Evaluation of Acceptability, Functionality, and Validity of a Passive Image-Based Dietary Intake Assessment Method in Adults and Children of Ghanaian and Kenyan Origin Living in London, UK
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
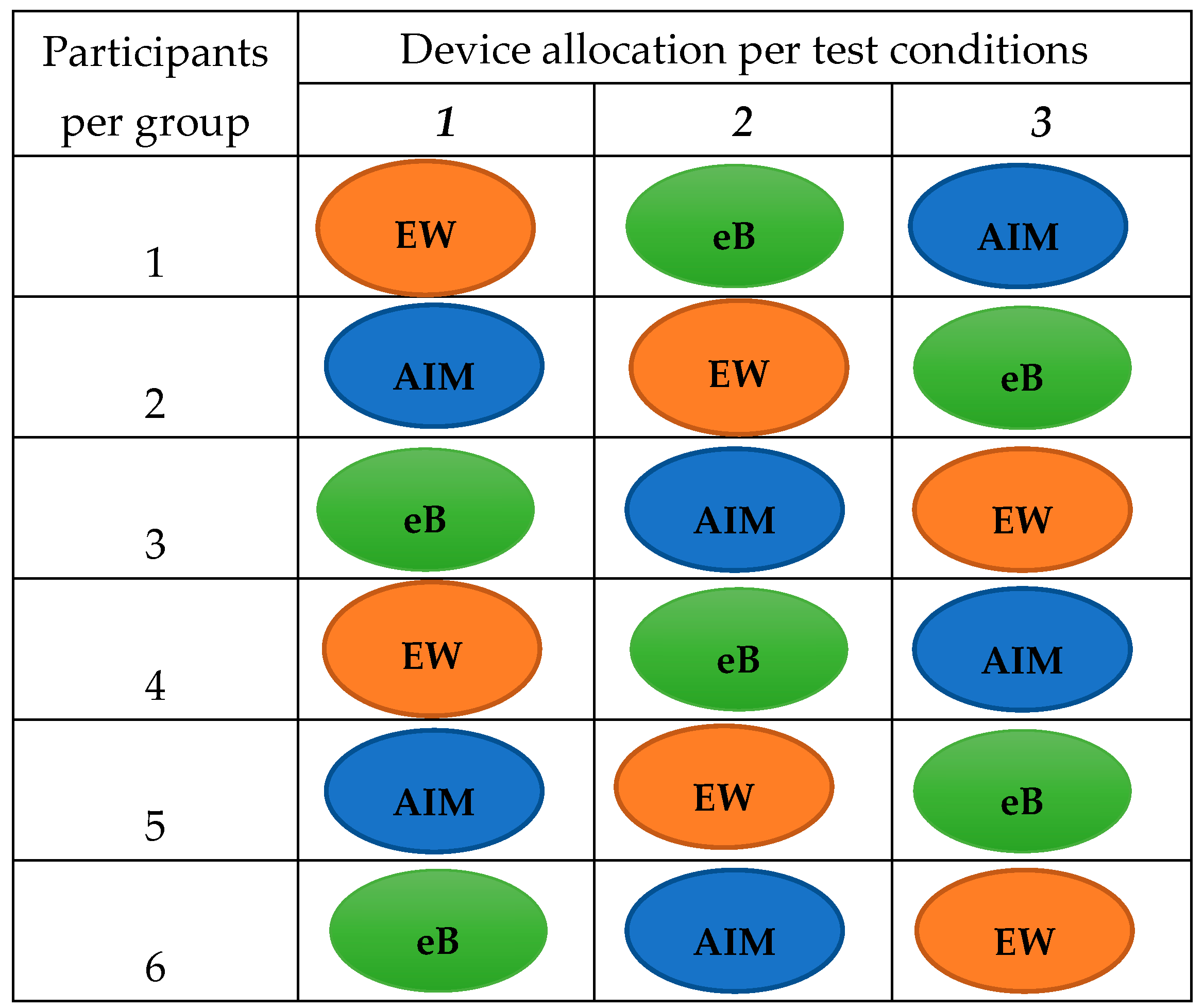
2.2. Study Design
2.3. Testing of the Devices in Adults
2.4. Testing of the Devices in Children
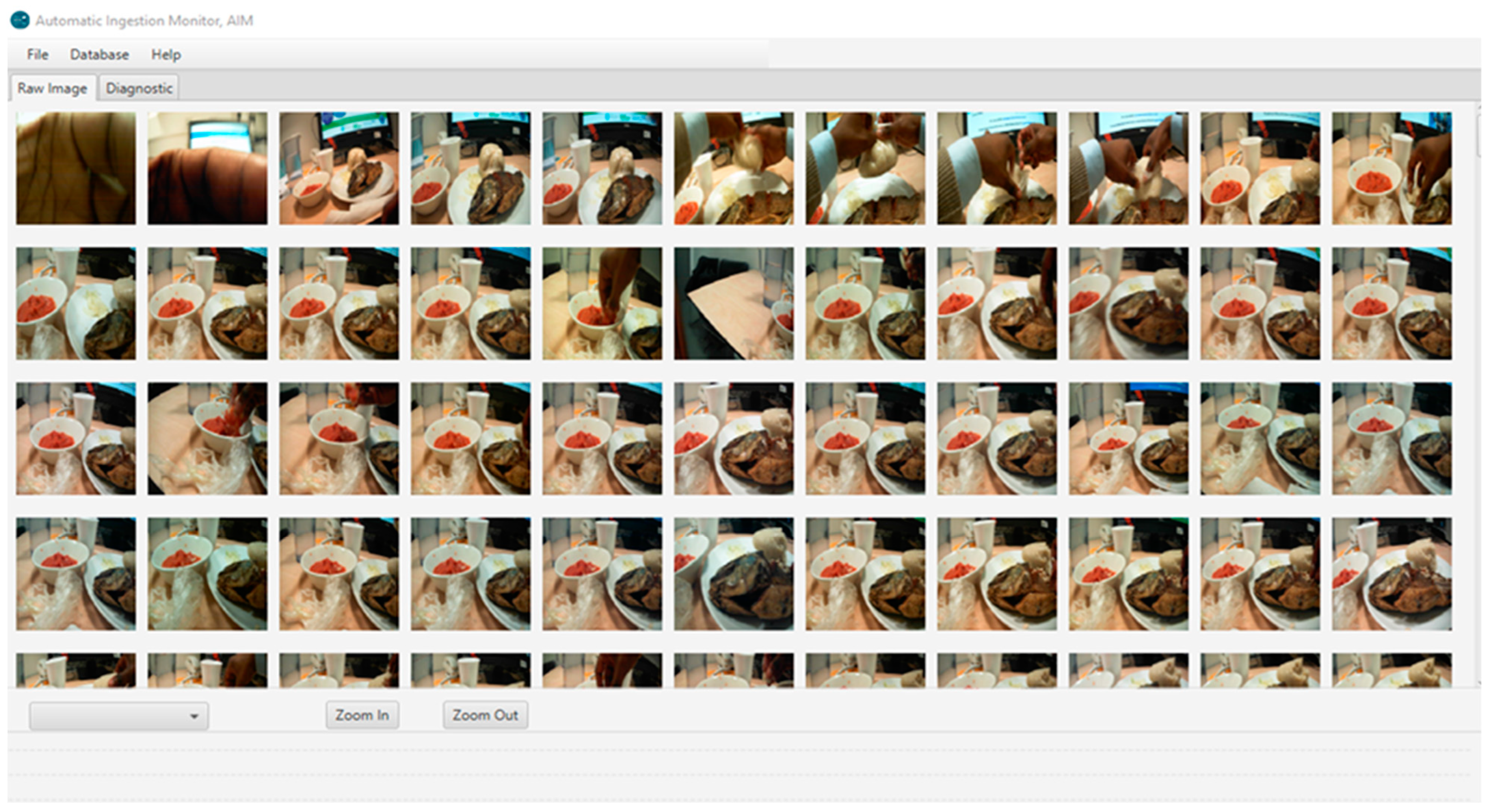
2.5. Assessment of the Functionality of the Camera Devices
2.6. Assessment of Portion Size of Food Captured on Images
2.7. Assessment of the Nutrient Content of Foods
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Assessment of Acceptability of the Devices
3.3. Assessment of the Functionality of the Devices
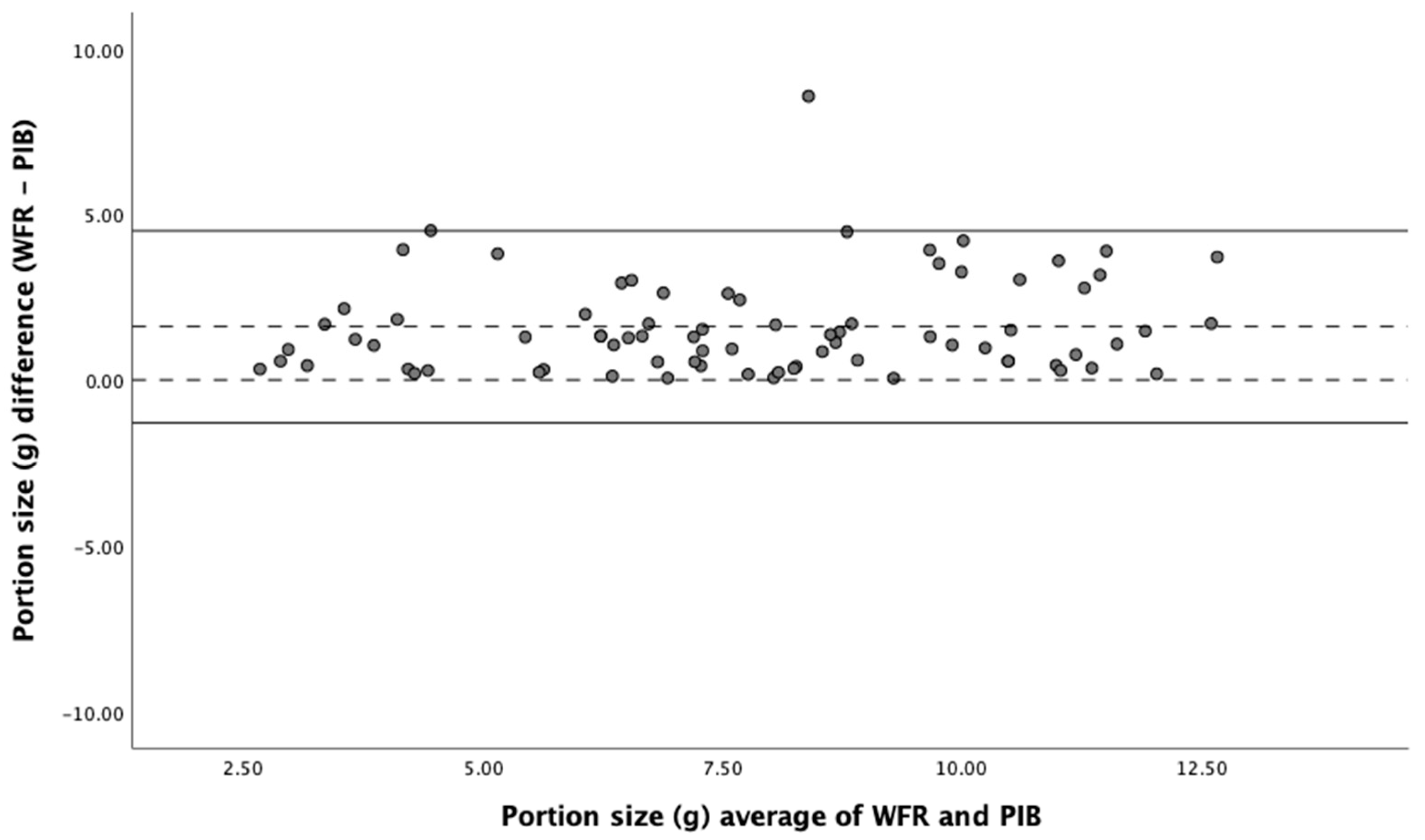
3.4. Assessment of the Validity of Food Portion Size Estimation
3.5. Assessment of the Validity of Nutrient Intake Estimation (Child Study)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Global Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.; Levin, C.; Hale, J.; Hutchinson, B. Economic effects of the double burden of malnutrition. Lancet 2020, 395, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.C.; Colaiezzi, B.A.; Bell, W.; Charrondiere, U.R.; Leclercq, C. Overcoming Dietary Assessment Challenges in Low-Income Countries: Technological