Factor Analysis of the Brazilian Questionnaire on Adherence to Ketogenic Dietary Therapy: Keto-Check
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Exploratory Factor Analysis
2.3. Confirmatory Factor Analysis
- Root mean square error of approximation (RMSEA), whose values below 0.08 indicate good model fit [53];
- NCP (estimated noncentrality parameter) is a measure used in statistical power analysis for hypothesis testing. It is typically used in the context of testing for the significance of the difference between two means, such as in a t-test. It was adjusted with 26 degrees of freedom and the test of approximate fit considered the null hypothesis RMSEA < 0.05 [54];
- NNFI (non-normed fit index), also known as the Tucker–Lewis Index (TLI), is a goodness-of-fit index used in structural equation modeling (SEM). The NNFI is a relative fit index that compares the fit of a hypothesized model to a baseline model. It is calculated as the difference in the chi-square values of the hypothesized model and the baseline model, divided by the degrees of freedom of the hypothesized model. An NNFI value of 1 indicates perfect fit, while values closer to 0 indicate poorer fit [55];
- Comparative Fit Index (CFI): values above 0.95 indicate good model fit [56];
- GFI (goodness-of-fit index), first proposed by Jöreskog and Sörbom (1981), generally, a GFI value of 0.90 or higher is considered indicative of good fit, although this threshold may vary depending on the specific research context [57].
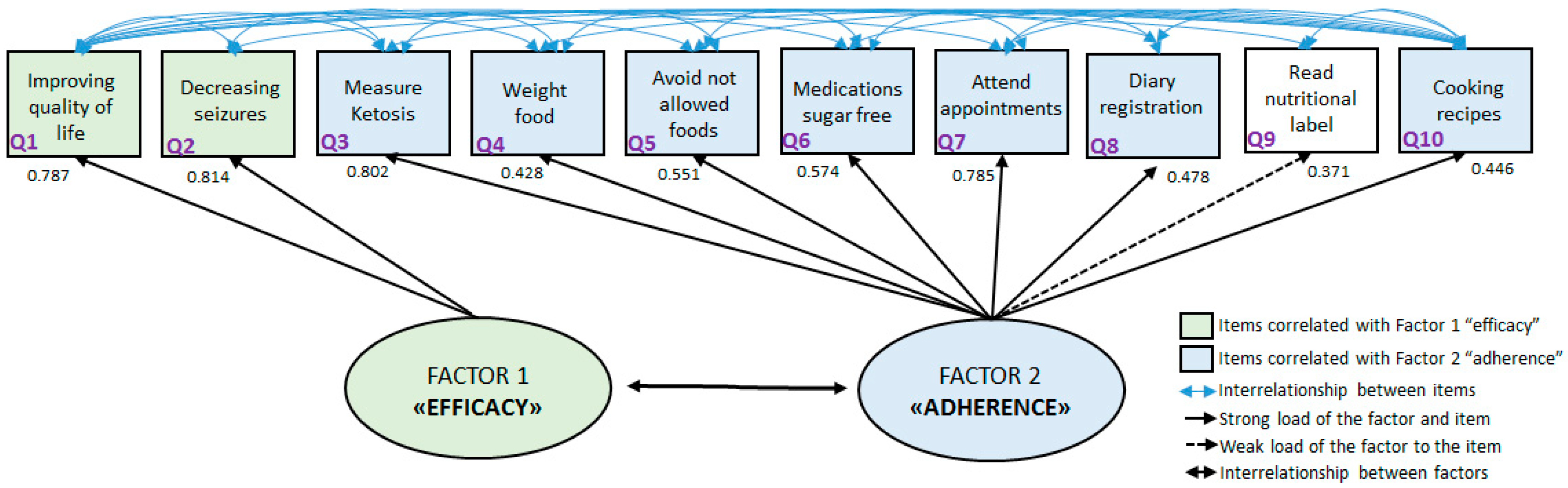
2.4. Graphical Presentation
2.5. Replicability Analysis
- Generalized H index (G-H): the H index evaluates how well the set of items represents the common factor. This value is between 0 and 1 and values greater than 0.80 suggest that the latent variable was well defined; that is, it tends to be stable across studies, while a variable with lower values tends to change across studies [52];
- Cronbach’s alpha and composite reliability: with values ranging from 0 to 1, with higher values indicating greater internal consistency. Cronbach’s alpha can be classified as when it reaches values greater than 0.8, “almost perfect”; from 0.61 to 0.8, “substantial”; from 0.41 to 0.6, “moderate”; between 0.21 and 0.4, “reasonable”; and less than 0.21, a “small” consistency is considered [58,59]. Composite reliability is a measure of internal consistency reliability that is similar to Cronbach’s alpha but is based on the concept of a latent variable model. It is calculated by taking the sum of the variances of the observed indicators and dividing it by the sum of the variances of the observed indicators plus the error variances [60];
- McDonald’s ordinal omega method: it is used when the scale items are measured on an ordinal or categorical scale. It is based on the concept of hierarchical factor models and provides an estimate of the proportion of variance in the observed scores that is due to a general underlying factor. Ordinal omega can range from 0 to 1, with higher values indicating greater internal consistency. It is recommended for use with Likert-type scales and other ordinal or categorical data [61].
