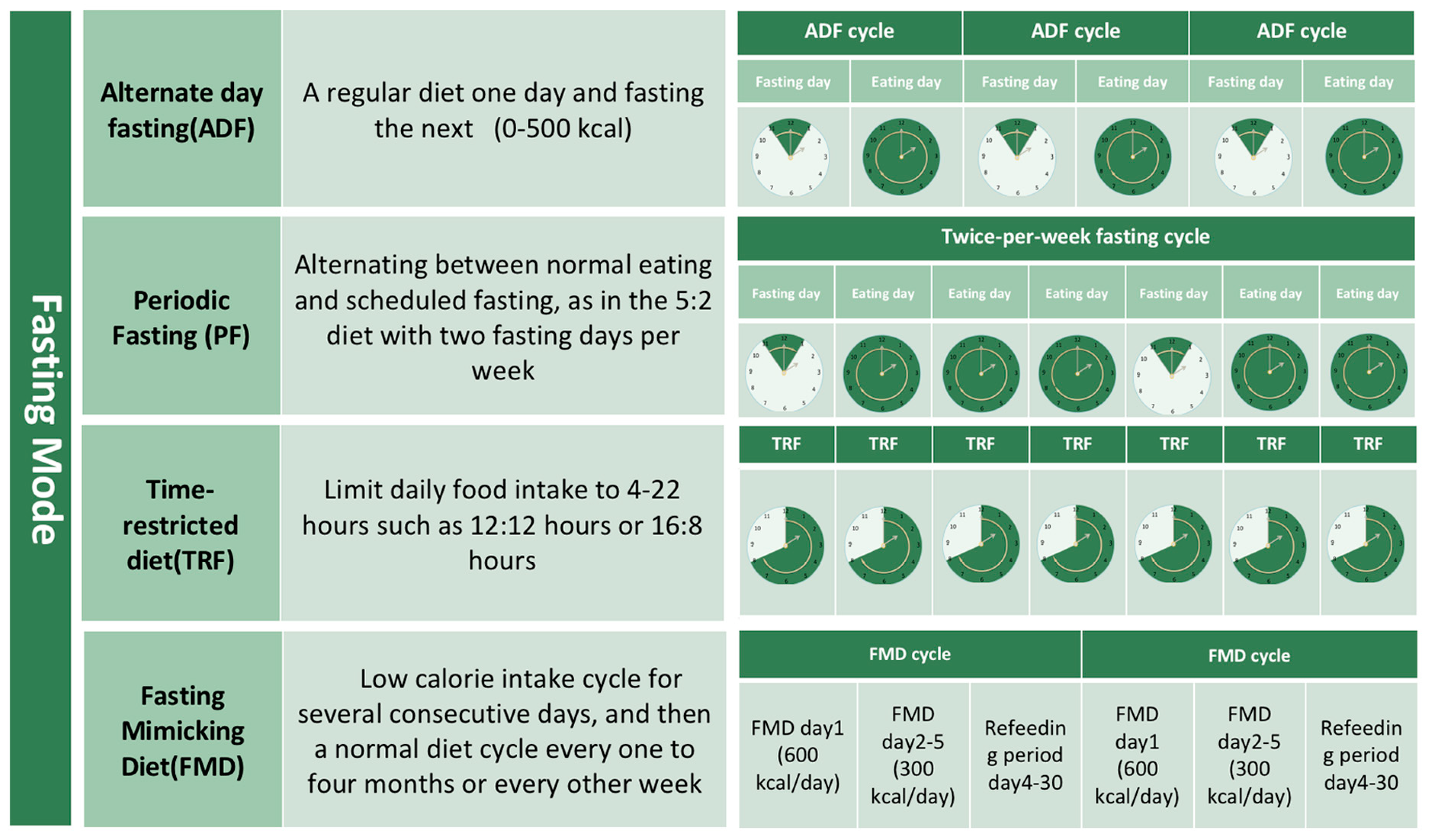
2. Intermittent Fasting (IF)
2.1. Concept and Modes
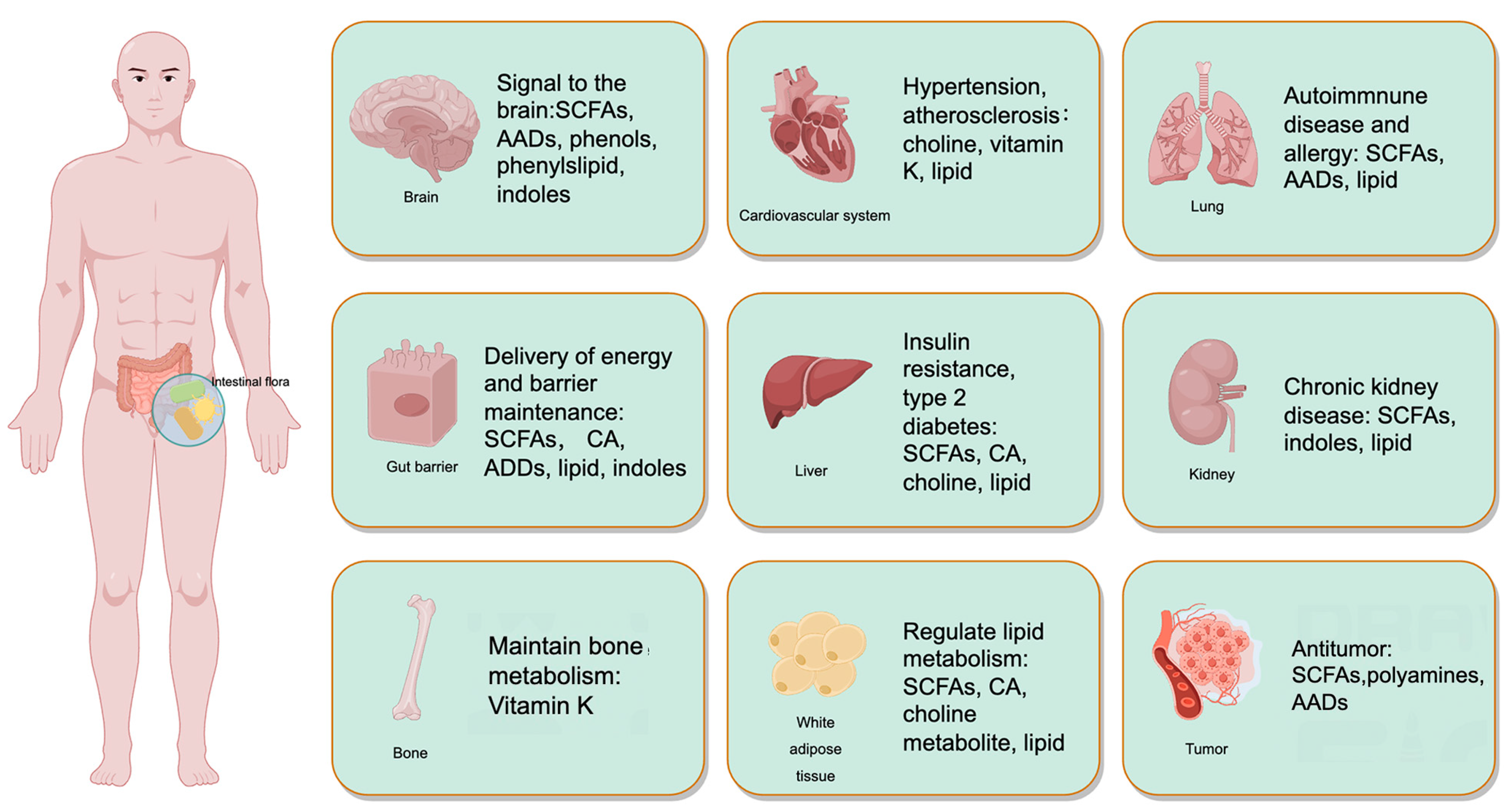
2.2. The Extensive Benefits of IF
2.3. Potential Harm of IF
2.3.1. Diet Quality and Energy Imbalance
2.3.2. Hormone Disorders
2.3.3. Dietary Patterns and Mental Health
2.3.4. Other Side Effect of IF
3. The Role of Intestinal Microflora in IF-Induced Improvement in Cognitive Protective Effect
3.1. The Changes of Microbial Metabolites Following IF
3.1.1. Short-Chain Fatty Acids (SCFAs)
3.1.2. Amino Acids and Derivatives
3.1.3. Bile Acid
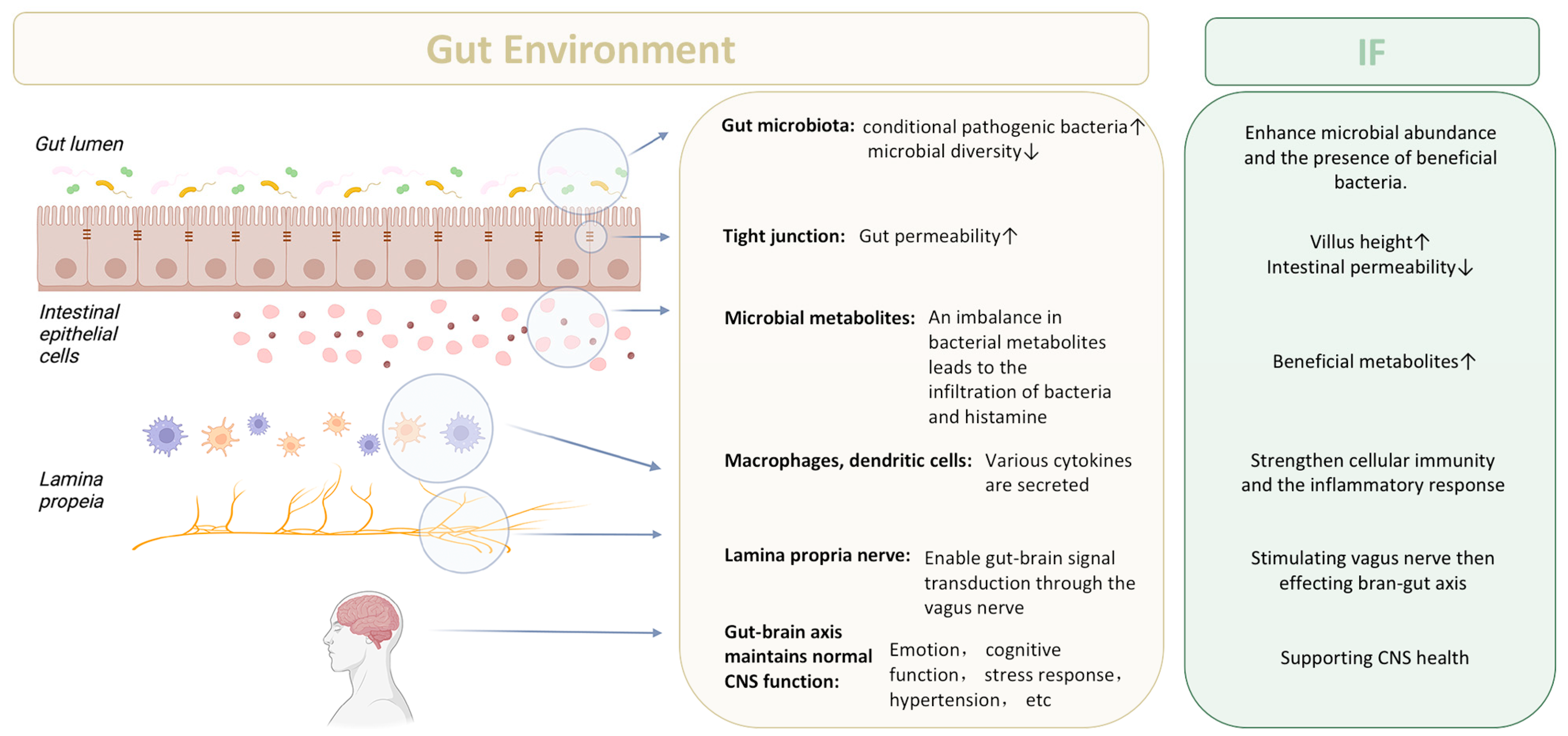
3.2. The Integrity of Intestinal Barrier
3.3. Vagal Nerve Signal
3.4. Geographical Evaluation of IF and Gut Microbiota Studies across Diverse Populations
4. The Potential Role of Gut Microbiota in IF-Mediated Neuroprotection
4.1. IF and Neurodegenerative Diseases
4.1.1. Alzheimer’s Disease
| Diseases | Outcomes of IF | Model System | Duration of Intervention | Key Findings |
|---|---|---|---|---|
| Alzheimer’s Disease | Exacerbated AD-like neurodegenerative changes | 5XFAD mice | 4 months | Every other fasting regimen increased inflammation and altered glutamatergic signaling, without affecting Aβ load [137]. |
| Enhanced Aβ clearance through autophagy | In vitro (neuronal toxicity) | Not specified | Caloric restriction and prolonged IF increased markers of autophagic activity and decreased markers of apoptosis [138]. | |
| Improvement in neuronal differentiation and memory | 3xTg-AD mice | 3 months | IF activated GSK-3β, leading to enhanced neuronal differentiation in the hippocampus and improved memory [139]. | |
| Neuroprotective effects; increased BDNF and NT3 | Type 2 diabetic rats | 3 months | IF increased levels of BDNF, NT3, serotonin, dopamine, and glutamic acid, showing potential protective effects [140]. | |
| Improved cognitive function and Aβ clearance | APP/PS1 double-transgenic mice | 5 months | IF restored AQP4 polarity, possibly through β-hydroxybutyrate, leading to reduced Aβ pathology [141]. | |
| Ameliorated cognitive deficits and reduced Aβ and tau pathologies | 3xTgAD mice | 7 or 14 months | Both CR and IF improved cognitive function, with CR showing reduced Aβ and tau levels [142]. | |
| Improved memory function and alleviated osteoarthritic symptoms | Ovariectomised rats induced with AD and OA | 6 weeks | IF with a high-protein diet showed neuroprotective effects, potentially through the gut–microbiota–metabolites–brain axis [143]. | |
| Improved cognitive function and metabolic disturbances | Ovariectomized rats infused with β-amyloid | 4 weeks | IF protected against memory loss and metabolic disturbances in estrogen-deficient rats [144]. | |
| Parkinson’s Disease | Neuroprotective; reduced dopaminergic neuronal loss and astroglial activation | MPTP mouse model | 2 weeks | Alternate-day fasting increased neurotrophic factors, suppressed motor impairments, and mitigated MPTP-induced dopaminergic neuronal loss [145]. |
| Exacerbated neuronal death and increased excitatory amino acids | C57BL/6J mice treated with rotenone | 28 days | IF in combination with neurotoxin exposure led to increased neuronal death, excitatory amino acids, and inflammatory lipids [146]. | |
| Huntington’s Disease | Enhanced mHTT clearance and promoted autophagy | YAC128 mice expressing cleavable mHTT | Not specified | Scheduled feeding paradigm reduced mHTT levels; fasting-induced autophagy remained functional despite impaired basal autophagy due to cleavable mHTT [147]. |
| Multiple Sclerosis | Investigate impact on MS during Ramadan | 80 adult MS patients (40 fasting, 40 non-fasting) in Isfahan, Iran | Ramadan period + 6 months follow-up | No significant changes in disability or clinical relapses [148]. |
| Determine feasibility of Time Restricted Eating (TRE) | 12 participants with RRMS | 8 weeks, 16 h fasting daily | TRE feasible and acceptable; exploratory results suggest further study warranted [149]. | |
| Assess intermittent caloric restriction on EAE (MS model) | Mice with EAE | 4th week post-immunization, two cycles of 3 days FMD + 4 days normal feeding | Decreased EAE severity, immune cell infiltration, and CNS demyelination; enhanced CNS recover [150]. | |
| Explore effects of IF on MS and EAE, focusing on gut microbiota | EAE mice and pilot clinical trial in MS patients | / | IF improved clinical course and pathology in EAE, altered gut flora and T cell profiles; similar effects in pilot clinical trial [47]. |
4.1.2. Parkinson’s Disease (PD)
4.1.3. Huntington Disease (HD)
4.1.4. Multiple Sclerosis (MS)
4.2. IF and Acute Central Nervous System Injury
4.2.1. Ischemic Stroke
4.2.2. Epilepsy
4.3. Perioperative Neurocognitive Dysfunction (PND)
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4EPS | 4-ethyphenyl sulfate |
| AD | Alzheimer’s disease |
| ADF | alternate day diet |
| ANS | autonomic nervous system |
| Aβ | amyloid-beta |
| CNS | central nervous system |
| CR | calorie restriction |
| CSF | cerebrospinal fluid |
| EAE | experimental autoimmune encephalomyelitis |
| ENS | intestinal nervous system |
| FMD | simulated fasting diet |
| GI | gastrointestinal |
| GIT | gastrointestinal tract |
| GLP-1 | glucagon-like peptide-1 |
| HD | Huntington’s disease |
| IER | immune effector response |
| IF | intermittent fasting |
| IL | interleukin |
| KD | ketogenic diet |
| LPSs | lipopolysaccharides |
| MS | multiple sclerosis |
| PD | Parkinson’s disease |
| PF | periodic fasting |
| PND | perioperative neurocognitive dysfunction |
| SCFAs | short-chain fatty acids |
| SHBG | sex hormone binding globulin |
| TRF | time restricted diet |
| VN | vagus nerve |
| cICCs | Cajal interstitial cell |
| αSyn | α-synuclein |
References
- Przybyłowicz, K.E.; Danielewicz, A. Eating Habits and Disease Risk Factors. Nutrients 2022, 14, 3143. [Google Scholar] [CrossRef]
- Mohan, V.; Unnikrishnan, R.; Shobana, S.; Malavika, M.; Anjana, R.; Sudha, V. Are excess carbohydrates the main link to diabetes & its complications in Asians? Indian J. Med. Res. 2018, 148, 531–538. [Google Scholar] [CrossRef]
- Flanagan, E.; Lamport, D.; Brennan, L.; Burnet, P.; Calabrese, V.; Cunnane, S.C.; de Wilde, M.C.; Dye, L.; Farrimond, J.A.; Lombardo, N.E.; et al. Nutrition and the ageing brain: Moving towards clinical applications. Ageing Res. Rev. 2020, 62, 101079. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef] [PubMed]
- Fanti, M.; Mishra, A.; Longo, V.D.; Brandhorst, S. Time-Restricted Eating, Intermittent Fasting, and Fasting-Mimicking Diets in Weight Loss. Curr. Obes. Rep. 2021, 10, 70–80. [Google Scholar] [CrossRef]
