Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People
Abstract
1. Introduction
- The International Working Group on Sarcopenia (IWGS): The IWGS proposed a definition in 2011: “Sarcopenia is defined as a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, and it is strictly correlated with physical disability, poor quality of life, and death”.
- The Foundation for the National Institutes of Health (FNIH): In collaboration with various experts, they established a working definition in 2014: “Low muscle mass as measured by any method, and low muscle strength and/or low physical performance”.
- Definition and Outcomes Consortium’s (SDOC) efforts, which involved a literature review, data analysis from multiple studies, and expert panel review to establish an evidence-based understanding of sarcopenia. They define sarcopenia as characterized by both weakness (defined by low grip strength) and slowness (defined by low usual gait speed) in older adults. These two components are considered important discriminators and predictors of various adverse health-related outcomes, including falls, self-reported mobility limitation, hip fractures, and mortality in community-dwelling older adults.
2. Part 1: Physiological Regulation of Muscle Metabolism and Growth
2.1. The Molecular Mechanisms behind Muscles Growth in Young Subjects: Exercise, Nutrients, and Hormones
2.2. Effects of Substrates and Exercise on Skeletal Muscle Protein Synthesis in Young, Middle-Aged Subjects
2.2.1. Effects of Proteins and Amino Acids
| Type of Food/Protein/AA | Subjects | Dose A: Either the Threshold or the Lowest Dose that Increased MPS | Dose B: Either the Highest Dose Tested or that Maximally Stimulated MPS | Exercise Status | Refs. |
|---|---|---|---|---|---|
| Whey protein | Y (≈21 y) males | >20 g | ≈20 to 40 g | +Ex | [71] |
| Whey protein | Y (20–22 y) males | 10 g | 40 g | −/+Ex | [54] |
| Whey protein | Y adults | 5–20 g | 20 g | −Ex | [72] |
| Combination of 5 Whey Protein and 1 Egg studies (original data recalculated to body weight.) | Y (22 y) males | 8 g | ≈20 g | −/+Ex | [73] |
| Milk protein + CHO | Y (27 y) | 15 g | 45 g | +Ex | [74] |
| Milk protein concentrate | Y (22 y) males | / | 38 g | −Ex | [69] |
| Egg protein | Y (22 y) males | 5–10 g | 20 g | +Ex | [72] |
| Egg protein | Y males | 5–10 g | 20 g | +Ex | [55] |
| Whey protein Hydrolysate (dose achieving the greatest stimulation of MPS in [40].) (=AA) | Y (23 y) males | ≈10 g (as AA) | / | +Ex | [40] |
| Casein (micellar) (dose achieving an intermediate stimulation of MPS in [40].) | Y (23 y) males | ≈10 g | / | +Ex | [40] |
| Soy Hydrolysate (=AA) (dose achieving the lowest stimulation of MPS in [40].) | Y (23 y) males | ≈10 g (as AA) | / | +Ex | [40] |
| Beef (beef contains ≈ 22% of weight as protein.) | M males | >113 g | >170 (further enhanced by exercise) g | −/+Ex | [56] |
| Mixed animal (beef) and vegetal protein | Y (≈ 30 y) males | 40 g | 40–70 g | −/+Ex | [57] |
| Cristalline EAA | Y (34 y) | 15 g (as EAA) | −Ex | [58] | |
| EAA + Leucine + CHO | Y (≈26 y) | ≈20 g (as EAA) (Published data are reported as 0.35 g/FFM (Free Fat Mass). Here, they have been recalculated per mean subject assuming that FFM equals LBM (Lean Body Mass).) | −Ex | [59,75] | |
| Mycoprotein concentrate (corresponding to a total of 70 g whole mycoprotein.) | Y (21 y) | 38 g | +Ex | [76] |
2.2.2. Effects of Exercise and Nutrition on Muscle Protein Synthesis and Accretion
2.2.3. Effect of Other Substrates
Glucose
Lipids and Ketones
Other Nutritional Interventions
2.3. Hormones and Related Drug Interventions
2.3.1. Insulin
2.3.2. Glucocorticoids
2.3.3. Human Growth Hormone (hGH) and IGF-1
2.3.4. Catecholamines
2.3.5. Estrogens
2.3.6. Androgens
2.4. Exercise
2.4.1. Resistance Exercise Can Be Further Classified into Two Main Categories:
2.4.2. Effects of Exercise in Conjunction with Nutrient Intake
2.4.3. Exercise–Insulin Interaction
3. Part 2. The Pathophysiology of Sarcopenia in Ageing: Metabolic Hormonal, Cardiovascular, and Functional Changes in the Elderly and the Effects of Nutrition, Exercise, and Other Factors
3.1. The Molecular Mechanisms behind Anabolic Resistance with Ageing
3.1.1. Intracellular Signaling
mTOR Kinase
AKT Kinase
3.1.2. Extracellular Signaling
3.2. Anabolic Resistance in Ageing: Human Studies
- An increased splanchnic “trapping” of the ingested substrates, henceforth reducing amino acid delivery to peripheral tissues, such as skeletal muscle.
- A decreased amino acid utilization by muscle, and/or the requirement for a greater AA load/delivery to stimulate appropriately PS in muscle, compatible with an anabolic-resistant state. In other words, the skeletal muscle in ageing might be less sensitive to lower (normal) levels of amino acids than that in young adults, and may thus require more protein to acutely stimulate muscle protein synthesis above rest, to achieve the required accretion of muscle proteins.
- A decrease in energy production otherwise required to sustain the energy-expensive PS.
- Altered protein digestion.
- A decrease in transluminal AA transport.
- An intestinal microbiota different from that of younger people.
3.2.1. Basal Skeletal Muscle Protein Turnover in Ageing
3.2.2. Skeletal Muscle Protein Turnover in Ageing in Response to Nutrition and Exercise
3.2.3. Anabolic Response in Skeletal Muscles of Aged People
- Data consistent with a normal anabolic response (= no resistance).
| Type of Food/Protein Tested | Subjects | Dose A: Either the Threshold or the Lowest Dose Increasing MPS | Dose B: Either the Highest Dose Tested or that Maximally Stimulating MPS | Exercise Status | Comment | Reference |
|---|---|---|---|---|---|---|
| Dose–response study | ||||||
| Liquid meals (recalculated from original data to weight and total protein intake over the test) | Healthy (62–75 y) males and females | 29 g | 115 g | −/+Ex | Max effect obtained at the lowest dose. MPS increases greater after exercise. | [211] |
| Sarcopenic vs. healthy elderly | ||||||
| Leucine-enriched whey protein | Sarcopenic (81 y) males | 21 g | / | −Ex | Similar increase in MPS in both groups | [214] |
| Healthy (69 y) males | ||||||
| Similar response to controls (= no resistance) | ||||||
| Whey protein isolate | Old (71 y) males | 10 g | 40 g | −/+Ex | Exercise enhanced max effect at 40 g protein | [218] |
| Beef (beef contains −22% of weight as protein) | O (68–70 y) vs. Y (34–41 y) | 113 g | 340 g | −Ex | [212,213] | |
| CHO + WP + Leu (Data recalculated for the subjects’ average weight (75 kg). The table reports the total of the EAA+Leu administered, which were fractionated in 6 doses every hour) | O (75 y) vs. Y (20 y) males | / | 72 g WP + 13.5 g Leu | +Ex | 30 min of moderate-intensity physical activity | [213] |
| EAA drink | Elderly (67 y) males and females | 15 g | / | −Ex | Retarded albeit still sustained response in the elderly; faster, short-lived one in the young over 3 h | [58] |
| Mixed animal (beef) and vegetal foods | Elderly (69 y) males and females | 35 g protein | 70 g protein | −Ex | ≈5x greater response of MPS at the higher protein dose. At the 35 g dose, the response in O was > 5x lower than that in Y (see below) | [216] |
| Mixed animal (beef) and vegetal foods | Y (31 y) males and females | 40–44 g protein | 66–70 g prot | −/+Ex | At the 36 g dose, the response in O was > 5x less than that in Y | [57] |
| Protein and CHO | O (75 y) males | 20 g | −Ex | [217] | ||
| Y (21 y) males | ||||||
| Decreased/Blunted/Delayed response (= anabolic resistance) | ||||||
| Combined analysis of Whey Protein (n = 5 studies) Egg (n = 1 study) (original data recalculated to body weight) | Elderly (≈71 y) males | 8 g | ≈32 g | −Ex | Delayed response in the older group | [73] |
| Young (≈22 y) males | 8 g | ≈20g | −Ex | |||
| Intact whey protein | O (≥70 y) | 5–20 g | ≥20 to 40 g | −/+Ex | In the resting elderly, the response of MPS plateaus at 20 g, at a lower value than in Y (40 g). Resistance exercise can increase MPS only in O but at greater protein intake. | [72,218] |
| Y (> 23 y) | 5–20 g | 20 g | −/+Ex | |||
| Crystalline EAA | Y (28) vs. O (70 y) (Similar response of MPS in the two groups) | 2.5 g (?) | 20–40 g (the highest dose (40 g) was tested only in the older group) | −/+Ex | [60] | |
| AA + Leucine | O (68–70 y) males and females | 15 (Published data are reported as 0.35 g/FFM (Free Fat Mass). Here, they have been recalculated per mean subject assuming the FFM individuals’ LBM (Lean Body Mass).) | / | −/+Ex −/+insuin | MPD = pre and post ex, with or without insulin | [59] |
| Leucine-enriched EAA | O (67) vs. Y (29) | 6.7 g EAA | / | −Ex | A higher leucine dose (41%) was necessary to match the increase in MPS of the O to that of the Y | [195] |
- Data consistent with an impaired response (=anabolic resistance)
3.2.4. Insulin Resistance
3.2.5. Resistance to the Anabolic Effects of Exercise
3.2.6. Deleterious Effects of Bed Rest in the Elderly
3.2.7. Effect of the Inflammatory State
3.2.8. Role of Blood Perfusion of Skeletal Muscle
3.3. Strategies to Counteract Anabolic Resistance in Ageing
3.3.1. The Effect of Complex Nutritional Supplements
3.3.2. Specific Effects of Leucine Addition
3.3.3. Exercise Strategies
3.3.4. Steps for Adaptation to Exercise
3.3.5. Caution and Contraindications to Exercise in the Elderly
3.3.6. Other Treatments
3.3.7. Optimizing Nutrition–Exercise Interaction in the Stimulation of Skeletal Muscle Anabolism in Ageing
3.3.8. Muscle and Bones