3. Results
4. Discussion
Strengths and Limitations
- Social factors: these include stress, limited practical and social support, a chaotic environment, and an unhealthy lifestyle;
- Patient-related factors: poor language skills, deficient planning and organizational abilities, emotional instability, concerns about illness, feelings of helplessness, memory deficits, depression, and negative affectivity;
- Health-system-related factors: inadequate interaction and communication between patients and healthcare professionals, perceived discrimination in the healthcare system based on race, ethnicity, education, or income, and limited access to healthcare services;
- Treatment and disease-related factors: beliefs in barriers, such as unpleasant taste or side effects, actual side effects of medication, high treatment costs, low confidence in using the therapy, and apprehensions regarding potential side effects.
- Social factors: enhanced adherence can be facilitated by an increased ability to follow norms, a positive intrafamilial relationship, strong family support, such as proximity to relatives, and the possibility of receiving adequate treatment at home;
- Patient-related factors: improved self-efficacy and self-control, a positive perception of general health, beliefs about the severity of the disease, knowledge about the purpose and effects of therapy, and recognition of disease symptoms;
- Health-system-related factors: A good relationship with healthcare professionals and improved communication, particularly in terms of receiving instructions about treatment;
- Treatment and disease-related factors: positive beliefs about treatment effectiveness, perception of the necessity for treatment, confidence in treatment safety, and recognition of the benefits of treatment.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.d.C.L.; Ferraris, C.; Catalano, G.; Guglielmetti, M.; Pasca, L.; Pezzotti, E.; Carpani, A.; Tagliabue, A. Ketosis and migraine: A systematic review of the literature and meta-analysis. Front. Nutr. 2023, 10, 1204700. [Google Scholar] [CrossRef]
- Varesio, C.; Grumi, S.; Zanaboni, M.P.; Mensi, M.M.; Chiappedi, M.; Pasca, L.; Ferraris, C.; Tagliabue, A.; Borgatti, R.; De Giorgis, V. Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol. Nutrients 2021, 13, 2057. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.C.; Hsu, F.-C.; Berrington, A.; Henry-Barron, B.; Vizthum, D.; Blair, L.; Kossoff, E.H.; Easter, L.; Whitlow, C.T.; Barker, P.B.; et al. Feasibility and Biological Activity of a Ketogenic/Intermittent-Fasting Diet in Patients With Glioma. Neurology 2021, 97, e953–e963. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Marson, A.G.; Tudur Smith, C.; Young, B.; Mills, S.J.; Cherry, M.G.; Jenkinson, M.D. Ketogenic diets as an adjuvant therapy for glioblastoma (KEATING): A randomized, mixed methods, feasibility study. J. Neuro-Oncol. 2020, 147, 213–227. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Murtagh, D.K.J.; Gilbertson, L.J.; Asztely, F.J.S.; Lynch, C.D.P. