A Double-Blind, Randomized, Placebo-Controlled Trial of the Effect of 1-Kestose on Defecation Habits in Constipated Kindergarten Children: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study Design
2.2. Ethical Considerations
2.3. DNA Extraction
2.4. 16S rRNA Gene-Sequence Analysis Using Next-Generation Sequencing (NGS)
2.5. Measurement of Short-Chain Fatty Acids (SCFAs)
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
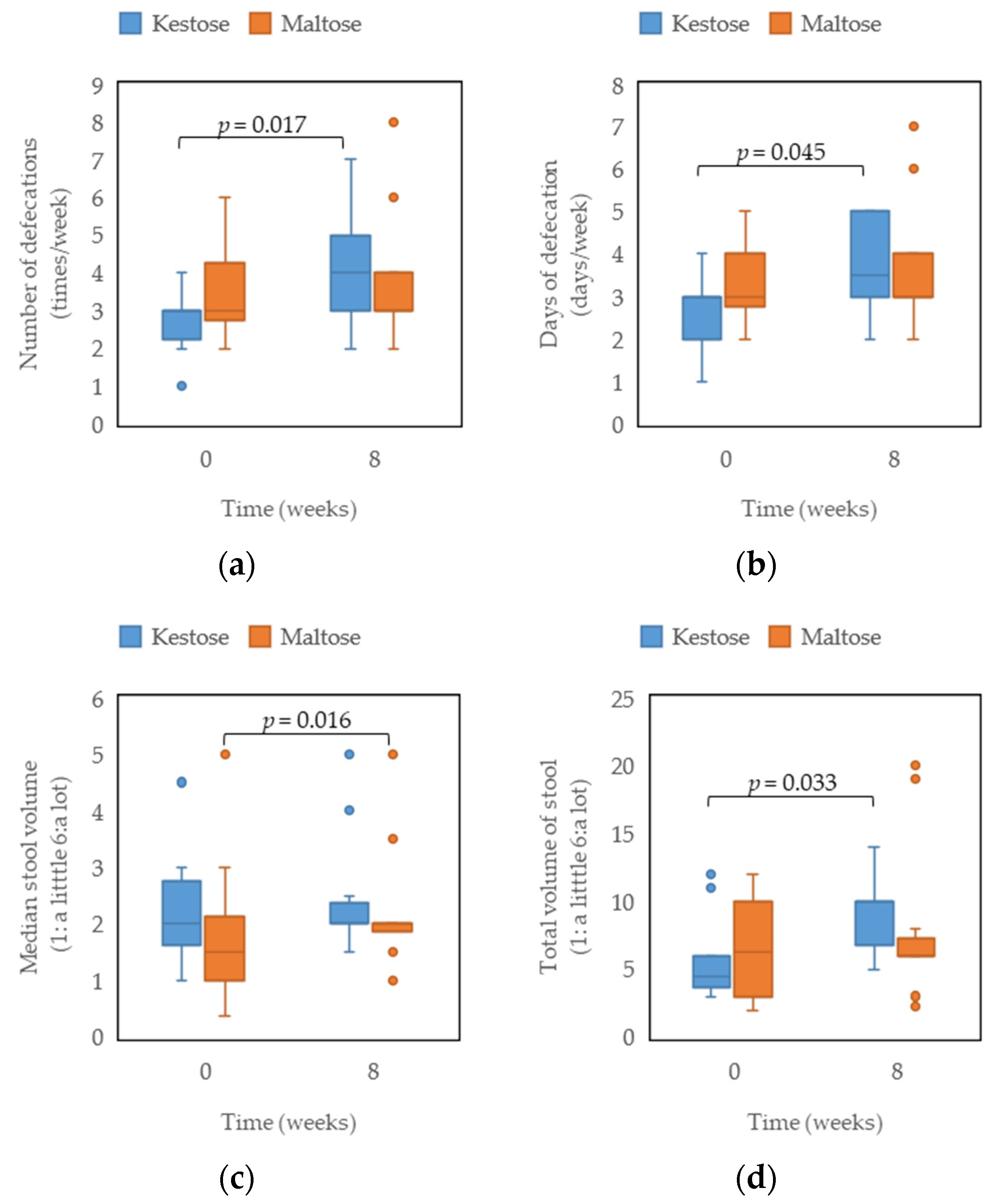
3.2. Defecation Diary
3.3. 16S rRNA Gene Metagenomic Analysis of Intestinal Microbiota before and after Kestose Ingestion
3.4. Concentrations of SCFAs in Stool in the Kestose Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bongers, M.E.J.; van Wijk, M.P.; Reitsma, J.B.; Benninga, M.A. Long-Term Prognosis for Childhood Constipation: Clinical Outcomes in Adulthood. Pediatrics 2010, 126, e156–e162. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Krogulska, A. The Significance of the Gut Microbiome in Children with Functional Constipation. Adv. Clin. Exp. Med. 2021, 30, 471–480. [Google Scholar] [CrossRef]
- Huang, R.; Hu, J. Positive Effect of Probiotics on Constipation in Children: A Systematic Review and Meta-Analysis of Six Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2017, 7, 153. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Closa-Monasterolo, R.; Ferré, N.; Castillejo-DeVillasante, G.; Luque, V.; Gispert-Llaurado, M.; Zaragoza-Jordana, M.; Theis, S.; Escribano, J. The Use of Inulin-Type Fructans Improves Stool Consistency in Constipated Children. A Randomised Clinical Trial: Pilot Study. Int. J. Food Sci. Nutr. 2017, 68, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.K.; Toporovski, M.S.; Tahan, S.; Neufeld, C.B.; de Morais, M.B. Dietary Fiber Mixture in Pediatric Patients with Controlled Chronic Constipation. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 297–302. [Google Scholar] [CrossRef]
- Ose, R.; Hirano, K.; Maeno, S.; Nakagawa, J.; Salminen, S.; Tochio, T.; Endo, A. The Ability of Human Intestinal Anaerobes to Metabolize Different Oligosaccharides: Novel Means for Microbiota Modulation? Anaerobe 2018, 51, 110–119. [Google Scholar] [CrossRef]
- Endo, A.; Nakamura, S.; Konishi, K.; Nakagawa, J.; Tochio, T. Variations in Prebiotic Oligosaccharide Fermentation by Intestinal Lactic Acid Bacteria. Int. J. Food Sci. Nutr. 2016, 67, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Fujii, T.; Hirano, K.; Maeno, S.; Tonozuka, T.; Sakamoto, M.; Ohkuma, M.; Tochio, T.; Endo, A. Characterization of Fructooligosaccharide Metabolism and Fructooligosaccharide-Degrading Enzymes in Human Commensal Butyrate Producers. Gut Microbes 2021, 13, 1869503. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Self, M.M.; Czyzewski, D.I.; Cejka, S.; Swank, P.R.; Shulman, R.J. Bristol Stool Form Scale Reliability and Agreement Decreases When Determining Rome III Stool Form Designations. Neurogastroenterol. Motil. 2016, 28, 443–448. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Hisada, T.; Endoh, K.; Kuriki, K. Inter- and Intra-Individual Variations in Seasonal and Daily Stabilities of the Human Gut Microbiota in Japanese. Arch. Microbiol. 