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Ackermans, L.L.G.C.; Rabou, J.; Basrai, M.; Schweinlin, A.; Bischoff, S.C.; Cussenot, O.; Cancel-Tassin, G.; Renken, R.J.; Gómez, E.; Sánchez-González, P.; et al. Screening, Diagnosis and Monitoring of Sarcopenia: When to Use Which Tool? Clin. Nutr. ESPEN 2022, 48, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of Sarcopenia in the World: A Systematic Review and Meta- Analysis of General Population Studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R. Effects of Aging on Muscle Fibre Type and Size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Schmid, A.; Sheikhzadeh, A.; Nordin, M.; Yoon, J.; Frankel, V. Effects of Aging on Type II Muscle Fibers: A Systematic Review of the Literature. J. Aging Phys. Act. 2007, 15, 336–348. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of Sarcopenia: Prevalence, Risk Factors, and Consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, C.W.; Prost, R.W.; Fitts, R.H.; Hunter, S.K. Bioenergetic Basis for the Increased Fatigability with Ageing. J. Physiol. 2019, 597, 4943–4957. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Fry, C.S.; Glynn, E.L.; Dreyer, H.C.; Dhanani, S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. Rapamycin Administration in Humans Blocks the Contraction-Induced Increase in Skeletal Muscle Protein Synthesis: Rapamycin Blocks Protein Synthesis in Human Muscle. J. Physiol. 2009, 587, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Francaux, M.; Demeulder, B.; Naslain, D.; Fortin, R.; Lutz, O.; Caty, G.; Deldicque, L. Aging Reduces the Activation of the MTORC1 Pathway after Resistance Exercise and Protein Intake in Human Skeletal Muscle: Potential Role of REDD1 and Impaired Anabolic Sensitivity. Nutrients 2016, 8, 47. [Google Scholar] [CrossRef]
- Léger, B.; Cartoni, R.; Praz, M.; Lamon, S.; Dériaz, O.; Crettenand, A.; Gobelet, C.; Rohmer, P.; Konzelmann, M.; Luthi, F.; et al. Akt Signalling through GSK-3beta, MTOR and Foxo1 Is Involved in Human Skeletal Muscle Hypertrophy and Atrophy. J. Physiol. 2006, 576, 923–933. [Google Scholar] [CrossRef]
- Di Camillo, B.; Eduati, F.; Nair, S.K.; Avogaro, A.; Toffolo, G.M. Leucine Modulates Dynamic Phosphorylation Events in Insulin Signaling Pathway and Enhances Insulin-Dependent Glycogen Synthesis in Human Skeletal Muscle Cells. BMC Cell. Biol. 2014, 15, 9. [Google Scholar] [CrossRef]
- Kakigi, R.; Yoshihara, T.; Ozaki, H.; Ogura, Y.; Ichinoseki-Sekine, N.; Kobayashi, H.; Naito, H. Whey Protein Intake after Resistance Exercise Activates MTOR Signaling in a Dose-Dependent Manner in Human Skeletal Muscle. Eur. J Appl. Physiol. 2014, 114, 735–742. [Google Scholar] [CrossRef]
- Jacquemin, V.; Furling, D.; Bigot, A.; Butler-Browne, G.S.; Mouly, V. IGF-1 Induces Human Myotube Hypertrophy by Increasing Cell Recruitment. Exp. Cell Res. 2004, 299, 148–158. [Google Scholar] [CrossRef]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef]
- Hansen, M.; Koskinen, S.O.; Petersen, S.G.; Doessing, S.; Frystyk, J.; Flyvbjerg, A.; Westh, E.; Magnusson, S.P.; Kjaer, M.; Langberg, H. Ethinyl Oestradiol Administration in Women Suppresses Synthesis of Collagen in Tendon in Response to Exercise: Oral Contraceptives and Tendon Collagen. J. Physiol. 2008, 586, 3005–3016. [Google Scholar] [CrossRef]
- Perrini, S.; Laviola, L.; Carreira, M.C.; Cignarelli, A.; Natalicchio, A.; Giorgino, F. The GH/IGF1 Axis and Signaling Pathways in the Muscle and Bone: Mechanisms Underlying Age-Related Skeletal Muscle Wasting and Osteoporosis. J. Endocrinol. 2010, 205, 201–210. [Google Scholar] [CrossRef]
- Chikani, V.; Ho, K.K.Y. Action of GH on Skeletal Muscle Function: Molecular and Metabolic Mechanisms. J. Mol. Endocrinol. 2014, 52, R107–R123. [Google Scholar] [CrossRef]
- West, D.W.D.; Phillips, S.M. Anabolic Processes in Human Skeletal Muscle: Restoring the Identities of Growth Hormone and Testosterone. Physician Sports Med. 2010, 38, 97–104. [Google Scholar] [CrossRef]
- Florini, J.R. Effects of Testosterone on Qualitative Pattern of Protein Synthesis in Skeletal Muscle. Biochemistry 1970, 9, 909–912. [Google Scholar] [CrossRef]
- Mauras, N.; Hayes, V.; Welch, S.; Rini, A.; Helgeson, K.; Dokler, M.; Veldhuis, J.D.; Urban, R.J. Testosterone Deficiency in Young Men: Marked Alterations in Whole Body Protein Kinetics, Strength, and Adiposity. J. Clin. Endocrinol. Metab. 1998, 83, 1886–1892. [Google Scholar] [CrossRef]
- Demling, R.H.; Orgill, D.P. The Anticatabolic and Wound Healing Effects of the Testosterone Analog Oxandrolone after Severe Burn Injury. J. Crit. Care 2000, 15, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone Physiology in Resistance Exercise and Training: The Up-Stream Regulatory Elements. Sports Med. 2010, 40, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Vierck, J. Satellite Cell Regulation Following Myotrauma Caused by Resistance Exercise. Cell Biol. Int. 2000, 24, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Woodhouse, L.; Casaburi, R.; Singh, A.B.; Bhasin, D.; Berman, N.; Chen, X.; Yarasheski, K.E.; Magliano, L.; Dzekov, C.; et al. Testosterone Dose-Response Relationships in Healthy Young Men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1172–E1181. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 292–301. [Google Scholar] [CrossRef]
- Tezze, C.; Amendolagine, F.I.; Nogara, L.; Baraldo, M.; Ciciliot, S.; Arcidiacono, D.; Zaramella, A.; Masiero, G.; Ferrarese, G.; Realdon, S.; et al. A Combination of Metformin and Galantamine Exhibits Synergistic Benefits in the Treatment of Sarcopenia. JCI Insight 2023, 8, e168787. [Google Scholar] [CrossRef] [PubMed]
- Malena, A.; Pennuto, M.; Tezze, C.; Querin, G.; D’Ascenzo, C.; Silani, V.; Cenacchi, G.; Scaramozza, A.; Romito, S.; Morandi, L.; et al. Androgen-Dependent Impairment of Myogenesis in Spinal and Bulbar Muscular Atrophy. Acta Neuropathol. 2013, 126, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389. [Google Scholar] [CrossRef]
- Solagna, F.; Tezze, C.; Lindenmeyer, M.T.; Lu, S.; Wu, G.; Liu, S.; Zhao, Y.; Mitchell, R.; Meyer, C.; Omairi, S.; et al. Pro-Cachectic Factors Link Experimental and Human Chronic Kidney Disease to Skeletal Muscle Wasting Programs. J. Clin. Investig. 2021, 131, e135821. [Google Scholar] [CrossRef]
- Smith, K.; Rennie, M.J. The Measurement of Tissue Protein Turnover. Baillière’s Clin. Endocrinol. Metab. 1996, 10, 469–495. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Rémond, D.; Van Loon, L.J.C. The Muscle Protein Synthetic Response to Food Ingestion. Meat Sci. 2015, 109, 96–100. [Google Scholar] [CrossRef]
- Wolfe, R.R. Regulation of Muscle Protein by Amino Acids. J. Nutr. 2002, 132, 3219S–3224S. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Bennet, W.M.; Connacher, A.A.; Scrimgeour, C.M.; Rennie, M.J. The Effect of Amino Acid Infusion on Leg Protein Turnover Assessed by L-[15N]Phenylalanine and L-[1-13C]Leucine Exchange. Eur. J. Clin. Investig. 2008, 20, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Barazzoni, R.; Zanetti, M.; Kiwanuka, E.; Tiengo, A. The Role of Substrates in the Regulation of Protein Metabolism. Baillière’s Clin. Endocrinol. Metab. 1996, 10, 511–532. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Nair, K.S. Differential Regulation of Protein Dynamics in Splanchnic and Skeletal Muscle Beds by Insulin and Amino Acids in Healthy Human Subjects. Diabetes 2003, 52, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential Amino Acids Are Primarily Responsible for the Amino Acid Stimulation of Muscle Protein Anabolism in Healthy Elderly Adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Regulation of Global and Specific MRNA Translation by Oral Administration of Branched-Chain Amino Acids. Biochem. Biophys. Res. Commun. 2004, 313, 423–427. [Google Scholar] [CrossRef]
- Ham, D.J.; Caldow, M.K.; Lynch, G.S.; Koopman, R. Leucine as a Treatment for Muscle Wasting: A Critical Review. Clin. Nutr. 2014, 33, 937–945. [Google Scholar] [CrossRef]
- May, M.E.; Buse, M.G. Effects of Branched-Chain Amino Acids on Protein Turnover. Diabetes Metab. Rev. 1989, 5, 227–245. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Jefferson, L.S. Signaling Pathways Involved in Translational Control of Protein Synthesis in Skeletal Muscle by Leucine. J. Nutr. 2001, 131, 856S–860S. [Google Scholar] [CrossRef]
- Buse, M.G.; Reid, S.S. Leucine. A Possible Regulator of Protein Turnover in Muscle. J. Clin. Investig. 1975, 56, 1250–1261. [Google Scholar] [CrossRef]
- De Bandt, J.-P. Leucine and Mammalian Target of Rapamycin–Dependent Activation of Muscle Protein Synthesis in Aging. J. Nutr. 2016, 146, 2616S–2624S. [Google Scholar] [CrossRef]
- Van Loon, L.J.C. Leucine as a Pharmaconutrient in Health and Disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Zanetti, M.; Barazzoni, R.; Vettore, M.; Michielan, F. Mechanisms of Postprandial Protein Accretion in Human Skeletal Muscle. Insight from Leucine and Phenylalanine Forearm Kinetics. J. Clin. Investig. 1996, 98, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.A.; Church, D.D.; Wolfe, R.R.; Ferrando, A.A.; Pasiakos, S.M. Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients 2020, 12, 2457. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar Muscle Protein Synthesis Rates Subsequent to a Meal in Response to Increasing Doses of Whey Protein at Rest and after Resistance Exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef]
- Moore, D.R.; Tang, J.E.; Burd, N.A.; Rerecich, T.; Tarnopolsky, M.A.; Phillips, S.M. Differential Stimulation of Myofibrillar and Sarcoplasmic Protein Synthesis with Protein Ingestion at Rest and after Resistance Exercise. J. Physiol. 2009, 587, 897–904. [Google Scholar] [CrossRef]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-Dependent Responses of Myofibrillar Protein Synthesis with Beef Ingestion Are Enhanced with Resistance Exercise in Middle-Aged Men. Appl. Physiol. Nutr. Metab. 2013, 38, 120–125. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.; Spencer, H.J.; Azhar, G.; Ferrando, A.A.; Wolfe, R.R. The Anabolic Response to a Meal Containing Different Amounts of Protein Is Not Limited by the Maximal Stimulation of Protein Synthesis in Healthy Young Adults. Am. J. Physiol. -Endocrinol. Metab. 2016, 310, E73–E80. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.-J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino Acid Ingestion Improves Muscle Protein Synthesis in the Young and Elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef]