- Stockman, M.-C.; Thomas, D.; Burke, J.; Apovian, C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Gudden, J.; Vasquez, A.A.; Bloemendaal, M. The Effects of Intermittent Fasting on Brain and Cognitive Function. Nutrients 2021, 13, 3166. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Whittaker, D.S.; Akhmetova, L.; Carlin, D.; Romero, H.; Welsh, D.K.; Colwell, C.S.; Desplats, P. Circadian modulation by time-restricted feeding rescues brain pathology and improves memory in mouse models of Alzheimer’s disease. Cell Metab. 2023, 35, 1704–1721.e6. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Hofer, U. Gut–brain axis in ageing. Nat. Rev. Microbiol. 2022, 20, 446. [Google Scholar] [CrossRef] [PubMed]
- Welton, S.; Minty, R.; O’Driscoll, T.; Willms, H.; Poirier, D.; Madden, S.; Kelly, L. Intermittent fasting and weight loss Systematic review. Can. Fam. Physician 2020, 66, 117–125. [Google Scholar] [PubMed]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical application of intermittent fasting for weight loss: Progress and future directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 81–94, Correction in Nat. Rev. Neurosci. 2020, 21, 63–80. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Karam, G.; Agarwal, A.; Sadeghirad, B.; Jalink, M.; Hitchcock, C.L.; Ge, L.; Kiflen, R.; Ahmed, W.; Zea, A.M.; Milenkovic, J.; et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: Systematic review and network meta-analysis. BMJ 2023, 380, e072003. [Google Scholar] [CrossRef]
- Pedersen, A.L.W.; Lindekilde, C.R.; Andersen, K.; Hjorth, P.; Gildberg, F.A. Health behaviours of forensic mental health service users, in relation to smoking, alcohol consumption, dietary behaviours and physical activity—A mixed methods systematic review. J. Psychiatr. Ment. Health Nurs. 2021, 28, 444–461. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Ortiz, R.M. Thyroid Hormone Regulation and Insulin Resistance: Insights From Animals Naturally Adapted to Fasting. Physiology 2017, 32, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Akasheh, R.T.; Kroeger, C.M.; Trepanowski, J.F.; Gabel, K.; Hoddy, K.K.H.; Kalam, F.; Cienfuegos, S.; Varady, K.A. Weight loss efficacy of alternate day fasting versus daily calorie restriction in subjects with subclinical hypothyroidism: A secondary analysis. Appl. Physiol. Nutr. Metab. 2020, 45, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Klein, S.; Holloszy, J.O.; Premachandra, B.N. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab. 2006, 91, 3232–3235. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Corapi, S.; Gabel, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of Intermittent Fasting on Reproductive Hormone Levels in Females and Males: A Review of Human Trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef]
- Kalam, F.; Akasheh, R.T.; Cienfuegos, S.; Ankireddy, A.; Gabel, K.; Ezpeleta, M.; Lin, S.; Tamatam, C.M.; Reddy, S.P.; Spring, B.; et al. Effect of time-restricted eating on sex hormone levels in premenopausal and postmenopausal females. Obesity 2023, 31 (Suppl. S1), 57–62. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, W.; Luo, C.; Sun, L.; Feng, T.; Zhang, Y.; Yin, Y.; Zhang, W. Maternal intermittent fasting in mice disrupts the intestinal barrier leading to metabolic disorder in adult offspring. Commun. Biol. 2023, 6, 30. [Google Scholar] [CrossRef]
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef]
- Alvarez Ruiz, E.M.; Gutiérrez-Rojas, L. Comorbidity of bipolar disorder and eating disorders. Rev. Psiquiatr. Salud Ment. 2015, 8, 232–241. [Google Scholar] [CrossRef]
- Altman, S.E.; Shankman, S.A. What is the association between obsessive–compulsive disorder and eating disorders? Clin. Psychol. Rev. 2009, 29, 638–646. [Google Scholar] [CrossRef]
- Barati, M.; Ghahremani, A.; Ahmadabad, A.H. Intermittent fasting: A promising dietary intervention for autoimmune diseases. Autoimmun. Rev. 2023, 22, 103408. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.; Kahles, F.; Liu, D.; Downey, J.; Koekkoek, L.L.; Roudko, V.; D’souza, D.; McAlpine, C.S.; Halle, L.; Poller, W.C.; et al. Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity 2023, 56, 783–796.e7. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Dorsett, Y.; Fontana, L.; Zhou, Y.; Piccio, L. Effects of dietary restriction on gut microbiota and CNS autoimmunity. Clin. Immunol. 2022, 235, 108575. [Google Scholar] [CrossRef] [PubMed]
- Saglam, D.; Colak, G.A.; Sahin, E.; Ekren, B.Y.; Sezerman, U.; Bas, M. Effects of Ramadan intermittent fasting on gut microbiome: Is the diet key? Front. Microbiol. 2023, 14, 1203205. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xia, K.; Dai, M.; Han, X.; Yuan, P.; Liu, J.; Liu, S.; Jia, F.; Chen, J.; Jiang, F.; et al. Intermittent fasting modulates the intestinal microbiota and improves obesity and host energy metabolism. NPJ Biofilms Microbiomes 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jasbi, P.; Bowes, D.A.; Dirks, B.; Whisner, C.M.; Arciero, K.M.; Poe, M.; Gu, H.; Gumpricht, E.; Sweazea, K.L.; et al. Exploratory analysis of one versus two-day intermittent fasting protocols on the gut microbiome and plasma metabolome in adults with overweight/obesity. Front. Nutr. 2022, 9, 1036080. [Google Scholar] [CrossRef]
- Khan, M.N.; Rana, M.I.; Ayyaz, A.; Khan, M.Y.; Imran, M. Intermittent fasting positively modulates human gut microbial diversity and ameliorates blood lipid profile. Front. Microbiol. 2022, 13, 922727. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Zhang, X.; Ma, M.; Xie, Z.; Pan, Q.; Ma, Z.; Peppelenbosch, M.P. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am. J. Clin. Nutr. 2021, 113, 1332–1342. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Frank, D.N.; Borengasser, S.J.; Ostendorf, D.M.; Ir, D.; Jambal, P.; Bing, K.; Wayland, L.; Siebert, J.C.; Bessesen, D.H.; et al. The Gut Microbiota during a Behavioral Weight Loss Intervention. Nutrients 2021, 13, 3248. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Roshanravan, N.; Alamdari, N.M.; Safaiyan, A.; Mosharkesh, E.; Hadi, A.; Barati, M.; Ostadrahimi, A. The interplay between fasting, gut microbiota, and lipid profile. Int. J. Clin. Pract. 2021, 75, e14591. [Google Scholar] [CrossRef] [PubMed]