2015, 197, 919–934. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the Gut Microbiota Composition between Obese and Non-Obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and next-Generation Sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Baaleman, D.F.; Tabbers, M.M.; Smidt, H.; Benninga, M.A. Nonpharmacologic Treatment for Children with Functional Constipation: A Systematic Review and Meta-Analysis. J. Pediatr. 2022, 240, 136–149.e5. [Google Scholar] [CrossRef] [PubMed]
- van der Schoot, A.; Drysdale, C.; Whelan, K.; Dimidi, E. The Effect of Fiber Supplementation on Chronic Constipation in Adults: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2022, 116, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; Bernal, M.-J.; Blasco, J.; Martínez, R.; Dalmau, J.; Ortuño, I.; Espín, B.; Vasallo, M.-I.; Gil, D.; Vidal, M.-L.; et al. Prebiotic Effect during the First Year of Life in Healthy Infants Fed Formula Containing GOS as the Only Prebiotic: A Multicentre, Randomised, Double-Blind and Placebo-Controlled Trial. Eur. J. Nutr. 2015, 54, 89–99. [Google Scholar] [CrossRef]
- Tochio, T.; Kadota, Y.; Tanaka, T.; Koga, Y. 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium Prausnitzii as Well as Bifidobacteria in Humans. Foods 2018, 7, 140. [Google Scholar] [CrossRef]
- Shibata, R.; Koga, Y.; Takahashi, M.; Murakami, Y.; Tochio, T.; Kadota, Y. In Children with Cow’s Milk Allergy, 1-Kestose Affects the Gut Microbiota and Reaction Threshold. Pediatr. Res. 2023. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The Effects of Inulin on Gut Microbial Composition: A Systematic Review of Evidence from Human Studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Fuentes, S.; Grievink, W.; van Niftrik, L.; Tindall, B.J.; Timmerman, H.M.; Rijkers, G.T.; Smidt, H. Characterization of Romboutsia Ilealis Gen. Nov., Sp. Nov., Isolated from the Gastro-Intestinal Tract of a Rat, and Proposal for the Reclassification of Five Closely Related Members of the Genus Clostridium into the Genera Romboutsia Gen. Nov., Intestinibacter Gen. Nov., Terrisporobacter Gen. Nov. and Asaccharospora Gen. Nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1600–1616. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, A.; Adachi, K.; Yamaguchi, Y.; Izawa, S.; Yamamoto, S.; Hijikata, Y.; Ebi, M.; Funaki, Y.; Ogasawara, N.; Goto, C.; et al. Gut Environment and Dietary Habits in Healthy Japanese Adults and Their Association with Bowel Movement. Digestion 2020, 101, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, R.; Jia, M.; Su, Y.; Zhu, W. Metatranscriptomic Analysis of Colonic Microbiota’s Functional Response to Different Dietary Fibers in Growing Pigs. Anim. Microbiome 2021, 3, 45. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, X.; Luo, T.; Wang, D.; Sun, Y.; Dai, J. Effects of Short-Term Dietary Fiber Intervention on Gut Microbiota in Young Healthy People. DMSO 2021, 14, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Marrs, T.; Jo, J.-H.; Perkin, M.R.; Rivett, D.W.; Witney, A.A.; Bruce, K.D.; Logan, K.; Craven, J.; Radulovic, S.; Versteeg, S.A.; et al. Gut Microbiota Development during Infancy: Impact of Introducing Allergenic Foods. J. Allergy Clin. Immunol. 2021, 147, 613–621.e9. [Google Scholar] [CrossRef]