Solutions Proposed by the International Dietary Data Expansion (INDDEX) Project. Nutrients 2017, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Charrondiere, U.R.; Bell, W. Measurement Errors in Dietary Assessment Using Self-Reported 24-Hour Recalls in Low-Income Countries and Strategies for Their Prevention. Adv. Nutr. 2017, 8, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Timon, C.M.; van den Barg, R.; Blain, R.J.; Kehoe, L.; Evans, K.; Walton, J.; Flynn, A.; Gibney, E.R. A review of the design and validation of web- and computer-based 24-h dietary recall tools. Nutr. Res. Rev. 2016, 29, 268–280. [Google Scholar] [CrossRef]
- Boushey, C.J.; Spoden, M.; Zhu, F.M.; Delp, E.J.; Kerr, D.A. New mobile methods for dietary assessment: Review of image-assisted and image-based dietary assessment methods. Proc. Nutr. Soc. 2017, 76, 283–294. [Google Scholar] [CrossRef]
- Jobarteh, M.L.; McCrory, M.A.; Lo, B.; Sun, M.; Sazonov, E.; Anderson, A.K.; Jia, W.; Maitland, K.; Qiu, J.; Steiner-Asiedu, M.; et al. Development and Validation of an Objective, Passive Dietary Assessment Method for Estimating Food and Nutrient Intake in Households in Low- and Middle-Income Countries: A Study Protocol. Curr. Dev. Nutr. 2020, 4, nzaa020. [Google Scholar] [CrossRef]