- Fujita, S.; Volpi, R. Nutrition and sarcopenia of ageing. Nutr. Res. Rev. 2004, 17, 69–76. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic Signaling Deficits Underlie Amino Acid Resistance of Wasting, Aging Muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Xu, Z.; Tan, Z.; Zhang, Q.; Gui, Q.; Yang, Y. The Effectiveness of Leucine on Muscle Protein Synthesis, Lean Body Mass and Leg Lean Mass Accretion in Older People: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kihata, A.; Naraba, H.; Kanda, N.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. Β-Hydroxy-β-methylbutyrate, Arginine, and Glutamine Complex on Muscle Volume Loss in Critically Ill Patients: A Randomized Control Trial. J. Parenter. Enter. Nutr. 2020, 44, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Xi, P. Regulation of Protein Metabolism by Glutamine: Implications for Nutrition and Health. Front. Biosci. 2011, 16, 578. [Google Scholar] [CrossRef]
- Svanberg, E.; Möller-Loswick, A.-C.; Matthews, D.E.; Körner, U.; Lundholm, K. The Effect of Glutamine on Protein Balance and Amino Acid Flux across Arm and Leg Tissues in Healthy Volunteers: Glutamine and Muscle Protein Anabolism. Clin. Physiol. 2001, 21, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Zachwieja, J.J.; Witt, T.L.; Yarasheski, K.E. Intravenous Glutamine Does Not Stimulate Mixed Muscle Protein Synthesis in Healthy Young Men and Women. Metabolism 2000, 49, 1555–1560. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater Stimulation of Myofibrillar Protein Synthesis with Ingestion of Whey Protein Isolate v. Micellar Casein at Rest and after Resistance Exercise in Elderly Men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef]
- Van Vliet, S.; Beals, J.W.; Holwerda, A.M.; Emmons, R.S.; Goessens, J.P.; Paluska, S.A.; De Lisio, M.; Van Loon, L.J.C.; Burd, N.A. Time-Dependent Regulation of Postprandial Muscle Protein Synthesis Rates after Milk Protein Ingestion in Young Men. J. Appl. Physiol. 2019, 127, 1792–1801. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Dunlop, M.V.; Machin, D.J.; Coelho, M.O.C.; Pavis, G.F.; Porter, C.; Murton, A.J.; Abdelrahman, D.R.; Dirks, M.L.; Stephens, F.B.; et al. A Mycoprotein-Based High-Protein Vegan Diet Supports Equivalent Daily Myofibrillar Protein Synthesis Rates Compared with an Isonitrogenous Omnivorous Diet in Older Adults: A Randomised Controlled Trial. Br. J. Nutr. 2021, 126, 674–684. [Google Scholar] [CrossRef]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The Response of Muscle Protein Synthesis Following Whole-Body Resistance Exercise Is Greater Following 40 g than 20 g of Ingested Whey Protein. Physiol. Rep. 2016, 4, e12893. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Phillips, S.M. Skeletal Muscle Protein Metabolism in the Elderly: Interventions to Counteract the “anabolic Resistance” of Ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. Ser. A 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Smeets, J.S.J.; Betz, M.W.; Senden, J.M.; Goessens, J.P.B.; Gijsen, A.P.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Dose-Response Effects of Dietary Protein on Muscle Protein Synthesis during Recovery from Endurance Exercise in Young Men: A Double-Blind Randomized Trial. Am. J. Clin. Nutr. 2020, 112, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient Signalling in the Regulation of Human Muscle Protein Synthesis: Nutrient Signalling and Muscle Protein Synthesis. J. Physiol. 2007, 582, 813–823. [Google Scholar] [CrossRef]
- West, S.; Monteyne, A.J.; Whelehan, G.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.A.; Blackwell, J.R.; Stephens, F.B.; Wall, B.T. Mycoprotein Ingestion within or without Its Wholefood Matrix Results in Equivalent Stimulation of Myofibrillar Protein Synthesis Rates in Resting and Exercised Muscle of Young Men. Br. J. Nutr. 2023, 130, 20–32. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The Digestion Rate of Protein Is an Independent Regulating Factor of Postprandial Protein Retention. Am. J. Physiol. -Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Cope, M.B.; Mukherjea, R.; Jennings, K.; Volpi, E.; et al. Soy-Dairy Protein Blend and Whey Protein Ingestion after Resistance Exercise Increases Amino Acid Transport and Transporter Expression in Human Skeletal Muscle. J. Appl. Physiol. 2014, 116, 1353–1364. [Google Scholar] [CrossRef]
- Tipton, K.D.; Wolfe, R.R. Exercise, Protein Metabolism, and Muscle Growth. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 109–132. [Google Scholar] [CrossRef]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An Abundant Supply of Amino Acids Enhances the Metabolic Effect of Exercise on Muscle Protein. Am. J. Physiol. -Endocrinol. Metab. 1997, 273, E122–E129. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Murphy, C.H.; Longland, T.M.; Phillips, S.M. Role of Protein and Amino Acids in Promoting Lean Mass Accretion with Resistance Exercise and Attenuating Lean Mass Loss during Energy Deficit in Humans. Amino Acids 2013, 45, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; Van Loon, L.J. Whey Protein Stimulates Postprandial Muscle Protein Accretion More Effectively than Do Casein and Casein Hydrolysate in Older Men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and Whey Exert Different Effects on Plasma Amino Acid Profiles, Gastrointestinal Hormone Secretion and Appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B.; Tipton, K.D.; Miller, S.L.; Wolf, S.E.; Wolfe, R.R. An Oral Essential Amino Acid-Carbohydrate Supplement Enhances Muscle Protein Anabolism after Resistance Exercise. J. Appl. Physiol. 2000, 88, 386–392. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Aarsland, A.A.; Sanford, A.P.; Wolfe, R.R. Stimulation of Net Muscle Protein Synthesis by Whey Protein Ingestion before and after Exercise. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E71–E76. [Google Scholar] [CrossRef]
- Reeds, P.J.; Burrin, D.G.; Davis, T.A.; Stoll, B. Amino Acid Metabolism and the Energetics of Growth. Arch. Anim. Nutr. 1998, 51, 187–197. [Google Scholar] [CrossRef]
- Smiles, W.J.; Hawley, J.A.; Camera, D.M. Effects of Skeletal Muscle Energy Availability on Protein Turnover Responses to Exercise. J. Exp. Biol. 2016, 219, 214–225. [Google Scholar] [CrossRef][Green Version]
- Hedden, M.P.; Buse, M.G. Effects of Glucose, Pyruvate, Lactate, and Amino Acids on Muscle Protein Synthesis. Am. J. Physiol. 1982, 242, E184–E192. [Google Scholar] [CrossRef]
- Goichon, A.; Coëffier, M.; Claeyssens, S.; Lecleire, S.; Cailleux, A.-F.; Bôle-Feysot, C.; Chan, P.; Donnadieu, N.; Lerebours, E.; Lavoinne, A.; et al. Effects of an Enteral Glucose Supply on Protein Synthesis, Proteolytic Pathways, and Proteome in Human Duodenal Mucosa. Am. J. Clin. Nutr. 2011, 94, 784–794. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Leese, G.P.; Smith, K.; Watt, P.W.; Nevill, A.; Rooyackers, O.; Wagenmakers, A.J.; Rennie, M.J. Effect of Oral Glucose on Leucine Turnover in Human Subjects at Rest and during Exercise at Two Levels of Dietary Protein. J. Physiol. 2000, 525 Pt 1, 271–281. [Google Scholar] [CrossRef]
- Staples, A.W.; Burd, N.A.; West, D.W.D.; Currie, K.D.; Atherton, P.J.; Moore, D.R.; Rennie, M.J.; Macdonald, M.J.; Baker, S.K.; Phillips, S.M. Carbohydrate Does Not Augment Exercise-Induced Protein Accretion versus Protein Alone. Med. Sci. Sports Exerc. 2011, 43, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Beelen, M.; Stellingwerff, T.; Pennings, B.; Saris, W.H.M.; Kies, A.K.; Kuipers, H.; Van Loon, L.J.C. Coingestion of Carbohydrate with Protein Does Not Further Augment Postexercise Muscle Protein Synthesis. Am. J. Physiol. -Endocrinol. Metab. 2007, 293, E833–E842. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.-J.; Bier, D.; Schoeller, D.; Wolfe, R.; Matthews, D.E.; Munro, H.N.; Young, V.R. Effects of Intravenous Glucose on Whole Body Leucine Dynamics, Studied With 1-13C-Leucine, in Healthy Young and Elderly Adults. J. Gerontol. 1984, 39, 673–681. [Google Scholar] [CrossRef]
- Heiling, V.J.; Campbell, P.J.; Gottesman, I.S.; Tsalikian, E.; Beaufrere, B.; Gerich, J.E.; Haymond, M.W. Differential Effects of Hyperglycemia and Hyperinsulinemia on Leucine Rate of Appearance in Normal Humans. J. Clin. Endocrinol. Metab. 1993, 76, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of Glycogen Availability on Human Skeletal Muscle Protein Turnover during Exercise and Recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.H.; Rittig, N.; Johannsen, M.; Møller, A.B.; Jørgensen, J.O.; Jessen, N.; Møller, N. Effects of 3-Hydroxybutyrate and Free Fatty Acids on Muscle Protein Kinetics and Signaling during LPS-Induced Inflammation in Humans: Anticatabolic Impact of Ketone Bodies. Am. J. Clin. Nutr. 2018, 108, 857–867. [Google Scholar] [CrossRef]
- Rodriguez, N.; Schwenk, W.F.; Beaufrere, B.; Miles, J.M.; Haymond, M.W. Trioctanoin Infusion Increases in Vivo Leucine Oxidation: A Lesson in Isotope Modeling. Am. J. Physiol. -Endocrinol. Metab. 1986, 251, E343–E348. [Google Scholar] [CrossRef]
- Haymond, M.W.; Tessari, P.; Beaufrere, B.; Rodriguez, N.; Bailey, J.; Miles, J.M. Effects of Parenteral Lipid on Leucine Metabolism: Dependence of Fatty Acid Chain Length. JPEN J. Parenter. Enter. Nutr. 1988, 12, 94S–97S. [Google Scholar] [CrossRef]
- Lang, C.H. Elevated Plasma Free Fatty Acids Decrease Basal Protein Synthesis, but Not the Anabolic Effect of Leucine, in Skeletal Muscle. Am. J. Physiol. -Endocrinol. Metab. 2006, 291, E666–E674. [Google Scholar] [CrossRef]