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kasperek, K.; Rękawek, P.; Piątkowska-Chmiel, I. The therapeutic role of ketogenic diet in neurological disorders. Nutrients 2022, 14, 1952. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Guglielmetti, M.; Neri, L.d.C.L.; Allehdan, S.; Mohsin Albasara, J.M.; Fareed Alawadhi, H.H.; Trentani, C.; Perna, S.; Tagliabue, A. A review of ketogenic dietary therapies for epilepsy and neurological diseases: A proposal to implement an adapted model to include healthy mediterranean products. Foods 2023, 12, 1743. [Google Scholar] [CrossRef]
- MacCracken, K.A.; Scalisi, J.C. Development and evaluation of a ketogenic diet program. J. Am. Diet. Assoc. 1999, 99, 1554–1558. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Krauss, G.L.; McGrogan, J.R.; Freeman, J.M. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology 2003, 61, 1789–1791. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971, 21, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, H.H.; Thiele, E.A. Low-glycemic-index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005, 65, 1810–1812. [Google Scholar] [CrossRef]
- Sabate, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Fawcett, J. Thoughts about meanings of compliance, adherence, and concordance. Nurs. Sci. Q. 2020, 33, 358–360. [Google Scholar] [CrossRef]
- Ferraris, C.; Guglielmetti, M.; Tamagni, E.; Trentani, C.; De Giorgis, V.; Pasca, L.; Varesio, C.; Ferraro, O.E.; Tagliabue, A. Use of Remote Monitoring by E-mail for Long-Term Management of the Classic Ketogenic Diet. Nutrients 2020, 12, 1833. [Google Scholar] [CrossRef]
- Pasca, L.; Ferraris, C.; Guglielmetti, M.; Varesio, C.; Totaro, M.; Trentani, C.; Marazzi, C.; Brambilla, I.; Ballante, E.; Armeno, M.; et al. Ketonemia variability through menstrual cycle in patients undergoing classic ketogenic diet. Front. Nutr. 2023, 10, 1188055. [Google Scholar] [CrossRef]
- Erkent, I.; Ilgaz, F.; Dericioglu, N. Difficulties in the implementation of the ketogenic diet in adult patients with refractory epilepsy. Epilepsy Behav. 2023, 144, 109234. [Google Scholar] [CrossRef]
- Armeno, M.; Verini, A.; Caballero, E.; Cresta, A.; Valenzuela, G.R.; Caraballo, R. Long-term effectiveness and adverse effects of ketogenic diet therapy in infants with drug-resistant epilepsy treated at a single center in Argentina. Epilepsy Res. 2021, 178, 106793. [Google Scholar] [CrossRef] [PubMed]
- Operto, F.F.; Labate, A.; Aiello, S.; Perillo, C.; de Simone, V.; Rinaldi, R.; Coppola, G.; Pastorino, G.M.G. The Ketogenic Diet in Children with Epilepsy: A Focus on Parental Stress and Family Compliance. Nutrients 2023, 15, 1058. [Google Scholar] [CrossRef]
- De Amicis, R.; Leone, A.; Pellizzari, M.; Foppiani, A.; Battezzati, A.; Lessa, C.; Tagliabue, A.; Ferraris, C.; De Giorgis, V.; Olivotto, S.; et al. Long-term follow-up of nutritional status in children with GLUT1 Deficiency Syndrome treated with classic ketogenic diet: A 5-year prospective study. Front. Nutr. 2023, 10, 1148960. [Google Scholar] [CrossRef]