- Fukui, H.; Xu, X.; Miwa, H. Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The Control and Consequences of Bacterial Fermentation in the Human Colon. J. Appl. Microbiol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Bäuerlein, M.; Frohberg, C.; Landschütze, V.; Gibson, G.R. A Double-Blind, Placebo-Controlled, Cross-over Study to Establish the Bifidogenic Effect of a Very-Long-Chain Inulin Extracted from Globe Artichoke (Cynara Scolymus) in Healthy Human Subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in Healthy Young Population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
| Relative Abundance Median (25th–75th %) | p | ||
|---|---|---|---|
| Kestose (n = 10) | |||
| 0 Weeks | 8 Weeks | ||
| Bifidobacterium | 22.2 (13.3–32.2) | 29.2 (17.2–42.9) | 0.074 |
| Blautia | 14.7 (8.7–18.0) | 13.1 (10.4–15.3) | 0.445 |
| Fusicatenibacter | 4.5 (2.3–6.3) | 5.4 (4.5–8.2) | 0.721 |
| Anaerostipes | 4.4 (2.6–6.7) | 3.0 (1.7–5.6) | 0.959 |
| Collinsella | 3.9 (0.2–6.3) | 4.7 (0.3–9.3) | 0.446 |
| Gemmiger | 3.6 (0.4–5.1) | 3.1 (0.4–5.4) | 0.953 |
| Anaerobutyricum | 3.2 (0.7–4.6) | 2.8 (0.3–3.6) | 0.646 |
| Streptococcus | 2.5 (2.1–5.4) | 1.6 (1.0–7.3) | 0.878 |
| Ruminococcus | 1.7 (0.3–3.6) | 1.5 (0.3–2.5) | 0.333 |
| Bacteroides | 1.6 (0.5–2.3) | 1.1 (0.1–3.6) | 0.799 |
| Agathobacter | 1.6 (0.0–2.4) | 0.9 (0.2–1.5) | 0.674 |
| Intestinibacter | 1.3 (0.4–1.9) | 0.3 (0.2–0.6) | 0.047 |
| Clostridium sensu stricto | 1.0 (0.3–2.3) | 0.5 (0.1–0.7) | 0.074 |
| Romboutsia | 0.9 (0.3–5.1) | 1.5 (0.6–3.3) | 1.000 |
| Lachnospiracea_incertae_sedis | 0.7 (0.3–1.9) | 0.6 (0.2–1.3) | 0.508 |
| Faecalibacterium | 0.6 (0.2–2.6) | 1.0 (0.6–4.2) | 0.441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, M.; Kadota, Y.; Shiko, Y.; Kawasaki, Y.; Sakurai, K.; Mori, C.; Shimojo, N. A Double-Blind, Randomized, Placebo-Controlled Trial of the Effect of 1-Kestose on Defecation Habits in Constipated Kindergarten Children: A Pilot Study. Nutrients 2023, 15, 3276. https://doi.org/10.3390/nu15143276
Takahashi M, Kadota Y, Shiko Y, Kawasaki Y, Sakurai K, Mori C, Shimojo N. A Double-Blind, Randomized, Placebo-Controlled Trial of the Effect of 1-Kestose on Defecation Habits in Constipated Kindergarten Children: A Pilot Study. Nutrients. 2023; 15(14):3276. https://doi.org/10.3390/nu15143276
Chicago/Turabian StyleTakahashi, Mayuko, Yoshihiro Kadota, Yuki Shiko, Yohei Kawasaki, Kenichi Sakurai, Chisato Mori, and Naoki Shimojo. 2023. "A Double-Blind, Randomized, Placebo-Controlled Trial of the Effect of 1-Kestose on Defecation Habits in Constipated Kindergarten Children: A Pilot Study" Nutrients 15, no. 14: 3276. https://doi.org/10.3390/nu15143276
APA StyleTakahashi, M., Kadota, Y., Shiko, Y., Kawasaki, Y., Sakurai, K., Mori, C., & Shimojo, N. (2023). A Double-Blind, Randomized, Placebo-Controlled Trial of the Effect of 1-Kestose on Defecation Habits in Constipated Kindergarten Children: A Pilot Study. Nutrients, 15(14), 3276. https://doi.org/10.3390/nu15143276