- Tsintzas, K.; Jones, R.; Pabla, P.; Mallinson, J.; Barrett, D.A.; Kim, D.-H.; Cooper, S.; Davies, A.; Taylor, T.; Chee, C.; et al. Effect of Acute and Short-Term Dietary Fat Ingestion on Postprandial Skeletal Muscle Protein Synthesis Rates in Middle-Aged, Overweight, and Obese Men. Am. J. Physiol. -Endocrinol. Metab. 2020, 318, E417–E429. [Google Scholar] [CrossRef]
- Nair, K.S.; Welle, S.L.; Halliday, D.; Campbell, R.G. Effect of Beta-Hydroxybutyrate on Whole-Body Leucine Kinetics and Fractional Mixed Skeletal Muscle Protein Synthesis in Humans. J. Clin. Investig. 1988, 82, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Beta-Hydroxy-Beta-Methylbutyrate Supplementation and Skeletal Muscle in Healthy and Muscle-Wasting Conditions: HMB Supplementation and Muscle. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite Β-hydroxy-β-methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. β-Alanine Supplementation Increased Physical Performance and Improved Executive Function Following Endurance Exercise in Middle Aged Individuals. J. Int. Soc. Sports Nutr. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H. Role of β-Alanine Supplementation on Muscle Carnosine and Exercise Performance. Med. Sci. Sports Exerc. 2010, 42, 1162–1173. [Google Scholar] [CrossRef]
- Parise, G.; Mihic, S.; MacLennan, D.; Yarasheski, K.E.; Tarnopolsky, M.A. Effects of Acute Creatine Monohydrate Supplementation on Leucine Kinetics and Mixed-Muscle Protein Synthesis. J. Appl. Physiol. 2001, 91, 1041–1047. [Google Scholar] [CrossRef]
- Tipton, K.D.; Ferrando, A.A. Improving Muscle Mass: Response of Muscle Metabolism to Exercise, Nutrition and Anabolic Agents. Essays Biochem. 2008, 44, 85–98. [Google Scholar] [CrossRef]
- Tessari, P.; Inchiostro, S.; Biolo, G.; Vincenti, E.; Sabadin, L. Effects of Acute Systemic Hyperinsulinemia on Forearm Muscle Proteolysis in Healthy Man. J. Clin. Investig. 1991, 88, 27–33. [Google Scholar] [CrossRef]
- Biolo, G.; Declan Fleming, R.Y.; Wolfe, R.R. Physiologic Hyperinsulinemia Stimulates Protein Synthesis and Enhances Transport of Selected Amino Acids in Human Skeletal Muscle. J. Clin. Investig. 1995, 95, 811–819. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Lee, J.L.; Dreyer, H.C.; Dhanani, S.; Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Sheffield-Moore, M.; Rasmussen, B.B.; Volpi, E. Insulin Stimulates Human Skeletal Muscle Protein Synthesis via an Indirect Mechanism Involving Endothelial-Dependent Vasodilation and Mammalian Target of Rapamycin Complex 1 Signaling. J. Clin. Endocrinol. Metab. 2010, 95, 3848–3857. [Google Scholar] [CrossRef]
- Tessari, P.; Inchiostro, S.; Biolo, G.; Marescotti, M.C.; Fantin, G.; Boscarato, M.T.; Merola, G.; Mantero, F.; Tiengo, A. Leucine Kinetics and the Effects of Hyperinsulinemia in Patients With Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 1989, 68, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Horber, F.F.; Haymond, M.W. Human Growth Hormone Prevents the Protein Catabolic Side Effects of Prednisone in Humans. J. Clin. Investig. 1990, 86, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-Induced Skeletal Muscle Atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Louard, R.J.; Bhushan, R.; Gelfand, R.A.; Barrett, E.J.; Sherwin, R.S. Glucocorticoids Antagonize Insulin’s Antiproteolytic Action on Skeletal Muscle in Humans. J. Clin. Endocrinol. Metab. 1994, 79, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Fryburg, D.A.; Gelfand, R.A.; Barrett, E.J. Growth Hormone Acutely Stimulates Forearm Muscle Protein Synthesis in Normal Humans. Am. J. Physiol. -Endocrinol. Metab. 1991, 260, E499–E504. [Google Scholar] [CrossRef]
- Haymond, M.W.; Horber, F.; De Feo, P.; Kahn, S.E.; Mauras, N. Effect of Human Growth Hormone and Insulin-Like Growth Factor I on Whole-Body Leucine and Estimates of Protein Metabolism. Horm. Res. 1993, 40, 92–94. [Google Scholar] [CrossRef]
- Kandalla, P.K.; Goldspink, G.; Butler-Browne, G.; Mouly, V. Mechano Growth Factor E Peptide (MGF-E), Derived from an Isoform of IGF-1, Activates Human Muscle Progenitor Cells and Induces an Increase in Their Fusion Potential at Different Ages. Mech. Ageing Dev. 2011, 132, 154–162. [Google Scholar] [CrossRef]
- Górecki, D.C.; Beręsewicz, M.; Zabłocka, B. Neuroprotective Effects of Short Peptides Derived from the Insulin-like Growth Factor 1. Neurochem. Int. 2007, 51, 451–458. [Google Scholar] [CrossRef]
- Qin, L.-L.; Li, X.-K.; Xu, J.; Mo, D.-L.; Tong, X.; Pan, Z.-C.; Li, J.-Q.; Chen, Y.-S.; Zhang, Z.; Wang, C.; et al. Mechano Growth Factor (MGF) Promotes Proliferation and Inhibits Differentiation of Porcine Satellite Cells (PSCs) by down-Regulation of Key Myogenic Transcriptional Factors. Mol. Cell. Biochem. 2012, 370, 221–230. [Google Scholar] [CrossRef]
- Matthews, D.E.; Pesola, G.; Campbell, R.G. Effect of epinephrine on amino acid and energy metabolism in humans. Am. J. Physiol. 1990, 258 Pt 1, E948–E956. [Google Scholar] [CrossRef]
- Schiefermeier, M.; Ratheiser, K.; Zauner, C.; Roth, E.; Eichler, H.; Matthews, D. Epinephrine Does Not Impair Utilization of Exogenous Amino Acids in Humans. Am. J. Clin. Nutr. 1997, 65, 1765–1773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kraenzlin, M.E.; Keller, U.; Keller, A.; Thélin, A.; Arnaud, M.J.; Stauffacher, W. Elevation of Plasma Epinephrine Concentrations Inhibits Proteolysis and Leucine Oxidation in Man via Beta-Adrenergic Mechanisms. J. Clin. Investig. 1989, 84, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Fryburg, D.A.; Gelfand, R.A.; Jahn, L.A.; Oliveras, D.; Sherwin, R.S.; Sacca, L.; Barrett, E.J. Effects of Epinephrine on Human Muscle Glucose and Protein Metabolism. Am. J. Physiol. -Endocrinol. Metab. 1995, 268, E55–E59. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.J.; Horan, M.A.; Little, R.A.; Rothwell, N.J. Anabolic Effects of Clenbuterol on Skeletal Muscle Are Mediated by Beta 2-Adrenoceptor Activation. Am. J. Physiol. -Endocrinol. Metab. 1992, 263, E50–E56. [Google Scholar] [CrossRef]
- Ryall, J.G.; Lynch, G.S. The Potential and the Pitfalls of β-Adrenoceptor Agonists for the Management of Skeletal Muscle Wasting. Pharmacol. Ther. 2008, 120, 219–232. [Google Scholar] [CrossRef]
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. [Google Scholar] [CrossRef]
- Boland, R.; Vasconsuelo, A.; Milanesi, L.; Ronda, A.C.; de Boland, A.R. 17beta-Estradiol Signaling in Skeletal Muscle Cells and Its Relationship to Apoptosis. Steroids 2008, 73, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-J.A.; McCormick, K.M.; Brazeau, D.A.; Brazeau, G.A. Estrogen Effects on Skeletal Muscle Insulin-like Growth Factor 1 and Myostatin in Ovariectomized Rats. Exp. Biol. Med. 2007, 232, 1314–1325. [Google Scholar] [CrossRef]
- Khadilkar, S.S. Musculoskeletal Disorders and Menopause. J. Obstet. Gynaecol. India 2019, 69, 99–103. [Google Scholar] [CrossRef]
- Hansen, M. Female Hormones: Do They Influence Muscle and Tendon Protein Metabolism? Proc. Nutr. Soc. 2018, 77, 32–41. [Google Scholar] [CrossRef]
- Aloia, J.F.; McGowan, D.M.; Vaswani, A.N.; Ross, P.; Cohn, S.H. Relationship of Menopause to Skeletal and Muscle Mass. Am. J. Clin. Nutr. 1991, 53, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-J.; Choi, Y.; Jung, S.-J.; Kwak, H.-B. Role of Exercise in Estrogen Deficiency-Induced Sarcopenia. J. Exerc. Rehabil. 2022, 18, 2–9. [Google Scholar] [CrossRef]
- Smith, G.I.; Yoshino, J.; Reeds, D.N.; Bradley, D.; Burrows, R.E.; Heisey, H.D.; Moseley, A.C.; Mittendorfer, B. Testosterone and Progesterone, But Not Estradiol, Stimulate Muscle Protein Synthesis in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.E.; Layman, D.K. Leucine Regulates Translation Initiation of Protein Synthesis in Skeletal Muscle after Exercise. J. Nutr. 2006, 136, 533S–537S. [Google Scholar] [CrossRef]
- Herman, J.R.; Rana, S.R.; Chleboun, G.S.; Gilders, R.M.; Hageman, F.C.; Hikida, R.S.; Kushnick, M.R.; Ragg, K.E.; Staron, R.S.; Toma, K. Correlation Between Muscle Fiber Cross-Sectional Area And Strength Gain Using Three Different Resistance-Training Programs In College-Aged Women. J. Strength Cond. Res. 2010, 24, 1. [Google Scholar] [CrossRef]
- Jones, E.J.; Bishop, P.A.; Woods, A.K.; Green, J.M. Cross-Sectional Area and Muscular Strength: A Brief Review. Sports Med. 2008, 38, 987–994. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-Related Differences in the Dose-Response Relationship of Muscle Protein Synthesis to Resistance Exercise in Young and Old Men: Age-Related Effects of Exercise on Muscle Anabolism. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef]
- Mayhew, D.L.; Kim, J.; Cross, J.M.; Ferrando, A.A.; Bamman, M.M. Translational Signaling Responses Preceding Resistance Training-Mediated Myofiber Hypertrophy in Young and Old Humans. J. Appl. Physiol. 2009, 107, 1655–1662. [Google Scholar] [CrossRef]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed Muscle Protein Synthesis and Breakdown after Resistance Exercise in Humans. Am. J. Physiol. -Endocrinol. Metab. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Pak-Loduca, J.; Hasten, D.L.; Obert, K.A.; Brown, M.B.; Sinacore, D.R. Resistance Exercise Training Increases Mixed Muscle Protein Synthesis Rate in Frail Women and Men >/=76 Yr Old. Am. J. Physiol. 1999, 277, E118–E125. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Zachwieja, J.J.; Bier, D.M. Acute Effects of Resistance Exercise on Muscle Protein Synthesis Rate in Young and Elderly Men and Women. Am. J. Physiol. 1993, 265, E210–E214. [Google Scholar] [CrossRef] [PubMed]
- Sheffield-Moore, M.; Paddon-Jones, D.; Sanford, A.P.; Rosenblatt, J.I.; Matlock, A.G.; Cree, M.G.; Wolfe, R.R. Mixed Muscle and Hepatic Derived Plasma Protein Metabolism Is Differentially Regulated in Older and Younger Men Following Resistance Exercise. Am. J. Physiol. -Endocrinol. Metab. 2005, 288, E922–E929. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Harber, M.P. Skeletal Muscle Hypertrophy After Aerobic Exercise Training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef]