- Ye, F.; Li, X.-J.; Jiang, W.-L.; Sun, H.-B.; Liu, J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: A meta-analysis. J. Clin. Neurol. 2015, 11, 26–31. [Google Scholar] [CrossRef] [PubMed]
- González-Lamuño, D.; Sánchez-Pintos, P.; Andrade, F.; Couce, M.L.; Aldámiz-Echevarría, L. Treatment adherence in tyrosinemia type 1 patients. Orphanet J. Rare Dis. 2021, 16, 256. [Google Scholar] [CrossRef]
- Mulder, H.A.; McIntire, G.L.; Wallace, F.N.; Poklis, J.L. Determination of patient adherence for duloxetine in urine. J. Anal. Toxicol. 2022, 46, 905–910. [Google Scholar] [CrossRef]
- Griva, K.; Nandakumar, M.; Ng, J.-A.H.; Lam, K.F.Y.; McBain, H.; Newman, S.P. Hemodialysis Self-management Intervention Randomized Trial (HED-SMART): A Practical Low-Intensity Intervention to Improve Adherence and Clinical Markers in Patients Receiving Hemodialysis. Am. J. Kidney Dis. 2018, 71, 371–381. [Google Scholar] [CrossRef]
- Cabral, A.C.; Lavrador, M.; Castel-Branco, M.; Figueiredo, I.V.; Fernandez-Llimos, F. Development and validation of a Medication Adherence Universal Questionnaire: The MAUQ. Int. J. Clin. Pharm. 2023, 45, 999–1006. [Google Scholar] [CrossRef]
- Gila-Díaz, A.; Arribas, S.M.; López de Pablo, Á.L.; López-Giménez, M.R.; Phuthong, S.; Ramiro-Cortijo, D. Development and validation of a questionnaire to assess adherence to the healthy food pyramid in spanish adults. Nutrients 2020, 12, 1656. [Google Scholar] [CrossRef]
- Esquivel Garzón, N.; Díaz Heredia, L.P. Validity and Reliability of the Treatment Adherence Questionnaire for Patients with Hypertension. Investig. Educ. Enferm. 2019, 37, e09. [Google Scholar] [CrossRef][Green Version]
- Hebel, S.; Kahn-Woods, E.; Malone-Thomas, S.; McNeese, M.; Thornton, L.; Sukhija-Cohen, A.; Patani, H.; Engeran, W.; Daughtridge, G. Brief Report: Discrepancies Between Self-Reported Adherence and a Biomarker of Adherence in Real-World Settings. J. Acquir. Immune Defic. Syndr. 2020, 85, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.A.; Eroglu, E.I.; Rooney, K.; Harper, C.; McClintock, S.; Franklin, J.; Markovic, T.P.; Seimon, R.V.; Sainsbury, A. Urine dipsticks are not accurate for detecting mild ketosis during a severely energy restricted diet. Obes. Sci. Pract. 2020, 6, 544–551. [Google Scholar] [CrossRef]
- Barone, B.; Rodacki, M.; Cenci, M.C.P.; Zajdenverg, L.; Milech, A.; Oliveira, J.E.P. de [Diabetic ketoacidosis in adults--update of an old complication]. Arq. Bras. Endocrinol. Metabol. 2007, 51, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Veggiotti, P.; Cusmai, R.; Bertoli, S.; Cardinali, S.; Dionisi-Vici, C.; Elia, M.; Lispi, M.L.; Sarnelli, C.; Tagliabue, A.; et al. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: An Italian multicentric experience. Epilepsy Res. 2002, 48, 221–227. [Google Scholar] [CrossRef]
- Amari, A.; Grace, N.C.; Fisher, W.W. Achieving and maintaining compliance with the ketogenic diet. J. Appl. Behav. Anal. 1995, 28, 341–342. [Google Scholar] [CrossRef][Green Version]