- Doulah, A.; Ghosh, T.; Hossain, D.; Imtiaz, M.H.; Sazonov, E. “Automatic Ingestion Monitor Version 2”—A Novel Wearable Device for Automatic Food Intake Detection and Passive Capture of Food Images. IEEE J. Biomed. Health Inform. 2020, 25, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Burke, L.E.; Baranowski, T.; Fernstrom, J.D.; Zhang, H.; Chen, H.C.; Bai, Y.; Li, Y.; Li, C.; Yue, Y.; et al. An exploratory study on a chest-worn computer for evaluation of diet, physical activity and lifestyle. J. Healthc. Eng. 2015, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Sun, J.; Tang, X. Single Image Haze Removal Using Dark Channel Prior. IEEE Trans. Pattern Anal. Mach. Intell. 2011, 33, 2341–2353. [Google Scholar] [PubMed]
- Fontana, J.M.; Farooq, M.; Sazonov, E. Automatic Ingestion Monitor: A Novel Wearable Device for Monitoring of Ingestive Behavior. IEEE Trans. Biomed. Eng. 2014, 61, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.A. Portion Photos of Popular Foods, 1st ed.; The American Dietetic Association: Providence, RI, USA; Center for Nutrition Education, University of Wisconsin, Stout: Menomonie, WI, USA, 1997; 128p. [Google Scholar]
- Murugu, D.; Kimani, A.; Mbelenga, E.; Mwai, J. Kenya Food Composition Tables; FAO: Rome, Italy, 2018. [Google Scholar]