- Küüsmaa, M.; Schumann, M.; Sedliak, M.; Kraemer, W.J.; Newton, R.U.; Malinen, J.-P.; Nyman, K.; Häkkinen, A.; Häkkinen, K. Effects of Morning versus Evening Combined Strength and Endurance Training on Physical Performance, Muscle Hypertrophy, and Serum Hormone Concentrations. Appl. Physiol. Nutr. Metab. 2016, 41, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; McGinley, C.; Lee, M.J.-C.; Saner, N.J.; Garnham, A.; Bishop, D.J. Interpretation of Exercise-Induced Changes in Human Skeletal Muscle MRNA Expression Depends on the Timing of the Post-Exercise Biopsies. Peer J. 2022, 10, e12856. [Google Scholar] [CrossRef] [PubMed]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.L.; Lehti, M.; Hulmi, J.J. Stimuli and Sensors That Initiate Skeletal Muscle Hypertrophy Following Resistance Exercise. J. Appl. Physiol. 2019, 126, 30–43. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/MTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. JND 2021, 8, 169–183. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Fry, C.S.; Drummond, M.J.; Gundermann, D.M.; Walker, D.K.; Glynn, E.L.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Mammalian Target of Rapamycin Complex 1 Activation Is Required for the Stimulation of Human Skeletal Muscle Protein Synthesis by Essential Amino Acids. J. Nutr. 2011, 141, 856–862. [Google Scholar] [CrossRef]
- Børsheim, E.; Tipton, K.D.; Wolf, S.E.; Wolfe, R.R. Essential Amino Acids and Muscle Protein Recovery from Resistance Exercise. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E648–E657. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Tipton, K.D.; Chinkes, D.L.; Wolf, S.E.; Wolfe, R.R. Independent and Combined Effects of Amino Acids and Glucose after Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Borsheim, E.; Wolf, S.E.; Sanford, A.P.; Wolfe, R.R. Acute Response of Net Muscle Protein Balance Reflects 24-h Balance after Exercise and Amino Acid Ingestion. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E76–E89. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Ferrando, A.A.; Phillips, S.M.; Doyle, D.; Wolfe, R.R. Postexercise Net Protein Synthesis in Human Muscle from Orally Administered Amino Acids. Am. J. Physiol. 1999, 276, E628–E634. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Wolf, S.E.; Sanford, A.P.; Wolfe, R.R. Ingestion of Casein and Whey Proteins Result in Muscle Anabolism after Resistance Exercise. Med. Sci. Sports Exerc. 2004, 36, 2073–2081. [Google Scholar] [CrossRef]
- English, K.L.; Paddon-Jones, D. Protecting Muscle Mass and Function in Older Adults during Bed Rest. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 34–39. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Nutrient Timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Kume, W.; Yasuda, J.; Hashimoto, T. Acute Effect of the Timing of Resistance Exercise and Nutrient Intake on Muscle Protein Breakdown. Nutrients 2020, 12, 1177. [Google Scholar] [CrossRef]
- Biolo, G.; Williams, B.D.; Fleming, R.Y.; Wolfe, R.R. Insulin Action on Muscle Protein Kinetics and Amino Acid Transport during Recovery after Resistance Exercise. Diabetes 1999, 48, 949–957. [Google Scholar] [CrossRef]
- Michel, J.-P.; Beattie, B.L.; Martin, F.C.; Walston, J. Oxford Textbook of Geriatric Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 2018; 1392p, ISBN 9780198701590. [Google Scholar]
- Janssen, I. The Epidemiology of Sarcopenia. Clin. Geriatr. Med. 2011, 27, 355–363. [Google Scholar] [CrossRef]
- Markofski, M.M.; Dickinson, J.M.; Drummond, M.J.; Fry, C.S.; Fujita, S.; Gundermann, D.M.; Glynn, E.L.; Jennings, K.; Paddon-Jones, D.; Reidy, P.T.; et al. Effect of Age on Basal Muscle Protein Synthesis and MTORC1 Signaling in a Large Cohort of Young and Older Men and Women. Exp. Gerontol. 2015, 65, 1–7. [Google Scholar] [CrossRef]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Aging Impairs Contraction-Induced Human Skeletal Muscle MTORC1 Signaling and Protein Synthesis. Skelet. Muscle 2011, 1, 11. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and Contractile Regulation of Human Skeletal Muscle Protein Synthesis and MTORC1 Signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Parkington, J.D.; Carambula, S.; Fielding, R.A. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1080–R1086. [Google Scholar] [CrossRef] [PubMed]
- Rivas, D.A.; Lessard, S.J.; Rice, N.P.; Lustgarten, M.S.; So, K.; Goodyear, L.J.; Parnell, L.D.; Fielding, R.A. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J. 2014, 28, 4133–4147. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Velloso, C.P. Regulation of Muscle Mass by Growth Hormone and IGF-I. Br. J. Pharmacol. 2008, 154, 557–568. [Google Scholar] [CrossRef]
- Zachwieja, J.J.; Yarasheski, K.E. Does Growth Hormone Therapy in Conjunction With Resistance Exercise Increase Muscle Force Production and Muscle Mass in Men and Women Aged 60 Years or Older? Phys. Ther. 1999, 79, 76–82. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Pruitt, L.; Reim, J.; Hintz, R.L.; Butterfield, G.; Hoffman, A.R.; Marcus, R. Effect of Recombinant Human Growth Hormone on the Muscle Strength Response to Resistance Exercise in Elderly Men. J. Clin. Endocrinol. Metab. 1994, 79, 1361–1366. [Google Scholar] [CrossRef]
- Cuneo, R.C.; Salomon, F.; Wiles, C.M.; Hesp, R.; Sönksen, P.H. Growth Hormone Treatment in Growth Hormone-Deficient Adults. I. Effects on Muscle Mass and Strength. J. Appl. Physiol. 1991, 70, 688–694. [Google Scholar] [CrossRef]
- Gibney, J.; Wallace, J.D.; Spinks, T.; Schnorr, L.; Ranicar, A.; Cuneo, R.C.; Lockhart, S.; Burnand, K.G.; Salomon, F.; Sonksen, P.H.; et al. The Effects of 10 Years of Recombinant Human Growth Hormone (GH) in Adult GH-Deficient Patients. J. Clin. Endocrinol. Metab. 1999, 84, 2596–2602. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Papadakis, M.A. Growth Hormone Replacement in Healthy Older Men Improves Body Composition but Not Functional Ability. Ann. Intern. Med. 1996, 124, 708. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.; Maghnie, M.; Colao, A.; Vlachaki, I.; Colombo, G. Cost-Consequence Analysis for Human Recombinant Growth Hormone (r-HGH) Treatment Administered via Different Devices in Children with Growth Hormone Deficiency in Italy. CEOR 2019, 11, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.; Tay, L.; Lim, J.P.; Leung, B.P.; Yeo, A.; Yew, S.; Ding, Y.Y.; Lim, W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 979–986. [Google Scholar] [CrossRef]
- Widajanti, N.; Soelistijo, S.; Hadi, U.; Thaha, M.; Aditiawardana; Widodo; Firdausi, H.; Nurina, Y.; Asikin, M.; Srinowati, H.; et al. Association between Sarcopenia and Insulin-Like Growth Factor-1, Myostatin, and Insulin Resistance in Elderly Patients Undergoing Hemodialysis. J. Aging Res. 2022, 2022, 1327332. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; Boreham, C.; Ennis, S.; Ryan, A.W.; De Vito, G. Genetic Associations with Aging Muscle: A Systematic Review. Cells 2019, 9, 12. [Google Scholar] [CrossRef]
- Bhasin, S. Testosterone Supplementation for Aging-Associated Sarcopenia. J. Gerontol. Ser. A 2003, 58, M1002–M1008. [Google Scholar] [CrossRef]
- Gharahdaghi, N.; Rudrappa, S.; Brook, M.S.; Idris, I.; Crossland, H.; Hamrock, C.; Abdul Aziz, M.H.; Kadi, F.; Tarum, J.; Greenhaff, P.L.; et al. Testosterone Therapy Induces Molecular Programming Augmenting Physiological Adaptations to Resistance Exercise in Older Men. J. Cachexia Sarcopenia Muscle 2019, 10, 1276–1294. [Google Scholar] [CrossRef] [PubMed]
- Gharahdaghi, N.; Rudrappa, S.; Brook, M.S.; Farrash, W.; Idris, I.; Aziz, M.H.A.; Kadi, F.; Papaioannou, K.; Phillips, B.E.; Sian, T.; et al. Pharmacological Hypogonadism Impairs Molecular Transducers of Exercise-induced Muscle Growth in Humans. J. Cachexia Sarcopenia Muscle 2022, 13, 1134–1150. [Google Scholar] [CrossRef]
- Borst, S.E. Interventions for Sarcopenia and Muscle Weakness in Older People. Age Ageing 2004, 33, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Trappe, T.; Williams, R.; Carrithers, J.; Raue, U.; Esmarck, B.; Kjaer, M.; Hickner, R. Influence of Age and Resistance Exercise on Human Skeletal Muscle Proteolysis: A Microdialysis Approach. J. Physiol. 2004, 554, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Welle, S.; Thornton, C.; Jozefowicz, R.; Statt, M. Myofibrillar Protein Synthesis in Young and Old Men. Am. J. Physiol. 1993, 264, E693–E698. [Google Scholar] [CrossRef] [PubMed]
- Welle, S.; Thornton, C.; Statt, M. Myofibrillar Protein Synthesis in Young and Old Human Subjects after Three Months of Resistance Training. Am. J. Physiol. 1995, 268, E422–E427. [Google Scholar] [CrossRef] [PubMed]
- Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effect of Age on in Vivo Rates of Mitochondrial Protein Synthesis in Human Skeletal Muscle. Proc. Natl. Acad. Sci. USA 1996, 93, 15364–15369. [Google Scholar] [CrossRef] [PubMed]
- Balagopal, P.; Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effects of Aging on in Vivo Synthesis of Skeletal Muscle Myosin Heavy-Chain and Sarcoplasmic Protein in Humans. Am. J. Physiol. 1997, 273, E790–E800. [Google Scholar] [CrossRef]
- Hasten, D.L.; Pak-Loduca, J.; Obert, K.A.; Yarasheski, K.E. Resistance Exercise Acutely Increases MHC and Mixed Muscle Protein Synthesis Rates in 78-84 and 23-32 Yr Olds. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E620–E626. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Welle, S.; Nair, K.S. Muscle Protein Synthesis in Younger and Older Men. JAMA 2002, 287, 317–318. [Google Scholar] [CrossRef]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and Aerobic Exercise Training Effects on Whole Body and Muscle Protein Metabolism. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E92–E101. [Google Scholar] [CrossRef]