- Kossoff, E.H.; Doerrer, S.S.; Turner, Z. How do parents find out about the ketogenic diet? Epilepsy Behav. 2012, 24, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Farasat, S.; Kossoff, E.H.; Pillas, D.J.; Rubenstein, J.E.; Vining, E.P.; Freeman, J.M. The importance of parental expectations of cognitive improvement for their children with epilepsy prior to starting the ketogenic diet. Epilepsy Behav. 2006, 8, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.d.C.L.; Sampaio, L.P. de B. Validation of ketogenic diet adherence questionnaire: Keto-check. Arq. Neuropsiquiatr. 2022, 80, 794–801. [Google Scholar] [CrossRef]
- Alanis Guevara, M.I.; García de Alba García, J.E.; López Alanis, A.L.; González Ojeda, A.; Fuentes Orozco, C. Estudio prospectivo de dieta Atkins modificada en epilepsia farmacorresistente de adultos: Efectividad, tolerabilidad y adherencia. Neurología, 2022; in press. [Google Scholar] [CrossRef]
- Klein, P.; Janousek, J.; Barber, A.; Weissberger, R. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav. 2010, 19, 575–579. [Google Scholar] [CrossRef]
- Schoeler, N.E.; Cross, J.H. Ketogenic diet therapy in infants with epilepsy. Paediatr. Child Health 2020, 30, 356–360. [Google Scholar] [CrossRef]
- Landry, M.J.; Crimarco, A.; Perelman, D.; Durand, L.R.; Petlura, C.; Aronica, L.; Robinson, J.L.; Kim, S.H.; Gardner, C.D. Adherence to Ketogenic and Mediterranean Study Diets in a Crossover Trial: The Keto-Med Randomized Trial. Nutrients 2021, 13, 967. [Google Scholar] [CrossRef]
- Sijtsma, K.; Pfadt, J.M. Part II: On the use, the misuse, and the very limited usefulness of cronbach’s alpha: Discussing lower bounds and correlated errors. Psychometrika 2021, 86, 843–860. [Google Scholar] [CrossRef]
- Sijtsma, K. On the use, the misuse, and the very limited usefulness of cronbach’s alpha. Psychometrika 2009, 74, 107–120. [Google Scholar] [CrossRef]
- Damásio, B.F. Contribuições da Análise Fatorial Confirmatória Multigrupo (AFCMG) na avaliação de invariância de instrumentos psicométricos. Psico-USF 2013, 18, 211–220. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: London, UK, 2012; ISBN 0205849571. [Google Scholar]
- Lorenzo-Seva, U.; Ferrando, P.J. FACTOR: A computer program to fit the exploratory factor analysis model. Behav. Res. Methods 2006, 38, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Seva, U.; Ferrando, P.J. Factor Analysis; Universitat Rovira i Virgili: Tarragona, Spain, 2022. [Google Scholar]
- Ferrando, P.J.; Lorenzo-Seva, U. Program FACTOR at 10: Origins, development and future directions. Psicothema 2017, 29, 236–240. [Google Scholar] [CrossRef]
- Lorenzo-Seva, U.; Van Ginkel, J.R. Multiple Imputation of missing values in exploratory factor analysis of multidimensional scales: Estimating latent trait scores. Analesps 2016, 32, 596. [Google Scholar] [CrossRef]
- Bartlett, M.S. A note on the multiplying factors for various chi square approximations. J. R. Stat. Soc. Ser. B 1954, 16, 296–298. [Google Scholar]
- KAISER, H. An index of factorial simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Timmerman, M.E.; Lorenzo-Seva, U. Dimensionality assessment of ordered polytomous items with parallel analysis. Psychol. Methods 2011, 16, 209–220. [Google Scholar] [CrossRef]