- Al Marzooqi, H.M.; Burke, S.J.; Al Ghazali, M.R.; Duffy, E.; Yousuf, M.H.S.A. The development of a food atlas of portion sizes for the United Arab Emirates. J. Food Compos. Anal. 2015, 43, 140–148. [Google Scholar] [CrossRef]
- Charrondiere, U.R. FAO/INFOODS Database (Version 2.0); Food and Agriculture Organization: Rome, Italy, 2012. [Google Scholar]
- McCance and Widdowson’s Composition of Food Integrated Dataset (CoFID). 2019. Available online: https://wwwgovuk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 30 January 2021).
- Stadlmayr, B.; Enujiugha, V.N.; Bayili, G.R.; Fagbohound, E.G.; Samb, B.; Addy, P.; Ingrid; Barikmo; Ouattara, F.; Oshaug, A.; et al. West African Food Composition Table; FAO: Rome, Italy, 2012. [Google Scholar]
- Forestfield Software Limited. Dietplan 7. 2020. Available online: http://wwwforesoftcouk/indexhtml (accessed on 2 February 2023).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Ekstrom, E.-C.; Hyder, S.M.Z.; Chowdhury, A.M.R.; Chowdhury, S.A.; Lönnerdal, B.; Habicht, J.-P.; Persson, L.Ã. Efficacy and trial effectiveness of weekly and daily iron supplementation among pregnant women in rural Bangladesh: Disentangling the issues. Am. J. Clin. Nutr. 2002, 76, 1392–1400. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef]
- IBM Service Corps. IBM SPSS Statistics. 2020. Available online: https://wwwibmcom/analytics/spss-statistics-software (accessed on 2 February 2023).