- Maifeld, A.; Bartolomaeus, H.; Löber, U.; Avery, E.G.; Steckhan, N.; Markó, L.; Wilck, N.; Hamad, I.; Šušnjar, U.; Mähler, A.; et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat. Commun. 2021, 12, 1970. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Bagheri, R.; Ashtary-Larky, D.; Wong, A.; Triki, R.; Hackney, A.C.; Laher, I.; Ben Abderrahman, A. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol. Behav. 2020, 225, 113090. [Google Scholar] [CrossRef]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutr. Health. 2020, 26, 79–85. [Google Scholar] [CrossRef]
- Mesnage, R.; Grundler, F.; Schwiertz, A.; Le Maho, Y.; de Toledo, F.W. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J. Nutr. Sci. 2019, 8, e36. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Yalinay, M.; Karakan, T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting. Turk. J. Gastroenterol. 2019, 30, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Zhang, J.; Liu, W.; Jing, W.; Lyu, B.; Yu, H.; Zhang, Z. Insoluble Dietary Fiber from Okara Combined with Intermittent Fasting Treatment Synergistically Confers Antiobesity Effects by Regulating Gut Microbiota and Its Metabolites. J. Agric. Food Chem. 2023, 71, 13346–13362. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Zhou, R.; Wang, M.; Xiang, W.; You, Z.; Li, M.; Tang, R.; Zheng, J.; Li, J.; et al. Gut microbiota and transcriptome dynamics in every-other-day fasting are associated with neuroprotection in rats with spinal cord injury. Front. Microbiol. 2023, 14, 1206909. [Google Scholar] [CrossRef]
- Teker, H.T.; Ceylani, T. Intermittent fasting supports the balance of the gut microbiota composition. Int. Microbiol. 2023, 26, 51–57. [Google Scholar] [CrossRef]
- Xia, J.; Guo, W.; Hu, M.; Jin, X.; Zhang, S.; Liu, B.; Qiu, H.; Wang, K.; Zhuge, A.; Li, S.; et al. Resynchronized rhythmic oscillations of gut microbiota drive time-restricted feeding induced nonalcoholic steatohepatitis alleviation. Gut Microbes 2023, 15, 2221450. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-X.; Hu, J.-Q.; Fu, W.; Zhong, J.; Cao, C.; Wang, C.-C.; Qi, S.-Q.; Zhang, X.-L.; Liu, G.-H.; Gao, Y.-D. Intermittent fasting protects against food allergy in a murine model via regulating gut microbiota. Front. Immunol. 2023, 14, 1167562. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, F.; Wang, Y.; Su, Y.; Verhaar, A.; Ma, Z.; Peppelenbosch, M.P. Investigating Ramadan Like Fasting Effects on the Gut Microbiome in BALB/c Mice. Front. Nutr. 2022, 9, 832757. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-Y.; Zhang, J.; Wang, J.; Wang, Y.; Li, Z.; Liao, Y.; Liao, Y.; Zhang, C.; Liu, Z.; Song, L.; et al. Intermittent fasting protects against Alzheimer’s disease in mice by altering metabolism through remodeling of the gut microbiota. Nat. Aging 2022, 2, 1024–1039. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Che, M.; Li, Y.; Feng, R.; Sun, C. Gut microbiota mediates the anti-obesity effect of intermittent fasting by inhibiting intestinal lipid absorption. J. Nutr. Biochem. 2023, 116, 109318. [Google Scholar] [CrossRef]
- Wu, J.; Man, D.; Shi, D.; Wu, W.; Wang, S.; Wang, K.; Li, Y.; Yang, L.; Bian, X.; Wang, Q.; et al. Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome. Nutrients 2022, 14, 5311. [Google Scholar] [CrossRef]
- Hernandez, A.R.; Watson, C.; Federico, Q.P.; Fletcher, R.; Brotgandel, A.; Buford, T.W.; Carter, C.S.; Burke, S.N. Twelve Months of Time-Restricted Feeding Improves Cognition and Alters Microbiome Composition Independent of Macronutrient Composition. Nutrients 2022, 14, 3977. [Google Scholar] [CrossRef]
- Gregor, A.; Huber, L.; Auernigg-Haselmaier, S.; Sternberg, F.; Billerhart, M.; Dunkel, A.; Somoza, V.; Ogris, M.; Kofler, B.; Longo, V.D.; et al. A Comparison of the Impact of Restrictive Diets on the Gastrointestinal Tract of Mice. Nutrients 2022, 14, 3120. [Google Scholar] [CrossRef]
- Xie, S.; Guan, C.; Huang, T.; Liu, Y.; Yuan, F.; Xu, D. Intermittent fasting promotes repair of rotator cuff injury in the early postoperative period by regulating the gut microbiota. J. Orthop. Translat. 2022, 36, 216–224. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, B.; Abo-Hamzy, T.; Nelson, J.W.; Ambati, C.S.R.; Petrosino, J.F.; Bryan, R.M.; Durgan, D.J. Restructuring the Gut Microbiota by Intermittent Fasting Lowers Blood Pressure. Circ. Res. 2021, 128, 1240–1254. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Loh, Y.J.; Yang, X.; Zhang, C. The effect of calorie intake, fasting, and dietary composition on metabolic health and gut microbiota in mice. BMC Biol. 2021, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, Y.; Luo, X.M.; Ma, Y.; Liu, J.; Wang, H. Intermittent Fasting Reshapes the Gut Microbiota and Metabolome and Reduces Weight Gain More Effectively Than Melatonin in Mice. Front. Nutr. 2021, 8, 784681. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, W.; Wang, J.; Yu, J.; Yang, L.-Q. Intermittent Fasting Improves Lipid Metabolism Through Changes in Gut Microbiota in Diet-Induced Obese Mice. Med. Sci. Monit. 2020, 26, e926789. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, H.; Xie, Z.; Wang, L.; Sun, Y.; Yang, H.; Hu, D.; Mao, Y. Time-Restricted Feeding Reduces the Detrimental Effects of a High-Fat Diet, Possibly by Modulating the Circadian Rhythm of Hepatic Lipid Metabolism and Gut Microbiota. Front. Nutr. 2020, 7, 596285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, Y.; Li, F.; Wang, Y.; Ma, Z.; Li, Z.; Su, J. The effects of daily fasting hours on shaping gut microbiota in mice. BMC Microbiol. 2020, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Esteve-Llorens, X.; Darriba, C.; Moreira, M.T.; Feijoo, G.; González-García, S. Towards an environmentally sustainable and healthy Atlantic dietary pattern: Life cycle carbon footprint and nutritional quality. Sci. Total. Environ. 2019, 646, 704–715. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Wei, H.; Yu, C.; Zhang, C.; Ren, Y.; Guo, L.; Wang, T.; Chen, F.; Li, Y.; Zhang, X.; Wang, H.; et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed. Pharmacother. 2023, 160, 114308. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Wang, B.; Wang, Y. Xiaoyao San ameliorates high-fat diet-induced anxiety and depression via regulating gut microbiota in mice. Biomed. Pharmacother. 2022, 156, 113902. [Google Scholar] [CrossRef]
- Su, S.; Chen, M.; Wu, Y.; Lin, Q.; Wang, D.; Sun, J.; Hai, J. Fecal microbiota transplantation and short-chain fatty acids protected against cognitive dysfunction in a rat model of chronic cerebral hypoperfusion. CNS Neurosci. Ther. 2023, 29, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wang, B.; Tian, P.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Daily intake of Lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation. Food Funct. 2021, 12, 2591–2604. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Xia, J. Stroke and Vascular Cognitive Impairment: The Role of Intestinal Microbiota Metabolite TMAO. CNS Neurol. Disord. Drug Targets 2023, 23, 102–121. [Google Scholar] [CrossRef]