- Ferrando, P.J.; Lorenzo-Seva, U. Assessing the quality and appropriateness of factor solutions and factor score estimates in exploratory item factor analysis. Educ. Psychol. Meas. 2018, 78, 762–780. [Google Scholar] [CrossRef]
- Browne, M.W.; Cudeck, R. Alternative ways of assessing model fit. In Testing Structural Equation Models; Bollen, K.A., Long, J.S., Eds.; Sage: Newcastle upon Tyne, UK, 1993; pp. 136–162. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Tucker, L.; Lewis, C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika 1973, 38, 1–10. [Google Scholar] [CrossRef]
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Jöreskog, K.G. LISREL: Analysis of linear structural relationships by maximum likelihood, instrumental variables, and least squares methods. In Advances in Factor Analysis and Structural Equation Models; Jöreskog, K.G., Sörbom, D., Eds.; Abt Books, Scientific Software; University of Michigan: Ann Arbor, Michigan, USA, 1981; pp. 23–51. [Google Scholar]
- Revorêdo, L.D.S.; Maia, R.S.; Torres, G.D.V.; Chaves Maia, E.M. O uso da técnica delphi em saúde: Uma revisão integrativa de estudos brasileiros. RACS 2015, 22, 16. [Google Scholar] [CrossRef]
- Cronbach, L.F. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Raykov, T. Estimation of composite reliability for congeneric measures. Appl. Psychol. Meas. 1997, 21, 173–184. [Google Scholar] [CrossRef]
- McDonald, T.J.W.; Cervenka, M.C. Ketogenic diets for adult neurological disorders. Neurotherapeutics 2018, 15, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, S.L.; Vining, E.P.; Quaskey, S.A.; Mellits, D.; Freeman, J.M. Efficacy of the ketogenic diet for intractable seizure disorders: Review of 58 cases. Epilepsia 1992, 33, 1132–1136. [Google Scholar] [CrossRef]
- Mady, M.A.; Kossoff, E.H.; McGregor, A.L.; Wheless, J.W.; Pyzik, P.L.; Freeman, J.M. The ketogenic diet: Adolescents can do it, too. Epilepsia 2003, 44, 847–851. [Google Scholar] [CrossRef]
- Jagadish, S.; Payne, E.T.; Wong-Kisiel, L.; Nickels, K.C.; Eckert, S.; Wirrell, E.C. The ketogenic and modified atkins diet therapy for children with refractory epilepsy of genetic etiology. Pediatr. Neurol. 2019, 94, 32–37. [Google Scholar] [CrossRef]
- Damásio, B.F.; Borsa, J.C.; da Silva, J.P. 14-item resilience scale (RS-14): Psychometric properties of the Brazilian version. J. Nurs. Meas. 2011, 19, 131–145. [Google Scholar] [CrossRef]
- Krousel-Wood, M.; Craig, L.S.; Peacock, E.; Zlotnick, E.; O’Connell, S.; Bradford, D.; Shi, L.; Petty, R. Medication adherence: Expanding the conceptual framework. Am. J. Hypertens. 2021, 34, 895–909. [Google Scholar] [CrossRef]
- Schoeler, N.E.; MacDonald, L.; Champion, H.; Cross, J.H.; Sander, J.W.; Sisodiya, S.M.; Horne, R. Assessing parents’ attitudes towards ketogenic dietary therapies. Epilepsy Behav. 2014, 39, 1–5. [Google Scholar] [CrossRef]

| Question | Average (from 0–5) | Confidence Interval (95%) | Variance | Distortion | Kurtosis |
|---|---|---|---|---|---|
| 1 | 3.862 | (3.53–4.19) | 1929 | −1.055 | −0.287 |
| 2 | 4.086 | (3.77–4.40) | 1734 | −1.303 | 0.329 |
| 3 | 4.276 | (4.02–4.53) | 1.114 | −1.594 | 1993 |