- Baumgartner, J.; Smuts, C.M.; Aeberli, I.; Malan, L.; Tjalsma, H.; Zimmermann, M.B. Overweight impairs efficacy of iron supplementation in iron-deficient South African children: A randomized controlled intervention. Int. J. Obes. 2012, 37, 24–30. [Google Scholar] [CrossRef]
- Lombard, M.J.; Steyn, N.P.; Charlton, K.E.; Senekal, M. Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr. J. 2015, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Judd, A.L.; Beck, K.L.; McKinlay, C.; Jackson, A.; Conlon, C.A. Validation of a Complementary Food Frequency Questionnaire to assess infant nutrient intake. Matern. Child Nutr. 2020, 16, e12879. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.F.; McNeill, G.; Tomany, J.O.; Simpson, J.A.; Peace, H.S.; Wei, L.; Grubb, D.A.; Bolton-Smith, C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: Use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003, 6, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Lemieux, S.; Lafrenière, J.; Laramée, C.; Robitaille, J.; Morisset, A.S. Validation of a self-administered web-based 24-hour dietary recall among pregnant women. BMC Pregnancy Childbirth 2018, 18, 112. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Hardie, L.J.; Frost, G.S.; Alwan, N.A.; Bradbury, K.E.; Carter, M.; Elliott, P.; Evans, C.E.L.; Ford, H.E.; Hancock, N.; et al. Validation of the Oxford WebQ Online 24-Hour Dietary Questionnaire Using Biomarkers. Am. J. Epidemiol. 2019, 188, 1858–1867. [Google Scholar] [CrossRef]
- Wark, P.A.; Hardie, L.J.; Frost, G.S.; Alwan, N.A.; Carter, M.; Elliott, P.; Ford, H.E.; Hancock, N.; Morris, M.A.; Mulla, U.Z.; et al. Validity of an online 24-h recall tool (myfood24) for dietary assessment in population studies: Comparison with biomarkers and standard interviews. BMC Med. 2018, 16, 136. [Google Scholar] [CrossRef]
- Boushey, C.J.; Spoden, M.; Delp, E.J.; Zhu, F.; Bosch, M.; Ahmad, Z.; Shvetsov, Y.B.; DeLany, J.P.; Kerr, D.A. Reported Energy Intake Accuracy Compared to Doubly Labeled Water and Usability of the Mobile Food Record among Community Dwelling Adults. Nutrients 2017, 9, 312. [Google Scholar] [CrossRef]
- Gemming, L.; Rush, E.; Maddison, R.; Doherty, A.; Gant, N.; Utter, J.; Ni Mhurchu, C. Wearable cameras can reduce dietary under-reporting: Doubly labelled water validation of a camera-assisted 24 h recall. Br. J. Nutr. 2015, 113, 284–291. [Google Scholar] [CrossRef]
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef]

| Characteristics | Adult Cohort (n = 18) | Child Cohort (n = 17) |
|---|---|---|
| Age 1 | 37 (20–71) | 9 (1–17) |
| Female | 13 (72.2) | 9 (53.0) |
| Male | 5 (27.8) | 8 (47.0) |
| Ghanaian origin | 14 (77.8) | 14 (82.4) |
| Kenyan origin | 4 (22.2) | 3 (17.6) |
| Adult Cohort (n = 18) | Child Cohort (n = 17) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics 1 | AIM | eButton | Ear-Worn 2 | p 3 | AIM | eButton | p 4 |