- Zhong, C.; Lu, Z.; Che, B.; Qian, S.; Zheng, X.; Wang, A.; Bu, X.; Zhang, J.; Ju, Z.; Xu, T.; et al. Choline Pathway Nutrients and Metabolites and Cognitive Impairment After Acute Ischemic Stroke. Stroke 2021, 52, 887–895. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—An emerging role for gut microbiome. Alzheimer’s Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Nie, K.; Li, Y.; Zhang, J.; Gao, Y.; Qiu, Y.; Gan, R.; Zhang, Y.; Wang, L. Distinct Bile Acid Signature in Parkinson’s Disease With Mild Cognitive Impairment. Front. Neurol. 2022, 13, 897867. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Sun, P.; Wang, M.; Li, Z.; Wei, J.; Liu, F.; Zheng, W.; Zhu, X.; Chai, X.; Zhao, S. Eucommiae cortex polysaccharides mitigate obesogenic diet-induced cognitive and social dysfunction via modulation of gut microbiota and tryptophan metabolism. Theranostics 2022, 12, 3637–3655. [Google Scholar] [CrossRef]
- Camacho-Barcia, L.; García-Gavilán, J.; Martínez-González, M.; Fernández-Aranda, F.; Galié, S.; Corella, D.; Cuenca-Royo, A.; Romaguera, D.; Vioque, J.; Alonso-Gómez, M.; et al. Vitamin K dietary intake is associated with cognitive function in an older adult Mediterranean population. Age Ageing 2022, 51, afab246. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Almeida, O.P. Effect of Vitamin B Supplementation on Cognitive Function in the Elderly: A Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 419–434. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef]
- He, P.; Yu, L.; Tian, F.; Zhang, H.; Chen, W.; Zhai, Q. Dietary Patterns and Gut Microbiota: The Crucial Actors in Inflammatory Bowel Disease. Adv. Nutr. 2022, 13, 1628–1651. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The association of diet, gut microbiota and colorectal cancer: What we eat may imply what we get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Yang, B.; Xiong, Z.; Lin, M.; Yang, Y.; Chen, Y.; Zeng, J.; Jia, X.; Feng, L. Astragalus polysaccharides alleviate type 1 diabetes via modulating gut microbiota in mice. Int. J. Biol. Macromol. 2023, 234, 123767. [Google Scholar] [CrossRef]
- May, K.S.; Hartigh, L.J.D. Modulation of Adipocyte Metabolism by Microbial Short-Chain Fatty Acids. Nutrients 2021, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-F.; Wang, C.-Y.; Wang, J.-H.; Wang, Q.-N.; Li, S.-J.; Wang, H.-O.; Zhou, F.; Li, J.-M. Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage. Nutrients 2022, 14, 1882. [Google Scholar] [CrossRef] [PubMed]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut–Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sun, Q.; Zheng, H.; Zhang, Y.; Wang, Y.; Liu, S.; Duan, L. Roseburia hominis Alleviates Neuroinflammation via Short-Chain Fatty Acids through Histone Deacetylase Inhibition. Mol. Nutr. Food Res. 2022, 66, e2200164. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.-Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: An integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut 2021, 71, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Contreras-Rodríguez, O.; Burokas, A.; Ortega-Sanchez, J.-A.; Blasco, G.; Coll, C.; Biarnés, C.; Castells-Nobau, A.; Puig, J.; et al. Obesity-associated deficits in inhibitory control are phenocopied to mice through gut microbiota changes in one-carbon and aromatic amino acids metabolic pathways. Gut 2021, 70, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Pascual, F.; Camilli, S.; Lockey, R.F.; Kolliputi, N. Mind-body connection: Metabolite 4-ethylphenyl linked to anxiety behavior and oligodendrocyte modification in autism spectrum disorder. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G422–G425. [Google Scholar] [CrossRef]
- Niu, H.; Zhou, X.; Gong, P.; Jiao, Y.; Zhang, J.; Wu, Y.; Lyu, L.; Liang, C.; Chen, S.; Han, X.; et al. Effect of Lactobacillus rhamnosus MN-431 Producing Indole Derivatives on Complementary Feeding-Induced Diarrhea Rat Pups Through the Enhancement of the Intestinal Barrier Function. Mol. Nutr. Food Res. 2022, 66, e2100619. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Byrne, C.D. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2023, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Arnoriaga-Rodríguez, M.; Martin, M.; de la Vega-Correa, L.; Zapata, C.; Burokas, A.; Blasco, G.; Coll, C.; Escrichs, A.; et al. Microbiota alterations in proline metabolism impact depression. Cell Metab. 2022, 34, 681–701.e10. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.; Buchmann, A.; Müller, S.T.; Volleberg, M.; Haynes, M.; Ghisleni, C.; Noeske, R.; Tuura, R.; Hasler, G. Evaluation of Prefrontal γ-Aminobutyric Acid and Glutamate Levels in Individuals With Major Depressive Disorder Using Proton Magnetic Resonance Spectroscopy. JAMA Psychiatry 2022, 79, 1209–1216. [Google Scholar] [CrossRef]
- Xia, H.; Chen, H.; Cheng, X.; Yin, M.; Yao, X.; Ma, J.; Huang, M.; Chen, G.; Liu, H. Zebrafish: An efficient vertebrate model for understanding role of gut microbiota. Mol. Med. 2022, 28, 161. [Google Scholar] [CrossRef]
- Fiaschini, N.; Mancuso, M.; Tanori, M.; Colantoni, E.; Vitali, R.; Diretto, G.; Rebenaque, L.L.; Stronati, L.; Negroni, A. Liver Steatosis and Steatohepatitis Alter Bile Acid Receptors in Brain and Induce Neuroinflammation: A Contribution of Circulating Bile Acids and Blood-Brain Barrier. Int. J. Mol. Sci. 2022, 23, 14254. [Google Scholar] [CrossRef]
- Guan, B.; Tong, J.; Hao, H.; Yang, Z.; Chen, K.; Xu, H.; Wang, A. Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases. Acta Pharm. Sin. B 2022, 12, 2129–2149. [Google Scholar] [CrossRef]
- Ren, Z.-L.; Li, C.-X.; Ma, C.-Y.; Chen, D.; Chen, J.-H.; Xu, W.-X.; Chen, C.-A.; Cheng, F.-F.; Wang, X.-Q. Linking Nonalcoholic Fatty Liver Disease and Brain Disease: Focusing on Bile Acid Signaling. Int. J. Mol. Sci. 2022, 23, 13045. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, F.; Ning, J.; Peng, R.; Shang, J.; Liu, H.; Shang, M.; Bao, X.-Q.; Zhang, D. Novel compound FLZ alleviates rotenone-induced PD mouse model by suppressing TLR4/MyD88/NF-κB pathway through microbiota–gut–brain axis. Acta Pharm. Sin. B 2021, 11, 2859–2879. [Google Scholar] [CrossRef]