- Balagopal, P.; Schimke, J.C.; Ades, P.; Adey, D.; Nair, K.S. Age Effect on Transcript Levels and Synthesis Rate of Muscle MHC and Response to Resistance Exercise. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E203–E208. [Google Scholar] [CrossRef]
- Karakelides, H.; Irving, B.A.; Short, K.R.; O’Brien, P.; Nair, K.S. Age, Obesity, and Sex Effects on Insulin Sensitivity and Skeletal Muscle Mitochondrial Function. Diabetes 2010, 59, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The Response of Muscle Protein Anabolism to Combined Hyperaminoacidemia and Glucose-Induced Hyperinsulinemia Is Impaired in the Elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef]
- Volpi, E.; Sheffield-Moore, M.; Rasmussen, B.B.; Wolfe, R.R. Basal Muscle Amino Acid Kinetics and Protein Synthesis in Healthy Young and Older Men. JAMA 2001, 286, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A High Proportion of Leucine Is Required for Optimal Stimulation of the Rate of Muscle Protein Synthesis by Essential Amino Acids in the Elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging Is Associated with Diminished Accretion of Muscle Proteins after the Ingestion of a Small Bolus of Essential Amino Acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal Muscle Strength Changes in Older Adults: Influence of Muscle Mass, Physical Activity, and Health. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B209–B217. [Google Scholar] [CrossRef] [PubMed]
- Degens, H. The Role of Systemic Inflammation in Age-Related Muscle Weakness and Wasting. Scand. J. Med. Sci. Sports 2010, 20, 28–38. [Google Scholar] [CrossRef]
- Toth, M.J.; Matthews, D.E.; Tracy, R.P.; Previs, M.J. Age-Related Differences in Skeletal Muscle Protein Synthesis: Relation to Markers of Immune Activation. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E883–E891. [Google Scholar] [CrossRef]
- Lang, C.H.; Frost, R.A.; Nairn, A.C.; MacLean, D.A.; Vary, T.C. TNF-Alpha Impairs Heart and Skeletal Muscle Protein Synthesis by Altering Translation Initiation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E336–E347. [Google Scholar] [CrossRef]
- Moreau, K.; Walrand, S.; Boirie, Y. Protein Redistribution From Skeletal Muscle to Splanchnic Tissue on Fasting and Refeeding in Young and Older Healthy Individuals. J. Am. Med. Dir. Assoc. 2013, 14, 696–704. [Google Scholar] [CrossRef]
- Volpi, E.; Mittendorfer, B.; Wolf, S.E.; Wolfe, R.R. Oral Amino Acids Stimulate Muscle Protein Anabolism in the Elderly despite Higher First-Pass Splanchnic Extraction. Am. J. Physiol. 1999, 277, E513–E520. [Google Scholar] [CrossRef] [PubMed]
- Arnal, M.A.; Mosoni, L.; Boirie, Y.; Houlier, M.L.; Morin, L.; Verdier, E.; Ritz, P.; Antoine, J.M.; Prugnaud, J.; Beaufrère, B.; et al. Protein Pulse Feeding Improves Protein Retention in Elderly Women. Am. J. Clin. Nutr. 1999, 69, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Gryson, C.; Walrand, S.; Giraudet, C.; Rousset, P.; Migné, C.; Bonhomme, C.; Le Ruyet, P.; Boirie, Y. “Fast Proteins” with a Unique Essential Amino Acid Content as an Optimal Nutrition in the Elderly: Growing Evidence. Clin. Nutr. 2014, 33, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Walrand, S.; Gryson, C.; Salles, J.; Giraudet, C.; Migné, C.; Bonhomme, C.; Le Ruyet, P.; Boirie, Y. Fast-Digestive Protein Supplement for Ten Days Overcomes Muscle Anabolic Resistance in Healthy Elderly Men. Clin. Nutr. 2016, 35, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Buffière, C.; Gaudichon, C.; Hafnaoui, N.; Migné, C.; Scislowsky, V.; Khodorova, N.; Mosoni, L.; Blot, A.; Boirie, Y.; Dardevet, D.; et al. In the Elderly, Meat Protein Assimilation from Rare Meat Is Lower than That from Meat That Is Well Done. Am. J. Clin. Nutr. 2017, 106, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.; Horstman, A.M.; Franssen, R.; Crombag, J.J.; Langer, H.; Bierau, J.; Respondek, F.; Van Loon, L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Snijders, T.; Linkens, A.M.; Hamer, H.M.; Van Kranenburg, J.; Van Loon, L.J. Ingestion of Casein in a Milk Matrix Modulates Dietary Protein Digestion and Absorption Kinetics but Does Not Modulate Postprandial Muscle Protein Synthesis in Older Men. J. Nutr. 2015, 145, 1438–1445. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Dhanani, S.; Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Jennings, K.; Rasmussen, B.B.; Volpi, E. A Moderate Acute Increase in Physical Activity Enhances Nutritive Flow and the Muscle Protein Anabolic Response to Mixed Nutrient Intake in Older Adults. Am. J. Clin. Nutr. 2012, 95, 1403–1412. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Jonkers, R.A.M.; Gleeson, B.G.; Beelen, M.; Meijer, K.; Savelberg, H.H.C.M.; Wodzig, W.K.W.H.; Dendale, P.; van Loon, L.J.C. Protein Supplementation before and after Exercise Does Not Further Augment Skeletal Muscle Hypertrophy after Resistance Training in Elderly Men. Am. J. Clin. Nutr. 2009, 89, 608–616. [Google Scholar] [CrossRef]
- Welle, S.; Thornton, C.A. High-Protein Meals Do Not Enhance Myofibrillar Synthesis after Resistance Exercise in 62- to 75-Yr-Old Men and Women. Am. J. Physiol. 1998, 274, E677–E683. [Google Scholar] [CrossRef]
- Symons, T.B.; Schutzler, S.E.; Cocke, T.L.; Chinkes, D.L.; Wolfe, R.R.; Paddon-Jones, D. Aging Does Not Impair the Anabolic Response to a Protein-Rich Meal. Am. J. Clin. Nutr. 2007, 86, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. J. Am. Dietic Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.F.; Verdijk, L.B.; Hamer, H.M.; Verlaan, S.; Luiking, Y.C.; Kouw, I.W.K.; Senden, J.M.; Van Kranenburg, J.; Gijsen, A.P.; Bierau, J.; et al. Both Basal and Post-Prandial Muscle Protein Synthesis Rates, Following the Ingestion of a Leucine-Enriched Whey Protein Supplement, Are Not Impaired in Sarcopenic Older Males. Clin. Nutr. 2017, 36, 1440–1449. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.; Choi, M.D.; Shin, Y.-A.; Schutzler, S.; Azhar, G.; Ferrando, A.A.; Wolfe, R.R.; Kim, I.-Y. The Anabolic Response to Dietary Protein Is Not Limited by the Maximal Stimulation of Protein Synthesis in Healthy Older Adults: A Randomized Crossover Trial. Nutrients 2020, 12, 3276. [Google Scholar] [CrossRef]
- Kiskini, A.; Hamer, H.M.; Wall, B.T.; Groen, B.B.L.; de Lange, A.; Bakker, J.A.; Senden, J.M.G.; Verdijk, L.B.; van Loon, L.J.C. The Muscle Protein Synthetic Response to the Combined Ingestion of Protein and Carbohydrate Is Not Impaired in Healthy Older Men. Age (Dordr) 2013, 35, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Verdijk, L.; Manders, R.J.; Gijsen, A.P.; Gorselink, M.; Pijpers, E.; Wagenmakers, A.J.; Van Loon, L.J. Co-Ingestion of Protein and Leucine Stimulates Muscle Protein Synthesis Rates to the Same Extent in Young and Elderly Lean Men. Am. J. Clin. Nutr. 2006, 84, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance Exercise Enhances Myofibrillar Protein Synthesis with Graded Intakes of Whey Protein in Older Men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Phillips, B.E.; Hill, I.; Greenhaff, P.; Lund, J.N.; Williams, J.P.; Rankin, D.; Wilkinson, D.J.; Smith, K.; Atherton, P.J. Human Skeletal Muscle Is Refractory to the Anabolic Effects of Leucine during the Postprandial Muscle-Full Period in Older Men. Clin. Sci. 2017, 131, 2643–2653. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Fujita, S.; Wolfe, R.R.; Mittendorfer, B.; Roy, M.; Rowe, V.L.; Volpi, E. Insulin Resistance of Muscle Protein Metabolism in Aging. FASEB J. 2006, 20, 768–769. [Google Scholar] [CrossRef]
- Groen, B.B.L.; Horstman, A.M.H.; Hamer, H.M.; De Haan, M.; Van Kranenburg, J.; Bierau, J.; Poeze, M.; Wodzig, W.K.W.H.; Rasmussen, B.B.; Van Loon, L.J.C. Increasing Insulin Availability Does Not Augment Postprandial Muscle Protein Synthesis Rates in Healthy Young and Older Men. J. Clin. Endocrinol. Metab. 2016, 101, 3978–3988. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Pennings, B.; Fry, C.S.; Dhanani, S.; Dillon, E.L.; Sheffield-Moore, M.; Volpi, E.; Rasmussen, B.B. Skeletal Muscle Protein Anabolic Response to Resistance Exercise and Essential Amino Acids Is Delayed with Aging. J. Appl. Physiol. 2008, 104, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.R.; Drummond, M.J.; Glynn, E.L.; Sattler, F.R.; Volpi, E. Aerobic Exercise Overcomes the Age-Related Insulin Resistance of Muscle Protein Metabolism by Improving Endothelial Function and Akt/Mammalian Target of Rapamycin Signaling. Diabetes. 2007, 56, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Reitelseder, S.; Dideriksen, K.; Agergaard, J.; Malmgaard-Clausen, N.M.; Bechshoeft, R.L.; Petersen, R.K.; Serena, A.; Mikkelsen, U.R.; Holm, L. Even Effect of Milk Protein and Carbohydrate Intake but No Further Effect of Heavy Resistance Exercise on Myofibrillar Protein Synthesis in Older Men. Eur. J. Nutr. 2019, 58, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.H.; Wolfe, R.R. Bedrest and Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 7–11. [Google Scholar] [CrossRef]
- Walker, D.K.; Dickinson, J.M.; Timmerman, K.L.; Drummond, M.J.; Reidy, P.T.; Fry, C.S.; Gundermann, D.M.; Rasmussen, B.B. Exercise, Amino Acids, and Aging in the Control of Human Muscle Protein Synthesis. Med. Sci. Sports Exerc. 2011, 43, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced Physical Activity in Young and Older Adults: Metabolic and Musculoskeletal Implications. Ther. Adv. Endocrinol. 2019, 10, 204201881988882. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Dickinson, J.M.; Fry, C.S.; Walker, D.K.; Gundermann, D.M.; Reidy, P.T.; Timmerman, K.L.; Markofski, M.M.; Paddon-Jones, D.; Rasmussen, B.B.; et al. Bed Rest Impairs Skeletal Muscle Amino Acid Transporter Expression, MTORC1 Signaling, and Protein Synthesis in Response to Essential Amino Acids in Older Adults. Am. J. Physiol. -Endocrinol. Metab. 2012, 302, E1113–E1122. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Paddon-Jones, D.; Hays, N.P.; Kortebein, P.; Ronsen, O.; Williams, R.H.; McComb, A.; Symons, T.B.; Wolfe, R.R.; Evans, W. EAA Supplementation to Increase Nitrogen Intake Improves Muscle Function during Bed Rest in the Elderly. Clin. Nutr. 2010, 29, 18–23. [Google Scholar] [CrossRef]