| Ease of use | 4.6 (3–5) a | 4.7 (4–5) a | 3.7 (1–5) b | 0.005 | 4.3 (3–5) | 4.4 (1–5) | 0.81 |
| Convenience | 4.4 (2–5) a | 4.5 (3–5) a | 3.7 (1–5) a | 0.04 | 4.3 (2–5) | 4.2 (2–5) | 0.86 |
| Likelihood of future use | 4.5 (3–5) a | 4.7 (3–5) a | 3.5 (1–5) b | 0.004 | 3.8 (1–5) | 3.9 (2–5) | 0.38 |
| Interference with eating 5 | -- | -- | -- | 1.6 (1–3) | 2.3 (1–5) | 0.49 | |
| Preferred device 6 | 28% | 67% | 5% | 58% | 42% | ||
| Adult Cohort (n = 18) | Child Cohort (n = 17) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics 1 | AIM | eButton | Ear-Worn 2 | p 3 | AIM | eButton | p 4 |
| Clarity of images | 3.9 (2–5) | 3.5 (2–5) | 4.2 (2–5) | 0.09 | 3.3 (2–4) | 3.7 (2–5) | 0.45 |
| Food plate visibility at eating onset | 3.9 (1–5) | 2.8 (1–5) | 3.9 (1–5) | 0.06 | 4.2 (3–5) | 3.3 (2–4) | 0.11 |
| Food plate visibility during eating | 3.7 (1–5) | 2.9 (1–5) | 3.9 (1–5) | 0.18 | 3.9 (2–5) | 3.7 (1–5) | 0.63 |
| Food plate visibility at the end of eating | 3.7 (1–5) | 3.1 (1–5) | 3.0 (1–5) | 0.32 | 3.7 (2–5) | 2.4 (1–4) | 0.18 |
| Weighed Food Record | Passive Image-Based | Pearson Correlation | Bland–Altman Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Food and Nutrient 1 | Mean | 95% CI | Mean | 95% CI | % Difference 2 | ICC 3 | 95% CI | Mean Difference 4 | 95% LOA | Bias | |
| Portion Size (g) | 12.2 | [10.7, 13.7] | 10.3 | [9.14, 11.4] | −14 | 0.75 | 0.75 | [0.28, 0.88] | 2.37 | −1.42 to 6.17 | 0.45 |
| Energy (Kcal) | 14.3 | [12.4, 16.2] | 11.8 | [10.4, 13.1] | −16 | 0.78 | 0.69 | [0.28, 0.80] | 3.07 | −1.77 to 7.92 | 0.43 |
| Protein (g) | 3.29 | [2.67, 3.90] | 2.89 | [2.37, 3.41] | −11 | 0.93 | 0.91 | [0.75, 0.96] | 0.60 | −0.33 to 1.54 | 0.19 |
| Fat (g) | 4.50 | [2.52, 6.47] | 2.89 | [2.10, 3.69] | −23 | 0.76 | 0.76 | [0.25, 0.91] | 1.09 | −1.30 to 2.39 | 0.11 |
| SFA (g) | 1.53 | [1.25, 1.81] | 1.11 | [0.89, 1.33] | −23 | 0.65 | 0.67 | [0.22, 0.86] | 0.47 | −0.63 to 1.57 | 0.31 |
| MUFA (g) | 2.28 | [1.85, 2.71] | 1.62 | [1.23, 2.01] | −23 | 0.74 | 0.77 | [0.40, 0.90] | 0.74 | −1.11 to 2.07 | 0.16 |
| PUFA (g) | 2.01 | [1.64, 2.37] | 1.43 | [1.15, 1.71] | −22 | 0.68 | 0.67 | [0.15, 0.87] | 0.62 | −0.82 to 12.1 | 0.28 |
| Fibre (g) | 2.15 | [1.85, 2.46] | 1.74 | [1.51, 1.97] | −17 | 0.71 | 0.68 | [0.16, 0.87] | 0.47 | −0.45 to 1.40 | 0.31 |
| CHO (mg) | 5.79 | [5.01, 6.58] | 4.58 | [4.23, 5.47] | −13 | 0.60 | 0.71 | [0.33, 0.88] | 1.48 | −0.64 to 3.59 | 0.39 |
| Sodium (mg) | 16.8 | [13.5, 20.1] | 12.7 | [9.72, 15.7] | −21 | 0.88 | 0.85 | [0.51, 0.94] | 4.71 | −4.59 to 14.0 | 0.09 |
| Potassium [27] | 17.5 | [14.4, 20.5] | 14.2 | [12.0, 16.4] | −16 | 0.91 | 0.80 | [0.45, 0.92] | 3.73 | −3.02 to 10.5 | 0.34 |
| Calcium (mg) | 6.38 | [5.46, 7.29] | 5.46 | [4.68, 6.24] | −12 | 0.77 | 0.79 | [0.46, 0.91] | 1.43 | −0.60 to 3.47 | 0.24 |