- Hu, R.; Li, S.; Diao, H.; Huang, C.; Yan, J.; Wei, X.; Zhou, M.; He, P.; Wang, T.; Fu, H.; et al. The interaction between dietary fiber and gut microbiota, and its effect on pig intestinal health. Front. Immunol. 2023, 14, 1095740. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Albaugh, V.L.; Neuhuber, W.L. Gut-brain communication and obesity: Understanding functions of the vagus nerve. J. Clin. Investig. 2021, 131, e143770. [Google Scholar] [CrossRef]
- Prescott, S.L.; Liberles, S.D. Internal senses of the vagus nerve. Neuron 2022, 110, 579–599. [Google Scholar] [CrossRef]
- Strader, A.D.; Woods, S.C. Gastrointestinal hormones and food intake. Gastroenterology 2005, 128, 175–191. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.B.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Bekkering, P.; Jafri, I.; van Overveld, F.J.; Rijkers, G.T. The intricate association between gut microbiota and development of type 1, type 2 and type 3 diabetes. Expert Rev. Clin. Immunol. 2013, 9, 1031–1041. [Google Scholar] [CrossRef]
- Zhao, Y.; Dua, P.; Lukiw, W.J. Microbial Sources of Amyloid and Relevance to Amyloidogenesis and Alzheimer’s Disease (AD). J. Alzheimer’s Dis. Parkinsonism. 2015, 5, 177. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lukiw, W.J. Alzheimer’s disease and the microbiome. Front. Cell. Neurosci. 2013, 7, 153. [Google Scholar] [CrossRef]
- Syed, A.K.; Boles, B.R. Fold modulating function: Bacterial toxins to functional amyloids. Front. Microbiol. 2014, 5, 401. [Google Scholar] [CrossRef]
- Hufnagel, D.A.; Tükel, C.; Chapman, M.R. Disease to dirt: The biology of microbial amyloids. PLoS Pathog. 2013, 9, e1003740. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Boles, B.R. Microbial amyloids—Functions and interactions within the host. Curr. Opin. Microbiol. 2013, 16, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Oli, M.W.; Otoo, H.N.; Crowley, P.J.; Heim, K.P.; Nascimento, M.M.; Ramsook, C.B.; Lipke, P.N.; Brady, L.J. Functional amyloid formation by Streptococcus mutans. Microbiology 2012, 158 Pt 12, 2903–2916. [Google Scholar] [CrossRef]
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Transmissible Proteins: Expanding the prion heresy. Cell 2012, 149, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Berti, V.; Murray, J.; Davies, M.; Spector, N.; Tsui, W.H.; Li, Y.; Williams, S.; Pirraglia, E.; Vallabhajosula, S.; McHugh, P.; et al. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J. Nutr. Health Aging 2015, 19, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Stefani, M.; Rigacci, S. Beneficial properties of natural phenols: Highlight on protection against pathological conditions associated with amyloid aggregation. BioFactors 2014, 40, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceuticals and amyloid neurodegenerative diseases: A focus on natural phenols. Expert Rev. Neurother. 2014, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef]
- Lazic, D.; Tesic, V.; Jovanovic, M.; Brkic, M.; Milanovic, D.; Zlokovic, B.V.; Kanazir, S.; Perovic, M. Every-other-day feeding exacerbates inflammation and neuronal deficits in 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Dis. 2020, 136, 104745. [Google Scholar] [CrossRef]
- Ntsapi, C.M.; Loos, B. Neurons die with heightened but functional macro- and chaperone mediated autophagy upon increased amyloid-ß induced toxicity with region-specific protection in prolonged intermittent fasting. Exp. Cell Res. 2021, 408, 112840. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Zhang, Y.; Wei, X.; Zang, J.; Liu, Y.; Wang, Y.; Gong, C.; Wei, W. Intermittent fasting promotes adult hippocampal neuronal differentiation by activating GSK-3β in 3xTg-AD mice. J. Neurochem. 2020, 155, 697–713. [Google Scholar] [CrossRef]
- Elesawy, B.H.; Raafat, B.M.; Al Muqbali, A.; Abbas, A.M.; Sakr, H.F. The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus. Brain Sci. 2021, 11, 242. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, Z.; Li, X.; Xing, A.; Jiang, C.; Chen, Y.; Shi, W.; An, L. Intermittent Fasting Protects against Alzheimer’s Disease Possible through Restoring Aquaporin-4 Polarity. Front. Mol. Neurosci. 2017, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Halagappa, V.K.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; LaFerla, F.M.; Mattson, M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shin, B.K. Intermittent fasting with a high-protein diet mitigated osteoarthritis symptoms by increasing lean body mass and reducing inflammation in osteoarthritic rats with Alzheimer’s disease-like dementia. Br. J. Nutr. 2022, 127, 55–67. [Google Scholar] [CrossRef]
- Shin, B.K.; Kang, S.; Kim, D.S.; Park, S. Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer’s disease-induced estrogen deficient rats. Exp. Biol. Med. 2018, 243, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Tatulli, G.; Mitro, N.; Cannata, S.M.; Audano, M.; Caruso, D.; D’arcangelo, G.; Lettieri-Barbato, D.; Aquilano, K. Intermittent Fasting Applied in Combination with Rotenone Treatment Exacerbates Dopamine Neurons Degeneration in Mice. Front. Cell. Neurosci. 2018, 12, 4. [Google Scholar] [CrossRef]
- Ojha, U.; Khanal, S.; Park, P.-H.; Hong, J.T.; Choi, D.-Y. Intermittent fasting protects the nigral dopaminergic neurons from MPTP-mediated dopaminergic neuronal injury in mice. J. Nutr. Biochem. 2023, 112, 109212. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Martin, D.D.O.; Schmidt, M.E.; Qiu, X.; Ladha, S.; Caron, N.S.; Skotte, N.H.; Nguyen, Y.T.N.; Vaid, K.; Southwell, A.L.; et al. Preventing mutant huntingtin proteolysis and intermittent fasting promote autophagy in models of Huntington disease. Acta Neuropathol. Commun. 2018, 6, 16. [Google Scholar] [CrossRef]
- Saadatnia, M.; Etemadifar, M.; Fatehi, F.; Ashtari, F.; Shaygannejad, V.; Chitsaz, A.; Maghzi, A.H. Short-term effects of prolonged fasting on multiple sclerosis. Eur. Neurol. 2009, 61, 230–232. [Google Scholar] [CrossRef]
- Wingo, B.C.; Rinker, J.R.; Green, K.; Peterson, C.M. Feasibility and acceptability of time-restricted eating in a group of adults with multiple sclerosis. Front. Neurol. 2022, 13, 1087126. [Google Scholar] [CrossRef]
- Bai, M.; Wang, Y.; Han, R.; Xu, L.; Huang, M.; Zhao, J.; Lin, Y.; Song, S.; Chen, Y. Intermittent caloric restriction with a modified fasting-mimicking diet ameliorates autoimmunity and promotes recovery in a mouse model of multiple sclerosis. J. Nutr. Biochem. 2021, 87, 108493. [Google Scholar] [CrossRef]