- Dillon, E.L.; Casperson, S.L.; Durham, W.J.; Randolph, K.M.; Urban, R.J.; Volpi, E.; Ahmad, M.; Kinsky, M.P.; Sheffield-Moore, M. Muscle Protein Metabolism Responds Similarly to Exogenous Amino Acids in Healthy Younger and Older Adults during NO-Induced Hyperemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1408–R1417. [Google Scholar] [CrossRef]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I. Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG) Nutritional Interventions to Improve Muscle Mass, Muscle Strength, and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef]
- Bukhari, S.S.I.; Phillips, B.E.; Wilkinson, D.J.; Limb, M.C.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Intake of Low-Dose Leucine-Rich Essential Amino Acids Stimulates Muscle Anabolism Equivalently to Bolus Whey Protein in Older Women at Rest and after Exercise. Am. J. Physiol. -Endocrinol. Metab. 2015, 308, E1056–E1065. [Google Scholar] [CrossRef] [PubMed]
- Borack, M.S.; Reidy, P.T.; Husaini, S.H.; Markofski, M.M.; Deer, R.R.; Richison, A.B.; Lambert, B.S.; Cope, M.B.; Mukherjea, R.; Jennings, K.; et al. Soy-Dairy Protein Blend or Whey Protein Isolate Ingestion Induces Similar Postexercise Muscle Mechanistic Target of Rapamycin Complex 1 Signaling and Protein Synthesis Responses in Older Men. J. Nutr. 2016, 146, 2468–2475. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.F.; Verdijk, L.B.; Hamer, H.M.; Verlaan, S.; Luiking, Y.; Kouw, I.W.K.; Senden, J.M.; van Kranenburg, J.; Gijsen, A.P.; Poeze, M.; et al. Impact of the Macronutrient Composition of a Nutritional Supplement on Muscle Protein Synthesis Rates in Older Men: A Randomized, Double Blind, Controlled Trial. J. Clin. Endocrinol. Metab. 2015, 100, 4124–4132. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino Acid Supplementation Increases Lean Body Mass, Basal Muscle Protein Synthesis, and Insulin-Like Growth Factor-I Expression in Older Women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Roschel, H.; Hayashi, A.P.; Fernandes, A.L.; Jambassi-Filho, J.C.; Hevia-Larraín, V.; De Capitani, M.; Santana, D.A.; Gonçalves, L.S.; De Sá-Pinto, A.L.; Lima, F.R.; et al. Supplement-Based Nutritional Strategies to Tackle Frailty: A Multifactorial, Double-Blind, Randomized Placebo-Controlled Trial. Clin. Nutr. 2021, 40, 4849–4858. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Brightwell, C.R.; Deer, R.R.; Graber, T.G.; Galvan, E.; Fry, C.S.; Volpi, E.; Rasmussen, B.B. Muscle Protein Anabolic Resistance to Essential Amino Acids Does Not Occur in Healthy Older Adults before or after Resistance Exercise Training. J. Nutr. 2018, 148, 900–909. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Volpi, E. Amino Acid Metabolism and Regulatory Effects in Aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 45–49. [Google Scholar] [CrossRef]
- Fiatarone Singh, M.A.; Bernstein, M.A.; Ryan, A.D.; O’Neill, E.F.; Clements, K.M.; Evans, W.J. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J. Nutr. Health Aging 2000, 4, 5–12. [Google Scholar]
- Conde Maldonado, E.; Marqués-Jiménez, D.; Casas-Agustench, P.; Bach-Faig, A. Effect of Supplementation with Leucine Alone, with Other Nutrients or with Physical Exercise in Older People with Sarcopenia: A Systematic Review. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2022, 69, 601–613. [Google Scholar] [CrossRef]
- Wall, B.T.; Hamer, H.M.; De Lange, A.; Kiskini, A.; Groen, B.B.L.; Senden, J.M.G.; Gijsen, A.P.; Verdijk, L.B.; Van Loon, L.J.C. Leucine Co-Ingestion Improves Post-Prandial Muscle Protein Accretion in Elderly Men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine Supplementation of a Low-Protein Mixed Macronutrient Beverage Enhances Myofibrillar Protein Synthesis in Young Men: A Double-Blind, Randomized Trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Gundermann, D.M.; Walker, D.K.; Reidy, P.T.; Borack, M.S.; Drummond, M.J.; Arora, M.; Volpi, E.; Rasmussen, B.B. Leucine-Enriched Amino Acid Ingestion after Resistance Exercise Prolongs Myofibrillar Protein Synthesis and Amino Acid Transporter Expression in Older Men. J. Nutr. 2014, 144, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Verdijk, L.B.; Van Der Hoeven, L.; Van Kranenburg, J.; Hartgens, F.; Wodzig, W.K.W.H.; Saris, W.H.M.; Van Loon, L.J.C. Prolonged Leucine Supplementation Does Not Augment Muscle Mass or Affect Glycemic Control in Elderly Type 2 Diabetic Men. J. Nutr. 2011, 141, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.S.; Tanaka, H. Exercise Prescription for the Elderly: Current Recommendations. Sports Med. 2001, 31, 809–818. [Google Scholar] [CrossRef]
- Lexell, J.; Taylor, C.C. Variability in Muscle Fibre Areas in Whole Human Quadriceps Muscle: Effects of Increasing Age. J. Anat. 1991, 174, 239–249. [Google Scholar]
- Dhillon, R.J.S.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; Izquierdo, M.; Serra-Rexach, J.A.; Santos-Lozano, A.; Lucia, A. Physical Exercise in the Old. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 1281–1304. ISBN 978-0-470-65071-4. [Google Scholar]
- Billot, M.; Calvani, R.; Urtamo, A.; Sánchez-Sánchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, M.N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. CIA 2020, 15, 1675–1690. [Google Scholar] [CrossRef]
- Roth, S.M.; Ferrell, R.F.; Hurley, B.F. Strength Training for the Prevention and Treatment of Sarcopenia. J. Nutr. Health Aging 2000, 4, 143–155. [Google Scholar]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and Optimal Management of Sarcopenia: A Review of Combined Exercise and Nutrition Interventions to Improve Muscle Outcomes in Older People. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [CrossRef]
- Monti, E.; Tagliaferri, S.; Zampieri, S.; Sarto, F.; Sirago, G.; Franchi, M.V.; Ticinesi, A.; Longobucco, Y.; Adorni, E.; Lauretani, F.; et al. Effects of a 2-year Exercise Training on Neuromuscular System Health in Older Individuals with Low Muscle Function. J. Cachexia Sarcopenia Muscle 2023, 14, 794–804. [Google Scholar] [CrossRef]
- Franchi, M.V.; Badiali, F.; Sarto, F.; Müller, P.; Müller, N.G.; Rehfeld, K.; Monti, E.; Rankin, D.; Longo, S.; Lund, J.; et al. Neuromuscular Aging: A Case for the Neuroprotective Effects of Dancing. Gerontology 2023, 69, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Exercise Training Guidelines for the Elderly. Med. Sci. Sports Exerc. 1999, 31, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, M.S.; Pollock, M.L. Prescription of Resistance Training for Health and Disease. Med. Sci. Sports Exerc. 1999, 31, 38–45. [Google Scholar] [CrossRef]
- Feigenbaum, M.S.; Pollock, M.L. Strength Training: Rationale for Current Guidelines for Adult Fitness Programs. Phys. Sportsmed. 1997, 25, 44–63. [Google Scholar] [CrossRef]
- Messier, S.P.; Dill, M.E. Alterations in Strength and Maximal Oxygen Uptake Consequent to Nautilus Circuit Weight Training: Research Quarterly for Exercise and Sport: Vol 56, No 4. Available online: https://www.tandfonline.com/doi/abs/10.1080/02701367.1985.10605339 (accessed on 24 August 2023).
- Gibbons, R.J.; Balady, G.J.; Beasley, J.W.; Bricker, J.T.; Duvernoy, W.F.; Froelicher, V.F.; Mark, D.B.; Marwick, T.H.; McCallister, B.D.; Thompson, P.D.; et al. ACC/AHA Guidelines for Exercise Testing. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J. Am. Coll. Cardiol. 1997, 30, 260–311. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise Standards for Testing and Training: A Scientific Statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Heath, E.H. ACSM’s Guidelines for Exercise Testing and Prescription, 7th Edition. Med. Sci. Sports Exerc. 2005, 37, 2018. [Google Scholar] [CrossRef]
- Verzola, D.; Barisione, C.; Picciotto, D.; Garibotto, G.; Koppe, L. Emerging role of myostatin and its inhibition in the setting of chronic kidney disease. Kidney Int. 2019, 95, 506–517. [Google Scholar] [CrossRef]
- Meynial-Denis, D.; Guerin, O.; Schneider, S.M.; Volkert, D.; Sieber, C.C. New Strategies to Fight against Sarcopenia at Old Age. J. Aging Res. 2012, 2012, 676042. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Dietary Quality Is Related to Frailty in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.M.; Spencer, H.J.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Protein Intake Distribution Pattern Does Not Affect Anabolic Response, Lean Body Mass, Muscle Strength or Function over 8 Weeks in Older Adults: A Randomized-Controlled Trial. Clin. Nutr. 2018, 37, 488–493. [Google Scholar] [CrossRef]
- Esmarck, B.; Andersen, J.L.; Olsen, S.; Richter, E.A.; Mizuno, M.; Kjaer, M. Timing of Postexercise Protein Intake Is Important for Muscle Hypertrophy with Resistance Training in Elderly Humans. J. Physiol. 2001, 535, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kouw, I.W.; Holwerda, A.M.; Trommelen, J.; Kramer, I.F.; Bastiaanse, J.; Halson, S.L.; Wodzig, W.K.; Verdijk, L.B.; Van Loon, L.J. Protein Ingestion before Sleep Increases Overnight Muscle Protein Synthesis Rates in Healthy Older Men: A Randomized Controlled Trial. J. Nutr. 2017, 147, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- CREA. Food Composition Tables (Tabelle di Composizione Degli Alimenti). National Researc Institute For foods and Nutrition (Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione, INRAN). Rome (Italy). 2019. Available online: http://sapermangiare.mobi/tabelle_alimenti.html (accessed on 1 August 2023).