| Magnesium (mg) | 6.79 | [5.76, 7.83] | 5.57 | [4.76, 6.38] | −16 | 0.84 | 0.78 | [0.31, 0.92] | 1.41 | −0.99 to 3.81 | 0.25 |
| Iron (mg) | 1.48 | [1.23, 1.73] | 1.21 | [1.00, 1.43] | −17 | 0.94 | 0.89 | [0.64, 0.96] | 0.29 | −0.21 to 0.80 | 0.18 |
| Zinc (mg) | 1.51 | [1.22, 1.80] | 1.25 | [1.00, 1.49] | −15 | 0.95 | 0.90 | [0.69, 0.96] | 0.33 | −0.19 to 0.85 | 0.19 |
| Copper [27] | 0.66 | [0.55, 0.76] | 0.53 | [0.45, 0.61] | −17 | 0.82 | 0.77 | [0.27, 0.91] | 0.15 | −0.08 to 0.37 | 0.24 |
| Niacin (mg) | 1.85 | [1.51, 2.18] | 1.57 | [1.25, 1.88] | −13 | 0.85 | 0.87 | [0.68, 0.94] | 0.46 | −0.33 to 1.25 | 0.14 |
| Carotene (µg) | 20.8 | [14.9, 26.6] | 16.1 | [11.6, 20.6] | −16 | 0.88 | 0.84 | [0.62, 0.93] | 5.93 | −6.91 to 18.8 | 0.29 |
| Folate (µg) | 6.22 | [4.98, 7.45] | 5.09 | [3.97, 6.22] | −16 | 0.79 | 0.86 | [0.67, 0.94] | 1.38 | −1.42 to 4.19 | 0.28 |
| Iodine (µg) | 2.15 | [1.71, 2.59] | 1.89 | [1.53, 2.23] | −9 | 0.74 | 0.75 | [0.36, 0.89] | 0.47 | −0.29 to 1.22 | 0.23 |
| Selenium (µg) | 2.56 | [2.10, 3.01] | 2.45 | [2.05, 2.85] | −4 | 0.71 | 0.80 | [0.54, 0.91] | 0.59 | −0.25 to 1.43 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jobarteh, M.L.; McCrory, M.A.; Lo, B.; Triantafyllidis, K.K.; Qiu, J.; Griffin, J.P.; Sazonov, E.; Sun, M.; Jia, W.; Baranowski, T.; et al. Evaluation of Acceptability, Functionality, and Validity of a Passive Image-Based Dietary Intake Assessment Method in Adults and Children of Ghanaian and Kenyan Origin Living in London, UK. Nutrients 2023, 15, 4075. https://doi.org/10.3390/nu15184075
Jobarteh ML, McCrory MA, Lo B, Triantafyllidis KK, Qiu J, Griffin JP, Sazonov E, Sun M, Jia W, Baranowski T, et al. Evaluation of Acceptability, Functionality, and Validity of a Passive Image-Based Dietary Intake Assessment Method in Adults and Children of Ghanaian and Kenyan Origin Living in London, UK. Nutrients. 2023; 15(18):4075. https://doi.org/10.3390/nu15184075
Chicago/Turabian StyleJobarteh, Modou L., Megan A. McCrory, Benny Lo, Konstantinos K. Triantafyllidis, Jianing Qiu, Jennifer P. Griffin, Edward Sazonov, Mingui Sun, Wenyan Jia, Tom Baranowski, and et al. 2023. "Evaluation of Acceptability, Functionality, and Validity of a Passive Image-Based Dietary Intake Assessment Method in Adults and Children of Ghanaian and Kenyan Origin Living in London, UK" Nutrients 15, no. 18: 4075. https://doi.org/10.3390/nu15184075
APA StyleJobarteh, M. L., McCrory, M. A., Lo, B., Triantafyllidis, K. K., Qiu, J., Griffin, J. P., Sazonov, E., Sun, M., Jia, W., Baranowski, T., Anderson, A. K., Maitland, K., & Frost, G. (2023). Evaluation of Acceptability, Functionality, and Validity of a Passive Image-Based Dietary Intake Assessment Method in Adults and Children of Ghanaian and Kenyan Origin Living in London, UK. Nutrients, 15(18), 4075. https://doi.org/10.3390/nu15184075