- Au, A.; Feher, A.; McPhee, L.; Jessa, A.; Oh, S.; Einstein, G. Estrogens, inflammation and cognition. Front. Neuroendocrinol. 2016, 40, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.-Y.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M.-Y. Pathological α-synuclein transmission initiates parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Autonomic involvement in Parkinson’s disease: Pathology, pathophysiology, clinical features and possible peripheral biomarkers. J. Neurol. Sci. 2012, 313, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Neural control of the gastrointestinal tract: Implications for Parkinson disease. Mov. Disord. 2008, 23, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Curr. Treat. Options Neurol. 2018, 20, 54. [Google Scholar] [CrossRef]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Maetzler, W.; Liepelt, I.; Berg, D. Progression of Parkinson’s disease in the clinical phase: Potential markers. Lancet Neurol. 2009, 8, 1158–1171. [Google Scholar] [CrossRef]
- Jellinger, K.A. Synuclein deposition and non-motor symptoms in Parkinson disease. J. Neurol. Sci. 2011, 310, 107–111. [Google Scholar] [CrossRef]
- Berryman, D.E.; Glad, C.A.M.; List, E.O.; Johannsson, G. The GH/IGF-1 axis in obesity: Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2013, 9, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Godau, J.; Knauel, K.; Weber, K.; Brockmann, K.; Maetzler, W.; Binder, G.; Berg, D. Serum insulinlike growth factor 1 as possible marker for risk and early diagnosis of parkinson disease. Arch. Neurol. 2011, 68, 925–931. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G. Alzheimer’s disease and insulin resistance: Translating basic science into clinical applications. J. Clin. Investig. 2013, 123, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Wan, R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005, 16, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.S.; Clark, J.; Wagenmakers, A.J. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu. Rev. Nutr. 2010, 30, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Bailey, D. Mechanisms governing the health and performance benefits of exercise. Br. J. Pharmacol. 2013, 170, 1153–1166. [Google Scholar] [CrossRef]
- Kurth, T.; Moore, S.C.; Gaziano, J.M.; Kase, C.S.; Stampfer, M.J.; Berger, K.; Buring, J.E. healthy lifestyle and the risk of stroke in women. Arch. Intern. Med. 2006, 166, 1403–1409. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef]
- van Praag, H. Neurogenesis and exercise: Past and future directions. NeuroMolecular Med. 2008, 10, 128–140. [Google Scholar] [CrossRef]
- Mattson, M.P. Interventions that improve body and brain bioenergetics for Parkinson’s disease risk reduction and therapy. J. Park. Dis. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; McManus, E.J.; Brinkhuis, M.; Romero-Ferrando, B. Time-Restricted Ketogenic Diet in Huntington’s Disease: A Case Study. Front. Behav. Neurosci. 2022, 16, 931636. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Metabolic Strategies in Healthcare: A New Era. Aging Dis. 2022, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.S.; Loh, D.H.; Wang, H.-B.; Tahara, Y.; Kuljis, D.; Cutler, T.; Ghiani, C.A.; Shibata, S.; Block, G.D.; Colwell, C.S. Circadian-based Treatment Strategy Effective in the BACHD Mouse Model of Huntington’s Disease. J. Biol. Rhythm. 2018, 33, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Loh, D.H.; Whittaker, D.S.; Cutler, T.; Howland, D.; Colwell, C.S. Time-Restricted Feeding Improves Circadian Dysfunction as well as Motor Symptoms in the Q175 Mouse Model of Huntington’s Disease. eNeuro 2018, 5, ENEURO.0431-17.2017. [Google Scholar] [CrossRef] [PubMed]
- McCourt, A.C.; O’Donovan, K.L.; Ekblad, E.; Sand, E.; Craufurd, D.; Rosser, A.; Sanders, D.; Stoy, N.; Rickards, H.; Wierup, N.; et al. Characterization of Gastric Mucosa Biopsies Reveals Alterations in Huntington’s Disease. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef]
- Moffitt, H.; McPhail, G.D.; Woodman, B.; Hobbs, C.; Bates, G.P. Formation of polyglutamine inclusions in a wide range of non-CNS tissues in the HdhQ150 knock-in mouse model of huntington’s disease. PLoS ONE 2009, 4, e8025. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Steinman, L.; Zamvil, S.S. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005, 26, 565–571. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Z.; Li, X.; Liang, Y.; Pei, S.; Hao, S.; Zhu, Q.; Yu, T.; Pei, Y.; Yuan, J.; et al. Cytoplasmic DNA sensing by KU complex in aged CD4(+) T cell potentiates T cell activation and aging-related autoimmune inflammation. Immunity 2021, 54, 632–647.e9. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; La Rocca, C.; De Candia, P.; Procaccini, C.; Colamatteo, A.; Micillo, T.; De Rosa, V.; Matarese, G. Metabolic control of immune tolerance in health and autoimmunity. Semin. Immunol. 2016, 28, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Mues, M.; Koutrolos, M.; Al Rasbi, Z.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4615–4622. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050. [Google Scholar] [CrossRef]
- Vieira, S.M.; Pagovich, O.E.; Kriegel, M.A. Diet, microbiota and autoimmune diseases. Lupus 2014, 23, 518–526. [Google Scholar] [CrossRef]
- Wu, H.-J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Piccio, L.; Stark, J.L.; Cross, A.H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 2008, 84, 940–948. [Google Scholar] [CrossRef]
- Henninger, N.; Kumar, R.; Fisher, M. Acute ischemic stroke therapy. Expert Rev. Cardiovasc. Ther. 2010, 8, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Prinz, I.; Silva-Santos, B.; Pennington, D.J. Functional development of γδ T cells. Eur. J. Immunol. 2013, 43, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, M.; Weymar, A.; Bernreuther, C.; Velden, J.; Arunachalam, P.; Steinbach, K.; Orthey, E.; Arumugam, T.V.; Leypoldt, F.; Simova, O.; et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 2012, 120, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef]
- Fann, D.Y.-W.; Santro, T.; Manzanero, S.; Widiapradja, A.; Cheng, Y.-L.; Lee, S.-Y.; Chunduri, P.; Jo, D.-G.; Stranahan, A.M.; Mattson, M.P.; et al. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp. Neurol. 2014, 257, 114–119. [Google Scholar] [CrossRef]
- Magdy, R.; Kishk, N.A.; Abokrysha, N.T.; Ramzy, G.M.; Rizk, H.I.; Hussein, M. Fasting and post fasting effect of Ramadan on different seizure types in patients with active epilepsy. Nutr. Neurosci. 2022, 25, 1100–1104. [Google Scholar] [CrossRef]
- Luo, A.; Yan, J.; Tang, X.; Zhao, Y.; Zhou, B.; Li, S. Postoperative cognitive dysfunction in the aged: The collision of neuroinflammaging with perioperative neuroinflammation. Inflammopharmacology 2019, 27, 27–37. [Google Scholar] [CrossRef]