- McKendry, J.; Currier, B.S.; Lim, C.; Mcleod, J.C.; Thomas, A.C.Q.; Phillips, S.M. Nutritional Supplements to Support Resistance Exercise in Countering the Sarcopenia of Aging. Nutrients 2020, 12, 2057. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, N.; Hongo, M.; Mizutani, Y.; Shimada, Y. Prevalence of Sarcopenia in Japanese Women with Osteopenia and Osteoporosis. J. Bone Miner. Metab. 2013, 31, 556–561. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Kiel, D.; Clemens, T.; Esser, K.; Orwoll, E.; O’Keefe, R.; Fielding, R. Forum on Bone and Skeletal Muscle Interactions: Summary of the Proceedings of an ASBMR Workshop. J. Bone Miner. Res. 2013, 28, 1857–1865. [Google Scholar] [CrossRef]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and Its Relationship with Bone Mineral Density in Middle-Aged and Elderly European Men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef]
- Kaji, H. Interaction between Muscle and Bone. J. Bone Metab. 2014, 21, 29–40. [Google Scholar] [CrossRef]
- Nunes, E.A.; Stokes, T.; McKendry, J.; Currier, B.S.; Phillips, S.M. Disuse-Induced Skeletal Muscle Atrophy in Disease and Nondisease States in Humans: Mechanisms, Prevention, and Recovery Strategies. Am. J. Physiol. -Cell Physiol. 2022, 322, C1068–C1084. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.-J.; Walrand, S.; Coxam, V. Muscle and Bone, Two Interconnected Tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, K.J.M.; McKenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front. Nutr. 2021, 8, 615849. [Google Scholar] [CrossRef] [PubMed]
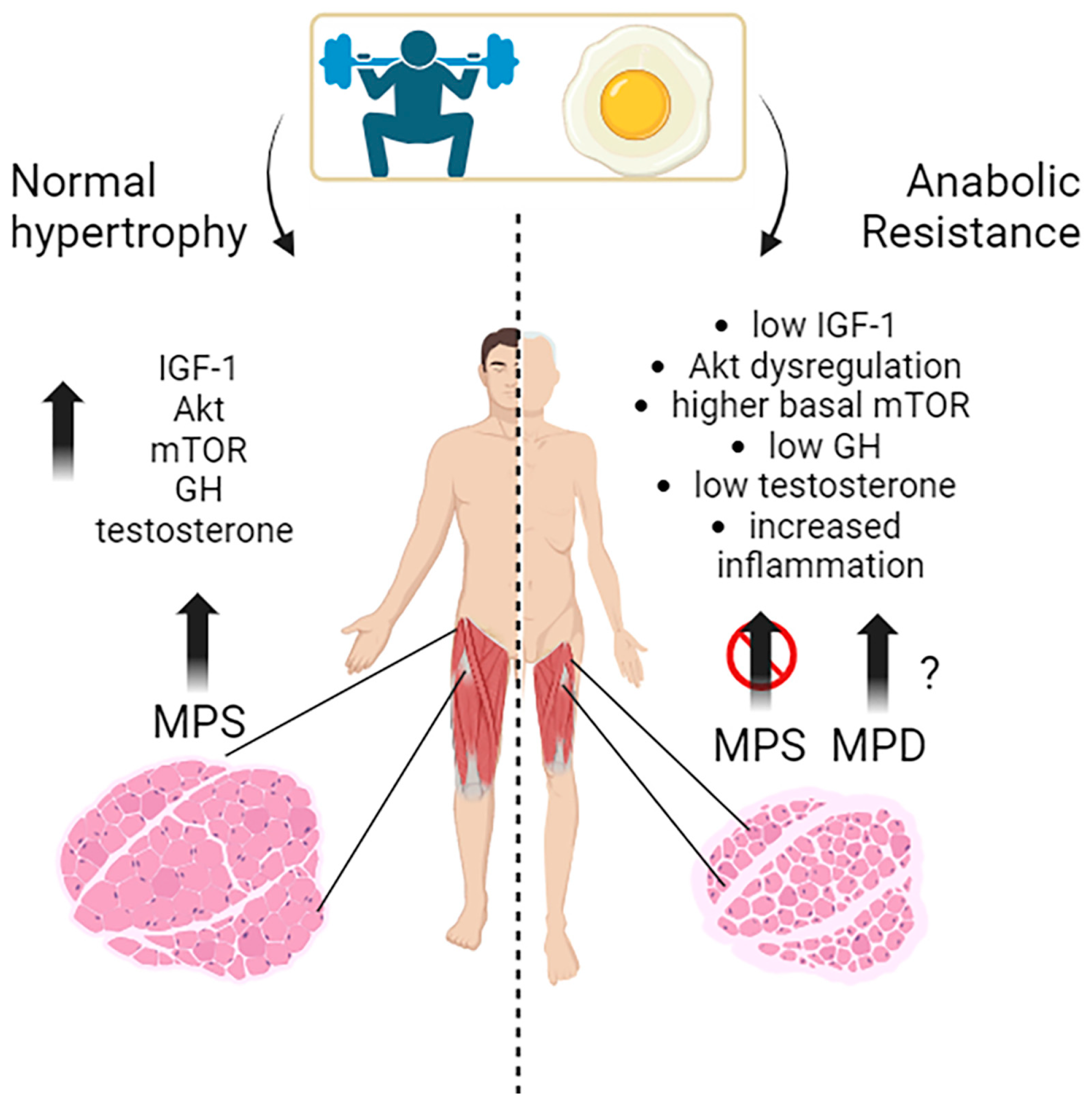
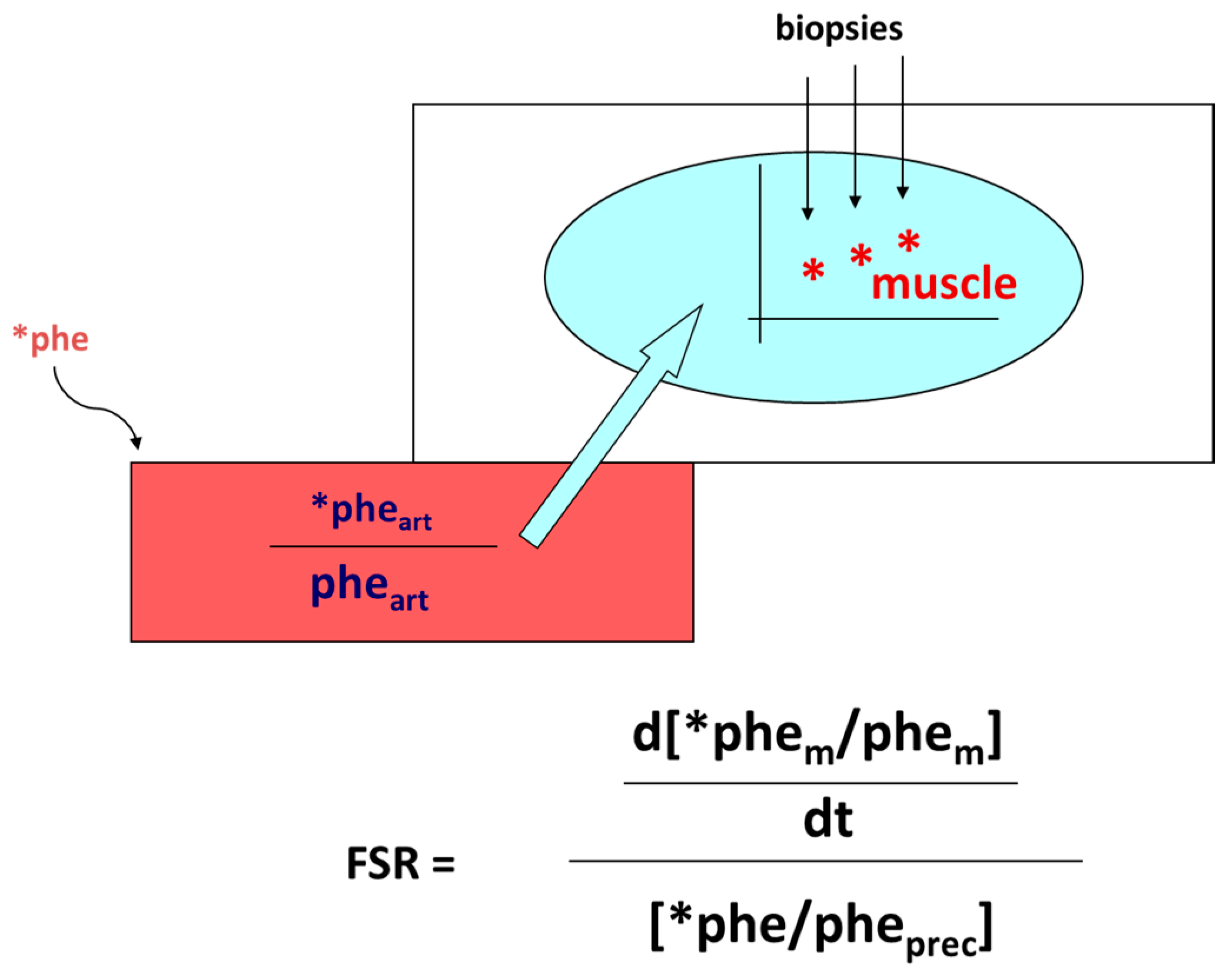

| 1. Consume a balanced diet rich in natural foods providing sufficient amounts of high-quality protein, to sustain (skeletal) muscle protein accretion; |
| 2. Keep/increase protein intake (preferentially of high quality) at ≥1.5 g/kg/day; |
| 3. Provide ≥ 30 g protein at each main meal, equally spread into the meals, to promote an optimal per meal stimulation of MPS; |
| 4. Prefer protein-rich natural foods over protein-rich supplements; |
| 5. Avoid as far as possible continuous 24 h nutrition; |
| 6. Provide sufficient energy; |
| 7. When adding supplements: |
| a. Use them only in specific cases; |
| b. Use EAA supplements preferentially rich in the BCAA; |
| c. Add leucine either to natural protein foods or to the AA supplements; |
| d. Consider that intake of protein supplements can proportionally lead to an involuntary reduction in the intake of protein-rich natural foods, thus partially offsetting their anabolic effect; |
| 8. When combined with exercise, assume nutrition taking into account: |
| a. The timing of administration: |
| i. pre-exercise; |
| ii. at the beginning (t = 0′) (preferred); |
| iii. or following exercise… |
| b. Amount and type of nutrition? |
| i. Natural protein-rich foods? And/or: |
| ii. Supplements; |
| 9. Consider other non-protein supplements: |
| 1. Creatine, PUFA (Polyunsaturated fatty acids). |
| Animal Foods | g | Calories |
| Cow whole milk | 909 | 582 |
| Ricotta from cow milk | 340 | 498 |
| Ricotta from buffalo milk | 286 | 606 |
| Low-fat, fresh cheese | 98 | 446 |
| High-fat, aged cheese | 89 | 347 |
| Chicken egg (n = 4.4) | 242 | 322 |
| Beef meat | 136 | 151 |
| Pork meat | 183 | 268 |
| Chicken breast | 131 | 131 |
| Bresaola | 94 | 142 |
| Turkey breast | 125 | 134 |
| Seabass (filets) | 141 | 210 |
| Fresh salmon | 163 | 302 |
| Vegetal Foods | g | Calories |
| Soybeans | 100 | 404 |
| Wheat meal | 273 | 927 |
| Corn meal | 345 | 1248 |
| Beans | 566 | 752 |
| Quinoa | 234 | 863 |
| Oatmeal | 238 | 917 |
| Rice | 450 | 1487 |
| Lentils | 475 | 436 |
| Chickpeas | 429 | 514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. https://doi.org/10.3390/nu15184073
Tezze C, Sandri M, Tessari P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients. 2023; 15(18):4073. https://doi.org/10.3390/nu15184073
Chicago/Turabian StyleTezze, Caterina, Marco Sandri, and Paolo Tessari. 2023. "Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People" Nutrients 15, no. 18: 4073. https://doi.org/10.3390/nu15184073
APA StyleTezze, C., Sandri, M., & Tessari, P. (2023). Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients, 15(18), 4073. https://doi.org/10.3390/nu15184073