| 4 | 4.828 | (4.66–5.00) | 0.505 | −4.683 | 21.242 |
| 5 | 4.362 | (4.06–4.66) | 1.576 | −1.820 | 1.720 |
| 6 | 4.319 | (4.04–4.60) | 1.372 | −1.812 | 2.079 |
| 7 | 4.914 | (4.83–5.00) | 0.130 | −5.536 | 36.301 |
| 8 | 4.431 | (4.16–4.70) | 1.245 | −2.228 | 3.880 |
| 9 | 4.836 | (4.68–4.99) | 0.416 | −4.752 | 23.136 |
| 10 | 4.707 | (4.52–4.89) | 0.587 | −3.277 | 10.945 |
| Question | Eigenvalue | Variance Ratio | Cumulative Proportion of Variance |
|---|---|---|---|
| 1 | 3.61200 | 0.36120 | 0.36120 |
| 2 | 2.10215 | 0.21022 | 0.57141 |
| 3 | 1.26059 | 0.12606 | |
| 4 | 0.89100 | 0.08910 | |
| 5 | 0.66426 | 0.06643 | |
| 6 | 0.55790 | 0.05579 | |
| 7 | 0.39421 | 0.03942 | |
| 8 | 0.33412 | 0.03341 | |
| 9 | 0.18375 | 0.01838 | |
| 10 | 0.00002 | 0.00000 |
| Question | % Variance Real Data | % Random Variance |
|---|---|---|
| 1 | 36.2051 | 21.9183 |
| 2 | 21.0499 | 17.5260 |
| 3 | 12.5966 | 14.9696 |
| 4 | 8.9175 | 13.0191 |
| 5 | 6.6575 | 11.0909 |
| 6 | 5.5924 | 9.1722 |
| 7 | 3.8519 | 7.2660 |
| 8 | 3.3287 | 5.2410 |
| 9 | 1.8008 | 2.9558 |
| Question | Factor 1 | Factor 2 | Commonality |
|---|---|---|---|
| 1 | 0.787 | 0.379 | 0.762 |
| 2 | 0.814 | 0.298 | 0.752 |
| 3 | 0.217 | 0.802 | 0.690 |
| 4 | −0.237 | 0.428 | 0.240 |
| 5 | −0.112 | 0.551 | 0.316 |
| 6 | 0.218 | 0.574 | 0.377 |
| 7 | 0.068 | 0.785 | 0.621 |
| 8 | −0.309 | 0.478 | 0.324 |
| 9 | −0.138 | 0.371 | 0.157 |
| 10 | −0.232 | 0.446 | 0.253 |
| Question | Factorial Loads | Error Variance | Error Squared Variance |
|---|---|---|---|
| 1 | 0.787 | 0.381 | 0.619 |
| 2 | 0.814 | 0.337 | 0.663 |
| 3 | 0.802 | 0.357 | 0.643 |
| 4 | 0.428 | 0.817 | 0.183 |
| 5 | 0.551 | 0.696 | 0.304 |
| 6 | 0.574 | 0.671 | 0.329 |
| 7 | 0.785 | 0.384 | 0.616 |
| 8 | 0.478 | 0.772 | 0.228 |
| 9 | 0.371 | 0.862 | 0.138 |
| 10 | 0.446 | 0.801 | 0.199 |
| Composite reliability | 0.857 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes Neri, L.d.C.; Ferraro, A.A.; Guglielmetti, M.; Fiorini, S.; Sampaio, L.P.d.B.; Tagliabue, A.; Ferraris, C. Factor Analysis of the Brazilian Questionnaire on Adherence to Ketogenic Dietary Therapy: Keto-Check. Nutrients 2023, 15, 3673. https://doi.org/10.3390/nu15173673
Lopes Neri LdC, Ferraro AA, Guglielmetti M, Fiorini S, Sampaio LPdB, Tagliabue A, Ferraris C. Factor Analysis of the Brazilian Questionnaire on Adherence to Ketogenic Dietary Therapy: Keto-Check. Nutrients. 2023; 15(17):3673. https://doi.org/10.3390/nu15173673
Chicago/Turabian StyleLopes Neri, Lenycia de Cassya, Alexandre Archanjo Ferraro, Monica Guglielmetti, Simona Fiorini, Letícia Pereira de Brito Sampaio, Anna Tagliabue, and Cinzia Ferraris. 2023. "Factor Analysis of the Brazilian Questionnaire on Adherence to Ketogenic Dietary Therapy: Keto-Check" Nutrients 15, no. 17: 3673. https://doi.org/10.3390/nu15173673
APA StyleLopes Neri, L. d. C., Ferraro, A. A., Guglielmetti, M., Fiorini, S., Sampaio, L. P. d. B., Tagliabue, A., & Ferraris, C. (2023). Factor Analysis of the Brazilian Questionnaire on Adherence to Ketogenic Dietary Therapy: Keto-Check. Nutrients, 15(17), 3673. https://doi.org/10.3390/nu15173673