- Scott, D.A.; Evered, L.A. Caring for the ageing mind. Anaesthesia 2019, 74, 271–273. [Google Scholar] [CrossRef]
- Jiang, X.; Gu, X.; Zhou, X.; Chen, X.; Zhang, X.; Yang, Y.; Qin, Y.; Shen, L.; Yu, W.; Su, D. Intestinal dysbacteriosis mediates the reference memory deficit induced by anaesthesia/surgery in aged mice. Brain Behav. Immun. 2019, 80, 605–615. [Google Scholar] [CrossRef]
- Marcucci, M.; Chan, M.T.V.; Smith, E.E.; Absalom, A.R.; Devereaux, P.J. Prevention of perioperative stroke in patients undergoing non-cardiac surgery. Lancet Neurol. 2023, 22, 946–958. [Google Scholar] [CrossRef]
- Bi, J.; Xu, Y.; Li, S.; Zhan, G.; Hua, D.; Tan, J.; Chi, X.; Xiang, H.; Guo, F.; Luo, A. Contribution of preoperative gut microbiota in postoperative neurocognitive dysfunction in elderly patients undergoing orthopedic surgery. Front. Aging Neurosci. 2023, 15, 1108205. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Li, S.; Li, S.; Wang, X.; Wang, Y.; Xie, Z.; Zhao, Y.; Zhang, J.; Luo, A. Gut Microbiome and Plasma Metabolome Signatures in Middle-Aged Mice With Cognitive Dysfunction Induced by Chronic Neuropathic Pain. Front. Mol. Neurosci. 2021, 14, 806700. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, Y.A.; Chapman, M.J.; Deane, A.M. Peri-operative nutrition. Anaesthesia 2016, 71 (Suppl. S1), 9–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Y.; Wu, M.; Hu, J.; Zhao, J.; Chen, X.; Liu, W.; Liu, K.; Li, C. Preoperative fasting confers protection against intestinal ischaemia/reperfusion injury by modulating gut microbiota and their metabolites in a mouse model. Br. J. Anaesth. 2022, 128, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhou, Z.; Li, X.; Xu, Q.; Zhou, B.; Yu, H.; Zhang, W.; Sun, Q.; Zhang, X.; Luo, X.; et al. Prognostic significance of preoperative nutritional status for postoperative acute kidney injury in older patients undergoing major abdominal surgery: A retrospective cohort study. Int. J. Surg. 2023. [Google Scholar] [CrossRef]
| Author, Year | Number of People | Fasting Plan | Sample Type | Microbiological Analysis Method | Main Findings |
|---|---|---|---|---|---|
| Duygu Saglam, 2023 | 12 adults | TRF for 29 days | Feces | 16s rRNA | Several gut bacteria including Firmicutes ↓ Escherichia and Shigella ↑ [34] |
| Xiangwei Hu, 2023 | 72 adults | PF(5:2 diet) lasting for three weeks | Feces | 16s rRNA | Parabacteroides distasonis and Bacteroides thetaiotaomicron ↑ [35] |
| Alex E Mohr, 2022 | 20 obese adults | Two groups, one fasted one day a week with six days of low calorie, the other fasted two days with five days of low calorie | Feces | 16s rRNA | Ruminal coccidiaceae ↑ [36] |
| Muhammad Nadeem Khan, 2022 | 14 women and 31 men | TRF for 26 days | Feces | 16s rRNA | Anti-inflammatory bacteria Lactobacillus and Bifidobacterium were favorably ↑, while pathogenic bacteria were ↓ [37] |
| Junhong, Su, 2021 | 34 overweight people | TRF for a month | Feces | 16s rRNA | Microbial diversity ↑ Especially Spriochetes from Clostridia ↑ [38] |
| Maggie A, 2021 | 34 overweight people | ADF for three months | Feces | 16s rDNA | Microbial species and abundance ↑ [39] |
| Guo Yi, 2021 | 39 patients with MS | 2-day modified IF for 8 weeks | Feces | 16s rRNA | The community structure of intestinal flora was not significantly affected Acidobacter ↓ [40] |
| Mohanmmadzaheh A, 2021 | 30 healthy examiners | Fasting in Ramadan(TRF) for a month | Fasting blood sample | 16s r RNA, qPCR | Bacteria that can degrade dietary ↓ [41] |
| Maifeld A, 2021 | 63 patients with METs | 12 Weeks, 300–350 kcal five days a week and modified DASH diet for the rest days | Feces | 16s rRNA gene | Changes in the diversity of the gut microbiome [42] |
| Hassane Z, 2020 | 30 obese males | Fasting in Ramadan(TRF) for a month | Blood sample | qPCR | Serum leptin level ↑, GLP- ↓, PYY ↓, CCK ↓ [43] |
| Gabel K, 2020 | 14 obese adults | TRF for 12 weeks | Feces | 16s rRNA gene | The systemic diversity of intestinal microbiota remains unchanged [44] |
| Mesnage R, 2019 | 15 healthy males | Buichinger fasting plan for 10 days with enema every two days | Feces | 16s rRNA gene amplification | Bacteria that can degrade dietary polysaccharides (such as Trichospiridae and Ruminococcaceae) ↓ [45] |
| Ozkul C, 2019 | 9 adults | Fating in Ramadan(TRF) for 29 days, | Feces | 16s rRNA gene, qPCR analysis | Coliphage and Bacteroides fragilis ↑ [46] |
| Cignarella F, 2018 | 16 patients with MS | ADF for 15 days, daily intake less than 500 kcal | Feces | 16s rRNA gene | Faecali bacterium ↑ [47] |
| Author, Year | Research Model | Fasting Plan | Sample Type | Microbiological Analysis Method | Main Findings |
|---|---|---|---|---|---|
| Sainan Wang, 2023 | 5-week-old C57 male mice | ADF | Feces | 16s rRNA gene | Firmicutes to Bacteroidetes ratio ↓ [48] |
| Junyu Wang,2023 | 2-month-old Sprague-Dawley rat | ADF | Feces | 16s rRNA gene | Variation in gut microbiota’s abundance and diversity with alternate-day fasting in injured rats [49]. |
| Hikmet Taner Teker, 2023 | 12-month-old Wistar male rat | TRF for 35 days | Cecum regions and their contents | 16s rRNA gene | The Firmicutes and Bacteroidetes ratio ↓, Proteobacteria ↓ [50] |
| Jiafeng Xia, 2023 | 6-week-old C57 male mice | Four groups were: unrestricted Western diet, time-restricted Western diet, unrestricted chow diet, and time-restricted chow diet | Feces | 16s rRNA gene | The TRF scheme restored the rhythmicity of genera such as Lactobacillus, Myxococcus and Acetobacter [51]. |
| Ruxue Ma, 2023 | 6–8-week-old Balb/c female mice | Three groups for four weeks: TRF, ADF, normal feeding | Feces | 16s rRNA gene | The expression of ZO- is higher in the ileum of two IF groups of mice. / hour fasting led to a reconfiguration of the gut microbiota. Alistipes and Rikenellaceae ↑ [52] |
| Junhong Su, 2022 | 6-week-old Balb/c male mice | TRF for 30 days | Feces | 16s rRNA | Lachnospiroceae and Ruminococcaceae ↓ [53] |
| Ruiyuan Pan, 2022 | 3-month-old 5xFAD mice | ADF until the mice grow to 5.5–6 months old | Feces | 16s rRNA gene | Firmicutes phylum ↑ Bacteroidetes ↓ Lactobacillus family ↑ [54] |
| Yang Hong, 2023 | 6-week-old C57 male mice | ADF | Feces | 16s rRNA gene | Lactobacilli and Verrucomicroniaceae ↑ [55] |
| Jingjing Wu, 2022 | 6-week-old C57 male mice | Short-term group: IF for two weeks; Long-term group: IF for 20 weeks | Feces | Non-targeted sequencing | Short-term IF: Colitogenic Bacteroides, Dopakuru and Akmansia ↑, Clostridium ruminant ↓. long term IF: Akmansia↓ and Lactobacillus ↑ [56] |
| Hernandez Abii R, 2022 | 8-month-old mice | TRF(Fasting 21 h a day) for 14 months | Feces | 16s rRNA gene amplification | Genus Leptomyces, Enteromonas and Eubacterium ↑ [57] |
| Andras G, 2022 | C57 male mice | IF group: fasting every other day for 28 days; FMD group: fasting four days a week for three weeks | Cecal contents | 16s rRNA gene | SCFAs level ↓ [58] |
| Shanshan Xie, 2022 | 12-week-old c57 male mice | Feces | 16s rRNA gene | The level of distant Bacteroides ↓ [59] | |
| Huanan Shi, 2021 | 5-week-old hypertensive rats | ADF for 10 weeks | Feces | 16s rRNA gene | Lactobacillus and Bifidobacterium ↑ [60] |
| Ziyi Zhang, 2021 | 8-week-old c57 male mice | PF(5:2 IF regimen) | Feces | 16s rRNA gene | Lactobacillus ↓ [61] |
| Jingliang Liu, 2021 | 6-week-old c57 male mice | 1. Control group; 2. Intermittent group; 3. Melatonin group; 4. Fasting with melatonin group | Feces | 16s rRNA gene amplification | Short-term IF: colitis cells, Bacteroides, Dopakuru and Akmansia ↑ , and Clostridium ruminant ↓. Long term IF:Lactobacillus ↑, Akmansia ↓ [62] |
| Ya Deng, 2020 | 3-week-old c57 male mice | ADF for a month | Feces | 16s rRNA gene amplification | The proportion of thick-wall bacteria and Bacteroides ↓ The relative abundance of intestinal flora [63] |
| Yuqian Ye, 2020 | 8-week-old Kunming male mice | Either a normal diet ad libitum, a high-fat diet ad libitum, or a high-fat diet restricted to TRF; for 8 weeks | Feces | 16s rRNA gene | Bacteroidetes ↑ Firmicutes ↓ [64] |
| Zhigang Liu, 2020 | 3-month-old db/db mice | NA | Feces | 16s rRNA gene | The integrity of intestinal barrier ↑ The level of SCFAs ↑ Intestinal microorganisms, and Lactobacillus ↑ Plasma LPS ↓ [65] |
| Linghao Li, 2020 | C57 male mice | NA | Feces | 16s rRNA | Akkermansia ↑ Alisma ↓ [66] |
| Cignarella F, 2018 | 7-week-old c57 BL/6 female mice | Fasting every other day for four weeks | Feces | 16s rRNA gene | Lactobacilli, Bacteroides and Prevostiae ↑ [47] |
| Eleni B, 2018 | 4-month-old db/db mice | Fasting every other day for seven months | Feces | 16s rRNA gene | The level of Bacteroides ↑, while Verruciformes ↓; The number of mucin, goblet cells, and villus length of intestinal mucosa in mice ↑, and plasma peptidoglycan ↓ [67] |
| Metabolites | Related Bacterium | Main Findings |
|---|---|---|
| SCFAs: acetic acid, propionic acid, butyric acid, isobutyric acid, etc. | Clostridium group of Chlamydomonas, including Eubacterium, Rosbergia, Fecal, etc. | 1. Butyric acid passed GPR109A/PPAR-γ/TLR4-NF-κ B signal pathway inhibits microglia-mediated neuroinflammation and enhances memory and cognitive performance in a correlated manner [71]. |
| 2. Supplementing SCFAs alleviated the manifestation of anxious and depression behaviors in mice [72] | ||
| 3. Chronic cerebral hypoperfusion resulted in the decrease in fecal acetic acid and propionic acid and the decrease in hippocampal acetic acid. After administration of FMT and SCFA, the above decrease was reversed by changing the structure and composition of fecal microbial community; FMT and SCFAs may alleviate the neuronal damage induced by chronic cerebral ischemia [73]. | ||
| 4. After young adult mice were transplanted into the microbiota of old mice, the expression of synaptic plasticity and neurotransmission proteins in the hippocampus decreased, and the microbiota producing SCFAs (Trichospiridae, Faecaceae and Ruminococcaceae) decreased [71] | ||
| 5. In VPA-induced autism model rats, the abundance of uric acid bacilli is high and the level of butyric acid is low. Lactobacillus suis CCFM 1076 helps to decrease the prevalence of uric acid bacteria and enhance the concentration of butyric acid [74] | ||
| Choline metabolites | Faecalibacterum prausznitzii | 1. TMAO is synthesized via the metabolic process of choline by intestinal microbes. The substance has the ability to traverse the blood-brain barrier and exert its effects on the central nervous system. The heightened level of focus will amplify the likelihood of experiencing unfavorable cardiovascular events [75]. |
| 2. Individuals who have elevated choline levels demonstrate a reduced susceptibility to cognitive impairment subsequent to an ischemic stroke [76]. | ||
| Cholic acid: cholate, porcine cholate, deoxycholate, etc. | Faecalibacterium praussznitzii, Lactobacillus, Bifidobacterium, etc. | 1. Individuals diagnosed with AD have reduced blood concentrations of CA, which is a primary BA. Conversely, these individuals show elevated levels of DCA, a secondary bile acid that is generated by bacteria [77]. |
| 2. There was a significant correlation seen between decreased concentrations of CDCA, CA, and UDCA, and the presence of PD-MCI [78]. | ||
| Amino acid derivatives | Faecalibacterium praussznitzii, Lactobacillus, Bifidobacterium, etc. | 1. Research investigations indicate that the suppression of intestinal ecological disturbances and the subsequent buildup of phenylalanine and isoleucine may effectively regulate neuroinflammation and alleviate cognitive impairment [79]. |
| 2. Obese diet mice showed cognitive dysfunction, accompanied by intestinal ecological disorder and Trp metabolic disorder [80]. | ||
| Vitamins: Vitamin K, Vitamin B12, thiamine, folic acid, etc. | NA | 1. There exists a positive correlation between elevated consumption of vitamin K via one’s diet and enhanced cognitive performance [81]. |
| 2. Elevated levels of plasma homocysteine have been illustrated to be positively correlated with an increased susceptibility to cognitive impairment and dementia [82]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Wang, X.; Li, Y.; Luo, A.; Zhao, Y.; Luo, X.; Li, S. Intermittent Fasting on Neurologic Diseases: Potential Role of Gut Microbiota. Nutrients 2023, 15, 4915. https://doi.org/10.3390/nu15234915
Guo M, Wang X, Li Y, Luo A, Zhao Y, Luo X, Li S. Intermittent Fasting on Neurologic Diseases: Potential Role of Gut Microbiota. Nutrients. 2023; 15(23):4915. https://doi.org/10.3390/nu15234915
Chicago/Turabian StyleGuo, Mingke, Xuan Wang, Yujuan Li, Ailin Luo, Yilin Zhao, Xiaoxiao Luo, and Shiyong Li. 2023. "Intermittent Fasting on Neurologic Diseases: Potential Role of Gut Microbiota" Nutrients 15, no. 23: 4915. https://doi.org/10.3390/nu15234915
APA StyleGuo, M., Wang, X., Li, Y., Luo, A., Zhao, Y., Luo, X., & Li, S. (2023). Intermittent Fasting on Neurologic Diseases: Potential Role of Gut Microbiota. Nutrients, 15(23), 4915. https://doi.org/10.3390/nu15234915







